Abstract
Purpose
To report the prevalence and characteristics of serrated polyps identified in a large, average-risk population undergoing screening computed tomographic (CT) colonography.
Materials and methods
This HIPAA-compliant retrospective study was approved by the Institutional Review Board of the University of Wisconsin School of Medicine and Public Health. The need for informed consent was waived. Nine thousand six hundred exams from 8289 patients were enrolled in a single institution CT colonography-based screening program (from 2004 to 2011) and were evaluated for the presence of nondiminutive serrated lesions and advanced adenomas. The prevalence and characteristics of these lesions were tabulated. Generalized estimating equation regressions of polyp characteristics that may contribute to visualization of serrated lesions were investigated, including polyp size, location, and morphologic appearance; histologic findings; and presence or absence of contrast material tagging.
Results
Nondiminutive serrated lesions (≥6mm) were seen at CT colonography-based screening with a prevalence of 3.1% (254 of 8289). Sessile serrated adenomas (SSAs) and traditional serrated adenomas (TSAs) constituted 36.8% (137 of 372) and 4.3% (16 of 372) of serrated lesions respectively; hyperplastic polyps (HPs) accounted for 58.9% (219 of 372 lesions). SSA and TSA tended to be large (mean size, 10.6 mm and 14.1 mm, respectively), with size categories and polyp subgroups significantly associated (p<0.0001). SSA tended to be proximal in location (91.2%, 125 of 137 lesions) and flat in morphologic appearance (39.4%, 54 of 137 lesions) compared with TSA and HP. The presence of high-grade dysplasia in serrated lesions was uncommon compared with advanced adenomas (one of 372 lesions versus 22 of 395 lesions, respectively; p<0.0001). Multivariate analysis showed that contrast material tagging markedly improved serrated polyp detection with an odds ratio of 40.4 (95% confidence interval: 10.1, 161.4).
Conclusion
Serrated lesions are seen at CT colonography-based screening with a nondiminutive prevalence of 3.1%. These lesions tend to be large, flat, and proximal in location. Adherent contrast material coating on these polyps aids in their detection, despite an often flat morphologic appearance.
Keywords: Colorectal cancer screening, colonic polyps, colorectal neoplasia, CT
Introduction
Serrated neoplasms of the colon and rectum represent a group of polyps with the potential to transform to colorectal cancer, distinct from the traditional adenoma-carcinoma pathway. These polyps comprise sessile serrated adenomas (SSAs, perhaps better known as sessile serrated polyps), traditional serrated adenomas (TSAs), and hyperplastic polyps (HPs) (1). Until recently, their future malignant potential was not clearly recognized (2). In particular, SSAs were frequently misclassified at histopathologic examination as benign HPs with no chance for future malignant transformation. It is now known that serrated neoplasms are a second polyp group with future malignant potential that is separate from the classic main pathway through adenomatous polyps. As a group, serrated polyps may account for up to 20% of sporadic colorectal cancers, where some lesions within the SSA subgroup and TSA subgroup can transform into cancer (3). True HPs remain innocuous in this new paradigm, without future malignant potential. These lesions may account for the surprising results seen in population studies in which investigators have reported decreased protective effects from colonoscopy screening for right-sided cancers compared with left-sided cancers (4). In addition, interval cancers in previously screened patients are four times more likely than noninterval cancers to show genetic signatures associated with the serrated neoplastic pathway (5). Such results point to the importance of these lesions, which may be missed or their clinical significance not truly recognized, leading to incomplete resection.
The nature of serrated lesions is becoming better defined through colonoscopy-based screening. SSA and TSA tend to be large lesions located in the right side of the colon. They are flat to sessile in nature, with indistinct edges and coloring similar to the adjacent mucosa. There can be an adherent overlying mucus cap (6,7).Conversely, the characteristics of such lesions identified at computed tomographic (CT) colonography-based screening have been poorly described to date. We know that although both colonoscopy and CT colonography can be used to detect colorectal polyps with a high degree of sensitivity and specificity, they have relatively different strengths in the detection of specific lesions related to factors such as morphologic appearance or location. The use of another screening modality such as CT colonography can help to increase the understanding of these lesions by allowing evaluation of them from a different perspective. The purpose of our study was to report the prevalence and characteristics of serrated adenomas identified in a large average-risk population undergoing CT colonography screening for colorectal cancer.
Materials and Methods
This Health Insurance Portability and Accountability Act-compliant retrospective study was approved by the institutional review board of the University of Wisconsin School of Medicine and Public Health. The need for informed consent was waived. Findings in 52 of 254 patients have been previously reported (8). The prior article dealt with the status of contrast material tagging on polyps with flat morphologic appearance, regardless of histologic findings, whereas in this article, we report on the polyp characteristics of a defined histologic group (ie, serrated polyps).
