Abstract
β2-adrenergic agonists (β-agonists) have been legally used in the U.S. for almost two decades to increase lean muscle mass in meat animals. Despite a cardiotoxic effect after high-dose exposure, there has been limited research on human β-agonist exposures related to meat consumption. We quantified urinary concentrations of ractopamine and zilpaterol, two FDA-approved β-agonist feed additives, and examined the extent to which the concentrations were associated with estimated usual meat intake levels. Overnight urine samples from 324 newly diagnosed breast cancer patients and spot urine samples from 46 lung cancer patients at the time of diagnosis, prior to treatment, were collected during 2006–2010 and 2014–2015, respectively. Urinary ractopamine and zilpaterol concentrations were measured by LC-MS/MS. Ractopamine and zilpaterol, respectively, were detected in 8.1% and 3.0% of the urine samples collected (n=370). Only 1.1% (n=4) of the urine samples had zilpaterol concentrations above the limit of quantification, with the mean value of 0.07 ng/mL in urine. The presence of detectable ractopamine and zilpaterol levels were not associated with meat consumption estimated from a food frequency questionnaire, including total meat (P=0.13 and 0.74, respectively), total red meat (P=0.72 and 0.74), unprocessed red meat (P=0.74 and 0.73), processed red meat (0.72 and 0.15), and poultry intake (P=0.67 for ractopamine). Our data suggest that the amount of meat-related exposure of β-agonists was low.
Keywords: β2-adrenergic agonists, ractopamine, zilpaterol, urine, cancer, meat consumption
Graphical Abstract

Introduction
Animal feed additives that could potentially cause adverse effects in humans exist at low concentrations, but widely in meat products.1,2 Although regulatory frameworks supporting the safe use of such additives are aimed at protecting human consumers, residue exposures are rarely studied at the population level.3,4 Since the mid to late 1980s, β2-adrenergic agonists (β-agonists), which are smooth muscle relaxants used clinically for bronchodilation, have been used both illicitly5,6 and legally7 in livestock feeds. These compounds alter the ratio in which dietary energy intake is partitioned between lean and fat tissue,8 and this “repartition” promotes leaner muscle and growth, resulting in increased profits. β-Agonists used in livestock differ greatly in terms of potency9 and bioavailability.10 Misuse of clenbuterol, a β-agonist with high oral potencies, in food animals has led to adverse effects in humans, including increasing heart rate and blood pressure, anxiety, palpitation, and skeletal muscle tremors after consumption of meats and livers containing β-agonists.11,12 Thus, clenbuterol has been banned worldwide for any growth uses in food animals.5,13
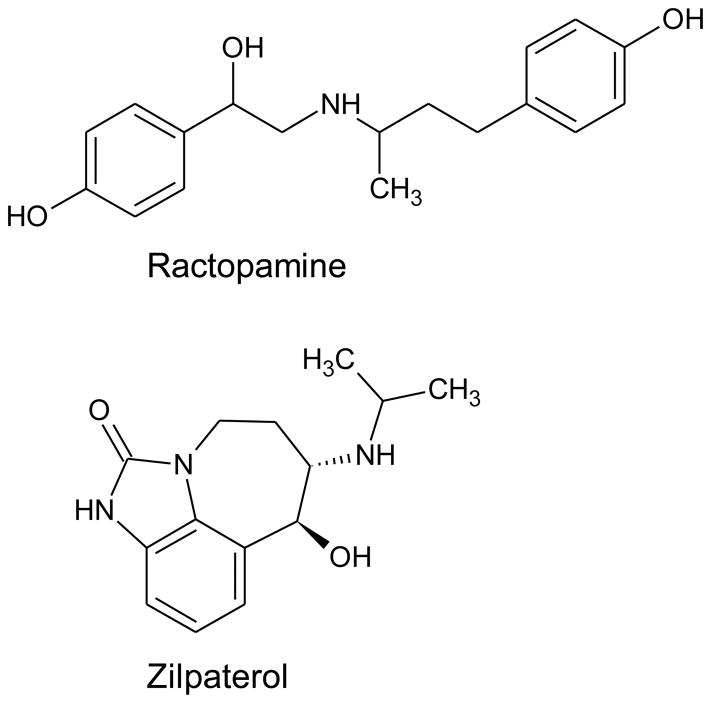
Currently two β-agonists have received approval by the U.S. Food and Drug Administration (FDA) for use as livestock feed additives: ractopamine hydrochloride (under trade names Paylean® for use with swine since 1999, Optaflexx® for cattle since 2003, and Topmax® for turkey since 2008) and zilpaterol hydrochloride (under trade name Zilmax® for cattle since 2006; Figure 1). Ractopamine and zilpaterol are polar β-agonists with lower oral bioavailability and shorter plasma half-lives than clenbuterol.10 It is estimated that 70–80% of commercially raised beef and pork in the U.S. are fed ractopamine or zilpaterol.14,15 Although the potencies of ractopamine and zilpaterol are much lower than clenbuterol,9 serious side effects, including mortality, have been attributed to these β-agonists in cattle and swine.15,16 Because 0- and 3-day withdrawal periods are required for ractopamine and zilpaterol, respectively, prior to slaughter, detectable residues of ractopamine remain quantifiable in pig muscle 5 to 7 days after an animal is slaughtered and in some edible organs for a longer period.17 A market survey of swine kidney showed that of 278 samples, 37% had detectable ractopamine residues (Shelver, unpublished data). For ractopamine, the US FDA allows up to 30, 50, and 100 ppb of ractopamine residue in raw muscles of cattle, hogs, and turkeys, respectively; for raw livers the allowable residue is 90, 150, and 450 ppb, respectively.18 For zilpaterol, the FDA allows 12 ppb of freebase equivalent in uncooked liver of cattle.19 In human, the Acceptable Daily Intake (ADI) values set by FDA are 1.25 μg/kg-body weight (BW)/day for ractopamine18 and 0.083 μg/kg-BW/day for zilpaterol19 (ADIs set by the United Nations/WHO are 0–1 and 0–0.04 μg/kg-BW/day, respectively)17 to avoid adverse cardiac effects. Hsieh et al. have established that cooking may not be able to completely degrade ractopamine.20 Thus, human exposure to residues of approved β-agonists is expected in countries for which approvals exist, but no published research has assessed exposure levels in these countries. In addition to cardiotoxicity, animal and preclinical data suggest these agents can lead to certain tumors and have effects on cell proliferation, if dosed at sufficient rates.21,22 Thus, understanding the exposures to residue levels in humans is important for research on risk assessment as well as examining potential health effects.
