Abstract
Introduction: Cannabis biosynthesizes Δ9-tetrahydrocannabinolic acid (THCA-A), which decarboxylates into Δ9-tetrahydrocannabinol (THC). There is growing interest in the therapeutic use of THCA-A, but its clinical application may be hampered by instability. THCA-A lacks cannabimimetic effects; we hypothesize that it has little binding affinity at cannabinoid receptor 1 (CB1).
Materials and Methods: Purity of certified reference standards were tested with high performance liquid chromatography (HPLC). Binding affinity of THCA-A and THC at human (h) CB1 and hCB2 was measured in competition binding assays, using transfected HEK cells and [3H]CP55,940. Efficacy at hCB1 and hCB2 was measured in a cyclic adenosine monophosphase (cAMP) assay, using a Bioluminescence Resonance Energy Transfer (BRET) biosensor.
Results: The THCA-A reagent contained 2% THC. THCA-A displayed small but measurable binding at both hCB1 and hCB2, equating to approximate Ki values of 3.1μM and 12.5μM, respectively. THC showed 62-fold greater affinity at hCB1 and 125-fold greater affinity at hCB2. In efficacy tests, THCA-A (10μM) slightly inhibited forskolin-stimulated cAMP at hCB1, suggestive of weak agonist activity, and no measurable efficacy at hCB2.
Discussion: The presence of THC in our THCA-A certified standard agrees with decarboxylation kinetics (literature reviewed herein), which indicate contamination with THC is nearly unavoidable. THCA-A binding at 10μM approximated THC binding at 200nM. We therefore suspect some of our THCA-A binding curve was artifact—from its inevitable decarboxylation into THC—and the binding affinity of THCA-A is even weaker than our estimated values. We conclude that THCA-A has little affinity or efficacy at CB1 or CB2.
Keywords: : cannabinoid receptors, Cannabis, pharmacodynamics, pharmacology, phytocannabinoids, THCA
Introduction
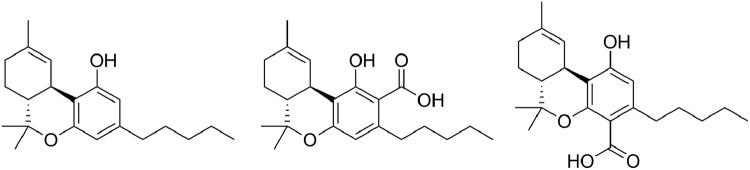
Cannabis biosynthesizes cannabinoids as carboxylic acids. The carboxylic acid of Δ9-tetrahydrocannabinol (THC) is Δ9-tetrahydrocannabinolic acid (THCA). Two isomers of THCA have been discovered, 2-COOH-THC (THCA-A) and 4-COOH-THC (THCA-B) (Fig. 1). Cannabis primarily biosynthesizes THCA-A,1 and this isomer has been the focus of most pharmacological studies. Conversely, THCA-B has greater stability and crystalizes more readily than THCA-A,2 so THCA-B became the molecule for modeling studies of cannabinoid receptors. According to the canonical cannabinoid biosynthesis pathway, olivetolic acid is prenylated into cannabigerolic acid (CBGA) with its carboxylic acid in an “A” position. The allylic rearrangement yielding THCA-B begs a mechanism.
FIG. 1.
Chemical structures of THC (left), THCA-A (center), and THCA-B (right). THC, Δ9-tetrahydrocannabinol; THCA-A, Δ9-tetrahydrocannabinolic acid A; THCA-B, 4-COOH-THC.
There is growing interest in the therapeutic use of THCA-A.3,4 The gray literature is immense: a simple Google search of “tetrahydrocannabinolic acid” plus “medical” returns 14,000 hits. The clinical application of THCA-A, however, is complicated by thermal instability—it readily decarboxylates into THC. This happens with heating (smoking and baking), as well as storage, at room temperature.
Studies on the “shelf life” of THCA-A are worth reviewing, for clinical purposes, as well as pharmacological research. Ethanol and olive oil extract approximately the same THCA-A/THC ratio from plant material,5,6 but THCA-A stability is greater in olive oil (78% of THCA-A remained after 10 days at 25°) than ethanol (only 33% remained).6 THCA-A is even less stable in hydroethanolic solvents.7 Other stability studies tested solvents used for laboratory reagents—methanol, chloroform, petroleum ether, and n-hexane.8,9 Decarboxylation rates depended upon temperature, with considerable losses at room temperature, and exposure to light accelerated the process. When stored for a month at refrigerator temperature (4°C), THCA-A decreased to between 91% (in methanol) and 68% (in chloroform) of initial levels. Losses still occurred at freezer temperature (−18°C).8
Hazekamp et al.10 demonstrated short-term stability in “cannabis tea.” They added quantified amounts of THCA-A and THC to boiled water. After 15 min of simmering above 55°C, they recovered 63% of THCA-A and only 17% of THC. However, THCA-A loss was substantial in cannabis tea stored at 4°C—decreasing to 71% of initial levels after 1 day. THCA-A rapidly decarboxylates if water is boiled after cannabis is added to it.11
Studies suggest that THCA-A may be more stable in herbal cannabis, where it is “hermetically sealed” within glandular trichomes, along with terpenoids which serve as protective antioxidants.
