Abstract
Functional genetic polymorphisms associated with Brain-Derived Neurotrophic Factor (BDNF) and serotonin (5-HTTLPR) have demonstrated associations with depression in interaction with environmental stressors. In light of evidence for biological connections between BDNF and serotonin, it is prudent to consider genetic epistasis between variants in these genes in the development of depressive symptoms. The current study examined the effects of val66met, 5-HTTLPR, and family environment quality on youth depressive symptoms in adolescence and young adulthood in a longitudinal sample oversampled for maternal depression history. A differential susceptibility model was tested, comparing the effects of family environment on depression scores across different levels of a cumulative plasticity genotype, defined as presence of both, either, or neither plasticity alleles (defined here as val66met Met and 5-HTTLPR ‘S’). Cumulative plasticity genotype interacted with family environment quality to predict depression among males and females at age 15. After age 15, however, the interaction of cumulative plasticity genotype and early family environment quality was only predictive of depression among females. Results supported a differential susceptibility model at age 15, such that plasticity allele presence was associated with more or less depressive symptoms depending on valence of the family environment, and a diathesis-stress model of gene-environment interaction after age 15. These findings, although preliminary because of the small sample size, support prior results indicating interactive effects of 5-HTTLPR, val66met, and environmental stress, and suggest that family environment may have a stronger influence on genetically susceptible women than men.
Keywords: depression, 5-HTTLPR, val66met, differential susceptibility, cumulative plasticity, early family environment
Depression is a complex, heterogeneous illness with both genetic and environmental risk factors. Gene-environment interaction (GxE) is a growing area of study that examines how the impact of environmental influences and particular genetic susceptibility factors are amplified by one another. In light of the relatively small effect sizes associated with individual genetic polymorphisms, researchers have increasingly considered the interplay between multiple genetic and environmental influences in the development of depression and other psychopathology (e.g., Grabe et al., 2012). The cumulative effects of genes with plausible functional connections and environmental triggers may better capture GxE effects than individual genetic risk factors alone (Belsky & Beaver, 2011).
One means of conceptualizing the combined effects of genetic risk is through cumulative genetic plasticity, or the idea that multiple genes in combination yield increased susceptibility to the environment (Belsky & Beaver 2011). In addition to considering multiple, plausibly linked genotypes, cumulative genetic plasticity models assume that genes provide susceptibility to both positive and negative environmental influences, rather than just risk of adverse outcomes. This approach is in keeping with the differential susceptibility model of GxE interaction, which suggests that particular genetic polymorphisms predispose an individual to increased sensitivity to environmental factors, which can yield positive or negative outcomes depending on the nature of environmental circumstances (Belsky & Pluess, 2009, 2013). This is in contrast to traditional diathesis-stress models, which suggest that biologically or genetically predisposed individuals are at increased risk of adverse outcomes in the face of negative environmental circumstances, but are unaffected by positive environmental circumstances (Monroe & Simons, 1991).
Selection of genes for consideration in models of cumulative genetic plasticity involved in depression should prioritize genes with plausible functional connections and demonstrated susceptibility to similar environmental factors, as well as established links with depression. Two genetic polymorphisms, brain-derived neurotrophic factor (BDNF) val66met and the serotonin transporter region 5-HTTLPR, have garnered considerable attention in GxE studies of depression. BDNF, a secretory protein, and serotonin (5-HT), a neurotransmitter, both influence neurogenesis and synaptic plasticity (Mattson, Maudsley, & Martin 2004), processes implicated in the development and maintenance of depression (Martinowich & Lu 2008). The neurotrophin hypothesis of depression posits that deficiencies in central BDNF lead to cell death in the hippocampus and prefrontal cortex, which in turn contribute to depression (Martinowich, Manji, & Lu 2007). In support of functional connectedness between BDNF and serotonin, BDNF has been found to influence the structural plasticity and survival of central serotonergic neurons in animal models (e.g., Eaton, Staley, Globus, & Whittemore 1995) and administration of BDNF enhances 5-HT neurotransmission and increases 5-HT metabolism in the brain (Mattson, Maudsley, & Martin 2004). Furthermore, there is evidence to suggest that BDNF may facilitate or perhaps mediate responsiveness to treatment with Selective Serotonin Reuptake Inhibitors (SSRIs; e.g., Duman & Monteggia 2006).
The two most commonly studied functional genetic polymorphisms associated with BDNF and serotonin in depression research are val66met and 5-HTTLPR, respectively (Risch et al., 2009; Verhagen et al., 2010). There are three allelic variants (or genotypes) associated with val66met: Val/Val, Val/Met, and Met/Met. The presence of a Met allele results in a valine to methionine substitution at codon 66, which interferes with trafficking of BDNF mRNA (Chiatruttini et al. 2009) and impairs the activity-dependent secretion of BDNF (Verhagen et al. 2010), such that carriers of the Met allele have reduced hippocampal levels of BDNF. The most commonly studied allelic variants associated with the serotonin gene region polymorphism 5-HTTLPR are: long/long (L/L), short/long (S/L), and short/short (S/S). The S allele is associated with lower transcriptional efficiency of serotonin (Lesch et al. 1996). In light of literature demonstrating biological associations between BDNF and serotonin, consideration of potential genetic epistasis between these two polymorphisms is prudent. A three-way interaction between 5-HTTLPR, BDNF val66met, and early adversity has been shown to predict depressive symptoms among children, as well as female adolescents and adults (Kaufman et al. 2006; Wichers et al. 2008). Similarly, 5HTTLPR, BDNF val66met, and past year life events were found to be predictive of depressive symptoms in a sample of elderly adults (Kim et al. 2007). Finally, in support of a functional relationship between BDNF val66met and 5-HTTLPR, healthy adult Korean subjects who were homozygous for the S allele of 5-HTTLPR and had a Met allele of the BDNF val66met polymorphism displayed lower serum levels of BDNF than those who had either or neither risk allele (Bhang, Ahn, & Choi 2011). Existing research on the relationship between environmental stress, BDNF val66met, and 5-HTTLPR has yielded some discrepant results, including unexpected null findings in a large longitudinal study of almost 1600 adolescents (Nederhof et al. 2010). Additionally, while most studies have demonstrated that the S and Met variants (those typically associated with risk in two-way GxE interactions) have yielded greatest risk (e.g., Drury et al., 2012; Karg, Burmeister, Shedden, & Sen, 2011), there have been some discrepancies, with the L and Val variants occasionally conferring heightened risk for depression (e.g., Grabe et al. 2012).