Study Population
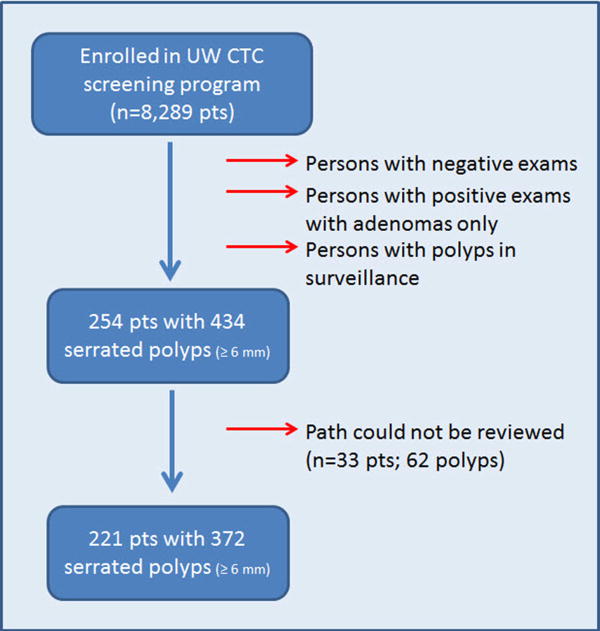
Between August 2004 and October 2011, data on 8289 consecutive patients who were enrolled in the University of Wisconsin CT colonography colorectal cancer screening program were extracted from a prospectively acquired database. Patients were referred to this program by primary care providers for the purpose of colorectal cancer screening; patients were considered average risk and were typically asymptomatic. High-risk patients or patients with a history of cancer, bowel resection, hereditary polyposis syndromes, or inflammatory bowel disease were excluded from the screening database and from consideration. Patients may have undergone an examination for initial screening for colorectal cancer (83.5%, 6918 of 8289 patients), for routine screening 5 years after a prior CT colonography screening examination with negative findings (13.5%, 1120 of 8289 patients), or for follow-up of a polyp detected with CT colonography in an imaging surveillance examination (3.0%, 251 of 8289 patients). In total, patients underwent 9600 examinations. From this group, patients (n=254) with pathologically proven serrated neoplasms that were 6 mm and larger in size, including all HPs, were extracted. SSAs and sessile serrated polyps were considered synonymous terms. Because the recognition and pathologic classification of serrated lesions has evolved during the study period, the histologic material of all lesions initially reported as SSAs and sessile serrated polyps, HPs 10 mm in size and larger, and 6-9 mm HPs that were located in the right side of the colon were reviewed by a gastrointestinal pathologist with 18 years of experience (K.A.M.). Ultimately, 62 serrated polyps in these subgroups (from a total of 434 serrated polyps) could not be reviewed, as the pathologic material was obtained outside of the parent institution. The summary flowchart for the final serrated polyp cohort is depicted in Figure 1. Besides the serrated polyps, all resected adenomas (≥ 6 mm) identified during this time period were also evaluated (n=989 polyps, 612 patients) to serve as a comparison group.
Figure 1. Flowchart for serrated polyp inclusion in study.
Extracted data fields included demographic information of age, sex, and body mass index of the patient, as well as polyp characteristics that included size, location, morphologic appearance, histologic findings, and presence of high-grade dysplasia (HGD) for each polyp. Size, as noted in the database (in millimeters), was determined at CT colonography according to longest linear dimension of the polyp assessed at combination two-dimensional (2D) and three-dimensional (3D) evaluation. Polyps 10 mm in size and larger were considered large polyps, whereas polyps 6-9 mm in size were considered small. Diminutive lesions (5 mm or smaller) were not reported at CT colonography in compliance with standard CT colonography reporting and data system, or C-RADS, classifications and guidelines.[9] Location was reported according to standard anatomic segments. The colon and rectum were divided into cecum, ascending colon, transverse colon, descending colon, sigmoid colon, and rectum. The flexures were not used as modifiers. For the purposes of this study, location was further condensed into the proximal colon (cecum and ascending and transverse colon) and distal colon (descending and sigmoid colon and rectum). Morphologic appearance was categorized as being sessile, flat, pedunculated, carpet, or other. Sessile lesions were dome-shaped polyps with a broad base of attachment to the colonic mucosa. Flat polyps were defined as plaque-like lesions raised 3 mm or less from the colonic surface. Pedunculated lesions were those with a defined stalk. Carpet lesions (superficial lateral spreading tumors) were flat lesions that were raised 3 mm or less and were larger than 3 cm in size. An “other” category included morphologic appearances not included in the previous groups. Within the adenoma group, if an adenoma was 10 mm in size and larger, had a villous component, or had HGD, it was deemed an advanced adenoma.
Because contrast material coating was not recorded in the database, the CT colonography images of all polyps with serrated histologic findings were reviewed by a radiologist trained in gastrointestinal imaging with 11 to 21 years of experience (D.H.K., M.G.L., or J.L.H.) for the presence or absence of adherent surface coating of the polyp by contrast material. If present, the thickness of the contrast material coating (in millimeters) was measured by using electronic calipers on the 2D source image. The orthogonal view that maximized the vertical thickness was used. Polyp window settings (window width, 2000 HU; window level 0 HU) were used.
Any polyp reported at therapeutic colonoscopy but not identified prospectively at CT colonography was deemed a CT colonography-occult lesion in the database. Size, morphologic appearance, and location were thus determined with colonoscopy. All CT-occult lesions (n=32) were reviewed on the 2D source series by one author (D.H.K., a radiologist trained in gastrointestinal imaging with 21 years of experience) to determine if these lesions could be detected in retrospect. If seen, the assessment for contrast material coating was conducted, including presence, absence, and thickness of the coating.
CT colonography protocol
The specific protocol regarding bowel preparation and colonic distention has been described in detail in previous reports (10-12) Additional details are provided in Appendix E1 (online).
Patients underwent imaging with (8-, 16-, or 64-section) multidetector CT scanners (General Electric, Waukesha Wis). A low dose protocol was used, with the aim of keeping the overall exposure less than 3-5 mSv. The following parameters included a section collimation of 1.25 mm with a 1-mm reconstruction interval, tube rotation of 0.5 seconds, noise index of 50, 30-150 mA, and 120 kVp.
