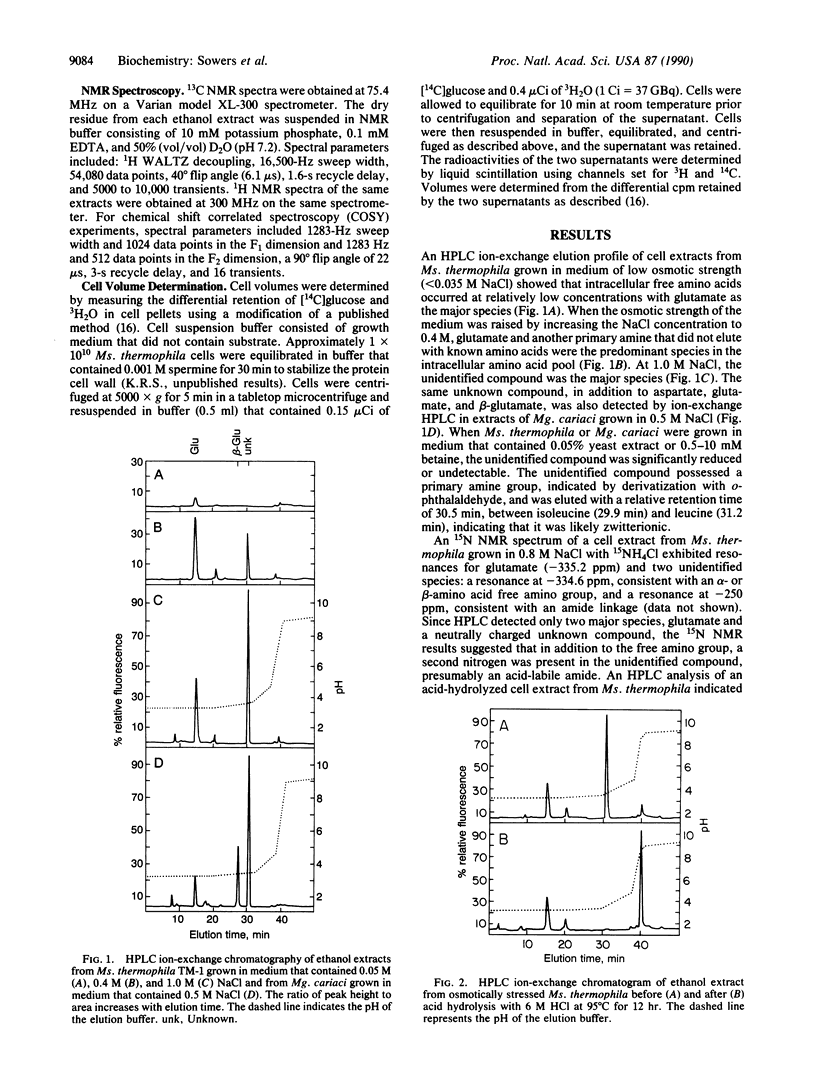
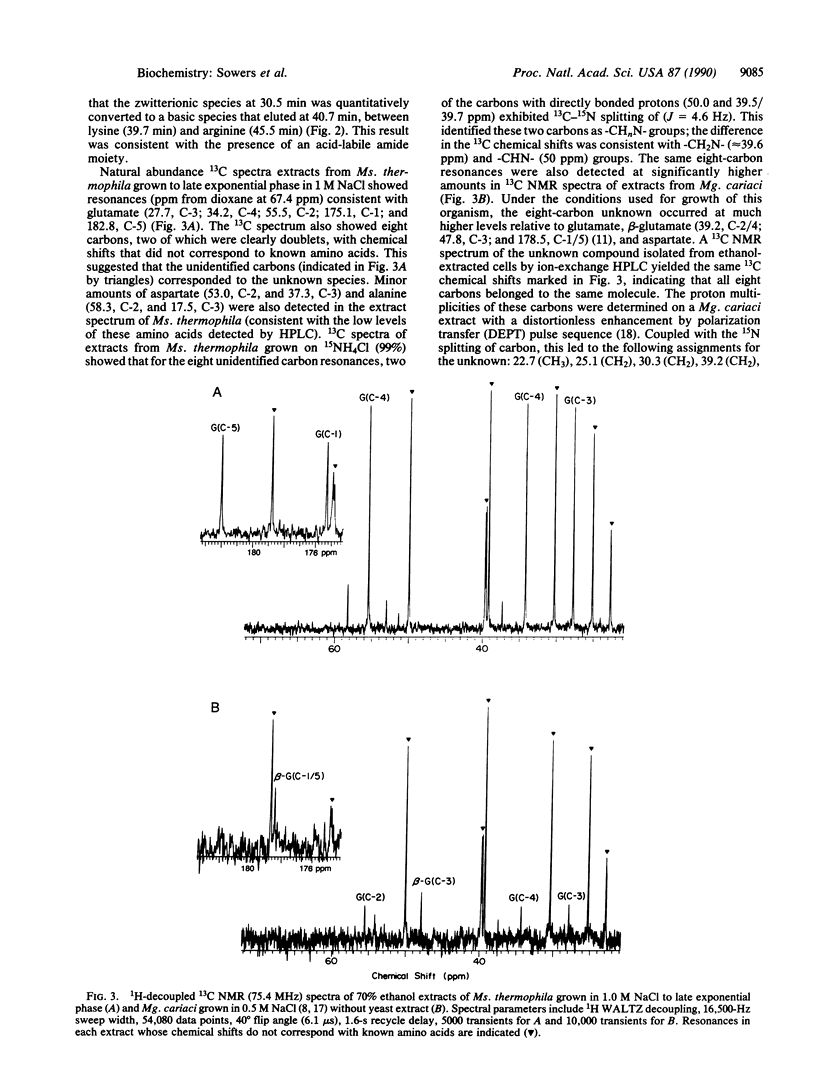
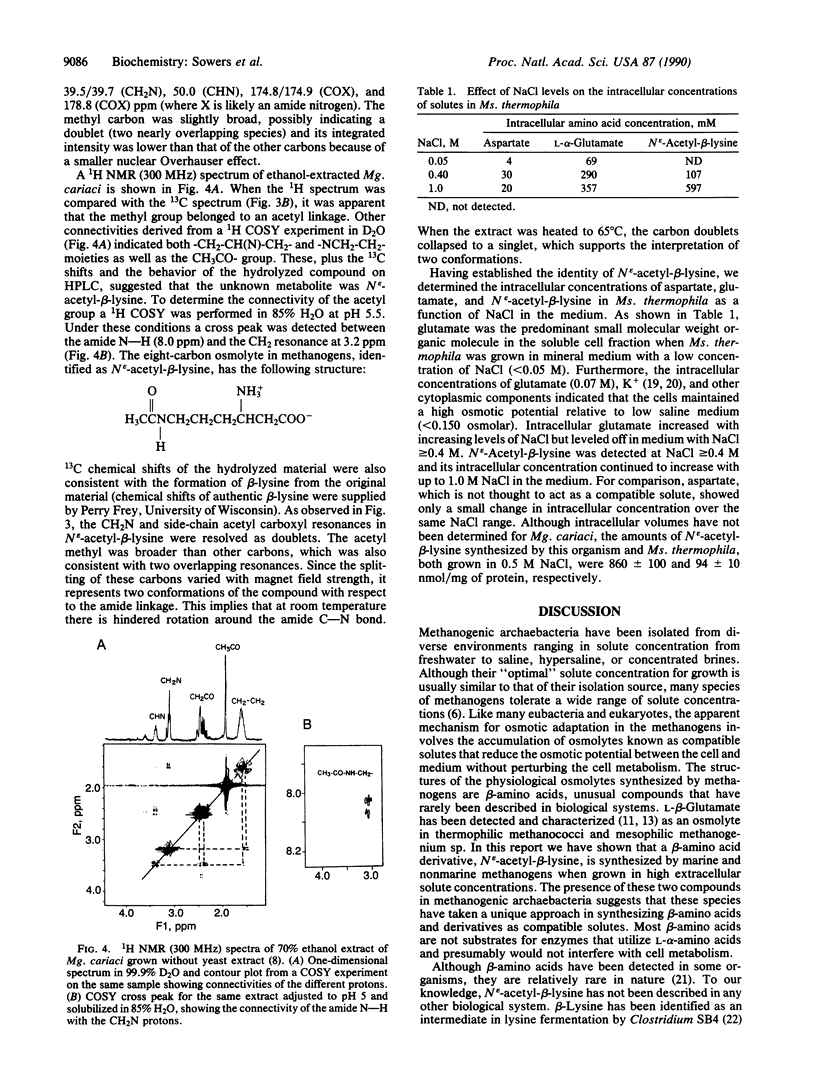
Abstract
Methanosarcina thermophila, a nonmarine methanogenic archaebacterium, can grow in a range of saline concentrations. At less than 0.4 M NaCl, Ms. thermophila accumulated glutamate in response to increasing osmotic stress. At greater than 0.4 M NaCl, this organism synthesized a modified beta-amino acid that was identified as N epsilon-acetyl-beta-lysine by NMR spectroscopy and ion-exchange HPLC. This beta-amino acid derivative accumulated to high intracellular concentrations (up to 0.6 M) in Ms. thermophila and in another methanogen examined--Methanogenium cariaci, a marine species. The compound has features that are characteristic of a compatible solute: it is neutrally charged at physiological pH and it is highly soluble. When the cells were grown in the presence of exogenous glycine betaine, a physiological compatible solute, N epsilon-acetyl-beta-lysine synthesis was repressed and glycine betaine was accumulated. N epsilon-acetyl-beta-lysine was synthesized by species from three phylogenetic families when grown in high solute concentrations, suggesting that it may be ubiquitous among the methanogens. The ability to control the biosynthesis of N epsilon-acetyl-beta-lysine in response to extracellular solute concentration indicates that the methanogenic archaebacteria have a unique beta-amino acid biosynthetic pathway that is osmotically regulated.