Cumulative genetic plasticity provides another means of explicating this relationship. Drury et al. (2012) utilized a cumulative plasticity approach to understanding the effects of positive and negative early caregiving environment on the social behaviors of young children on the basis of their possessing both 5-HTTLPR S and BDNF val66met Met alleles, one of these alleles, or neither of these alleles. Their results indicated that youth with both plasticity alleles demonstrated the most appropriate social behaviors under conditions of a positive caregiving environment, and the most inappropriate social behaviors under conditions of negative caregiving environment, relative to their peers with only one or either plasticity allele. These findings are in keeping with growing bodies of literature supporting independent differential susceptibility of 5-HTTLPR and BDNF val66met, such that individuals with the S and Met allelic variants demonstrate better outcomes under positive environmental conditions, and worse outcomes under negative environmental conditions, than do individuals without these alleles (e.g., Gunnar et al. 2012). Family environment and parental caregiving have far-reaching consequences for the mental health and well-being of offspring that span into adulthood. Depressive symptoms, in particular, are predicted by parental psychopathology, parent-child relationship quality, and parental discord (Davies & Cummings, 2006; Weich, Patterson, Shaw, & Stewart-Brown, 2009; Weissman et al., 1987). Youth with certain biological or genetic predispositions may at be particularly heightened sensitivity to both positive and negative family environments. The current study sought to explore whether genetic susceptibility associated with BDNF val66met and 5-HTTLPR confers heightened risk for depressive symptoms in interaction with a multi-method measurement of family environment quality, on a positive-negative continuum.
The present study sought to improve upon several important conceptual and methodological limitations that have restricted prior research on the interaction between BDNF val66met, 5-HTTLPR, and environmental stressors. In an effort to address issues related to problematic reliance on retrospective self-report measures of early life stress and cross-sectional designs, the current investigation utilized a longitudinal sample spanning ages 15 to 25. Family environment quality was measured using a composite of various interview and questionnaire measures by youth, mothers, and fathers at youth age 15. Youth depressive symptoms were sampled at ages 15, 20, and again between ages 22–25, to allow for testing of the persistence of GxE effects in adolescence and young adulthood. Additionally, the study provided a direct comparison of differential susceptibility and diathesis stress models of GxE interaction.
Prior research has failed to adequately account for possible stratification of the relationship between 5-HTTLPR, BDNF val66met, and stress by gender. GxE studies involving both 5-HTTLPR and BDNF val66met independently have occasionally demonstrated differential effects for men and women (e.g., Eley et al. 2004; Brummett et al. 2008, Verhagen et al. 2010). One of the only existing studies providing evidence for a three-way interaction between 5-HTTLPR, BDNF val66met, and early adversity among adolescents/adults utilized a female-only sample (Wichers et al, 2008); while a mixed gender sample failed to demonstrate the expected two or three-way interactions (Nederhof et al. 2010). The current study examined whether the significant relationship between val66met, 5-HTTLPR, and environmental stress previously demonstrated among children and females replicated in a male adolescent/young adult sample.
In accordance with previous work, an interaction between plasticity allele presence (neither, either, or both plasticity alleles, defined here as val66met Met and 5-HTTLPR S) and family environment quality was predicted. It was expected that results would support a differential susceptibility model of GxE interaction, such that plasticity allele presence would be associated with relatively higher depression scores under conditions of adverse family environment quality, and lower depression scores under conditions of positive family environment quality.
Method
Participants
The current study includes 363 youth (140 males, 223 females) originally drawn from over 7,000 mothers and their offspring born between 1981 and 1984 participating in the Mater-University Study of Pregnancy (MUSP) birth cohort in Brisbane, Australia (Keeping et al. 1989). From the original study, 815 mother-offspring pairs were selected for study at age 15 on the basis of a wide range of exposure to maternal depression, over-sampling for maternal depression relative to the general population (complete sampling details described elsewhere, see Hammen & Brennan 2001). The current sample includes youth who were part of the age 15 study for children at risk for depression, were also participants at the age 20 follow-up, and who were recruited for blood sampling between 22–25. Due to procedural issues unrelated to the current analyses, somewhat fewer samples of 5-HTTLPR were genotyped than BDNF val66met, so the sample is constrained by availability of both genotypes.
Participants retained at age 20 did not differ from those identified at youth age 15 on history of maternal depression by youth age 15 (χ2 (1,815) = 3.60, p = 0.06), depressive symptoms at age 15 (t(693) = .35, p = .72), or family environment quality (t(813) = −.85, p = .40). Youth not participating at age 20 were more likely to be male (χ2(1,815) = 8.71, p = 0.001).
Participants who completed genotyping procedures at ages 22–25 did not differ from non-genotyped participants participating at age 20 on history of maternal depression by youth age 15 (χ2(1,663) = .63, p = .24) or family environment quality (t(661) = −.77, p = .44), although males were less likely to participate in genotyping than were females (χ2(1,663) = 23.22, p < .001), and youth participating in genotyping had higher depressive scores at age 20 (t(631) = 2.06, p = .04).
Median family income in the final sample fell in the working and lower middle class. The sample was predominantly white (92% white, 1.5% Asian, 6 % biracial, .5% other/not reported).
Procedure
Youth, their mothers, and available fathers completed semi-structured interviews and questionnaires independently in their homes at youth age 15. At age 20, youth and their mothers completed interviews and questionnaires in their homes. Participants were contacted between ages 22 and 25 about participation in the genotyping study. Participants who agreed to the blood collection were sent consent forms, questionnaires, a blood collection pack, and instructions to have blood drawn at a local pathology lab. Genotyping information is described below. All procedures were approved by Institutional Review Boards of the University of Queensland, University of California, Los Angeles, and Emory University. Participants provided written informed consent (or parental consent and youth assent in the case of the age 15 procedures) and were compensated for their time.
Measures
Family Environment Quality
Family environment quality was measured using a composite score consisting of multiple interview and self-report measures administered to youth, mothers, and fathers at youth age 15, probing for conditions up to the past year. The composite score is reflective of ongoing marital and parent-child relational dynamics, with final composite scores reflecting a continuum of positive to negative environment. Correlations with measures of family conditions and early adversity at age 5 (r = .23, p <.001) and family relationships at age 20 (r =.31, p <.001) in this sample suggest that this measure is reflective of ongoing family conditions.