Pathologic analysis
All initially reported SSAs, large HPs (≥ 10 mm), and right-sided HPs (6-9 mm) were reevaluated. According to standard University of Wisconsin Department of Pathology protocol, whole polyp specimens had been bisected for evaluation if larger than 0.7 cm in size. If the polyp was larger than 1.2 cm, the polyp was then serially sectioned and submitted. The slides were directly reviewed by a gastrointestinal fellowship-trained pathologist (K.A.M.) and categorized according to the World Health Organization classifications for serrated neoplasms (1). SSAs met criteria when they were cytologically bland, they contained serration at the base of the crypt, and they demonstrated dilation and/or abnormal shape at the base of the crypts, including L-shape and inverted T-shape architecture. Traditional serrated adenomas met criteria when they were lined by columnar epithelium with abundant nonmucinous eosinophilic cytoplasm, they contained hyperchromatic elongated nuclei, they exhibited ectopic and/or abortive crypts at right angles to the main crypt, and they had a filiform architecture.
Statistical analysis
All analyses were performed on a per-polyp basis. For CT colonography polyp visibility comparisons, generalized estimating equation models were used to account for potential correlation due to clustering of multiple polyps per patient. A robust sandwich variance estimator was used, with working independence correlation structure and a binomial family link. Ninety-five percent confidence intervals based on the robust standard error estimates were obtained. Summary descriptive measures (mean values, standard deviations, quartiles, minimum values, and maximum values) were obtained for continuous variables (age at CT colonography and polyp size), stratified by polyp type group. Means ± standard deviations were reported. Frequency counts and percentages were obtained for categorical variables (polyp size: 6-9mm or ≥ 10mm; polyp location: proximal or distal; morphologic appearance: sessile, flat, pedunculated, carpet, or other; and HGD at histologic examination: HGD or no HGD), which were also stratified by polyp type. The Kruskal-Wallis and the Fisher exact test were used to investigate differences between polyp type groups for continuous and discrete variables, respectively. To assess polyp type characteristics, we tabulated polyp morphologic appearance, location, and dichotomized size against polyp type. No pairwise comparisons were performed.
We used generalized estimating equation models to assess whether polyp characteristics (location, size, presence of contrast material tag, contrast material thickness, morphologic appearance, histologic findings) were associated with polyp visibility at CT colonography. We fitted separate univariable models for each predictor and computed odds ratios and corresponding 95% confidence intervals. Variables with P values less than .2 at univariable analysis were included in the multivariable model.
A P value less than 0.05 (two sided) was the criterion for statistical significance. For all statistical analyses and graphics, R version 2.1.3 software was used (R Foundation, Vienna, Austria) (13).
Results
Two hundred fifty-four patients with 434 serrated polyps 6 mm in size and larger were identified from this CT colonography screening cohort, constituting an overall prevalence of 3.1% (254 of 8289 patients). After excluding HPs, 114 patients (prevalence of 1.4%) harbored an SSA or TSA. From the original cohort, 62 pathologic polypectomy specimens from 33 patients were unavailable for reassessment of the original histologic findings and were thus excluded from the polyp subanalysis. A final study cohort of 221 patients with 372 polyps was used in serrated polyp characteristic subanalyses. Coincident with this time period, 989 adenomas (6 mm and larger) from 612 patients were removed (prevalence of 7.4%; 612 of 8289 patients). Three hundred ninety-five adenomas (40.0%) in 319 patients met the criteria for advanced adenoma status and served as one comparative group for this study. The demographics of each population are summarized in Table 1.
Table 1. Study Demographics.
| Demographic* | Serrated polyps(n=221 pts) | Adenomas(n=612 pts) | Advanced Adenomas** (n=319 pts) |
|---|---|---|---|
| Age (STD) | 58.1 ± 7.2 | 59.8 ± 8.8 | 61.1 ± 8.8 |
| M:F | 112:109 | 357:255 | 194:125 |
| BMI | 29.0±6.7 | 28.9 ±6.2 | 29.1± 6.3 |
Does not include a group of patients in CTC surveillance with isolated subcentimeter polyps of unknown histology
Are a subset of the total adenoma group. Defined as an adenoma either ≥ 10 mm, exhibits either tubulovillous or villous histology, or harbors high grade dysplasia
Regarding the serrated polyps, SSAs constituted 36.8% of this group (137 of 372 polyps), whereas TSAs accounted for 4.3% (16 of 372 polyps). HPs contributed to the largest segment of serrated polyps at 58.9% (219 of 372 polyps). Although nearly two thirds of the cohort had a solitary serrated lesion (144 of 221 patients, 65.2%), it was not uncommon for multiple serrated polyps to be present, with 34.8% of patients (77 of 221) harboring at least two or more serrated polyps. Of the group with multiple polyps, the mean number of resected serrated lesions was three (range, two to 11 lesions).