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. D., Simpson J. R. Water relations of sugar-tolerant yeasts: the role of intracellular polyols. J Gen Microbiol. 1972 Oct;72(3):589–591. doi: 10.1099/00221287-72-3-589. [DOI] [PubMed] [Google Scholar]
- Costilow R. N., Rochovansky O. M., Barker H. A. Isolation and identification of beta-lysine as an intermediate in lysine fermentation. J Biol Chem. 1966 Apr 10;241(7):1573–1580. [PubMed] [Google Scholar]
- Cover W. H., Martinez R. J., Rittenberg S. C. Permeability of the boundary layers of Bdellovibrio bacteriovorus 109J and its bdelloplasts to small hydrophilic molecules. J Bacteriol. 1984 Feb;157(2):385–390. doi: 10.1128/jb.157.2.385-390.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989 Mar;53(1):121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W. Beta-amino acids: mammalian metabolism and utility as alpha-amino acid analogues. Annu Rev Biochem. 1986;55:855–878. doi: 10.1146/annurev.bi.55.070186.004231. [DOI] [PubMed] [Google Scholar]
- Henrichs S. M., Cuhel R. Occurrence of beta-Aminoglutaric Acid in Marine Bacteria. Appl Environ Microbiol. 1985 Aug;50(2):543–545. doi: 10.1128/aem.50.2.543-545.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Measures J. C. Role of amino acids in osmoregulation of non-halophilic bacteria. Nature. 1975 Oct 2;257(5525):398–400. doi: 10.1038/257398a0. [DOI] [PubMed] [Google Scholar]