The measures comprising the family environment quality variable included interviewer-rated scores of youth and mother responses to the UCLA Chronic Stress Interview (Hammen et al. 1987), a semi-structured measure of objective conditions covering quality of functioning in the following domains: mother’s relationship with the youth, mother’s marital/romantic relationship, and youth’s relationship with immediate family members in at least the past six months. Interviewer scores in each of these areas ranged from exceptionally good to exceptionally poor. Additional items used in the family environment quality variable include the satisfaction subscale of the Dyadic Adjustment (DAS; Spanier 1976), which assesses overall relationship quality, and the Modified Conflict Tactics Scale (MCTS; Pan, Neidig, & O’Leary 1994), which assesses frequency of physical and psychological coercion between romantic partners. The DAS and MCTS were completed by mothers and available fathers. Youth also reported on quality of parent-child interactions in the revised Children’s Report of Parental Behaviors Inventory (CRPBI; Schludermann & Schludermann, unpublished manuscript 1988). The subscales of the CRPBI used were parental acceptance versus rejection and psychological control versus psychological autonomy. Each of the 11 scores used to form the family environment quality variable was standardized across the entire age 15 sample. An average family environment summary score was then formed for each participant (α = .78), with summary scores ranging from −1.25 to 1.99 (M = −0.01, SD = .57), with negative scores reflecting positive family functioning, and positive scores reflecting relatively higher family discord. Cronbach’s alpha (.79) indicated good internal consistency of the measures comprising this scale. The full measure construction has been described in full elsewhere (Hammen, Brennan, Keenan-Miller, Hazel, & Najman 2010).
Depressive Symptoms
Self-reported depressive symptoms at ages 15, 20, and 22–25 were assessed using the Beck Depression Inventory-II (BDI-II; Beck, Steer, & Brown, 1996), a well-validated and widely used measure of severity of depressive symptoms. Coefficient alpha reliability for the current sample was α = .92 at age 15, α = .93 at age 20, and α = .94 at ages 22–25.
Maternal Depression Diagnoses
Maternal depression was measured using the Structured Clinical Interview for DSM-IV Axis-I Disorders, Patient Edition (SCID; First, Spitzer, Gibbons, & Williams 1997), a reliable and well-validated semi-structured clinical interview assessing the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) criteria for adult Axis I psychopathology. In the current analyses, mothers’ current or lifetime depressive disorders (weighted kappas = 0.87 and 0.84, respectively) at youth age 15 defined maternal depression history. Of the 815 mothers included in the youth age 15 interview, 357 (43.8%) had a past or current depressive disorder (Major Depressive Disorder or dysthymia).
Genotyping
Blood samples were initially delivered to the Genetic Epidemiological Laboratory of the Queensland Institute of Medical Research (QIMR) in Brisbane, Australia.
Genotyping for 5-HTTLPR was conducted at QIMR; only 384 randomly selected samples were genotyped due to cost considerations. The 43 bp deletion polymorphism was genotyped by agarose gel analysis of PCR products spanning the central portion of repeats in the 5HTTLPR. PCR employed Qiagen enzyme and buffer except for the use of 30% deazaguanine and with 10 cycles of Touchdown protocol beginning at 67C and finishing at 62C with a further 32 cycles. Samples were subject to independent duplicate PCR with primer set 1 (acgttggatgTCCTGCATCCCCCAT, acgttggatgGCAGGGGGGATACTGCGA, lower case sequence is non-templated) yielding products of 198 and 154 bp for L and S versions and primer set 2 (acgttggatgTCCTGCATCCCCCAT, acttggatgGGGGATGCTGGAAGGGC) for products of 127 and 83 bp. A majority of samples underwent triplicate gel analysis. A minimum of two independent results in agreement was required for inclusion, which gave a final call rate of 96.4%. To estimate accuracy duplicate samples were genotyped for 829 individuals in a different study using these procedures, with discordance rates of 0.36%.
In view of evidence of variants of the L allele designated as LA and LG (SNP rs25531) in which LG and S are functionally similar (Hu, Zhu, Lipsky, & Goldman 2004), analyses were performed reclassifying LG variants as S. After reclassification, genotype frequencies were: L/L = .32, L/S = .46, and S/S = .22, which was in Hardy-Weinberg equilibrium, χ2(1, 381) = 1.61, p = 0.20.
For the BDNF val66met analyses, aliquots of DNA were shipped to UCLA for processing at the Social Genomics Core of the USC/UCLA Biodemography Center. Individual status on the BDNF val6mmet polymorphism was assayed by a commercial TaqMan Genotyping Assay (Applied Biosystems, Foster City, CA) performed on an iCycler real-time PCR instrument (BioRad, Hercules, CA) following the manufacturer’s specified protocol, as described in Cole et al. (2010). Test-retest reliability of duplicated specimens yielded a total genotyping error rate < 1%. In the present sample genotype frequencies were: Val/Val = .62, Val/Met = .34, Met/Met = .04, which was in Hardy-Weinberg equilibrium, χ2(1, 444) = .13, p = 0.72.
Cumulative Plasticity Genotype
A cumulative plasticity genotype was constructed in order to compare the relative effects of presence of plasticity alleles of neither, either, or both of the 5HTTLPR or BDNF genotypes, in keeping with models of cumulative plasticity and previously published work (Belsky & Beaver 2011; Drury et al. 2012). Youth who were L homozygotes and Val homozygotes were classified as “0/none,” youth who were either S or Met carriers were classified as “1/either,” and youth carrying both S and Met alleles were classified as “2/both.”
Statistical Analyses
As the sample was oversampled for maternal depression, history of maternal depression by youth age 15 was included as a covariate in all analyses. Gender was included either as a covariate or an interaction term, depending on the model. Parental self-reported race was also included as a covariate in all analyses as a proxy for participant race (which was not assessed), in light of reported stratifications of genotypes by racial groups. Analyses were conducted using hierarchical linear regression models in IBM SPSS Statistics Software Version 20. Regions of significance testing was conducted using the interactive calculator at quantpsy.org (Preacher, Curran, & Bauer, 2006).
Results
Three hundred and sixty-three individuals had valid genotype data and scores on relevant predictor and outcome variables. Genotype groups did not differ by the proportion of mothers reporting a history of depression by offspring age 15 (χ2(2, 363) = 1.42, p = .49), family environment quality (F(2,362) = 1.50, p = .23), BDI score at 15 (F(2,349) = 1.84, p = .16), 20 (F(2, 349) = 1.84, p = .16), or 22–25 (F(2, 332) = .62, p = .54), or race (χ2 (4, 361) = 7.25, p = .12); however, there was some stratification by gender (χ2 (2,363) = 6.07, p =.05;). Among the male participants, 29 (21%) had no plasticity alleles, 65 (46%) had either plasticity allele, and 46 (33%) had both plasticity alleles. Among the female participants, 34 (15%) had no plasticity alleles, 133 (60%) had either plasticity allele, and 56 (25%) had both plasticity alleles.