Figure 1.
Chemical structures of ractopamine and zilpaterol
Here, we evaluated human exposures to ractopamine and zilpaterol residues by quantifying each β-agonist in pre-surgical urine samples collected from a group of breast and lung cancer patients with data on usual meat intake. We measured these β-agonists in urine as a means of assessing exposure, as analytical methods with very high sensitivity have been established for animal urine testing23–25 and because previous studies have established that surveillance of human urine allows assessment of human exposure to β-agonist residues in food.26 In addition, we examined whether the detectable concentrations of the β-agonist were associated with meat intake levels. We hypothesized that individuals with higher levels of meat intake, including red meat and poultry, were more likely to have detectable urinary concentrations of the β-agonists used as feed additives than those with lower levels of meat intake.
Methods
Study patients
Breast cancer patients included in this study were participants in the Women’s Health after Breast Cancer (ABC) Study, a hospital-based prospective cohort study. ABC participants were women with incident breast cancer treated at Roswell Park Cancer Institute (RPCI) and initially enrolled in the Institute’s Data Bank and BioRepository (DBBR). Detailed methods of the DBBR have published elsewhere.27 Briefly, 423 early-stage (0 to IIIa), non-metastatic breast cancer patients were recruited between March 17, 2006 and April 22, 2010. The initial goal of the study was to examine determinants of weight gain after breast cancer diagnosis. As part of the DBBR protocol, a set of standardized questionnaires was administered at diagnosis to collect information on demographic, lifestyle factors, dietary intake, use of supplemental vitamins, and prescription and non-prescription drug use. Clinical data were abstracted and anthropometric measures were obtained by trained staff. Overnight urine samples were collected on the morning of surgery at the time of diagnosis and 12-months post diagnosis. Participants were instructed to void just before going to bed in the evening or at 11 pm and collect all urine passed overnight until the first void in the morning. Urine samples were brought in within about 3–4 hours of collection and aliquoted as unfractionated samples and stored in −80 ºC freezers until analysis. For the current study, we included ABC participants contributing urine samples at the time of diagnosis, who also had food frequency questionnaire (FFQ) data from DBBR (n=324).
Lung cancer patients were also recruited via the DBBR protocol. A total of 46 pre-surgical patients were recruited between March 2014 and January 2015. We included this group of patients because the recruitment period provided an opportunity to study more recent exposure compared to the ABC Study, as the use of β-agonists may have been more common in livestock feeds. No restrictions were applied for lung cancer stage or histology to maximize the number of individuals recruited. The DBBR questionnaire was administered and a spot urine sample was collected at the RPCI thoracic clinic. Urine samples were aliquoted as unfractionated samples and stored in −80 ºC freezers on the same day of collection. Written informed consent was obtained from both the breast and lung cancer patients. The study was approved by the institutional review board at RPCI.
Laboratory assays
Material and sample preparation
Laboratory analysis of urinary β-agonists was performed at the USDA-ARS Biosciences Research Laboratory. The study protocol was approved by the Institutional Biosafety Committee. Sample aliquots (10 mL) were shipped via overnight delivery on dry-ice to the USDA laboratory; upon receipt, all samples were stored at −80 °C. Liqua-Trol human urinalysis control was obtained from KOVA International Inc. (Garden Grove, CA). d6-ractopamine and d7-zilpaterol were obtained from Toronto Research Chemicals (Toronto, Ontario, Canada). β-Glucuronidase/aryl sulfatase from Patella vulgata was purchased from Sigma-Aldrich (St. Louis, MO). Ractopamine hydrochloride was a gift from Elanco, Greenfield, IN. Ractopamine glucuronide was synthesized and purified as previously described.28 Zilpaterol hydrochloride was a gift from Houchest-Rousell (Clinton, NJ). SELECTRASORB™ CLEAN-UP® C18 solid phase extraction media was purchased from United Chemical Technologies, Inc. (Bristol, PA). PTFE syringe filters were purchased from Grace Davidson Discovery Sciences (Deerfield, IL).
To 2 mL of control urine or incurred samples, 100 μL (20 ng/mL) of deuterated internal standard (ractopamine and zilpaterol), 250 units of β-glucuronidase/aryl sulfatase, and 80 μL of 2M ammonium acetate, pH 5.2 were added. Samples were then mixed, and incubated at 37 °C for 16 h with constant shaking at 50 rpm. Matrix-matched calibration standards were prepared from control urine by the sequential addition of enzyme, buffer, and 100 μL of working β-agonist standard as free-base equivalents for final concentrations of 1, 2, 10, 20, 100, and 200 ng/mL. To validate the activity of the β-glucuronidase solution 100 μL of a 31.7 ng/mL ractopamine glucuronide solution was added to 2 mL of control urine containing enzyme, buffer, and ractopamine internal standard as described above. Enzyme activity was validated with each sample set run. After the 16-h hydrolysis period, 160 μL of 2M sodium carbonate, 100 mg of sodium chloride, and 100 mg of C18 sorbent were added to each sample. Samples were subsequently extracted with ethyl acetate (1 mL × 3), followed by centrifuging at 3,000 × g for 10 min. The supernatant was separated, placed in 7-mL tubes and evaporated under a stream of nitrogen. The residue was reconstituted in 200 μL of 20% aqueous acetonitrile containing 0.1% formic acid and passed through a 0.45 μm PTFE filter. The solution was stored at −20 °C in a LC-MS vial within a silanized vial insert until analyzed.
LC-MS/MS analysis
Sample analysis was conducted on a Waters Acquity UPLC system in conjunction with a Waters triple quadrupole mass spectrometer. Sample aliquots (10 μL) were injected onto an ACQUITY UPLC™ BEH C18 column (1.7 μm, 2.1 × 50 mm; Waters, Milford, MA) equipped with a VanGuard pre-column (1.7 μm, 2.1 × 5 mm; Waters, Milford, MA). The autosampler was maintained at 4 °C and the chromatography guard and analytical columns at 45 °C. The binary gradient system consisted of solvent A, 5% MeOH/H2O containing 0.01% formic acid and solvent B, 100% MeOH containing 0.01% formic acid. Solvent program was 0 to 1.9 min 0% B → 100% B; 1.9 to 3.4 min 100% B; 3.4 to 3.41 min 100% B → 0% B; 3.41 to 7 min 0% B at a flow rate of 0.4 mL/min.