Table 2 demonstrates various polyp-related characteristics between SSAs, TSAs, HPs, and advanced adenomas. Regarding size, SSAs and TSAs (the serrated subgroups with malignant potential) tended to be larger lesions (mean size of SSAs, 10.6 mm; mean size of TSAs, 14.1 mm) compared with HPs (mean size, 7.2 mm). Size category and serrated polyp group were significantly associated (p< .0001). SSAs and TSAs constituted a higher frequency of serrated polyps in the large category (≥ 10 mm; SSAs, 70 of 137 lesions [51.1%]; and TSAs, eight of 16 lesions [50.0%]) than did HPs (19 of 219 polyps, 8.7%). Similarly, statistically significant associations were seen between polyp location and serrated lesion group (p< .0001). SSAs were overwhelmingly located in the proximal colon (125 of 137 lesions, 91.2%), whereas only a minority of TSAs (four of 16 lesions, 25.0%) and HPs (19 of 219 lesions; 8.7%) demonstrated a proximal location. Finally, morphologic appearance was associated with polyp group (p< .0001). SSAs were more often flat in morphologic appearance (54 of 137 lesions, 39.4%) than were TSAs (three of 16 lesions, 18.8%) or HPs (41 of 219 polyps; 18.7%). (Fig. 2)
Table 2. Polyp characteristics at CT Colonography.
| Characteristic | SSA/P(n=137 polyps) | TSA(n=16 polyps) | HP(n=219 polyps) | Advanced Adenomas**(n=395 polyps) |
|---|---|---|---|---|
| Size | ||||
| Average (± STD) | 10.6 ±5.1 | 14.1 ±11.1 | 7.2 ±1.7 | 14.6 ± 8.9 |
| Large (No. ≥ 10 mm) | 70 | 8 | 19 | 350 |
| Small (No. 6-9 mm) | 67 | 8 | 200 | 45 |
| Location | ||||
| Prox. colon (C/A/T)* | 125 (21/79/25) | 4 (0/2/2 ] | 19 (7/2/10) | 181 (55/84/42) |
| Dist. colon (D/S/R)* | 12 (4/6/2) | 12 (1/6/5) | 200 (16/129/55) | 214 (32/115/67) |
| Morphology | ||||
| Sessile | 62 | 9 | 139 | 164 |
| Flat | 54 | 3 | 41 | 47 |
| Pedunculated | 13 | 3 | 22 | 162 |
| Carpet | 2 | 1 | 0 | 9 |
| Other | 6 | 0 | 17 | 13 |
| Histology | ||||
| High Grade Dysplasia | 0 | 1 | 0 | 22 |
Proximal colon includes cecum (C), ascending(A), transverse (T). Distal colon includes descending (D), sigmoid (S), rectum (R)
n=171 with villous component.
Figure 2.
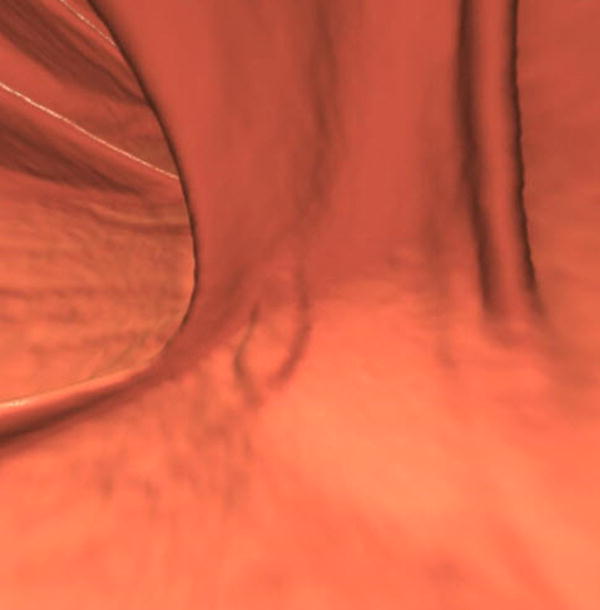
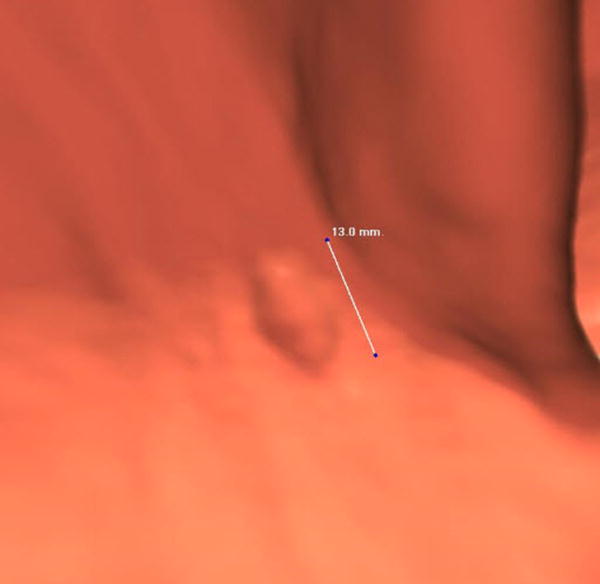
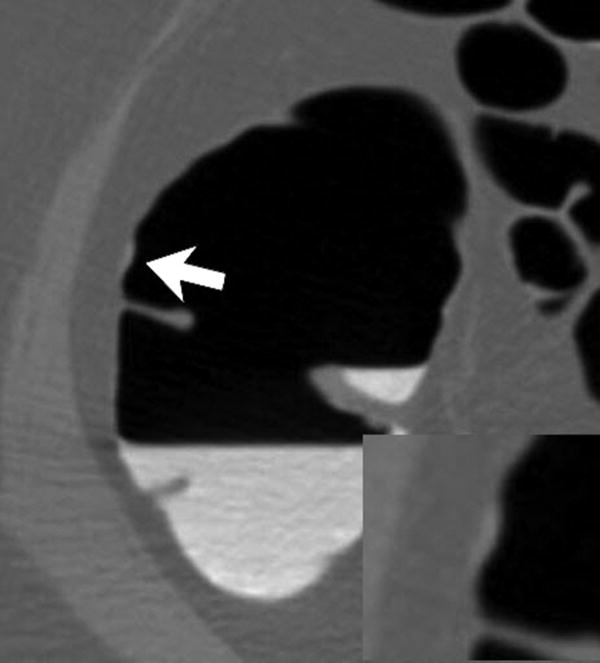
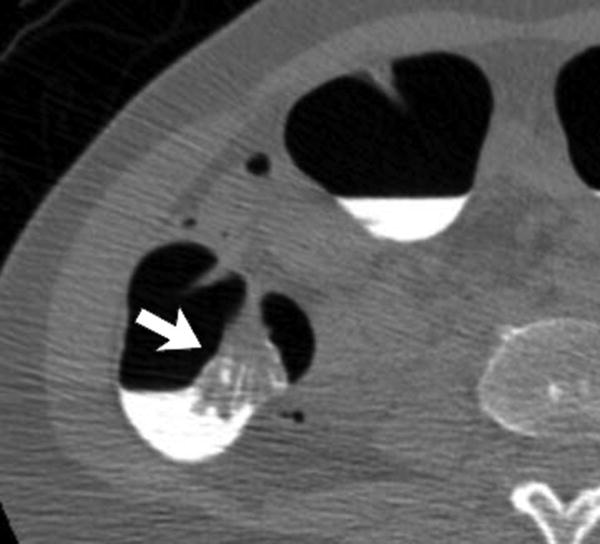
Three different patients underwent screening CTC and had a right-sided SSA. Each patient has a corresponding optical colonoscopy image (A, D, G), 3D CTC endoluminal image (B, E, H), and a 2D CTC axial image (C, F, I). The optical colonoscopy images nicely depict the flat, subtle nature of these large right-sided polyps. The 3D CTC endoluminal images show the appearance at imaging that led to the polypectomy at colonoscopy. Notice that the polyps are more prominent and protruding, as compared with the colonoscopic appearance. Axial 2D CTC images demonstrate how the 3D appearance represents a combination of a flat polyp and the overlying adherent tagging contrast agent (arrows). Magnified images (insets in F and I) better depict the subtle soft tissue thickening underneath the contrast agent.
