Abstract
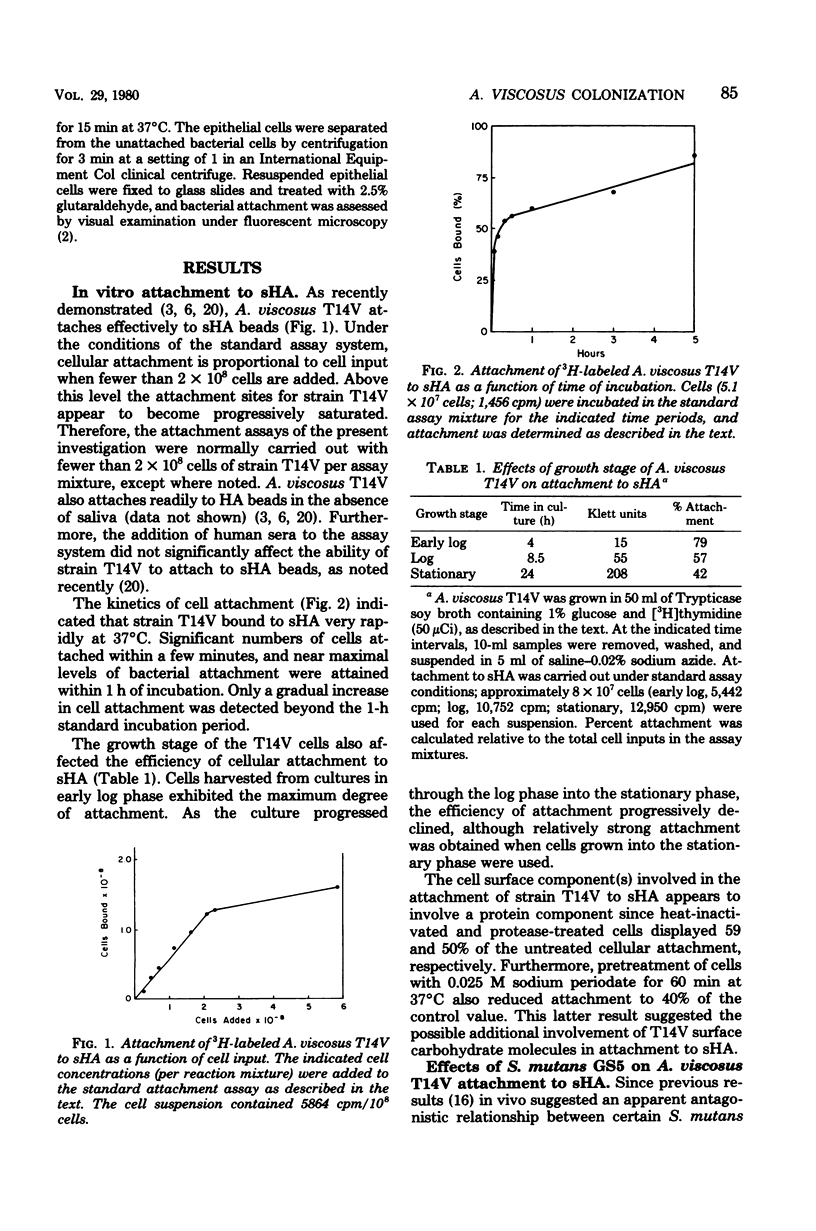
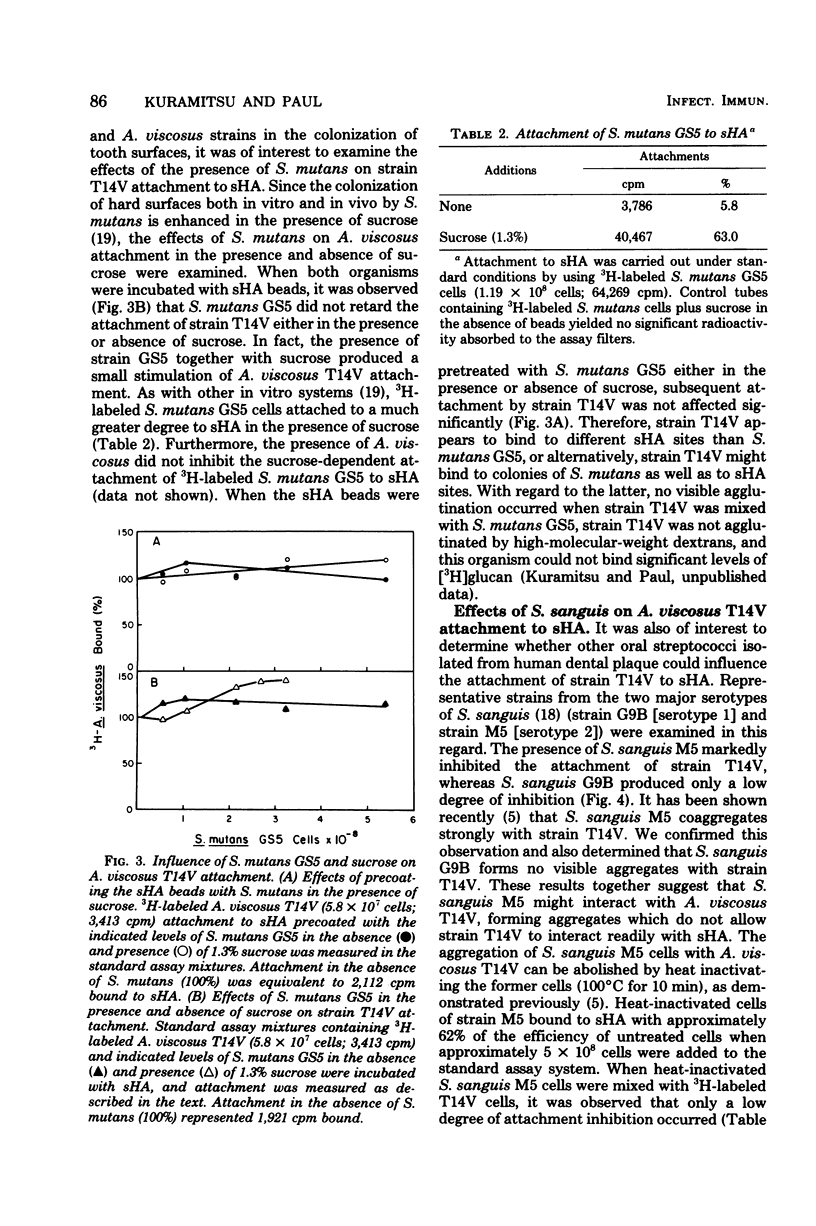
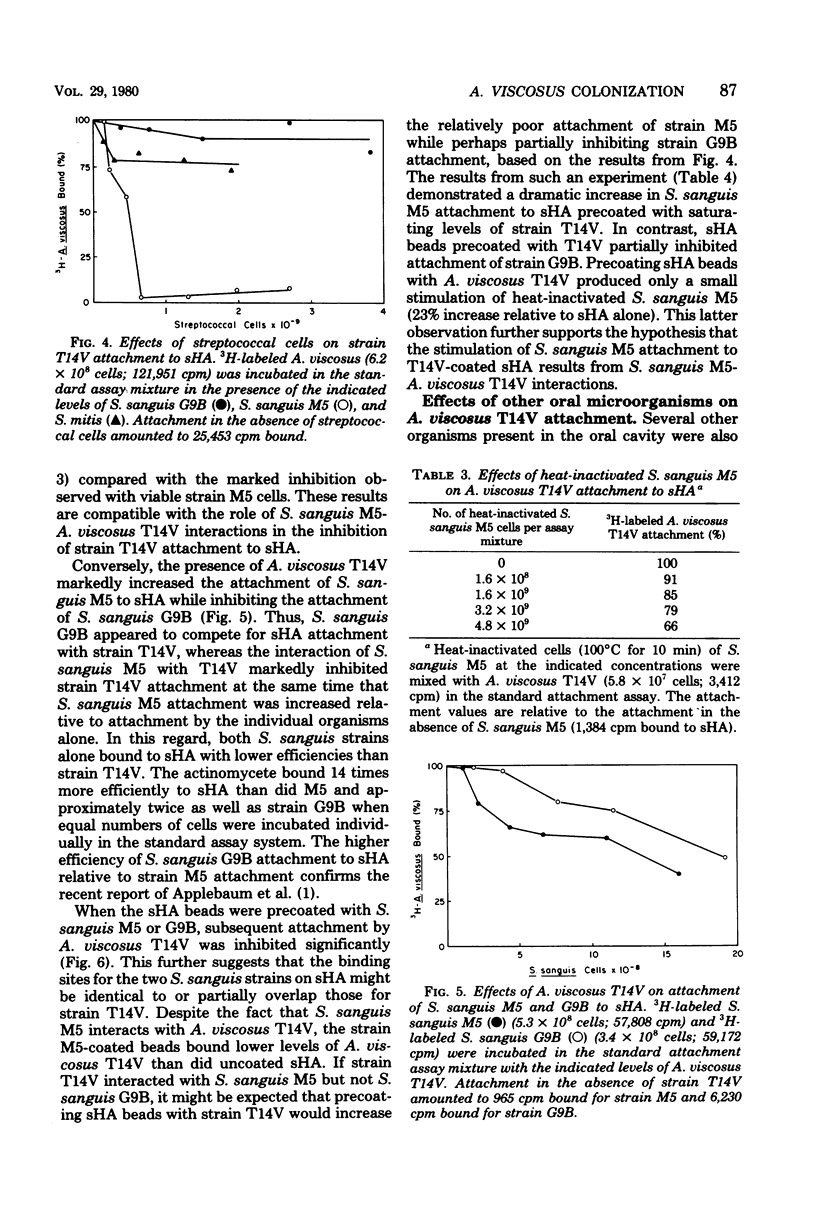
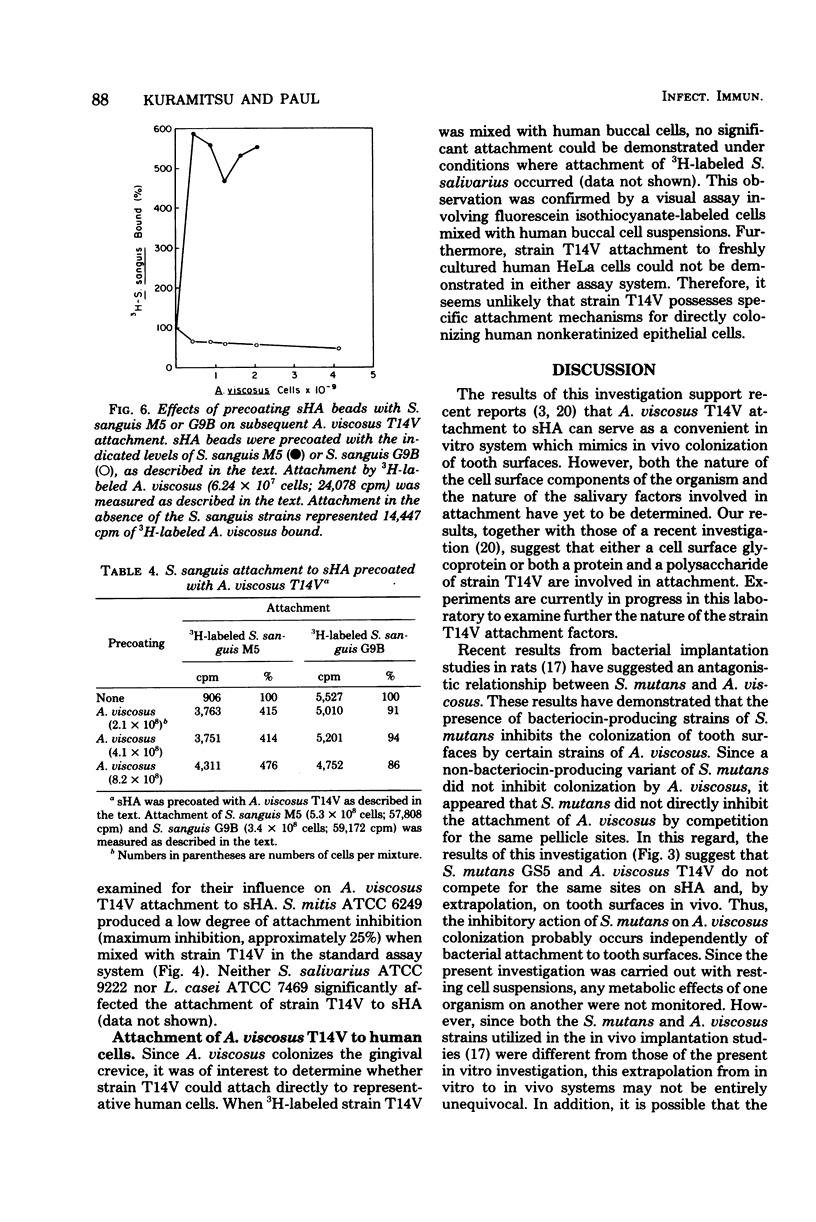
The effects of several microbial residents of human oral cavities on the attachment of Actinomyces viscosus T14V to tooth surfaces were assessed by using the saliva-coated hydroxyapatite (sHA) in vitro system. Attachment to sHA by A. viscosus T14V was not inhibited in the presence of Streptococcus mutans GS5, either in the presence or absence of sucrose. Precoating sHA beads with S. mutans in the presence of sucrose also did not retard strain T14V attachment. However, the presence of Streptococcus sanguis M5 during strain T14V attachment markedly inhibited the interaction of A. viscosus T14V with sHA, whereas another S. sanguis strain, strain G9B, produced relatively weak inhibition. The inhibitory effect of S. sanguis M5 appeared to result from the direct interaction of this organism with A. viscosus T14V. Conversely, the presence of strain T14V markedly increased the attachment of S. sanguis M5 to sHA while partially inhibiting the attachment of S. sanguis G9B. Moreover, sHA beads precoated with A. viscosus T14V bound much higher levels of S. sanguis M5 relative to uncoated sHA, whereas S. sanguis G9B attachment was only weakly inhibited. It was also observed that sHA beads precoated with either S. sanguis M5 or G9B inhibited subsequent attachment