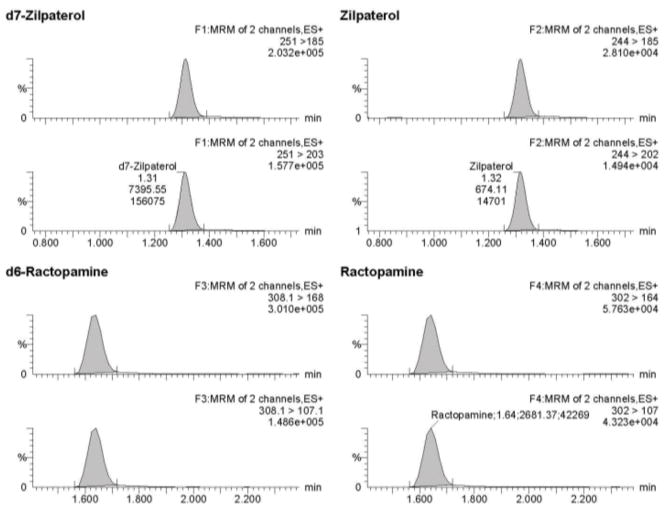
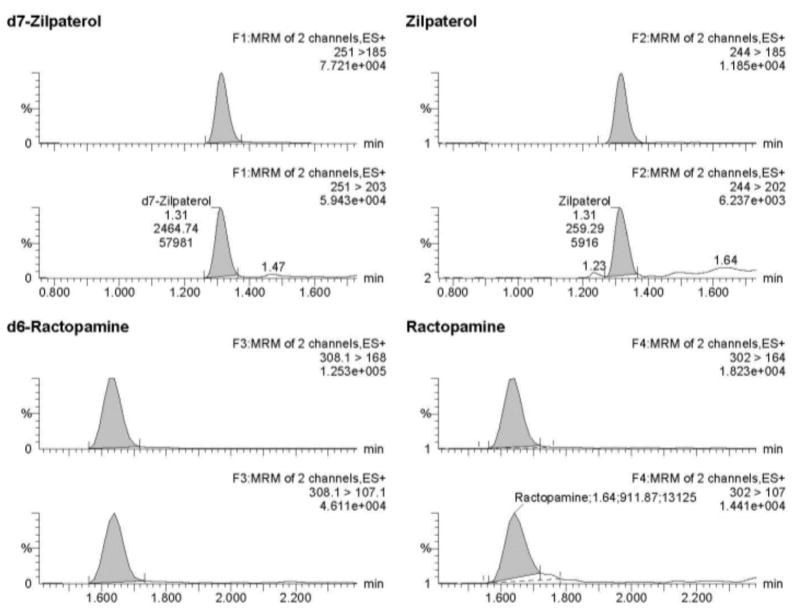
Data were acquired, processed and quantified using MassLynx™ 4.1 with TargetLynx™ systems (Waters Corporation, Milford, MA). Mass spectrometric conditions for ractopamine, zilpaterol, ractopamine-d6, and zilpaterol-d7 were optimized by direct infusion using electrospray ionization in the positive mode. To this end, optimal precursor ion, product ions, and the optimum collision energies and cone voltage were collected using AutoTune Wizard with the MassLynx™ 4.1 software. The desolvation temperature was set at 500 °C and the source temperature was set at 150 °C. Nitrogen, used as the cone gas, was set at 50 L/hr, desolvation gas flow was set at 800 L/hr and the collision gas flow of argon was set at 0.18 mL/min. Ions were monitored in the multiple reaction monitoring mode. Quantitation of ractopamine and zilpaterol were based on ion transitions of m/z 302 →164, and 244 →185 respectively. Qualification ion transitions were m/z 302 → 107 and 244 →202 for ractopamine and zilpaterol, respectively. Parameters for multiple reaction monitoring are listed in Table 1 and typical mass chromatograms are shown in Figure 2. Unknown concentrations were determined by LC-MS/MS using a matrix-matched standard curve with ractopamine-d6 and zilpaterol-d7 serving as internal standards; linear regressions were established with 1/x weighting. The limit of detections (LOD) and limit of quantifications (LOQ) were calculated based on slope and standard deviation of intercept using the mean of three calibration curves. Concentrations above LOQ were reported only for samples with a signal-to-noise >10. The mean coefficients for linearity of calibration curves were 0.9968 for ractopamine and 0.9996 for zilpaterol (n = 35). The efficiency of converting ractopamine-glucuronide into ractopamine was 93.8% with a CV of 12.4% (n=34, omitting one sample due to mis-spiked concentration). Recoveries of ractopamine and zilpaterol from samples fortified to final concentrations of 1, 10, and 30 ng/mL were 78% to 120% with CVs of less than 10% (Table 2).
Table 1.
Parameters for multiple reaction monitoring
| Compound | Parent (m/z) | Product ion (m/z) | Collision energy (eV) | Cone voltage (V) |
|---|---|---|---|---|
| Ractopamine | 302 | 164 | 15 | 30 |
| 107 | 35 | 30 | ||
| Zilpaterol | 244 | 202 | 20 | 45 |
| 185 | 25 | 45 |
Figure 2.
MS/MS mass chromatograms for ractopamine and zilpaterol. Panel A represents 1 ng/mL of ractopamine and zilpaterol in 20% acetonitril/0.1% formic acid; Panel B represents final concentration of 1ng/mL of ractopamine and zilpaterol from urine extract (original urine concentration 0.1 ng/mL).
Table 2.
Validation data of ractopamine and zilpaterol in urine
| Drug added (ng/mL) | Recovery (%, n=6) | Intra-assay repeatability (% CV, n=6) | Recovery (%, n=35) | Inter-assay reproducibility (% CV, n=35) |
|---|---|---|---|---|
| Ractopamine | ||||
| 0.1 | 119.7 | 5.3 | 93.2 | 8.4 |
| 1 | 89.3 | 5.9 | 85.3 | 8.5 |
| 3 | 79.5 | 3.2 | 92.2 | 9.6 |
| Zilpaterol | ||||
| 0.1 | 77.5 | 5.3 | 91.3 | 6.6 |
| 1 | 89.4 | 1.3 | 95.9 | 3.2 |
| 3 | 78.4 | 1.8 | 96.0 | 6.7 |
Urine creatinine was measured on Vitros Fusion 5.1. Clinical Chemistry Analyzer (Ortho Clinical Diagnostics) using a slide method at RPCI Clinical Laboratories.