Compared with advanced adenomas (the adenoma subgroup most likely to undergo malignant transformation), serrated lesions differed markedly regarding the presence HGD (P < .0001). HGD was not seen in the SSA subcohort, and one polyp showed HGD in the TSA group, whereas it was seen in 5.6% of advanced adenomas (22 of 395 lesions). Advanced adenomas were distributed throughout the colon with a slight distal predominance (54.2%, 214 of 395 lesions). Advanced adenomas most often appeared as sessile (41.5%, 164 of 395 lesions) or pedunculated (41.0%, 162 of 395 lesions), whereas SSAs were either sessile (45.3%, 62 of 137 lesions) or flat (39.4%, 54 of 137 lesions). TSAs typically appeared as sessile lesions (56.3%, nine of 16 lesions). (Fig. 3) Carpet morphologic appearance constituted a minority of appearances for all polyp groups, with 2.3% of adenomas (nine of 395) and 1.4% of SSAs (two of 137 lesions) with such morphologic appearance.
Figure 3.
56-year-old female at screening CTC with a proven TSA. (A,B) 2D CTC axial images in the supine and prone positions demonstrate a large TSA (arrow). Often, when large, they can have a frond-like appearance at CTC. (C) 3D endoluminal CTC image demonstrates contrast coating (white). (D) Colonoscopic image confirms this large macro-lobulated TSA.
CT colonography-occult serrated lesions constituted 20.9 % of the combined SSA and TSA groups (32 of 153 lesions) in this series. CT colonography-occult lesions represent the additional serrated polyps that were seen at colonoscopy during polypectomy but not reported at initial CT colonography for patients who were referred from CT colonography for positive examination findings. The mean size of these lesions was 11.2 mm ± 8.4, although 59.4% (19 of 32 lesions) were in the sub-centimeter (6-9 mm) category. Most were proximal in location (96.9%, 31 of 32 lesions) and were either sessile (37.5%, 12 of 32 lesions) or flat (37.5%, 12 of 32 lesions) in morphologic appearance. For comparison, a smaller number of advanced adenomas (9.1%, 36 of 395 lesions) were CT colonography-occult lesions, leading to a statistically significant difference with serrated lesions (P = .0004). Review of the missed serrated lesions at CT colonography shows that 14 of 32 lesions (43.8%) could be seen in retrospect, while 56.2% remained truly occult.
Generalized estimating equation regressions of potentially predictive polyp factors regarding whether a serrated lesion was detected or occult showed that histologic findings (P = .15) location (P = .06), and contrast material coating (P < .0001) met inclusion for the multivariate model. In the multivariate analysis (with the effect of clustering taken into account), only contrast material coating remained significant (P < .0001), with an odds ratio of 40.4 (95% confidence interval: 10.1, 161.4) for serrated polyp detection when present.
Overall, oral contrast material coating of the mucosal surface of a polyp was present on 85.0% of SSAs and TSAs (130 of 153 lesions) (Fig. 2). By excluding those with a thin film (n=24) of contrast material, the mean thickness of the contrast material coating was 1.8 mm ± 0.9. There was a marked difference in coating between serrated lesions detected with CT colonography and the CT colonography-occult group at 96.7% (117 of 121 lesions) and 40.6% (13 of 32 lesions), respectively (P < .0001).