by strain T14V. These results suggest the possibility that the attachment sites for both S. sanguis strains partially overlap those for A. viscosus T14V. Streptococcus mitis weakly inhibited attachment of strain T14V to sHA, and both Streptococcus salivarius and Lactobacillus casei had little effect on attachment. In addition, both a radioisotope attachment assay and fluorescent microscopy demonstrated no significant attachment of A. viscosus T14V to human epithelial cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelbaum B., Golub E., Holt S. C., Rosan B. In vitro studies of dental plaque formation: adsorption of oral streptococci to hydroxyaptite. Infect Immun. 1979 Aug;25(2):717–728. doi: 10.1128/iai.25.2.717-728.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelt M. A., Duncan J. L. Adherence of group A streptococci to human epithelial cells. Infect Immun. 1978 Apr;20(1):200–208. doi: 10.1128/iai.20.1.200-208.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecher S. M., van Houte J., Hammond B. F. Role of colonization in the virulence of Actinomyces viscosus strains T14-Vi and T14-Av. Infect Immun. 1978 Nov;22(2):603–614. doi: 10.1128/iai.22.2.603-614.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burckhardt J. J., Guggenheim B. Increased smooth-surface caries incidence in gnotobiotic rats immunized with Actinomyces viscosus. Caries Res. 1980;14(1):56–59. doi: 10.1159/000260435. [DOI] [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson T. Salivary glycoproteins. Composition and adsorption to hydroxylapatite in relation to the formation of dental pellicles and calculus. Acta Odontol Scand. 