Assessment of meat intake
Average daily meat intake (g/d) was estimated from a self-administered FFQ adapted from the Fred Hutchinson Cancer Research Center’s GSEL-FFQ, which has been validated against multiple 24-h dietary recalls and 4-day food records.29 The FFQ queried both usual frequency (never, <1/month, 1/month, 2–3/month, 1/week, 2/week, 3–4/week, 5–6/week, 1/day, and 2+/day) and portion size (S, M, L) for approximately 125 food items, including meat and poultry, during the prior year. Intake levels for each food item were calculated as grams/day from the frequency and portion sizes specified in the FFQ. For the purpose of this study, we defined unprocessed meat as beef, pork, and lamb, which were queried together with ham as one food item. Processed meats, including lunchmeats, bacon, sausages, bratwursts, chorizo, salami, and hot dogs were queried from 6 food items. We summed the intake levels of unprocessed meat and processed meats to derive total red meat intake. Poultry intake was queried from two food items: “fried chicken, including nuggets and tenders” and “roasted, stewed, grilled, or broiled chicken and turkey,” and hence did not include processed poultry. Liver, i.e., chicken liver and organ meats, was queried from 1 food item. Subsequently, we derived total meat intake as the summation of total red meat, poultry, and liver intake levels. We did not examine liver intake as a separate category because this food was consumed by too few participants. Meat intake values were missing for 2 (0.6%) breast cancer patients and 22 (48%) lung cancer patients who did not provide FFQ data. The analysis on the association between urinary β-agonists concentrations and usual meat intake was restricted to 346 patients with the intake data.
Statistical analysis
Descriptive statistics were calculated for baseline characteristics (sex, age, race, and education) and meat intake levels for the two study patients groups (n=370). We reported the number of individuals with urinary β-agonists concentrations (i) greater than LOD values but equal or lower than LOQ values and (ii) greater than LOQ values. Means and standard deviations (SD) of ractopamine and zilpaterol concentrations in urine were reported for samples with the concentrations greater than LOQ values. We assessed the association of meat intake levels with urinary β-agonists concentrations using logistic regression. For each meat intake category, patients were grouped into above and below median of intake based on the median values in the respective groups. Comparing patients with above median intake to those with below median intake, odds ratios (ORs) and 95% confidence intervals (CIs) were estimated for detectable (i.e., measured concentrations > LOD) versus non-detectable values of the β-agonists. All statistical analyses were performed using Stata 12 (College Station, TX).
Results
Demographic characteristics of the breast and lung cancer patients are shown in Table 3. The estimated meat intakes were similar between the two patient groups: 80.5 g/d in the breast cancer patients and 76.9 g/d in the lung cancer patients. Approximately two-thirds of the consumption was red meat and one-third was poultry. Among total red meat intake, approximately half was unprocessed meat.
Table 3.
Characteristics and estimated meat intake levelsa of breast and lung cancer patients
| Characteristic | Breast cancer patients (n=324) | Lung cancer patients (n=46) |
|---|---|---|
| Sex (female) | 324 (100%) | 19 (41.3%) |
| Age (years) | ||
| <45 | 45 (13.9%) | 0 (0%) |
| 45 – 59 | 148 (45.7%) | 14 (30.5%) |
| 60 – 74 | 109 (33.6%) | 25 (54.3%) |
| ≥75 | 17 (5.2%) | 7 (15.2%) |
| Missing | 5 (1.5%) | 0 (0%) |
| Race | ||
| Caucasian | 300 (92.6%) | 43 (93.5%) |
| African American | 19 (5.9%) | 1 (2.1%) |
| Others | 5 (1.5%) | 2 (4.4%) |
| Education | ||
| Less than high school | 10 (3.1%) | 2 (4.4%) |
| High school | 80 (24.7%) | 7 (15.2%) |
| Greater than high school | 231 (71.3%) | 12 (26.1%) |
| Missing | 3 (0.9%) | 25 (54.3%) |
| Total meat intake (g/d) | ||
| Mean ± SD | 80.5 ± 47.4 | 76.9 ± 53.1 |
| Median (Q1–Q3) | 74.9 (46.8–106.2) | 64.3 (37.6–111.5) |
| Total red meat intake (g/d) | ||
| Mean ± SD | 58.5 ± 40.0 | 56.8 ± 40.0 |
| Median (Q1–Q3) | 50.6 (30.1–78.9) | 54.6 (21.9–85.6) |
| Unprocessed meat intake (g/d) | ||
| Mean ± SD | 30.0 ± 27.4 | 25.4 ± 20.7 |
| Median (Q1–Q3) | 23.9 (8.6–40.5) | 29.8 (5.2–39.9) |
| Processed meat intake (g/d) | ||
| Mean ± SD | 29.1 ± 22.4 | 31.4 ± 26.2 |
| Median (Q1–Q3) | 23.3 (14.3–37.4) | 25.3 (11.3–48.8) |
| Poultry intake (g/d) | ||
| Mean ± SD | 22.3 ± 16.8 | 18.1 ± 16.0 |
| Median (Q1–Q3) | 18.4 (9.2–30.4) | 12.3 (4.5–31.3) |
SD, standard deviation
Estimates were based on 322 breast cancer patients and 24 lung cancer patients with FFQ data.
For the ractopamine and zilpaterol analysis, method LODs were 0.26 and 0.10 ng/mL and LOQs were 0.79 and 0.30 ng/mL, respectively. These corresponded to urine concentrations of 0.026, 0.01, 0.079, and 0.030 ng/mL when adjusted to the original volume (Table 4). Among all urine samples, 8.1% (n=30) had ractopamine concentrations between the LOD and LOQ, but no sample had concentrations above the LOQ. For zilpaterol, 1.9% (n=7) of samples had concentrations between the LOD and LOQ and 1.1% (n=4) had concentrations above the LOQ. The mean concentration of zilpaterol in these samples was 0.07 ng/mL (0.17 ng/μg urine creatinine). The majority of samples with detectable concentrations belonged to the breast cancer patients (93% for ractopamine and 100% for zilpaterol; data not shown).
Table 4.