Studies suggest that THCA-A may be more stable in herbal cannabis, where it is “hermetically sealed” within glandular trichomes, whose gland heads contain up to 10% terpenoids.12 Terpenoids are potent antioxidants,13 protect living plants from thermal and oxidative stress,14 and likely inhibit the oxidative decarboxylation of THCA-A. Decarboxylation kinetics have been measured by heating herbal cannabis in undescribed conditions,15 in a nitrogen atmosphere,16 in sealed glass bottles,17 or cardboard boxes.18 Collectively, these studies showed that THCA-A decarboxylated within minutes at temperatures above 80°C. At room temperature in glass bottles with limited exposure to light, THCA-A dropped to 80% of initial levels after 25 months. At refrigerator (4°C) temperatures, 94.7% of THCA-A was still present.
THCA-A “shelf life” may be extended in hashish, where gland heads are mechanically detached and compacted to minimize exposure to light and oxygen.8,11,19 Baker et al.20 measured THCA and THC in seized materials, all approximately the same age. The THCA/THC ratio in hashish (mean 3.08) was greater than herbal material (mean 1.96).
The growing clinical interest in THCA-A is due, in part, to its perceived lack of cannabimimetic effects.15,21,22 This may be due to a lack of binding affinity at cannabinoid receptor type one (CB1). Affinity studies of THCA-A at CB1 report disparate results—equal to THC23 or 25-fold weaker than THC24 or lacking affinity.25,26 As we elaborate in the Discussion section, this incongruence is best explained by THCA-A contaminated by THC. Similarly, Edery et al.21 demonstrated very weak psychoactivity in rhesus monkeys, which they discounted as some THCA-A decarboxylating into THC during the course of the experiment.
In this current study, we measured the affinity of THCA-A at CB1, as well as cannabinoid receptor subtype two (CB2), as well as the efficacy of THCA-A at CB1 and CB2. We used a certified reference standard (meeting ISO17025 guidelines), but high performance liquid chromatography (HPLC) revealed that our THCA-A reference standard was contaminated by 2% THC. In this study we show that the only binding or efficacy detected for THCA-A is consistent with the level of THC contamination contained in the sample.
Methods
THCA-A was purchased from Cayman Chemical (lot no. 0466688) as a 1 mg/mL solution in acetonitrile. THC was purchased from THC Pharm GmbH (lot no. S12-003, delivered as a solid resin in a glass syringe) and dissolved at 31.6 mM in nitrogen-purged absolute ethanol. Materials were stored at −20°C (THCA-A) or −80°C (THC) before experimentation. Purity was assessed by reverse-phase HPLC using a Phenomenex™ C18 Gemini column (5 μm, 4.60×250 mm) on a Thermo Scientific UltiMate 3000 HPLC. A linear gradient of 65–100% MeOH (ca. 1%/min) in H2O with 0.1% formic acid was used.
Competition binding assays27 for hCB1 and hCB2 were performed by incubating either THC or THCA-A with membranes from HEK (human embryonic kidney) 293 cells transfected with either hCB1 or hCB2 receptors as previously described.28,29 Transfected HEK 293 cells were grown to 90–100% confluence in 175 cm2 flasks and harvested in ice-cold phosphate buffered saline (PBS) with 5 mM EDTA. Cells were centrifuged at 200×g for 10 min and frozen at −80°C until required. Cell pellets were thawed with Tris-sucrose buffer (50 mM Tris-HCl, pH 7.4, 200 mM sucrose, 5 mM MgCl2, 2.5 mM EDTA) and homogenized with a glass homogenizer. The homogenate was centrifuged at 1000×g for 10 min at 4°C and the pellet discarded. The supernatant was then centrifuged at 27,000×g for 30 min at 4°C. The final pellet was resuspended in a minimal volume of Tris-sucrose buffer and aliquoted to avoid repeated freeze–thaw cycles.
Protein concentration was determined using the DC Protein Assay Kit (Bio-Rad, Hercules, CA) following the manufacturers' protocol. Membranes (10 μg/point for CB1 and 5 μg/point for CB2) were resuspended in binding buffer (50 mM HEPES, 1 mM MgCl2, 1 mM CaCl2, 0.2% [w/v] bovine serum albumin [BSA; ICP Bio], pH 7.4) and incubated with [3H]-CP55,940 (2.5 nM; PerkinElmer, Waltham, MA; two different lots were used 175 and 150.2 Ci mmol−1) and a range of THC or THCA-A concentrations at 30°C for 60 min. THC and THCA-A were diluted directly from stocks to 40 μM in binding buffer supplemented with acetonitrile and ethanol, respectively, to match vehicle conditions with each compound. The compounds were then serially diluted in silanized vessels, maintaining ethanol and acetonitrile levels constant through the dilution series. These 4× dilution series were then added to a v-bottom 96-well plate with radioligand and cell membranes such that the final 1× concentration contained both 0.04% ethanol and 0.36% acetonitrile. These solvents were matched in the vehicle conditions.