Depressive Symptoms
Age 15
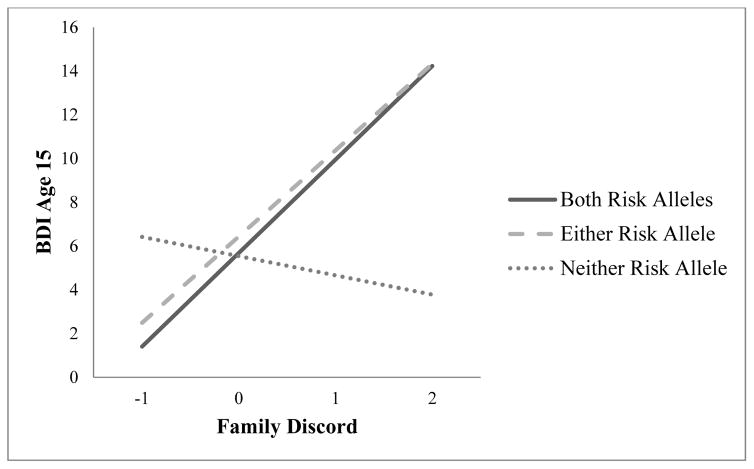
Family environment quality, cumulative plasticity genotype, and their interaction were used to predict BDI at age 15 controlling for maternal history of depression by youth age 15, race, and gender. The overall regression was significant, (F(6, 343) = 7.62, p < .001, R2 Adj = .10, p = .02). The two-way interaction term was significant (b = 2.01, SE = .85, p = .02), as illustrated in Figure 1, while neither cumulative plasticity genotype nor family environment quality was independently significant in the model (see Table 2 for all regression terms).
Fig. 1.
Effect of family environment quality on age 15 BDI scores, by cumulative plasticity genotype (positive numbers indicate increasing familial discord)
Table 2.
Differential susceptibility/diathesis stress indices for depressive symptoms outcomes by age.
| RoS X | Simple Slopes at +/− 2SD X |
RoS Z | Simple Slopes at all levels of Z |
Type I Error Control |
Nonlinear Terms | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | −2SD FD | Mean FD | +2SD FD | Lower Bound | Upper Bound | 0 Risk Alleles | Either Allele | Both Alleles | p value XZ | Adj. p XZ | X2 | ZX2 | R2 Δ | |
| Age 15 | −.69 | .98 | −2.48* | −0.17 | 2.14* | −8.03 | .33 | 1.05 | 3.07*** | 5.1*** | .017 | .0165 | 1.99 | −.59 | .01 |
| Age 20, Women | −1.14 | .18 | −4.13* | 1.27 | 6.68** | −3.13 | .57 | .07 | 4.65*** | 9.24*** | .007 | .05 | 1.09 | 1.30 | .02 |
| Age 22–25, Women | −1.43 | .08 | −3.69* | 0.28 | 3.14† | −1.01 | 1.08 | −2.59 | 1.56 | 5.71*** | .007 | .025 | −3.13 | 3.37† | ,02 |
Note: RoS, regions of significance; PoI, proportion of interaction; FD, family discord; X, Family Discord; Z, cumulative genetic susceptibility. RoS on X indicates that outside of noted bounds there is a significant effect of cumulative genetic plasticity on depressive symptoms; simple slopes of the effect of cumulative genetic plasticity on depressive symptoms at +/1 2 SD of FD are also presented. RoS on Z indicates that outside of noted bounds there is a significant effect of family discord on depressive symptoms; simple slopes of the effect of family discord on depressive symptoms at all levels of cumulative genetic plasticity are also presented. Type I error control presents noted p value for the interaction (family discord * cumulative genetic plasticity) term, and adjusted p value threshold.
p<.05
p <.01
p<.001
p<.07
To examine whether the relationship between cumulative plasticity genotype and family environment quality was consistent across genders, a three-way interaction between family environment quality, cumulative plasticity genotype, and gender, controlling for maternal depression history and race, was conducted. The interaction was not significant (b = .02, SE = .83, p = .28).
Age 20
The two-way interaction between family environment quality and cumulative plasticity genotype, controlling for maternal depression and participant gender and race, was not significant in predicting BDI at age 20 (b = 1.85, SE = 1.19, p = .12).
In order to test for consistency of the relationship between cumulative plasticity genotype and family environment quality across gender, these three terms and their two- and three-way interactions were used to predict BDI at age 20 controlling for maternal history of depression by youth age 15, participant gender, and race. The overall regression was significant F(9, 323) = 7.45, p < .001, R2 Adj = .15, p = .04. The three-way interaction term was significant, b = 2.83, SE = 1.14, p = .04.
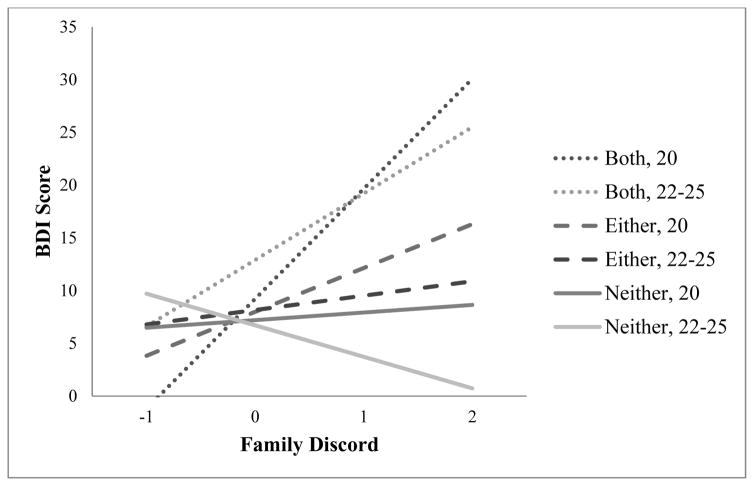
Simple effects tests revealed that the interaction of cumulative plasticity genotype and family environment quality was significant in predicting age 20 BDI among women (b = 4.62, SE = 1.70, p = .007; see Table 2 and illustration in Figure 2), but not men (b = −1.55, SE = 1.56, p = .32). Comparing these effects demonstrated a significant difference between the effect of the interaction between cumulative plasticity genotype and family environment quality on age 20 BDI scores in men and women (t(248) = 2.67, p = .008).
Fig. 2.
Effect of family environment quality on BDI scores, by cumulative plasticity genotype at ages 20 and 22–25, among women only (positive numbers indicate increasing familial discord)
Ages 22–25
Identical analyses to those conducted with age 20 BDI scores were repeated with age 22–25 BDI scores. Results mirrored those of age 20, such that the two-way interaction between family environment quality and cumulative plasticity genotype was not significant (b = 1.90, SE = 1.07, p = .08), while a three-way interaction with gender was significant (F(9, 352) = 4.41, p < .001, R2 Adj = .08, p = .03; b = −4.57, SE = −4.57, p = .03).
Simple effects tests revealed a significant interaction between cumulative plasticity genotype and family environment quality among women (b = 4.22, SE = 1.54, p = .007; see Table 2) but not men (b = −.43, SE = 1.44, p = .77), which were significantly different from each other (t(276) = 2.20, p = .03).