Method LOD and LOQ values, percentages of samples above these values, and concentrations of ractopamine and zilpaterol in the study population
| β-agonist compound | Number of urine samples (N) | LOD (ng/μL) | LOQ (ng/μL) | N (%) with values between LOD and LOQ | N (%) with values >LOQ | Concentrations (ng/mL)a,b | Concentrations (ng/μg urine creatinine)a |
|---|---|---|---|---|---|---|---|
| Ractopamine | 370 | 0.026 | 0.079 | 30 (8.1%) | 0 (0%) | – | – |
| Zilpaterol | 370 | 0.010 | 0.030 | 7 (1.9%) | 4 (1.1%) | 0.07 ± 0.05 | 0.17 ± 0.28 |
LOD, limit of detection; LOQ, limit of quantification.
Mean ± SD in the samples with concentrations > LOQ
Corrected for the concentration factor from the original urine volume.
Table 5 shows the association of detectable (versus non-detectable) ractopamine and zilpaterol concentrations with estimated meat intake levels. Compared to patients with lower meat intake levels (below median), detectable urinary ractopamine concentrations were less likely to be observed among patients with higher meat intake levels (above median; for total meat, OR=0.54, 95% CI=0.24–1.20). This association was not statistical significant at the 0.05 level and the same pattern was also observed for the other meat intake categories, i.e., total red meat, unprocessed meat, processed meat, and poultry. However, for zilpaterol, detectable urinary concentrations were more likely to be observed among patients with higher meat intake levels, compared to patients with lower meat intake levels (for total meat, OR=1.22, 95% CI=0.10–1.41), except for processed meat (OR=0.37, 95% CI=0.10–1.41). None of the associations for zilpaterol were significant.
Table 5.
Association between detectable β-agonist compounds and estimated meat intake (n=346)
| Meat intake | Below/above mediana | Ractopamine | Zilpaterol | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Det./ND | OR (95% CI) | P-value | Det./ND | OR (95% CI) | P-value | ||
| Total meat | Below | 18/156 | 1.00 | 5/169 | 1.00 | ||
| Above | 10/162 | 0.54 (0.24–1.20) | 0.13 | 6/166 | 1.22 (0.37–4.08) | 0.74 | |
| Total red meat | Below | 15/159 | 1.00 | 5/169 | 1.00 | ||
| Above | 13/159 | 0.87 (0.40–1.88) | 0.72 | 6/166 | 1.22 (0.37–4.08) | 0.74 | |
| Unprocessed meat | Below | 15/160 | 1.00 | 5/170 | 1.00 | ||
| Above | 13/158 | 0.88 (0.41–1.90) | 0.74 | 6/165 | 1.24 (0.37–4.13) | 0.73 | |
| Processed meat | Below | 15/159 | 1.00 | 8/166 | 1.00 | ||
| Above | 13/159 | 0.87 (0.40–1.88) | 0.72 | 3/169 | 0.37 (0.10–1.41) | 0.15 | |
| Poultry | Below | 15/157 | 1.00 | ||||
| Above | 13/161 | 0.85 (0.39–1.83) | 0.67 | – b | |||
Det., detectable; ND, non-detectable; OR, odds ratio; CI, confidence interval
The median values are, for total meat intake, 74.9 g/d in breast cancer patients and 64.3 g/d in lung cancer patients; for total red meat intake, 50.6 g/d in breast cancer patients and 54.6 g/d in lung cancer patients; for unprocessed meat intake, 23.9 g/d in breast cancer patients and 29.8 g/d in lung cancer patients; for processed meat intake, 23.3 g/d in breast cancer patients and 25.3 g/d in lung cancer patients.
Not calculated because zilpaterol is only approved in cattle.
Discussion
To our knowledge, this is the first study reporting an assessment of urinary concentrations of FDA-approved β-agonist feed additives in a group of meat consumers from the United States, a country in which both ractopamine and zilpaterol are approved livestock feed additives. Ractopamine residues have been investigated in urine collected from individuals (n = 21) in Taiwan;30 negative findings in Taiwan are not surprising since β-agonists are banned from use in food animals in the country. In this study, in urine samples from two groups of patients with newly-diagnosed breast and lung cancer, detectable levels of ractopamine or zilpaterol occurred, albeit in a low number of samples and at very low concentrations. Because neither ractopamine nor zilpaterol are used in human medicine, nor are they generally available to US consumers, the detection of urinary residues suggests that exposures likely occurred through the consumption of meat. A possibility for the observation of low urinary concentrations of β-agonists is that not all the meat consumed by the participants contained a meaningful level of β-agonist residue. Data reporting concentrations of retail meat products for β-agonist residues are sparse. According to the USDA Food Safety and Inspection Service (FSIS), the percentages of meat products containing β-agonist residues were low.1,2 In 2013, 14 cattle liver (mean=36 ppb), 1 cattle muscle (2.6 ppb), and 6 swine liver (mean=32 ppb) samples were detected with ractopamine, with an overall detection rate of 1.2% (21 out of 1,231 cattle and 575 swine samples).1 No violation, i.e., a concentration of ractopamine ≥90 ppb for cattle liver, ≥30 ppb for cattle muscle, and ≥150 ppb for swine liver,18 was found. In 2014, 36 cattle liver (mean=41 ppb), 5 cattle muscle (mean=9.5 ppb), and 3 swine liver (mean=39 ppb) samples were detected with ractopamine; a single violation was found in cattle liver (ractopamine concentration= 128 ppb).2 The overall detection rate for ractopamine was 1.9% (45 out of 1,561 cattle and 775 swine samples). Zilpaterol was not tested in 2013; however, in 2014 there was one violative sample of cattle muscle (concentrations not quantified). A possible limitation of the residue surveillance program is that the number of samples tested, although statistically designed, is minute relative to the amount of meat in the U.S. market. Due to the fact that US consumers have available a variety of meat products from a diverse number of sources (organic, home-raised, commercially raised, processed and non-processed products, etc.), variation associated with using urinary biomarkers to assess feed-related β-agonist exposure within any given individual can be large. Thus, to investigate β-agonist exposures of clinical significance using urine, multiple urine samples collected from a large number of individuals over time may be needed.