GF/C Harvest Plates (PerkinElmer) were presoaked in 0.1% polyethylenimine and then washed with 200 μL ice-cold wash buffer (50 mM HEPES pH 7.4 500 mM NaCl, 0.1% BSA) before filtration of samples and then three additional 200 μL washes in ice-cold wash buffer. Harvest plates were dried overnight at 24°C, 50 μL of scintillation fluid (IRGASAFE PLUS; PerkinElmer) was added to each well, and plates were read 30 min later for 2 min per well in a MicroBeta TriLux (PerkinElmer).
Competition binding curves were fit by nonlinear regression using one site competition binding with GraphPad Prism 6.0. Dissociation constant in a competition binding assay (Ki) was determined from half maximal inhibitory concentration (IC50) using previously established Kd of 2.5 nM (CB1) or 3 nM (CB2), respectively, (unpublished data). Binding experiments for CB1 and CB2 were performed five times in triplicate. pKi values are expressed as mean±standard error of the mean (SEM). For THCA-A full binding curves could not be established (because it failed to fully displace [3H]-CP55,940 at concentrations up to 10 μM); therefore, we estimated percentage displacement at 10 μM. This was converted to an approximate IC50 by assuming a hill slope of 1 and utilizing the following equation: 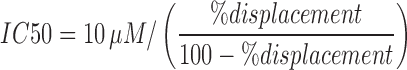 and then to approximate Ki using the Cheng–Prusoff equation:
and then to approximate Ki using the Cheng–Prusoff equation: 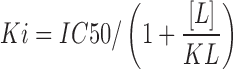
Efficacy at hCB1 and hCB2 was investigated in a cyclic adenosine monophosphase (cAMP) assay, to determine if THCA-A could inhibit forskolin-stimulated cAMP (i.e., act as an agonist) or prevent the inhibition produced by EC90 concentrations of CP55,940 (i.e., act as an antagonist). Cellular cAMP levels were measured as previously described.28 Briefly, the pcDNA3L-His-CAMYEL plasmid (ATCC, Manassas, VA) was transfected into HEK 293-hCB1 or hCB2 cells using linear polyethylenimine (molecular weight 25 kDa; Polysciences, Warrington, PA). After 24 h transfection cells were replated in poly-d-lysine (0.05 mg mL−1 in PBS; Sigma-Aldrich, St Louis, MO) coated 96 Well Solid White Flat Bottom Polystyrene TC-Treated Microplates (Corning) at a density of 55,000–80,000 cells per well. After 24 h, cells were serum-starved in Hank's balanced salt solution (HBSS) containing 1 mg mL−1 BSA, pH 7.4 for 30 min before assay. Five minutes before the addition of drug or vehicle dissolved in HBSS plus 1 mg mL−1 BSA cells were treated with 5 μM Coelenterazine-h (Nanolight Technology). Emission signals were detected simultaneously at 460/25 nM (RLuc) and 560/25 nM (YFP), immediately following drug addition, with a LUMIstar plate reader (BMG) at 37°C. Raw data are presented as an inverse bioluminescence resonance energy transfer (BRET) ratio of emission at 460/535 nM, so that an increase in ratio correlates with an increase in cAMP production. Area under the curve analysis was carried out using GraphPad Prism, and values normalized to forskolin (100%) and vehicle (0%). t-Tests (GraphPad Prism) were utilized to determine if THCA-A significantly altered the response in the presence or absence of CP55,940.