Additional Criteria for Gene-Environment Interaction
In accordance with previously established guidelines of testing true gene-environment interaction (e.g., Belsky, Bakersman-Kranenberg, & van Ijzendoorn, 2007), the genetic susceptibility factor was not related to the environmental predictor (r = .05, p = .31), suggesting that G–E correlation did not account for the observed findings. Additionally, the genetic susceptibility factor was not significantly associated with BDI at age 15 (r = −.01, p = .74), 20 (r = .02, p = .74), or 22–25 (r = −.01, p = .93), ruling out the possibility of main effect of genotype. Finally, the specificity of the model was confirmed in tests replacing the susceptibility factors and outcomes.
Differential Susceptibility vs. Diathesis-Stress
In order to determine whether the observed interactions were better accounted for by differential susceptibility or diathesis stress, five additional tests were conducted at each time point (Roisman et al., 2012). First, regions of significance (Ros) on X (family environment) and accompanying simple slopes tests were conducted in order to determine whether significant effects of cumulative genetic susceptibility on depressive symptoms existed at both high and low ends of family environment quality (within +/− 2 SD). Effects at both ends of family environment would indicate differential susceptibility, whereas effects under only negative family environment conditions would indicate diathesis stress. Second, RoS on Z (genetic susceptibility) and accompanying simple slopes tests were conducted in order to determine whether significant effects of family environment quality on depressive symptoms existed at each level of cumulative genetic plasticity (no plasticity alleles, either plasticity allele, or both plasticity alleles; within +/− 1 SD of family environment quality). This is not a direct test of differential susceptibility vs. diathesis-stress, but rather an indication of whether the observed genetic effects are cumulative in nature (Belsky, Bakersman-Kranenberg, & van Ijzendoorn, 2007). Third, a Proportion of Interaction (PoI) index was constructed to determine the proportion of the GxE interaction falling above and below the mean of family environmental quality. Indices falling between .4 and .6 suggest that roughly half of the interaction falls above and below the mean and are considered indicative of differential susceptibility, while indices outside of that range are more in keeping with diathesis-stress. Fourth, adjustments to the thresholds for significance of the interaction tests were made in accordance with a Bonferonni-type procedure in order to control for Type I error-rates. Finally, interaction models including nonlinear terms (x2 and zx2) were tested; significance of nonlinear terms would serve as evidence against differential susceptibility.
Age 15
RoS on X and accompanying simple slopes tests revealed significant effects of cumulative genetic susceptibility on depressive symptoms at both high and low levels of family environment (within +/− 2 SD, or −1.14 to 1.14; see Table 3 for complete results), in support of differential susceptibility over diathesis stress. The RoS on Z test revealed significant effects of family environment on depressive symptoms for participants having either or both (but not neither) risk alleles. Simple slopes of allele groups 1(either) and 2(both) did not differ significantly from each other (t(186) = −.22, p = .83), but each differed significantly from that of group 0(neither) (0 vs 1: t(120) = −3.24, p = .002; 0 vs 2: t(120) = −3.23, p = .002). The PoI index (.42) was consistent with differential susceptibility. Next, adjustments to the p-value were made in accordance with the Type I error critique presented by Roisman et al., 2012; the observed p value (.017) slightly exceeded the adjusted threshold (.0165). Nonlinear terms were non-significant.
Age 20 BDI (Women only)
The RoS on X and accompanying simple slope tests revealed significant effects of cumulative genetic plasticity on depressive symptoms at high and low ends of family environment quality, however, the lower bound (−1.14) was essentially equivalent to −2 SD (−1.15 to 1.21), indicating that few cases would be affected by the low (positive) end of family environment quality; thus supporting diathesis stress. The RoS on Z test revealed significant effects of family environment on depressive symptoms for participants having either or both (but not neither) risk alleles. Simple slopes of allele groups 0 (neither) and 1(either) did not differ significantly from each other (t(66) = −1.36, p = .18). The 2(both) allele group differed significantly from the 0 allele group (t(66) = −2.56, p = .01), and trended towards differing significantly from the 1 allele group (t(98) = −1.79, p = .08). These results indicate significant effects of family environment on depressive symptoms with presence of either or both susceptibility alleles, with stronger effects in the presence of both alleles. The PoI, .72, indicated support for diathesis stress over differential susceptibility. The observed p value (.007) remained significant with the adjusted threshold (.05). Nonlinear terms were non-significant.
Age 22–25 BDI (Women only)
The RoS on X and accompanying simple slope tests revealed significant and marginally significant effects of cumulative genetic plasticity on depressive symptoms at high and low ends of family environment, however, the lower bound exceeded −2 SD (−1.15 to 1.21), demonstrating support for diathesis stress over differential susceptibility. The RoS on Z test revealed significant effects of family environment on depressive symptoms for participants having both but not either or neither risk alleles. The simple slope of the 2(both) allele group differed significantly from that of the 0(neither) group (t(66) = −2.68, p = .009) and the 1(either) allele group (t(110) = −2.11, p = .04). The simple slope of the 1 allele group, however, did not differ significantly from that of the 0 group (t(66) = −1.40, p = .17), in support of cumulative over singular effects of the genotypes. The PoI index (78) supported diathesis stress. The observed p value (.007) remained significant with the adjusted threshold (.025). Nonlinear terms were non-significant.
Discussion
The current study examined the interactive effects of cumulative plasticity genotype of BDNF val66met and 5-HTTLPR (defined as presence of neither, either, or both 5-HTTLPR S and val66met Met alleles) and family environment quality on depressive symptoms in a longitudinal sample of youth at ages 15, 20, and 22–25. Consistent with hypotheses and previous research, there was a significant effect of the interaction between cumulative plasticity genotype and family relationship quality on self-reported depressive symptoms at youth age 15 in the present sample (e.g., Drury et al. 2012). At ages 20 and 22–25 the observed GxE interaction was only significant among women, a finding which is consistent with previous evidence of gender differences of GxE interactions involving both BDNF val66met and 5-HTTLPR (e.g., Eley et al. 2004; Brummett et al. 2008).