Dietary exposure to β-agonist residues has been hypothesized to have broad negative implications on human health and well-being.31 Acute adverse effects associated with β-agonists in general, and especially those used in human therapy, are well known and are predictable.32,33 They include increased heart rate and blood pressure, anxiety, palpitation and skeletal muscle tremor. These adverse effects, and others, have been noted in the instances of food poisoning that have occurred in Europe and Asia caused by clenbuterol residues in illicitly-treated animals.5,34–36 In addition to heart and vascular smooth muscle tissues, β2-receptors are also located in many organs including the intestine, breast, and lung and bronchus. Although experimental data showed that ractopamine and zilpaterol are not mutagenic or genotoxic, exposures in mice consistently increases the incidence of uterine leiomyoma, which is an overgrowth of smooth muscle and connective tissue in the uterus, through non-genotoxic mechanisms.21 β-Agonists, in general, may promote cell proliferation and tumor growth through signaling the cyclic adenosine monophosphate (cAMP) and mitogen-activated protein kinase (MAPK) pathways.22,37 In addition, based on in silico models, ractopamine has been hypothesized to act as an endocrine disruptor by activating estrogen receptor (ER)-α-mediated gene transcription,38 an important pathway of breast carcinogenesis. This hypothesis needs further research taking other pharmacokinetic factors, such as bioavailability, into account, as the potency of ractopamine to ER is several orders less than estrogen.38 Epidemiological research investigating clinical use of β-agonists and β-blockers would be able to shed light on whether β-agonists are associated with human breast cancer development and outcomes. However, the evidence is inconsistent between these two types of medications. Studies suggest that conditions requiring β-agonists as treatments, such as asthma, are not associated with breast cancer risk,39 while individuals who use β-blockers (versus non-users) had a lower risk of breast cancer among healthy women and mortality among breast cancer patients.40 Because the potencies and exposure levels of these medications are much higher than the exposure to β-agonist residues from meat intake, as suggested by our data, it may be difficult to observe direct effects from the low-does, albeit long-term exposure from diet.
In humans, β-agonists (e.g. ractopamine) containing phenolic hydroxyl groups, are metabolized in the liver and intestine through glucuronidation and sulfation by UDP-glucuronosyltransferase (UGT) 1A6 and 1A9 and sulfotransferase (SULT1A3).21,41 According to Smith and Rodewald,42 less than 5% of the total ractopamine dose in humans is excreted in urine as parent ractopamine, with the balance eliminated as mono glucuronide and mono sulfate conjugates. Thus, at the low levels of ractopamine encountered as a residue in meat, one would expect almost no free ractopamine to be present in urine. Consequently, we measured total urinary β-agonist residue after enzymatic hydrolysis of sulfate and glucuronide conjugates.24 Even with hydrolysis of conjugates, parent ractopamine and zilpaterol were detected in only 41 of 370 samples analyzed, with only 4 samples containing quantifiable residue. Although conjugation serves to hasten the elimination of xenobiotics in humans, potential health risks due to low-dose exposure(s) to β-agonist residues should not be dismissed.43 Free-form β-agonists can be attracted to organs with a high density of β2-receptor, such as the lung.44 In addition, the rate and catalytic activity of conjugation are likely to vary by polymorphisms of UGT and SULT genes.45,46 More data are needed to reveal tissue- and organ-specific exposure and potential high-risk populations.
Ractopamine and zilpaterol are both metabolized quickly relative to other β-agonists which have caused human toxicities after illicit use.10 For example, ractopamine has a half-life of approximately 4 hours in blood.10,42,47 In humans orally dosed with 40 mg of ractopamine, approximately 33% of the total dose was excreted in urine (mostly as conjugates) within 6 hours, and 45.7% of the total dose was excreted in urine by 24 hours.21,42 Thus, a significant portion of any ractopamine residue present in consumed meat would be expected to be excreted in the overnight urine, which was collected from 11 pm to the morning of the next day in the breast cancer patients of the ABC Study. In the enrolled lung cancer patients, on the other hand, spot urine collected during the day, i.e., random specimen, might have been less ideal than overnight or 24-hour urine collections.48 It is also unclear whether the observed detectable concentrations from the breast cancer patients but not the lung cancer patients are due to differences in urine collection methods, amount of residues in the meat consumed, or sample sizes. We were also unable to determine the effects that cancer itself might have had on ractopamine or zilpaterol metabolism and their urinary excretion patterns. Thus, a well-controlled study in which healthy individuals ingest similar quantities of β-agonist residue is needed to examine the utility of different urine collection methods for assessing dietary β-agonist exposure.
We did not observe a clear relationship between urinary concentrations of β-agonists and estimated average daily meat consumption. An important limitation of this design was that the meat intake levels available in the study samples were estimated by an FFQ. The FFQ queried food intake during the preceding year, with the purpose of estimating ordinary food intakes in relation to long-term health outcomes. Also, several questions in the FFQ probed different food items, such as unprocessed pork, beef, and lamb intake, in a single question. Thus, we were unable to distinguish which of the combined items a patient was consuming. Studies interested in examining β-agonist exposures should consider assaying these compounds directly in urine samples and not rely on FFQ data as a surrogate. It remains unknown whether meat intake levels estimated by a 24-hour dietary recall or use of a food diary the day of, or before, urine collection would be able to show an association with urinary β-agonist concentrations, a short-term biomarker of exposure. The use of these dietary assessment tools would be important to show a direct association of β-agonists exposure and meat intake.
Assuming that industry use trends are correct and that a high proportion of commercially raised beef and pork are fed ractopamine or zilpaterol,14,15 it is possible that the participants of this study were exposed to residual β-agonists in meat. This supposition is supported by the fact that ractopamine was detected, but not at quantifiable levels, in approximately 8% of 370 urine samples and zilpaterol was detected in 3% of the samples. The low detection rates and even lower rates of quantifiable residues broadly support the procedures used by the US FDA Center for Veterinary Medicine to establish maximum residue levels in food animals. That is, the US regulatory framework seems –at least in the case of β-agonists– to be successful in minimizing exposures to residue. Future research on general population is warranted, as this study population is patients with early-stage breast or lung cancer, although the patients were contacted shortly after their diagnosis and urine was collected before surgery and treatment.
In conclusion, among the studied populations of early-stage breast and lung cancer patients with reported average meat intake levels, the concentrations of β-agonists used as feed additives (ractopamine and zilpaterol) were mostly non-detectable or below the method limit of quantitation. Although we did not observe statistically significant associations between estimated meat intakes and β-agonists in our sample, growth promoters including beta-agonists have been, and likely will continue to be, used in meat animals. Thus, human exposure and potential adverse outcomes due to the consumption of meat products warrant continued monitoring and research.