1968 May;26(1):3–21. doi: 10.3109/00016356809004577. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Moreno E. C., Spinell D. M. Model delineating the effects of a salivary pellicle on the adsorption of Streptococcus miteor onto hydroxyapatite. Infect Immun. 1976 Oct;14(4):1109–1112. doi: 10.1128/iai.14.4.1109-1112.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Selective bacterial adherence to oral epithelial surfaces and its role as an ecological determinant. Infect Immun. 1971 Apr;3(4):567–573. doi: 10.1128/iai.3.4.567-573.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman J. D., Van Houte J., Gibbons R. J. Sorption of bacteria to human enamel powder. Arch Oral Biol. 1970 Sep;15(9):899–903. doi: 10.1016/0003-9969(70)90163-9. [DOI] [PubMed] [Google Scholar]
- Jordan H. V., Hammond B. F. Filamentous bacteria isolated from human root surface caries. Arch Oral Biol. 1972 Sep;17(9):1333–1342. doi: 10.1016/0003-9969(72)90166-5. [DOI] [PubMed] [Google Scholar]
- Jordan H. V., Keyes P. H., Bellack S. Periodontal lesions in hamsters and gnotobiotic rats infected with actinomyces of human origin. J Periodontal Res. 1972;7(1):21–28. doi: 10.1111/j.1600-0765.1972.tb00627.x. [DOI] [PubMed] [Google Scholar]