Results
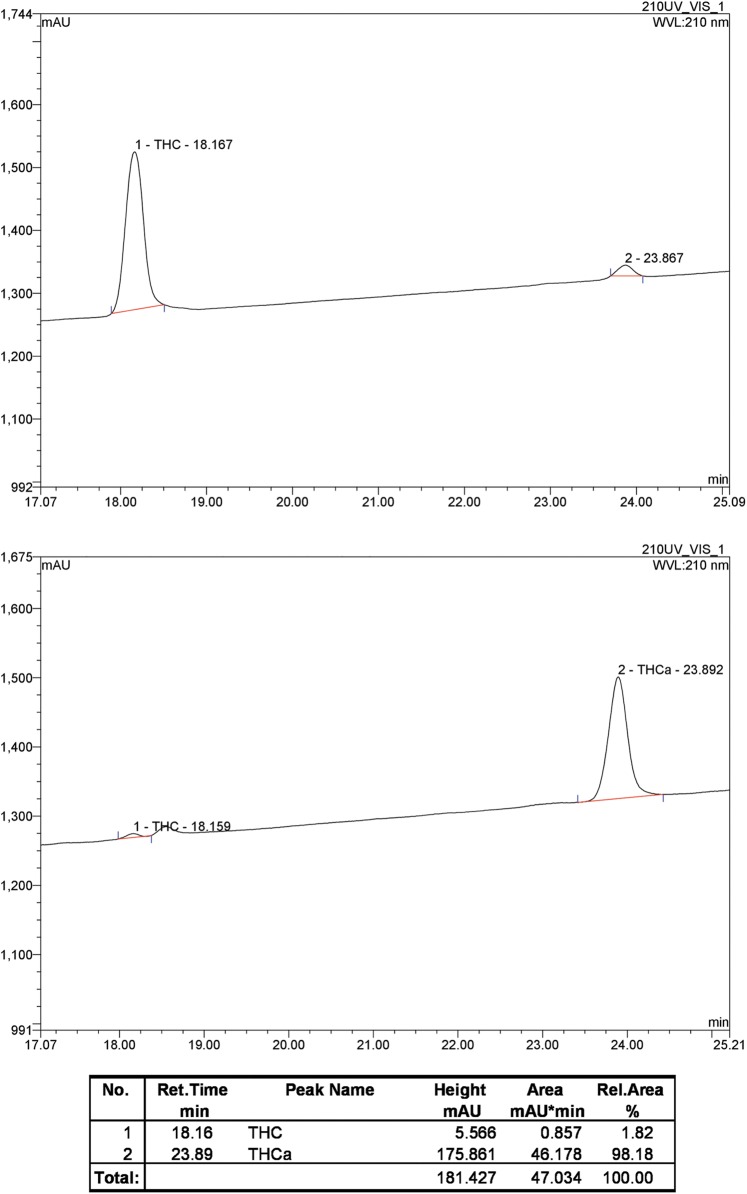
HPLC revealed the presence of 2% THC (THCRT=18.2 min) in the THCA-A sample (THCA-ART=23.9 min), established by correlation of retention time with an authentic sample of THC. The THC and THCA-A peaks were correlated to their respective molecular ions by electrospray ionization (ESI) mass spectrometry (THC m/z=338.1, [M+Na]1+ requires 337.5; THCA-A m/z=359.0, [M+H]1+ requires 359.5). Chromatograms of the two samples are illustrated in Figure 2.
FIG. 2.
Analytical RP-HPLC of THCA-A sample (lower panel) and THC standard (upper panel); absorbance detected at 210 nm. HPLC, high performance liquid chromatography.
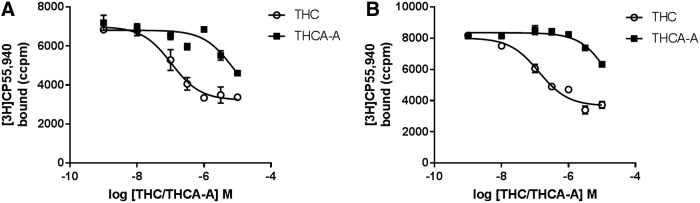
To determine if THCA-A could bind to the orthosteric binding site of hCB1 or hCB2, competition displacement assays were carried out (Fig. 3). Full displacement could not be achieved with THCA-A concentrations up to 10 μM. At 10 μM THCA-A produced a small but significant displacement at both hCB1 (62%±3%) and hCB2 (40%±8%). This level of displacement was insufficient to fully define competition binding curves, but would equate to approximate pKi's of 5.5 (3.1 μM) and 4.9 (12.5 μM), respectively. For comparative purposes competition binding assays were carried out with THC. THC fully displaced [3H]CP55,940 with mean pKi=7.3±0.03 (hCB1; 50 nM) and −7.0±0.04 (hCB2; 100 nM).
FIG. 3.
Binding affinity of THCA-A and THC illustrated in competition binding curves against [3H]CP55,940. CB1 on the left (A) and CB2 on the right (B). Data are representative data from a single experiment and data points represent mean±SEM for triplicate data points. CB1, cannabinoid receptor subtype one; CB2, cannabinoid receptor subtype two; SEM, standard error of the mean.