Acknowledgments
The authors wish to acknowledge the staff of the RPCI Data Bank and BioRepository Shared Resource (DBBR) for urine and questionnaire data collection. The skillful technical assistance provided by Michael Woodworth and Missy Berry at USDA is greatly appreciated. The Women’s Health after Breast Cancer (ABC) Study was supported by Susan G. Komen Breast Cancer Foundation (BCTR104906), Breast Cancer Research Foundation, the US Army Medical Research and Materiel Command (DoD W81XWH0610401), and RPCI Alliance Foundation. DBBR is supported by RPCI’s Cancer Center Support Grant from the National Cancer Institute (P30CA016056).
Footnotes
Declaration: There are no competing financial interests for all authors.
References
- 1.USDA Food Safety and Inspection Service. United States National Residue Program for Meat, Poultry, and Egg Products. 2013 Residue Sample Results. 2015 [Google Scholar]
- 2.USDA Food Safety and Inspection Service. United States National Residue Program for Meat, Poultry, and Egg Products. 2014 Residue Sample Results. 2015 [Google Scholar]
- 3.Baynes RE, Dedonder K, Kissell L, Mzyk D, Marmulak T, Smith G, et al. Health concerns and management of select veterinary drug residues. Food Chem Toxicol. 2016;88:112–22. doi: 10.1016/j.fct.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 4.Mason S. Tissue residues and withdrawal times. In: Riviere JE, editor. Comparative Pharmacokinetics, Principles, Techniques & Applications. 2. West Sussex, UK: Wiley Blackwell; 2011. pp. 413–23. [Google Scholar]
- 5.Kuiper HA, Noordam MY, van Dooren-Flipsen MM, Schilt R, Roos AH. Illegal use of beta-adrenergic agonists: European Community. J Anim Sci. 1998;76:195–207. doi: 10.2527/1998.761195x. [DOI] [PubMed] [Google Scholar]
- 6.As Beef Cattle Become Behemoths. Who Are Animal Scientists Serving? [Accessed February 26, 2016];The Chronicle of Higher Education. 2012 at http://chronicle.com/article/As-Beef-Cattle-Become/131480/
- 7.Sillence MN. Technologies for the control of fat and lean deposition in livestock. Vet J. 2004;167:242–57. doi: 10.1016/j.tvjl.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 8.Dalrymple RH, Baker PK, Gingher PE, Ingle DL, Pensack JM, Ricks CA. A repartitioning agent to improve performance and carcass composition of broilers. Poult Sci. 1984;63:2376–83. doi: 10.3382/ps.0632376. [DOI] [PubMed] [Google Scholar]
- 9.Smith DJ, Turberg MP, Burnett TJ, Dalidowicz J, Thomson TD, Anderson DB. Relative safety of clenbuterol and ractopamine residues in edible tissues of hogs. Proceedings: the 17th International Pig Veterinary Society Congress; June 2–5, 2002; Ames, Iowa. 2002. p. 194. [Google Scholar]
- 10.Smith DJ. The pharmacokinetics, metabolism, and tissue residues of beta-adrenergic agonists in livestock. J Anim Sci. 1998;76:173–94. doi: 10.2527/1998.761173x. [DOI] [PubMed] [Google Scholar]
- 11.Ramos F, Silveira I, Silva JM, Barbosa J, Cruz C, Martins J, et al. Proposed guidelines for clenbuterol food poisoning. The American journal of medicine. 2004;117:362. doi: 10.1016/j.amjmed.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 12.Brambilla G, Loizzo A, Fontana L, Strozzi M, Guarino A, Soprano V. Food poisoning following consumption of clenbuterol-treated veal in Italy. JAMA. 1997;278:635. [PubMed] [Google Scholar]
- 13.Serratosa J, Blass A, Rigau B, Mongrell B, Rigau T, Tortades M, et al. Residues from veterinary medicinal products, growth promoters and performance enhancers in food-producing animals: a European Union perspective. Rev Sci Tech. 2006;25:637–53. [PubMed] [Google Scholar]
- 14.Centner TJ, Alvey JC, Stelzleni AM. Beta agonists in livestock feed: status, health concerns, and international trade. J Anim Sci. 2014;92:4234–40. doi: 10.2527/jas.2014-7932. [DOI] [PubMed] [Google Scholar]
- 15.Loneragan GH, Thomson DU, Scott HM. Increased mortality in groups of cattle administered the beta-adrenergic agonists ractopamine hydrochloride and zilpaterol hydrochloride. PLoS One. 2014;9:e91177. doi: 10.1371/journal.pone.0091177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchant-Forde JN, Lay DC, Jr, Pajor EA, Richert BT, Schinckel AP. The effects of ractopamine on the behavior and physiology of finishing pigs. J Anim Sci. 2003;81:416–22. doi: 10.2527/2003.812416x. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. Residue evaluation of certain veterinary drugs. Joing FAO/WHO Expert Committee on Food Additives; Meeting 2010 – Evaluation of data on ractopamine residues in pig tissues; 2010. [Google Scholar]
- 18.Food and Drug Administration. Code of Federal Regulations - 21CFR556.570. Ractopamine. 2015 ( http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=556.570)
- 19.Food and Drug Administration. Code of Federal Regulations - 21CFR556.765. Zilpaterol. 2015 ( http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=556.765)
- 20.Hsieh MK, Chang SK, Lan PY, Chou CC. Studies of heat stability of ractopamine in water and soy cauce. Taiwan Vet J. 2011;37:111–8. (in Chinese) [Google Scholar]
- 21.INCHEM. Ractopamine. WHO FOOD ADDITIVES SERIES. :53. http://wwwinchemorg/documents/jecfa/jecmono/v53je08htm-pha.