- Kuramitsu H. K. Properties of a mutant of Streptococcus mutans altered in glucosyltransferase activity. Infect Immun. 1976 Feb;13(2):345–353. doi: 10.1128/iai.13.2.345-353.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Schauer S. V. Studies on the bacterial components which bind Streptococcus sanguis and Streptococcus mutans to hydroxyapatite. Arch Oral Biol. 1975 Sep;20(9):609–615. doi: 10.1016/0003-9969(75)90082-5. [DOI] [PubMed] [Google Scholar]
- Minah G. E., Loesche W. J. Sucrose metabolism in resting-cell suspensions of caries associated and non-caries-associated dental plaque. Infect Immun. 1977 Jul;17(1):43–54. doi: 10.1128/iai.17.1.43-54.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers A. H., van der Hoeven J. S., Mikx F. H. Inhibition of Actinomyces viscosus by bacteriocin-producing strains of Streptococcus mutans in the dental plaque of gnotobiotic rats. Arch Oral Biol. 1978;23(6):477–483. doi: 10.1016/0003-9969(78)90080-8. [DOI] [PubMed] [Google Scholar]
- Rosan B. Antigens of Streptococcus sanguis. Infect Immun. 1973 Feb;7(2):205–211. doi: 10.1128/iai.7.2.205-211.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler T. T., Clark W. B., Birdsell D. C. Adherence of Actinomyces viscosus T14V and T14AV to hydroxyapatite surfaces in vitro and human teeth in vivo. Infect Immun. 1979 Sep;25(3):1066–1074. doi: 10.1128/iai.25.3.1066-1074.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