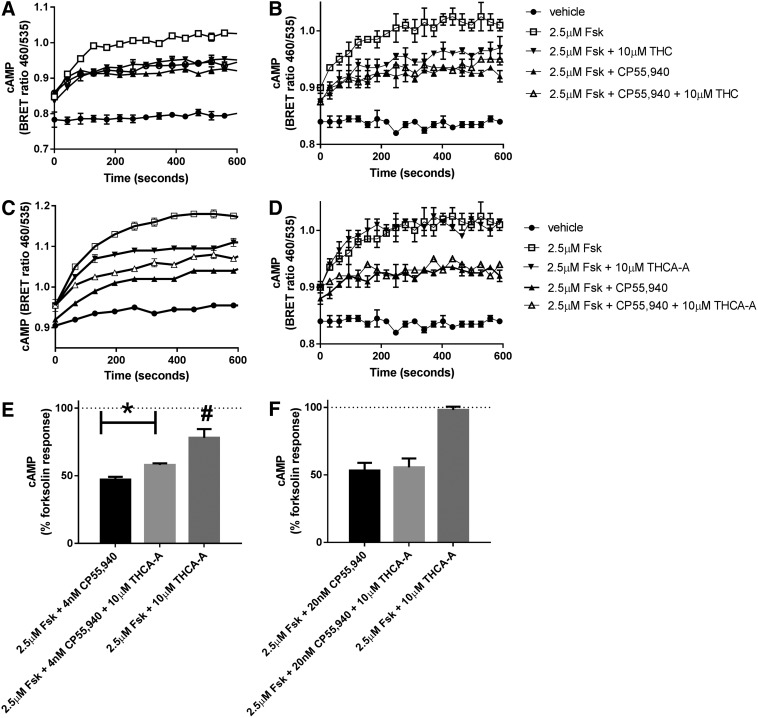
To determine if THCA-A could activate or block cannabinoid receptors, cAMP assays were carried out using a BRET biosensor as previously described.28 As both cannabinoid receptors are predominantly guanine nucleotide-binding protein subunit i (Gi) linked and therefore couple to the inhibition of cAMP, cAMP levels were increased with forskolin and then the ability of 10 μM THCA-A to alter cAMP was investigated in the presence and absence of the cannabinoid agonist CP55,940 at approximate EC90 concentrations (4 nM at hCB1 and 20 nM at hCB2). For comparison inhibition of forskolin mediated cAMP by 10 μM THC was also carried out. cAMP levels were measured for 9.9 min (595 sec), and then data were analyzed by an Area under the curve analysis, normalized to forskolin (100% and vehicle 0%).
As shown in Figure 4, at hCB1 CP55,940 inhibited forskolin stimulated cAMP to 50%±3.5%. In the presence of 10 μM THCA-A this stimulation was partially but significantly reversed to 59%±1.8% (p=0.0065 by paired t-test, n=5). On its own THCA-A produced a small but significant inhibition to 79%±5% (t-test compared to 100% p=0.015). At hCB2, CP55,940 inhibited to 53%±6%, which was unaltered in the presence of THCA-A (55%±7% p=0.6, n=6). Consistent with this, THCA-A alone produced no inhibition of forskolin mediated cAMP (99%±2%, p=0.64, n=6). As expected, THC (10 μM) produced equivalent inhibition of cAMP to that produced by CP55,940, acting as an efficacious agonist in this assay.
FIG. 4.
Efficacy of THCA-A and THC in cAMP assays, CB1 on the left, CB2 on the right. (A–D) Show representative images of the biosensor traces of single experiments carried out in duplicate. (A, B) Show that THC (10 μM) inhibits cAMP at both CB1 (left) and CB2 (right). (C, D) Show the same assay carried out with THCA-A. THCA-A can be seen to inhibit forskolin mediated cAMP alone and to partially antagonize the ability of CP55,940 to inhibit cAMP at CB1, but has no effect under equivalent conditions at CB2. The lower panels (E, F) are summary data for the area under the curve analyses for all replicate assays combined (n=5 for CB1 or n=6 for CB2). *p=0.0065 by paired t-test, n=5. #p=0.015 t-test compared to 100%. cAMP, cyclic adenosine monophosphase.
Discussion
Although there is growing interest in THCA-A among clinicians,3,4 decarboxylation studies suggest that contamination with THC is nearly unavoidable.5–11,15–20 The instability of THCA-A also hampers its pharmacological exploration.21 “How can anybody do an experiment if the compound likes to convert into something else just by sitting around, and the ‘something else’ has all kinds of activities?” (R. Mechoulam, personal communication, January 2017). Our study optimized in vitro stability by keeping THCA-A in acetonitrile. In vitro assays of THC can also be hampered by solubility issues. Our results with THC (Ki=50 nM at hCB1) indicate that THC did not fall out of solution. Because THCA-A is more water soluble than THC,10 we concluded that our results with THCA-A were not due to solubility issues.
THCA-A's lack of psychoactivity makes it attractive in some circles. Moreno-Sanz30 hypothesized that lack of cannabimimetic effects is due to restricted access to the central nervous system (CNS). Others have explored the affinity and efficacy of THCA-A at cannabinoid receptors. Rosenthaler et al.23 determined a Ki of 23.4 nM for THCA-A at hCB1, nearly equivalent to their measure of Ki of 35.6 nM for THC. This is in agreement with a meta-analysis of THC at hCB1, which reported a mean Ki of 25.1±0.39 nM (n=16 studies).31
Verhoeckx et al.24 determined a Ki of 890 nM for THCA-A at hCB1. They also determined a Ki of 3.5 nM for THC at hCB1, which is sevenfold greater than the meta-analytic mean. Applying this multiplier to their Ki of THCA-A would produce a Ki broadly consistent with our results. Ahmed et al.25 simply stated “no activity” for THCA-A at CB1, without a Ki value. Husni et al.26 determined a Ki of 1292 nM for THCA-A at CB1. Although they illustrate an incorrect structure for THCA-A, their results do indeed apply to THCA-A (M. Radwan, personal communication, January 2017).