Discussion
Our study clearly demonstrates that serrated neoplasms are identified at CT colonography screening. The lesions in the important SSA and TSA subgroups that have future malignant potential tend to be larger, flat in morphologic appearance, and located in the proximal colon. In contrast, the HP, which believed not to harbor risk for malignant transformation, tends to be subcentimeter in size and distal in location. In our study, TSAs are uncommon lesions, constituting 4.3% of all nondiminutive serrated polyps (16 of 372 polyps). The results corroborate the experience at colonoscopy (14-16) and increase the confidence in the developing narrative regarding the characteristics of these lesions.
There is increasing consensus that these lesions are more difficult to detect than adenomatous polyps with any modality, whether colonoscopy (17) or CT colonography. As such, it is likely that these lesions may have accounted for the decreased protective effect of colonoscopy screening for right-sided cancers (4). At colonoscopy, these lesions are less conspicuous, demonstrating an appearance similar to the colonic mucosa and exhibiting a flatter morphologic appearance (7,16). Often, there is an obscuring overlying mucin coating, which can paradoxically be used to detect these lesions (7). Furthermore, difficulties in measured pull-back and maneuvering of the colonoscope owing to the proximal location of these lesions may further impede the detection of serrated polyps. In response, advanced techniques such as chromoendoscopy have been used to help in the detection of these polyps. Similarly, for CT colonography, there have been questions raised regarding the ability of this screening imaging modality to allow detection of these lesions. In particular, it has been assumed that CT colonography would cause serrated polyps to be missed, because of the flat appearance and the lack of optical cues as are present at colonoscopy (18,19).
The results of our study show that CT colonography can indeed allow detection of these lesions, with a prevalence of 3.1% (254 of 8289 patients) for polyps 6 mm in size and larger. At first glance, it may appear that this rate is discrepant from that in some colonoscopy series in which a serrated lesion prevalence is reported to range from 12% to 21%, suggesting that CT colonography results in too many missed lesions (20-23). However, it is important to note that colonoscopic series include serrated polyps of all sizes, including diminutive lesions. For example, Min et al reported a rate of 11.9% of patients (110 of 926) who harbored 150 serrated polyps. Most serrated polyps were diminutive in size (107 of 150, 71.3%) while only seven of 150 (4.7%) were of large size-10 mm and larger. [21] Because the reporting threshold for CT colonography is set at 6 mm as established in the CT colonography reported and data system, or C-RADS (9), in our study, diminutive polyps believed to be clinically insignificant are effectively excluded. Thus, given this caveat, the results are likely in line with those of previous colonoscopy series. Furthermore, the prevalence of 1.4% (114 of 8289 patients) seen in our series for SSAs and TSAs (which represent the true serrated target lesions for screening) mirrors the rate seen in colonoscopic series such as that of Lash et al (n=179 111), which demonstrated a prevalence of 1.2% for SSA lesions (24). Finally, previously reported 5-year outcomes data from this CT colonography population support the fact that large numbers of serrated lesions are not missed where the interval cancer rate is low, calculated at 0.2 cancers per 1000 years of follow-up for people undergoing routine screening after receiving negative CT colonography findings (10).
The results of the present study also point to potential reasons why these lesions can be detected despite the flatter nature of these polyps. From the CT colonography literature and from clinical experience, it has become evident that the tagging contrast agents used in CT colonography bowel preparation to identify residual stool and fluid have an additional property that aids in polyp detection. With at least some CT colonography bowel regimens, there is an observed phenomenon where the contrast material adheres to the surface of a colonic polyp yet washes away from the adjacent mucosa (8,25). It is theorized that the barium in the contrast agent preparation may admix with the overlying adherent mucin on these lesions to highlight these lesions. This contrast agent property greatly aids in CT colonography interpretation, particularly in the identification and characterization of polyps that are often are only minimally raised from the mucosal surface (8). This is a main reason why such lesions can be detected despite the lack of mucosal optical cues present at colonoscopy and underscores the fact that CT colonography detection is a fundamentally different process than what is used at colonoscopy, despite visual similarities with the 3D endoluminal view (26). Our study showed that among SSA and TSA lesions, which hold the potential for future malignant transformation, most (85%) demonstrated contrast material coating. Of the various polyp factors, the presence of contrast material tagging was predictive of lesion visibility at multivariate analysis. Interestingly, the thickness of the contrast agent coating does not appear to affect whether the polyp is visualized or not, suggesting that even a thin film is enough to draw the radiologist's attention to the lesion.
However, there is no argument that these lesions are often less conspicuous and therefore more difficult to detect. As compared with the adenomatous CT colonography-occult polyps, the rate of CT colonography-occult lesions for serrated lesions is twice as high. It is important to realize that this measure does not represent the test sensitivity for serrated lesions, as we do not know the true false-negative rate of our series, which would require external confirmatory validation with colonoscopy for all of the patients who had normal findings at CT colonography examination. This surrogate measure, however, does give an indication of the relative difficulty of detecting these lesions when compared with adenomas and underscores the need for quality training for correct interpretation (27,28). In our experience, these lesions are more subtle than adenomas; in some cases, may resemble tagged adherent stool initially, but on close inspection, they have a thin rim of soft-tissue attenuation beneath the tag. With proper training, our series suggests that rates could potentially decrease, given that nearly half of the lesions occult at CT colonography can be detected in retrospect.