Follow up tests revealed that, contrary to study hypotheses, the observed GxE effects were supportive of differential susceptibility only at age 15. At age 15, genetically susceptible youth fared better (exhibited fewer depressive symptoms) under conditions of positive family environment and fared worse (exhibited more depressive symptoms) under conditions of negative family environment than their non-susceptible peers. Among women at ages 20 and 22–25, the observed interactions were more in keeping with a diathesis-stress model, such that genetically susceptible youth were at increased risk of depressive symptoms under negative conditions of the family environment but were relatively unaffected by positive family environment. The age 15 findings are consistent with prior evidence suggesting that allelic variants that have persisted in sizable portions in the population confer sensitivity, rather than merely risk, to environmental factors, particularly early in life (e.g., Drury et al. 2012; Gunnar et al. 2012). The present findings may indicate that genetic susceptibility to positive aspects of the family environment diminishes over time, or that the current study lacked the sample size necessary to detect differential susceptibility effects associated with the positive ranges of family environment at ages 20 and 22–25. Future work on genetic epistasis between 5-HTTLPR and BDNF val66met should continue to test both differential susceptibility and diathesis-stress models, as preliminary evidence suggests that both are viable forms of GxE effects involving these genotypes. Furthermore, while the present study tested presence of either, both, or neither susceptibility alleles (S and Met), future studies with much larger samples should attend to possible differences between heterozygosity (met/val, S/L) and homozygosity (met/met, S/S) for these alleles.
While the presence of both plasticity alleles conferred the strongest and most consistent effects of family environment quality on depressive symptoms at ages 20 and 22–25, tests of the simple effects revealed that at age 15, the effects of family environment quality on depressive symptoms were not significantly different among youth possessing either one or both plasticity alleles. Overall, the study supports cumulative effects of BDNF val66met and 5HTTLPR, and is consistent with prior research indicating biological connectedness between serotonin and BDNF (Duman & Monteggia 2006; Martinowich & Lu, 2008). Unfortunately, given sample size limitations, the present study is unable to determine whether the cumulative effects demonstrated are better represented by an additive or multiplicative model. Prior research in this area has demonstrated both interactive (e.g., Kaufman et al. 2006; Wichers et al. 2008) and additive (Aguilera et al. 2009) influences of BDNF val66met and 5-HTTLPR on depression and related outcomes, and this remains an important area of future research.
The present findings suggest a possible gender difference in genetic susceptibility to early family environment, which is consistent with literature demonstrating effects of 5-HTTLPR × environmental stress in women but not men (e.g., Eley et al. 2004). One prior study demonstrating an interactive effect of 5-HTTLPR and BDNF val66met genotypes and environmental stress on depression utilized an all-female sample (Wichers et al. 2008), and a mixed gender study failed to replicate any of the expected effects (Nederhof et al. 2010). The present results may shed light on these discrepancies, suggesting the possibility that cumulative genetic susceptibility to family environment may be at least more pronounced among, if not exclusive to, women,. The current results, however, should be interpreted with caution in light of the restrictively small sample size, and further investigation of possible gender differences in larger sample sizes is warranted. Notably, both men and women demonstrated genetic susceptibility to family environment quality at age 15, when, presumably, most men and women were living at home and thus concurrently exposed to the assessed aspects of familial environment, but only women continued to show these effects over time. The cause of this shift is unknown, but there are several possible explanations: women may be more susceptible to the social environment over time; women may have more continuing exposure to their family of origin, whereas men may have removed themselves from negative family influences in young adulthood; or, women may have been more likely to select into social environments in their young adulthood that mirrored those of their adolescent years, thus placing them at continued susceptibility to environmental factors. The nature of environmental stress may also produce different effects on men and women. The present family environment quality variable is primarily a reflection of social stressors and there is some evidence to suggest that women are more susceptible to social or interpersonal stress than are men (Shih, Eberhart, Hammen, & Brennan 2006). It is possible that non-social stressors would not produce the same gender difference in influence on depression scores.
The present study is strengthened by its use of a multi-informant, multi-method assessment of family environment, which spans a continuum of positive to negative conditions. This measure is an improvement over single-measure self-reports of family environment or early adversity taken in adulthood, which have yielded problematic discrepancies in previous GxE research (McGuffin, Alsabban, & Uher 2011). Additionally, the study is strengthened by its consideration of genetic susceptibility to positive and negative family environment and direct comparison of differential susceptibility and diathesis stress models of GxE interaction.
Although further clarification of GxE interaction in depression is needed before results such as those presently discussed bear direct clinical application, several potential implications exist. Studies such as this one may help identify youth who are particularly susceptible to both the positive and negative effects of family social environment during adolescence. It may be the case that while women’s genetic susceptibility to the family environment heightens risk for depressive symptoms (which are well-established to occur at higher frequencies among women, starting in adolescence), men may be at greater risk or susceptibility for other outcomes, such as externalizing symptoms. Treatment strategies may ultimately be informed by individuals’ genetic propensity for environmental influence on a wide array of psychological outcomes.
When considering the present findings, it is important to bear in mind that family environment quality is not purely a measure of the environment; rather, it is in part susceptible to and representative of genetic and biological influences (Kendler & Baker, 2007). Although results of the present study indicate that the genetic susceptibility factor studied here was not correlated with family environment quality (r = .05, p = .31), genetically-influenced traits undoubtedly contributed to the parental social and interactional styles measured. Similarly, the present sample was over-selected for exposure to maternal depression; which serves as both a marker of genetic risk and an environmental stressor or trigger in the development of youth depression (Rice, Harold, & Thapar, 2002). Depressed mothers exhibit impairments in parenting that elevate risk of depression, including more negative/hostile exchanges, disengagement, and fewer positive social interactions than non or never depressed mothers (Lovejoy, Graczyk, O’Hare, & Neuman, 2000). The fact that maternal depression status and family environment are dual risks should theoretically serve to increase exposure to both genetic and environmental risk factors, and thus ought not diminish or negate the observed findings. Nevertheless, it is important to bear in mind that the measured environmental conditions are not free of genetic influence.
Several limitations of the current study should be acknowledged. First, the sample was quite small for genetic analyses, and the current results should be viewed as preliminary evidence requiring replication in a larger sample. Nonetheless, within the dataset, the GxE findings were consistent at different time points, lending strength to the results. The current sample was also high risk with regards to history of maternal depression (although this was controlled for), which may not necessarily generalize to unselected samples. Additionally, although the measurement of family environment was strengthened by its utilization of questionnaire and interview reports by mother, father, and youth, this measurement was administered at age 15 and therefore not as strong as concurrent samples of early family environment. Finally, the sample was predominantly white, and therefore results may not generalize to non-white samples due to established differences in genotyping among racial groups.
The current study adds to prior evidence of cumulative effects of 5-HTTLPR, BDNF val66met, and family environment on depressive symptoms. Furthermore, it extends these findings to suggest the possibility of differential susceptibility, such that the identified plasticity alleles can yield better or worse outcomes in early adolescence, depending upon the nature of familial environment. Finally, the current results suggest that the BDNF val66met and 5-HTTLPR genotypes, at least in exposure to family environment, may differentially influence depression symptoms in men and women in young adulthood. The present study’s use of a longitudinal design and multi-method assessment of family environment demonstrates methodological improvement over previous studies of genetic epistasis between 5-HTTLPR and BDNF val66met, and underscores the importance of considering both genetic and environmental factors in the development of depression.