The reason for these disparate results cannot be easily explained. The methods used in these studies are compared in Table 1. Methodological details not supplied in original publications were obtained through personal communications (S. Rosenthaler, April 2015; K. Verhoeckx, April 2017; M. Radwan, January 2017). Affinity values among different studies may vary according to radioligand, CB species, and expression model, but these methodological factors rarely generate statistical differences.31 More likely, some THCA-A decarboxylated in these studies. The two studies that reported affinity23,24 did not authenticate the purity of their THCA-A reagents, whereas the two studies that reported no affinity25,26 authenticated THCA-A with HPLC and nuclear magnetic resonance (NMR) (authentication confirmed by M. Radwan, personal communication, January 2017).
Table 1.
Methodological Comparison of Five Δ9-Tetrahydrocannabinolic Acid A Affinity Studies
| Radioligand | CB species | Expression model | THCA-A source | |
|---|---|---|---|---|
| Rosenthaler et al.23 | [3H]CP55,940 | hCB1 | Sf9 cells | THC Pharm GmbH, synthetic, 1 mg/mL in methanol |
| Verhoeckx et al.24 | n.d.a | hCB1 | Sf9 cells | Extracted from plant material, in ethanol |
| Ahmed et al.25 | [3H]CP55,940 | rCB1 | Brain membranes | Extracted from plant material, in hexane |
| Husni et al.26 | [3H]CP55,940 | rCB1 | Brain membranes | Extracted from plant material, in hexane |
| This study | [3H]CP55,940 | hCB1 | HEK293 cells | Cayman Chemical, synthetic, 1 mg/mL in acetonitrile |
K. Verhoeckx (personal communication, April 2017) reports “outsourcing” the affinity part of their study and could not recall the specific radioligand used in the assay.
THCA-A showed little affinity at hCB1 in our competition binding assays. On the basis of 60% displacement at 10 μM, a Ki of 3 μM can be estimated, making it broadly comparable to that of cannabidiol (Ki=2.2 μM31)—a decidedly non-cannabimimetic ligand. At hCB2, THCA-A slightly displaced [3H]CP55,940 in binding assays—less than that produced at CB1, reaching 40% displacement at 10 μM, consistent with an estimated Ki of 12.5 μM. In comparison, THC showed 62-fold greater affinity at hCB1 and 125-fold greater affinity at hCB2.
Despite our use of a certified reference standard (meeting ISO17025 guidelines), the reagent nevertheless contained 2% THC. Numerous web sites advertise “crystalline THCA” and claim 99% to 100% purity. These gray market sources pose legal barriers regarding international shipment, lack ISO17025 standards, and await purity authentication. Hypothetically these products contain THCA-B, which crystalizes more readily than THCA-A.2 THCA-B also demonstrates greater thermal stability than THCA-A,32–34 so it may be worth investigating. However, only two studies have quantified THCA-A and THCA-B content in a variety of Cannabis landraces,33,34 so the prevalence of THCA-B is relatively unknown.
Given the susceptibility of THCA-A to lose its carboxylic acid moiety, contamination by THC may be difficult to avoid, as lamented by pharmacologists evaluating THCA-A.21 At a concentration of 10 μM THCA-A, the reagent contained ∼200 nM THC. In our hCB1 competition binding assay, THCA-A displacement at 10 μM approximated THC displacement at 200 nM (Fig. 3). We therefore suspect that some of our THCA-A binding curve was artifact—from its inevitable decarboxylation into THC—and the binding affinity of THCA-A at hCB is even weaker than our estimated values.
Consistent with low affinity, THCA-A showed low efficacy at hCB1. THCA-A (10 μM) produced a small but significant inhibition of forskolin cAMP, consistent with agonist activity. Due to solubility issues and the low potency of this compound, sufficiently high concentrations to determine the extent of agonism were not possible without reaching unacceptably high levels of solvent. As is consistent with a weak agonist, THCA-A slightly antagonized the effect produced by an EC90 concentration of CP55,940. Regarding hCB2, THCA-A produced no significant effect in cAMP assays.