The discrepancy in HGD rate between serrated lesions and the adenomas is an interesting finding, although the meaning of this observation is unclear. Given that dysplasia is the pathologic bridge to carcinoma, the lower rate of HGD in serrated polyps identified in this series could portend that malignant transformation is less likely than an adenoma for a given size, or conversely, that the high-grade transition state is a temporal shorter window for serrated lesions. Regardless, there is evidence that the conversion from benign serrated polyp to cancer occurs over a longer time frame, with the difference in the mean age of patients with serrated polyps and patients with microsatellite instability high, or MSI-H, cancers to be around 15 years (24).
A major strength of this study involves the pathologic reevaluation of the polypectomy specimens. As opposed to relying on pathologic reports, which were generated during a time when the recognition and classification for serrated lesions were changing, the direct review of the histologic specimens allowed application of the most recent criteria for these lesions by a pathologist with training in gastrointestinal examination. Thus, this series likely represents a more accurate reflection of serrated polyps that are present in average-risk individuals undergoing screening. Without this pathologic review, it is likely that many SSAs would have been misclassified as benign HPs, particularly for the large right-sided lesions.
Our study has a number of limitations. This study represents a retrospective analysis, with inherent biases associated with this study design. In addition, it represents a single-institution experience, which may limit generalization, although the enrolled screening population (which accounts for 97% of the study cohort) is large, and the demographic composition of this population is typical. Finally, the reproducibility of the measurement of the thickness of the contrast material coating is tempered by the fact that often, it is a small measurement made by one observer.
In conclusion, we describe the experience of serrated lesions seen at CT colonography-based colorectal cancer screening. The target lesions of interest (SSAs and TSAs) tend to be large, flat, and proximal in location. CT colonography can be used to detect these lesions by using the property of contrast material coating on the surface of these polyps, despite their flat morphologic appearance.
Supplementary Material
Advances in Knowledge.
The prevalence of serrated lesions ≥ 6 mm in an average risk CTC screening population is 3.1%. The lesions tend to be large, flat, and proximal in location.
Implication for Patient Care.
Knowledge of serrated polyp characteristics should improve recognition and removal of these important target lesions at CT colonography.
Acknowledgments
This research is supported in part by the National Institutes of Health National Cancer Institute (grant 1R01CA144835-01), the American Cancer Society Mentored Research Scholar Grant in Applied and Clinical Research (grant MRSG-13-144-01-CPHPS), and the University of Wisconsin Institute for Clinical and Translational Research through the National Center for Advancing Translational Sciences (grant UL1TR000427)
Dr. Pickhardt is co-founder of VirtuoCTC, and shareholder in SHINE and Cellectar Biosciences; Dr. Kim is co-founder of VirtuoCTC, a consultant for Viatronix, and on the medical advisory board for Digital Artforms; Dr. Lubner receives grants from GE Medical, Neuwave Medical, and Philips; Dr. Hinshaw is a stockholder in Neuwave Medical and Cellectar Biosciences; Dr. Munoz del Rio is a consultant for Lippincott, Williams, and Wilkins;
Footnotes
All other authors have no relevant financial disclosures
Appendix: (See additional file labeled Appendix E1)
References
- 1.Snover DC, Ahnen D, Burt R, et al. In: WHO classification of tumours of the digestive system. 4. Bosman FT, Carneiro F, Hruban RH, editors. Lyon: IARC; 2010. [Google Scholar]
- 2.Snover DC, Jass JR, Fenoglio-Preiser C, Batts KP. Serrated polyps of the large intestine - A morphologic and molecular review of an evolving concept. Am J Clin Pathol. 2005;124:380–91. doi: 10.1309/V2EP-TPLJ-RB3F-GHJL. [DOI] [PubMed] [Google Scholar]
- 3.Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113–30. doi: 10.1111/j.1365-2559.2006.02549.x. [DOI] [PubMed] [Google Scholar]
- 4.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of Colonoscopy and Death From Colorectal Cancer. Ann Intern Med. 2009;150:1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 5.Sawhney MS, Farrar WD, Gudiseva S, Nelson DB, Lederle FA, Rector TS, et al. Microsatellite instability in interval colon cancers. Gastroenterology. 2006;131:1700–5. doi: 10.1053/j.gastro.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 6.Biemer-Huttmann AE, Walsh MD, McGuckin MA, Ajioka Y, Watanabe H, Leggett BA, et al. Immunohistochemical staining patterns of MUC1, MUC2, MUC4, and MUC5AC mucins in hyperplastic polyps, serrated adenomas, and traditional adenomas of the colorectum. J Histochem Cytochem. 1999;47:1039–47. doi: 10.1177/002215549904700808. [DOI] [PubMed] [Google Scholar]