- Perroud B., Le Rudulier D. Glycine betaine transport in Escherichia coli: osmotic modulation. J Bacteriol. 1985 Jan;161(1):393–401. doi: 10.1128/jb.161.1.393-401.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. E., Lesage S., Roberts M. F. Beta-aminoglutaric acid is a major soluble component of Methanococcus thermolithotrophicus. Biochim Biophys Acta. 1989 Sep 15;992(3):320–326. doi: 10.1016/0304-4165(89)90091-3. [DOI] [PubMed] [Google Scholar]
- Robertson D. E., Noll D., Roberts M. F., Menaia J. A., Boone D. R. Detection of the osmoregulator betaine in methanogens. Appl Environ Microbiol. 1990 Feb;56(2):563–565. doi: 10.1128/aem.56.2.563-565.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. E., Roberts M. F., Belay N., Stetter K. O., Boone D. R. Occurrence of beta-glutamate, a novel osmolyte, in marine methanogenic bacteria. Appl Environ Microbiol. 1990 May;56(5):1504–1508. doi: 10.1128/aem.56.5.1504-1508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers K. R., Gunsalus R. P. Adaptation for growth at various saline concentrations by the archaebacterium Methanosarcina thermophila. J Bacteriol. 1988 Feb;170(2):998–1002. doi: 10.1128/jb.170.2.998-1002.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreeland R. H. Mechanisms of halotolerance in microorganisms. Crit Rev Microbiol. 1987;14(4):311–356. doi: 10.3109/10408418709104443. [DOI] [PubMed] [Google Scholar]
- Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N. Living with water stress: evolution of osmolyte systems. Science. 1982 Sep 24;217(4566):1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]