Verhoeckx et al.24 also measured efficacy, and their results correlated with ours—THCA-A at hCB1 showed no influence on cAMP production. However, THC showed no efficacy in their hands, either. Husni et al.26 used a different efficacy assay: agonist-stimulated [35S]GTPγS-binding in mouse brain membranes. The EC50 concentration of THC was 269 nM, whereas THCA-A was >10,000 nM. Lack of affinity and efficacy of THCA-A at hCB1 seems consistent with in vivo studies, where THCA-A lacked cannabimimetic activity in rodents and primates.15,21,22
Many questions regarding THCA-A remain unanswered. For example, an in vivo study of rats and shrews showed that antiemetic effects by THCA-A were blocked by rimonabant.22 This suggests a CB1-mediated mechanism, yet the authors reported that THCA-A did not induce CB1 agonist effects such as hypothermia or reduced motor activity. THCA-A is a promiscuous ligand and targets many molecular targets.30 However, its clinical usefulness, and its amenity to pharmacological analysis, may be hampered by its instability.
Abbreviations Used
- BRET
bioluminescence resonance energy transfer
- BSA
bovine serum albumin
- cAMP
cyclic adenosine monophosphase
- CB1
cannabinoid receptor subtype one
- CB2
cannabinoid receptor subtype two
- h
human
- HBSS
Hank's balanced salt solution
- HEK 293
human embryonic kidney cell line 293
- HPLC
high performance liquid chromatography
- IC50
half maximal inhibitory concentration
- Ki
dissociation constant in a competition binding assay
- PBS
phosphate buffered saline
- THC
Δ9-tetrahydrocannabinol
- THCA-A
Δ9-tetrahydrocannabinolic acid A
- THCA-B
4-COOH-THC
Acknowledgments
The authors gratefully acknowledge two anonymous reviewers for improving our article and personal communications by THCA-A researchers (S. Rosenthaler, K. Verhoeckx, and M. Radwan).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Mechoulam R, Ben-Zvi Z, Yagnitinsky B, et al. . A new tetrahydrocannabinolic acid. Tetrahedron Lett. 1969;10:2339–2341 [DOI] [PubMed] [Google Scholar]
- 2.Rosenqvist E, Ottersen T. The crystal and molecular structure of Δ9-tetrahydrocannabinolic acid B. Acta Chem Scand B. 1975;29:379–384 [DOI] [PubMed] [Google Scholar]
- 3.Russo E. Cannabis and epilepsy: an ancient treatment returns to the fore. Epilepsy Behav. 2016. December 15 [Epub ahead of print] [DOI] [PubMed]
- 4.Sulak D, Saneto R, Goldstein B. The current status of artisanal cannabis for the treatment of epilepsy in the United States. Epilepsy Behav. 2017. February 18 [Epub ahead of print] [DOI] [PubMed]
- 5.Romano LL, Hazekamp A. Cannabis oil: chemical evaluation of an upcoming cannabis-based medicine. Cannabinoids. 2013;1:1–11 [Google Scholar]
- 6.Citti C, Ciccarella G, Braghiroli D, et al. . Medicinal cannabis: principal cannabinoids concentration and their stability evaluated by a high performance liquid chromatography coupled to diode array and quadrupole time of flight mass spectrometry method. J Pharm Biomed Anal. 2016;128:201–209 [DOI] [PubMed] [Google Scholar]
- 7.Peschel W. Quality control of traditional cannabis tinctures: pattern, markers, and stability. Sci Pharm. 2016;84:567–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith RN, Vaughan CG. The decomposition of acidic and neutral cannabinoids in organic solvents. J Pharm Pharmacol. 1977;29:286–290 [DOI] [PubMed] [Google Scholar]
- 9.Veress T, Szanto JI, Leisztner L. Determination of cannabinoid acids by high-performance liquid chromatography of their neutral derivatives formed by thermal decarboxylation: I. Study of the decarboxylation process in open reactors. J Chromatogr A. 1990;520:339–347 [Google Scholar]
- 10.Hazekamp A, Bastola K, Rashidi H, et al. . Cannabis tea revisited: a systematic evaluation of the cannabinoid composition of cannabis tea. J Ethnopharmacol. 2007;113:85–90 [DOI] [PubMed] [Google Scholar]
- 11.De Zeeuw RA, Malingre TM, Merkus FW. Delta-1-tetrahydrocannabinolic acid, an important component in the evaluation of cannabis products. J Pharm Pharmacol. 1972;24:1–6 [DOI] [PubMed] [Google Scholar]
- 12.Potter D. The propagation, characterisation and optimisation of Cannabis sativa L. as a phytopharmaceutical. Doctoral thesis, King's College, London, 2009 [Google Scholar]
- 13.Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. 2011;163:1344–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Copolovici LO, Filella I, Llusià J, et al. . The capacity for thermal protection of phyotosynthetic electron transport varies for different monoterpenes in Quercus ilex. Plant Physiol. 2005;139:485–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamauchi T, Shoyama Y, Aramaki H, et al. . Tetrahydrocannabinolic acid a genuine substance of tetrahydrocannabinol. Chem Pharm Bull (Tokyo). 1967;15:1075–1076 [DOI] [PubMed] [Google Scholar]