Table 1.
Multiple regression analyses to predict G × E interactions with family environment quality
| BDI Age 15 | BDI Age 20 (Women Only) | BDI Age 22–25 (Women Only) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| b | SE | p | b | SE | p | b | SE | p | |
| Race | −.07 | .54 | .90 | .68 | 1.07 | .53 | −.12 | 1.01 | .92 |
| Maternal Depression | .23 | .68 | .74 | 3.47 | 1.23 | .005** | 2.66 | 1.13 | .02* |
| Gender (0 = male, 1 = female) | −1.21 | .66 | .07 | ||||||
| Family Environment | 1.10 | 1.10 | .32 | −.02 | 2.12 | .99 | −2.73 | 1.99 | .17 |
| Genotype | −.12 | .49 | .81 | 1.13 | .93 | .23 | 1.37 | .86 | .11 |
| FE × Gene | 2.01 | .85 | .017* | 4.62 | 1.70 | .007** | 4.22 | 1.54 | .007** |
Acknowledgments
The authors greatly appreciate the work of the project coordinators, Robyne LeBrocque, Cheri Dalton Comber, Sascha Hardwicke, and our interview staff. The authors also wish to thank the staff at the Genetic Epidemiological Laboratory of the Queensland Institute of Medical Research: Professor Nick Martin (Head) for cooperation, access, and facilitating QIMR activity, Michael James and Leanne Ryan for 5-HTTLPR and rs25531 genotyping, and Megan Campbell and Dixie Stratham for coordinating genetic data collection and analysis. Finally, the authors express thanks to the original MUSP principals, William Bor, MD, Michael O’Callaghan, MD, and Professor Gail Williams. The 5-HTTLPR assays were devised by Dr. M. R. James and carried out by his research staff, Leanne Ryan and Troy Dumenil at Queensland Institute of Medical Research.
This research was supported by National Institute of Mental Health R01 MH052239 to Brennan, Hammen, and Najman. The authors declare that they have no conflicts of interest.
References
- Aguilera M, Arias B, Wichers M, Barrantes-Vidal N, Moya J, Villa H, van Os J, Ibanez MI, Ruiperez MA, Ortet G, Fañanás L. Early adversity and 5-HTT/BDNF genes: new evidence of gene-environment interactions on depressive symptoms in a general population. Psychological Medicine. 2009;39(9):1425–1432. doi: 10.1017/S0033291709005248. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. The Beck Depression Inventory. 2. San Antonio, Texas: The Psychological Corporation; 1996. [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH. For better and for worse: differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16(6):300–304. doi: 10.1111/j.1467-8721.2007.00525.x. [DOI] [Google Scholar]
- Belsky J, Beaver KM. Cumulative-genetic plasticity, parenting and adolescent self-regulation. Journal of Child Psychology & Psychiatry. 2011;52(5):619–626. doi: 10.1111/j.1469-7610.2010.02327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond Diathesis-Stress: Differential Susceptibility to Environmental Influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond risk, resilience, and dysregulation: Phenotypic plasticity and human development. Development and Psychopathology. 2013;25:1243–1261. doi: 10.1017/S095457941300059X. [DOI] [PubMed] [Google Scholar]
- Bhang S, Ahn J, Choi S. Brain-derived neurotrophic factor and serotonin transporter gene-linked promoter region genes alter serum levels of brain-derived neurotrophic factor in humans. Journal of Affective Disorders. 2011;128(3):299–304. doi: 10.1016/j.jad.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Brummett BH, Boyle SH, Siegler IC, Kuhn CM, Ashley-Koch A, Jonassaint CR, Zuchner S, Collins A, Williams RB. Effects of environmental stress and gender on associations among symptoms of depression and the serotonin transporter gene linked polymorphic region (5-HTTLPR) Behavior Genetics. 2008;38(1):34–43. doi: 10.1007/s10519-007-9172-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaruttini C, Vicario A, Li Z, Baj G, Braiuca P, Wu Y, Lee FS, Gardossi L, Baraban M, Tongiorgi E. Dendritic trafficking of BDNF mRNA is mediated by translin and blocked by the G196A (Val66Met) mutation. PNAS Proceedings of the National Academy of Sciences of the United States of America. 2009;106(38):16481–16486. doi: 10.1073/pnas.0902833106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Arevalo JM, Takahasji R, Sloan EK, Lutgendorf SK, Sood AK, Sheridan JF, Seeman TE. Computational identification of gene-social environment interaction at the human IL6 locus. Proceedings of the National Academy of Science. 2010;107(12):5681–5686. doi: 10.1073/pnas.0911515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PT, Cummings EM. Interparental Discord, Family Process, and Developmental Psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology, Risk, Disorder, and Adaptation. Hoboken, NJ: John Wiley & Sons, Inc; 2006. pp. 86–121. [Google Scholar]
- Drury SS, Gleason MM, Theall KP, Smyke AT, Nelson CA, Fox NA, Zeanah CH. Genetic sensitivity to the caregiving context: the influence of 5httlpr and BDNF val66met on indiscriminate social behavior. Physiology & Behavior. 2012;106(5):728–735. doi: 10.1016/j.physbeh.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biological Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Eaton MJ, Staley JK, Globus MY, Whittemore SR. Developmental regulation of early serotonergic neuronal differentiation: the role of brain-derived neurotrophic factor and membrane depolarization. Developmental Biology. 1995;170(1):169–182. doi: 10.1006/dbio.1995.1205. [DOI] [PubMed] [Google Scholar]
- Eley TC, Sugden K, Corsico A, Gregory AM, Sham P, McGuffin P, Plomin R, Craig IW. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Molecular Psychiatry. 2004;9:908–915. doi: 10.1038/sj.mp.4001546. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. Washington, D.C: American Psychiatric Press; 1995. [Google Scholar]
- Grabe HJ, Schwahn C, Mahler J, Appel K, Schulz A, Spitzer C, Fenske K, Barnow S, Freyberger HJ, Teumer A, Petersmann A, Biffar R, Rosskopf D, Ulrich J, Völzke H. Genetic epistasis between the brain-derived neurotrophic factor Val66Met polymorphism and the 5-HTT promoter polymorphism moderates the susceptibility to depressive disorders after childhood abuse. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2012;36(2):264–270. doi: 10.1016/j.pnpbp.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Wenner JA, Thomas KM, Glatt CE, McKenna MC, Clark AG. The brain-derived neurotrophic factor Val66Met polymorphism moderates early deprivation effects on attention problems. Development and Psychopathology. 2012;24(4):1215–1223. doi: 10.1017/S095457941200065X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C, Adrian C, Gordon D, Burge D, Jaenicke C, Hiroto D. Children of depressed mothers: maternal strain and symptom predictors of dysfunction. Journal of Abnormal Psychology. 1987;96:190–198. doi: 10.1037/0021-843X.96.3.190. [DOI] [PubMed] [Google Scholar]