- 7.Tadepalli US, Feihel D, Miller KM, Itzkowitz SH, Freedman JS, Kornacki S, et al. A morphologic analysis of sessile serrated polyps observed during routine colonoscopy. Gastrointest Endosc. 2011;74:1360–8. doi: 10.1016/j.gie.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Kim DH, Hinshaw JL, Lubner MG, del Rio AM, Pooler BD, Pickhardt PJ. Contrast coating for the surface of flat polyps at CT colonography: a marker for detection. Eur Radiol. 2014;24:940–6. doi: 10.1007/s00330-014-3095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zalis ME, Barish MA, Choi JR, Dachman AH, Fenlon HM, Ferrucci JT, et al. CT colonography reporting and data system: A consensus proposal. Radiology. 2005;236:3–9. doi: 10.1148/radiol.2361041926. [DOI] [PubMed] [Google Scholar]
- 10.Kim DH, Pooler BD, Weiss JM, Pickhardt PJ. Five year colorectal cancer outcomes in a large negative CT colonography screening cohort. Eur Radiol. 2012;22:1488–94. doi: 10.1007/s00330-011-2365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim DH, Pickhardt PJ, Hanson ME, Hinshaw JL. CT Colonography: Performance and Program Outcome Measures in an Older Screening Population. Radiology. 2010;254:493–500. doi: 10.1148/radiol.09091478. [DOI] [PubMed] [Google Scholar]
- 12.Pickhardt PJ. Screening CT colonography: How I do it. Am J Roentgenol. 2007;189:290–8. doi: 10.2214/AJR.07.2136. [DOI] [PubMed] [Google Scholar]
- 13.R Development Core Team. R: A language and environment for statistical computing R Foundation of Statistical Computing. Vienna, Austria: 2014. http://wwwR-projectorg. [Google Scholar]
- 14.Carr NJ, Mahajan H, Tan KL, Hawkins NJ, Ward RL. Serrated and non-serrated polyps of the colorectum: their prevalence in an unselected case series and correlation of BRAF mutation analysis with the diagnosis of sessile serrated adenoma. J Clin Pathol. 2009;62:516–8. doi: 10.1136/jcp.2008.061960. [DOI] [PubMed] [Google Scholar]
- 15.Higuchi T, Sugihara K, Jass JR. Demographic and pathological characteristics of serrated polyps of colorectum. Histopathology. 2005;47:32–40. doi: 10.1111/j.1365-2559.2005.02180.x. [DOI] [PubMed] [Google Scholar]
- 16.Rustagi T, Rangasamy P, Myers M, Sanders M, Vaziri H, Wu GY, et al. Sessile serrated adenomas in the proximal colon are likely to be flat, large and occur in smokers. World J Gastroenterol. 2013;19:5271–7. doi: 10.3748/wjg.v19.i32.5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hetzel JT, Huang CS, Coukos JA, Omstead K, Cerda SR, Yang S, et al. Variation in the Detection of Serrated Polyps in an Average Risk Colorectal Cancer Screening Cohort. Am J Gastroenterol. 2010;105:2656–64. doi: 10.1038/ajg.2010.315. [DOI] [PubMed] [Google Scholar]
- 18.East JE. Proximal serrated polyp detection at colonoscopy: the best of times … the worst of times? Gastrointest Endosc. 2012;75:521–4. doi: 10.1016/j.gie.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Leggett B, Whitehall V. Role of the Serrated Pathway in Colorectal Cancer Pathogenesis. Gastroenterology. 2010;138:2088–100. doi: 10.1053/j.gastro.2009.12.066. [DOI] [PubMed] [Google Scholar]
- 20.Alvarez C, Andreu M, Castells A, Quintero E, Bujanda L, Cubiella J, et al. Relationship of colonoscopy-detected serrated polyps with synchronous advanced neoplasia in average-risk individuals. Gastrointest Endosc. 2013;78:333. doi: 10.1016/j.gie.2013.03.003. -+ [DOI] [PubMed] [Google Scholar]
- 21.Min YW, Lee JH, Lee SH, Park DI, Han DS, Rhee PL, et al. Prevalence of proximal colon serrated polyps in a population at average risk undergoing screening colonoscopy: A multicenter study. Clin Res Hepatol Gastroenterol. 2012;36:604–8. doi: 10.1016/j.clinre.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 22.Forsberg AM, Kjellstrom L, Agreus L, Andreasson AN, Nyhlin H, Talley NJ, et al. Prevalence of colonic neoplasia and advanced lesions in the normal population: a prospective population-based colonoscopy study. Scand J Gastroenterol. 2012;47:184–90. doi: 10.3109/00365521.2011.647062. [DOI] [PubMed] [Google Scholar]
- 23.Gao QY, Tsoi KKF, Hirai HW, Wong MCS, Chan FKL, Wu JCY, et al. Serrated Polyps and the Risk of Synchronous Colorectal Advanced Neoplasia: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2015;110:501–10. doi: 10.1038/ajg.2015.49. [DOI] [PubMed] [Google Scholar]
- 24.Lash RH, Genta RM, Schuler CM. Sessile serrated adenomas: prevalence of dysplasia and carcinoma in 2139 patients. J Clin Pathol. 2010;63:681–6. doi: 10.1136/jcp.2010.075507. [DOI] [PubMed] [Google Scholar]
- 25.Pickhardt PJ, Lam VP, Weiss JM, Kennedy GD, Kim DH. Carpet Lesions Detected at CT Colonography: Clinical, Imaging, and Pathologic Features. Radiology. 2014;270:435–43. doi: 10.1148/radiol.13130812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim DH, Pickhardt PJ. Radiologists should read CT colonography. Gastrointest Endosc Clin NA. 2010;20:259–69. doi: 10.1016/j.giec.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Liedenbaum MH, Bipat S, Bossuyt PMM, Dwarkasing RS, de Haan MC, Jansen RJ, et al. Evaluation of a Standardized CT Colonography Training Program for Novice Readers. Radiology. 2011;258:477–87. doi: 10.1148/radiol.10100019. [DOI] [PubMed] [Google Scholar]
- 28.Taylor SA, Halligan S, Burling D, Morley S, Bassett P, Atkin W, et al. CT colonography: effect of experience and training on reader performance. Eur Radiol. 2004;14:1025–33. doi: 10.1007/s00330-004-2262-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.