- 16.Doorenbos NJ, Fetterman PS, Quimby MW, et al. . Cultivation, extraction and analysis of Cannabis sativa L. Ann N Y Acad Sci. 1971;191:3–14 [Google Scholar]
- 17.Turner CE, Hadley KW, Fetterman PS, et al. . Constituents of Cannabis sativa L. IV: stability of cannabinoids in stored plant material. J Pharm Sci. 1973;62:1601–1605 [DOI] [PubMed] [Google Scholar]
- 18.Yotoriyama M. The decrease of tetrahydrocannabinolic acid (THCA) in Cannabis leaves during storage. Eisei Kagaku. 1980;26:505–3. [Google Scholar]
- 19.Lindholst C. Long term stability of cannabis resin and cannabis extracts. Aust J Forensic Sci. 2010;42:181–190 [Google Scholar]
- 20.Baker PB, Taylor BJ, Gough TA. The tetrahydrocannabinol and tetrahydrocannabinolic acid content of cannabis products. J Pharm Pharmacol. 1981;33:369–372 [DOI] [PubMed] [Google Scholar]
- 21.Edery H, Grunfeld Y, Porath G, et al. . Structure-activity relationships in the tetrahydrocannabinol series. Arzneimittelforschung. 1972;22:1995–2003 [PubMed] [Google Scholar]
- 22.Rock EM, Kopstick RL, Limebeer CL, et al. . Tetrahydrocannabinolic acid reduces nausea-induced conditioned gaping in rats and vomiting in Suncus murinus. Br J Pharmacol. 2013;170:641–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenthaler S, Pöhn B, Kolmanz C, et al. . Differences in receptor binding affinity of several phytocannabinoids do not explain their effects on neural cell cultures. Neurotoxicol Teratol. 2014;46:49–56 [DOI] [PubMed] [Google Scholar]
- 24.Verhoeckx KC, Korthout HA, van Meeteren-Kreikamp AP, et al. . Unheated Cannabis sativa extracts and its major compound THC-acid have potential immuno-modulating properties not mediated by CB1 and CB2 receptor coupled pathways. Int Immunopharmacol. 2006;6:656–665 [DOI] [PubMed] [Google Scholar]
- 25.Ahmed SA, Ross SA, Slade D, et al. . Cannabinoid ester constituents from high-potency Cannabis sativa. J Nat Prod. 2008;71:536–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Husni AS, McCurdy CR, Radwan MM, et al. . Evaluation of phytocannabinoids from high potency Cannabis sativa using in vitro bioassays to determine structure-activity relationships for cannabinoid receptor 1 and cannabinoid receptor 2. Med Chem Res. 2014;23:4295–4300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redmond WJ, Cawston EE, Grimsey NL, et al. . Identification of N-arachidonoyl dopamine as a highly biased ligand at cannabinoid CB1 receptors. Br J Pharmacol. 2016;173:115–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cawston EE, Redmond WJ, Breen CM, et al. . Real-time characterization of cannabinoid receptor 1 (CB1) allosteric modulators reveals novel mechanism of action. Br J Pharmacol. 2013;170:893–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grimsey NL, Goodfellow CE, Dragunow M, et al. . Cannabinoid receptor 2 undergoes Rab5-mediated internalization and recycles via a Rab11-dependent pathway. Biochim Biophys Acta. 2011;1813:1554–1560 [DOI] [PubMed] [Google Scholar]
- 30.Moreno-Sanz G. Can you pass the acid test? Critical review and novel therapeutic perspectives of Δ9-tetrahydrocannabinolic acid A. Cannabis Cannabinoid Res. 2016;1:124–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McPartland JM, Glass M, Pertwee RG. Meta-analysis of cannabinoid ligand binding affinity and cannabinoid receptor distribution: interspecies differences. Br J Pharmacol. 2007;152:583–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turner CE, Hadley KW, Henry J, et al. . Constituents of Cannabis sativa L. VII: use of silyl derivatives in routine analysis. J Pharm Sci. 1974;63:1872–1876 [DOI] [PubMed] [Google Scholar]
- 33.Brenneisen R, ElSohly MA. Chromatographic and spectroscoic profiles of Cannabis of different origins: part 1. J Forensic Sci. 1988;33:1385–1404 [PubMed] [Google Scholar]
- 34.Hanuš LO, Meyer SM, Muñoz E, et al. . Phytocannabinoids: a unified critical inventory. Nat Prod Rep. 2016;33:135–7. [DOI] [PubMed] [Google Scholar]
References
Cite this article as: McPartland JM, MacDonald C, Young M, Grant PS, Furkert DP, Glass M (2017) Affinity and efficacy studies of tetrahydrocannabinolic acid A at cannabinoid receptor types one and two, Cannabis and Cannabinoid Research 2:1, 87–95, DOI: 10.1089/can.2016.0032.