- Hammen C, Brennan PA. Depressed adolescents of depressed and nondepressed mothers: tests of an interpersonal impairment hypothesis. Journal of Consulting and Clinical Psychology. 2001;69(2):284–294. doi: 10.1037//0022-006x.69.2.284. doi:1037/0022-006X.69.2.284. [DOI] [PubMed] [Google Scholar]
- Hammen C, Brennan PA, Keenan-Miller D, Hazel NA, Najman JM. Chronic and acute stress, gender, and serotonin transporter gene-environment interactions in predicting depression symptoms in youth. Journal of Child Psychology & Psychiatry. 2010;51(2):180–187. doi: 10.1111/j.1469-7610.2009.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Zhu G, Lipsky R, Goldman D. HTTLPR allele expression is codominant, correlating with gene effects on fMRI and SPECT imaging intermediate phenotypes, and behavior. Biological Psychiatry. 2004;55(suppl 1):191S. [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Archives of General Psychiatry. 2011;68(5):444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Yang B, Douglas-Palumberi H, Grasso D, Lipschitz D, Houshyar S, Krystal JH, Gelernter J. Brain-derived neurotrophic factor-5-HTLPR gene interactions and environmental modifiers of depression in children. Biological Psychiatry. 2006;59(8):673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Keeping JD, Najman JM, Morrison J, Western JS, Andersen MJ, Williams GM. A prospective longitudinal study of social, psychological, and obstetrical factors in pregnancy: response rates and demographic characteristics of the 8,556 respondents. British Journal of Obstetrics and Gynecology. 1989;96:289–297. doi: 10.1111/j.1471-0528.1989.tb02388.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Baker JH. Genetic influences on measures of the environment: a systematic review. Psychological Medicine. 2007;37:615–626. doi: 10.1017/S0033291706009524. [DOI] [PubMed] [Google Scholar]
- Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Kim YH, Yoon JS. Interactions between life stressors and susceptibility genes (5-HTTLPR and BDNF) on depression in Korean elders. Biological Psychiatry. 2007;62:423–428. doi: 10.1016/j.biopsych.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527. doi: 10.1126/science/274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lovejoy MC, Graczyk PA, O’Hare E, Neuman G. Maternal depression and parenting behavior: A meta-analytic review. Clinical Psychology Review. 2000;20(5):561–592. doi: 10.1016/s0272-7358(98)00100-7. http://dx.doi.org/10.1016/S0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Lu B. Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology Reviews. 2008;33:73–83. doi: 10.1038/sj.npp.1302571. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nature Neuroscience. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. TRENDS in Neuroscience. 2004;27(10):589–594. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- McGuffin P, Alsabban S, Uher R. The truth about genetic variation in the serotonin transporter gene and response to stress and medication. The British Journal of Psychiatry. 2011;198:424–427. doi: 10.1192/bjp.bp.110.085225. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Simons AD. Diathesis-stress theories in the context of life stress research: Implications for the depressive disorders. Psychological Bulletin. 1991;110:406–425. doi: 10.1037/0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]
- Nederhof E, Bouma EMC, Oldehinkel AJ, Ormel J. Interaction between childhood adversity, brain-derived neurotrophic factor val/met and serotonin transporter polymorphism on depression: the TRAILS study. Biological Psychiatry. 2010;68(2):209–212. doi: 10.1016/j.biopsych.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Pan H, Neidig P, O’Leary KD. Male-female aggressor-victim differences in the factor structure of the Modified Conflict Tactics Scale. Journal of Interpersonal Violence. 1994;9:366–382. doi: 10.1177/088626094009003006. [DOI] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis? Journal of Educational and Behavioral Statistics. 2006;31:437–448. doi: 10.3102/10769986031004437. [DOI] [Google Scholar]
- Rice F, Harold GT, Thapar A. Assessing the effects of age, sex, and shared environment on the genetic aetiology of depression in childhood and adolescence. Journal of Child Psychiatry and Psychology. 2002;43(8):1039–1051. doi: 10.1111/1469-7610.00231. [DOI] [PubMed] [Google Scholar]
- Roisman GI, Newman DA, Fraley RC, Haltigan JD, Groh AM, Haydon KC. Distinguishing differential susceptibility from diathesis stress: Recommendations for evaluating interaction effects. Development and Psychopathology. 2012;24:389–409. doi: 10.1017/S0954579412000065. [DOI] [PubMed] [Google Scholar]
- Shih JH, Eberhart N, Hammen C, Brennan PA. Differential exposure and reactivity to interpersonal stress predict sex differences in adolescent depression. Journal of Clinical Child and Adolescent Psychology. 2006;35:103–115. doi: 10.1207/s15374424jccp3501_9. [DOI] [PubMed] [Google Scholar]
- Spanier GB. Measuring dyadic adjustment: new scales for assessing the quality of marriage and similar dyads. Journal of Marriage and the Family. 1976;38:15–28. doi: 10.2307/530547. [DOI] [Google Scholar]
- Verhagen M, van der Meij A, van Deurzen PA, Janzing JG, Arias-Vasquez A, Buitelaar JK, Franke B. Meta-analysis of the BDNF Val66Met polymorphism in major depressive disorder: effects of gender and ethnicity. Molecular Psychiatry. 2010;15(3):260–271. doi: 10.1038/mp.2008.109. [DOI] [PubMed] [Google Scholar]
- Weich S, Patterson J, Shaw R, Stewart-Brown S. Family relationship in childhood and common psychiatric disorders in later life: systematic review of prospective studies. The British Journal of Psychiatry. 2009;194:392–398. doi: 10.1192/bjp.bo.107.042515. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Gammon G, John K, Merikangas KR, Warner V, Prusoff BA, Sholomskas D. Children of Depressed Parents: Increased Psychopathology and Early Onset of Major Depression. Archives of General Psychiatry. 1987;44(10):847–853. doi: 10.1001/archpsyc.1987.01800220009002. [DOI] [PubMed] [Google Scholar]
- Wichers M, Kenis G, Jacobs N, Mengelers R, Derom C, Vlietnick R, van Os J. The BDNF Val(66)Met × 5-HTTLPR × child adversity interaction and depressive symptoms: an attempt at replication. American Journal of Medical Genetics Branch of Neuropsychiatric Genetics. 2008;147B(1):120–123. doi: 10.1017/S0033291709005248. [DOI] [PubMed] [Google Scholar]