- 22.Bruzzone A, Sauliere A, Finana F, Senard JM, Luthy I, Gales C. Dosage-dependent regulation of cell proliferation and adhesion through dual beta2-adrenergic receptor/cAMP signals. FASEB J. 2014;28:1342–54. doi: 10.1096/fj.13-239285. [DOI] [PubMed] [Google Scholar]
- 23.Shelver WL, Thorson JF, Hammer CJ, Smith DJ. Depletion of urinary zilpaterol residues in horses as measured by ELISA and UPLC-MS/MS. J Agric Food Chem. 2010;58:4077–83. doi: 10.1021/jf904253t. [DOI] [PubMed] [Google Scholar]
- 24.Smith DJ, Shelver WL. Tissue residues of ractopamine and urinary excretion of ractopamine and metabolites in animals treated for 7 days with dietary ractopamine. J Anim Sci. 2002;80:1240–9. doi: 10.2527/2002.8051240x. [DOI] [PubMed] [Google Scholar]
- 25.Shelver WL, Smith DJ. Tissue residues and urinary excretion of zilpaterol in sheep treated for 10 days with dietary zilpaterol. J Agric Food Chem. 2006;54:4155–61. doi: 10.1021/jf060552m. [DOI] [PubMed] [Google Scholar]
- 26.Guddat S, Fussholler G, Geyer H, Thomas A, Braun H, Haenelt N, et al. Clenbuterol - regional food contamination a possible source for inadvertent doping in sports. Drug Test Anal. 2012;4:534–8. doi: 10.1002/dta.1330. [DOI] [PubMed] [Google Scholar]
- 27.Ambrosone CB, Nesline MK, Davis W. Establishing a cancer center data bank and biorepository for multidisciplinary research. Cancer Epidemiol Biomarkers Prev. 2006;15:1575–7. doi: 10.1158/1055-9965.EPI-06-0628. [DOI] [PubMed] [Google Scholar]
- 28.Smith DJ, Feil VJ, Huwe JK, Paulson GD. Metabolism and disposition of ractopamine hydrochloride by turkey poults. Drug Metab Dispos. 1993;21:624–33. [PubMed] [Google Scholar]
- 29.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Annals of Epidemiology. 1999;9:178–87. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 30.Liou SH, Yang GC, Wang CL, Chiu YH. Monitoring of PAEMs and beta-agonists in urine for a small group of experimental subjects and PAEs and beta-agonists in drinking water consumed by the same subjects. J Hazard Mater. 2014;277:169–79. doi: 10.1016/j.jhazmat.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 31.European Food Safety Authority (EFSA) Scientific opinion of the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) on a request from the European Community on the safety evaluation of ractopamine. EFSA J. 2009;1041:1–52. [Google Scholar]
- 32.Reed CE. Adrenergic bronchodilators: pharmacology and toxicology. J Allergy Clin Immunol. 1985;76:335–41. doi: 10.1016/0091-6749(85)90650-5. [DOI] [PubMed] [Google Scholar]
- 33.Spangler DL. Review of side effects associated with beta agonists. Ann Allergy. 1989;62:59–62. [PubMed] [Google Scholar]
- 34.Shiu TC, Chong YH. A cluster of clenbuterol poisoning associated with pork and pig offal in Hong Kong. Publ Health Epidemiol Bull. 2001;10:14–7. [Google Scholar]
- 35.Wu ML, Deng JF, Chen Y, Chu WL, Hung DZ, Yang CC. Late diagnosis of an outbreak of leanness-enhancing agent-related food poisoning. Am J Emerg Med. 2013;31:1501–3. doi: 10.1016/j.ajem.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Yan H, Xu D, Meng H, Shi L, Li L. Food poisoning by clenbuterol in China. Qual Assur Safety Crop Foods. 2015;7:27–35. [Google Scholar]
- 37.Mills SE. Biological basis of the ractopamine response. J Anim Sci. 2002;80:E28–E32. [Google Scholar]
- 38.McRobb FM, Kufareva I, Abagyan R. In silico identification and pharmacological evaluation of novel endocrine disrupting chemicals that act via the ligand-binding domain of the estrogen receptor alpha. Toxicol Sci. 2014;141:188–97. doi: 10.1093/toxsci/kfu114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vojtechova P, Martin RM. The association of atopic diseases with breast, prostate, and colorectal cancers: a meta-analysis. Cancer Causes Control. 2009;20:1091–105. doi: 10.1007/s10552-009-9334-y. [DOI] [PubMed] [Google Scholar]
- 40.Childers WK, Hollenbeak CS, Cheriyath P. beta-Blockers Reduce Breast Cancer Recurrence and Breast Cancer Death: A Meta-Analysis. Clin Breast Cancer. 2015;15:426–31. doi: 10.1016/j.clbc.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Ko K, Kurogi K, Davidson G, Liu MY, Sakakibara Y, Suiko M, et al. Sulfation of ractopamine and salbutamol by the human cytosolic sulfotransferases. J Biochem. 2012;152:275–83. doi: 10.1093/jb/mvs073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith DJ, Rodewald JM. Urinary excretion of ractopamine and its conjugated metabolites by humans. 1994. Unpublished report on study No. T4V759404 from Lilly Research Laboratories, A Division of Eli Lilly and Company, Greenfield, IN, USA. Submitted to WHO by Elanco Animal Health, Division of Eli Lilly and Company, Indianapolis, IN, USA. [Google Scholar]
- 43.Ginsberg G, Rice DC. Does rapid metabolism ensure negligible risk from bisphenol A? Environ Health Perspect. 2009;117:1639–43. doi: 10.1289/ehp.0901010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang W, Mills SE. Quantitative analysis of beta-adrenergic receptor subtypes in pig tissues. J Anim Sci. 2002;80:963–70. doi: 10.2527/2002.804963x. [DOI] [PubMed] [Google Scholar]
- 45.Liu W, Ramirez J, Gamazon ER, Mirkov S, Chen P, Wu K, et al. Genetic factors affecting gene transcription and catalytic activity of UDP-glucuronosyltransferases in human liver. Hum Mol Genet. 2014;23:5558–69. doi: 10.1093/hmg/ddu268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomae BA, Rifki OF, Theobald MA, Eckloff BW, Wieben ED, Weinshilboum RM. Human catecholamine sulfotransferase (SULT1A3) pharmacogenetics: functional genetic polymorphism. J Neurochem. 2003;87:809–19. doi: 10.1046/j.1471-4159.2003.02027.x. [DOI] [PubMed] [Google Scholar]
- 47.Hunt TL. Cardiovascular activity and safety of ractopamine hydrochloride: determination of a no-effect dose. 1994. Unpublished report on study No. T4V-LC-ERAA from Pharmaco LSR, Austin, Texas 78704, USA. Submitted to WHO by Elanco Animal Health, Division of Eli Lilly and Company, Indianapolis, IN, USA. [Google Scholar]
- 48.Witte EC, Lambers Heerspink HJ, de Zeeuw D, Bakker SJ, de Jong PE, Gansevoort R. First morning voids are more reliable than spot urine samples to assess microalbuminuria. J Am Soc Nephrol. 2009;20:436–43. doi: 10.1681/ASN.2008030292. [DOI] [PMC free article] [PubMed] [Google Scholar]