Abstract
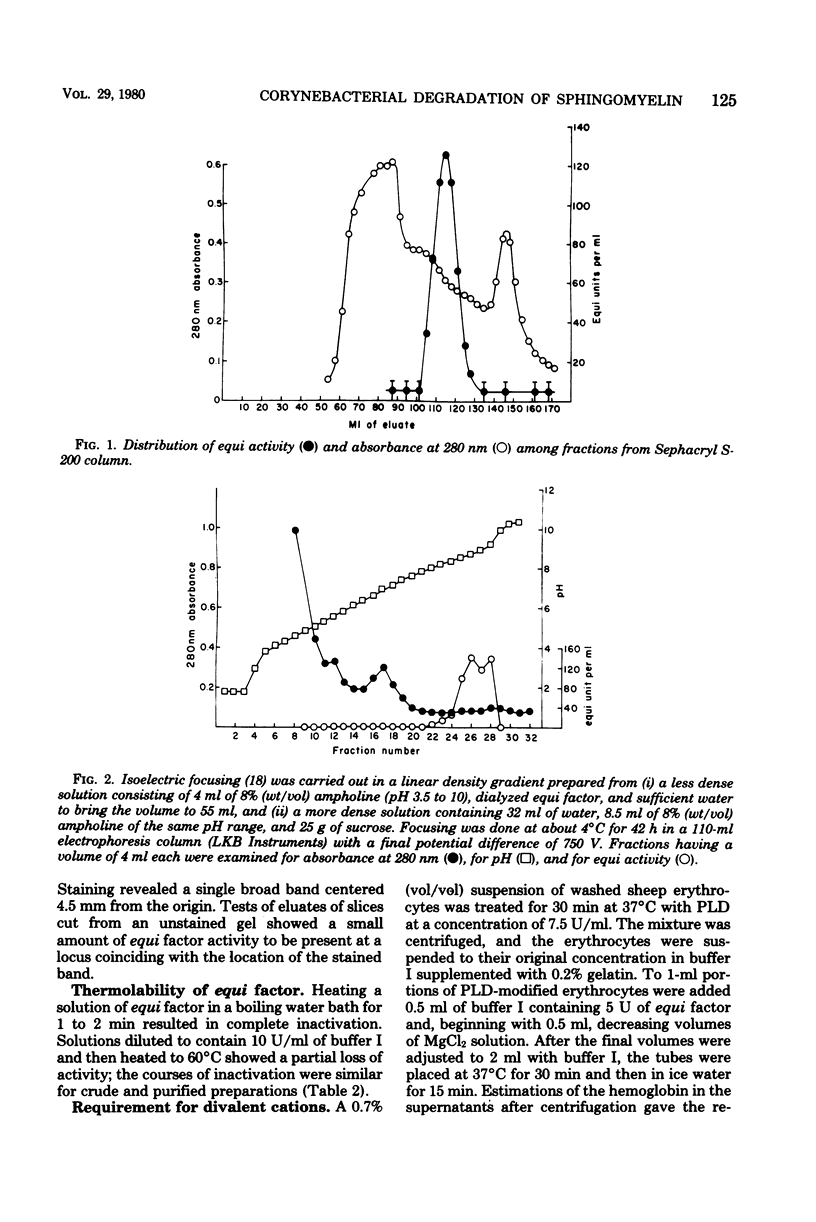
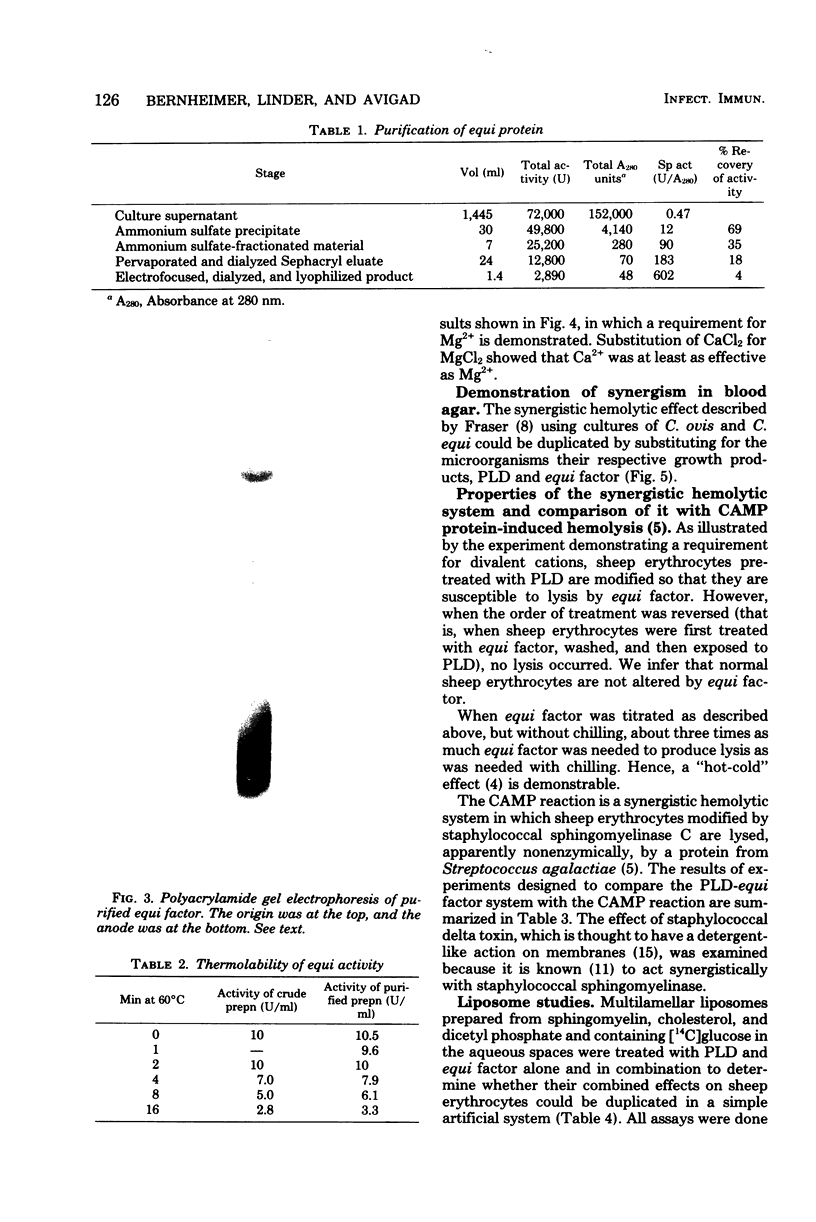
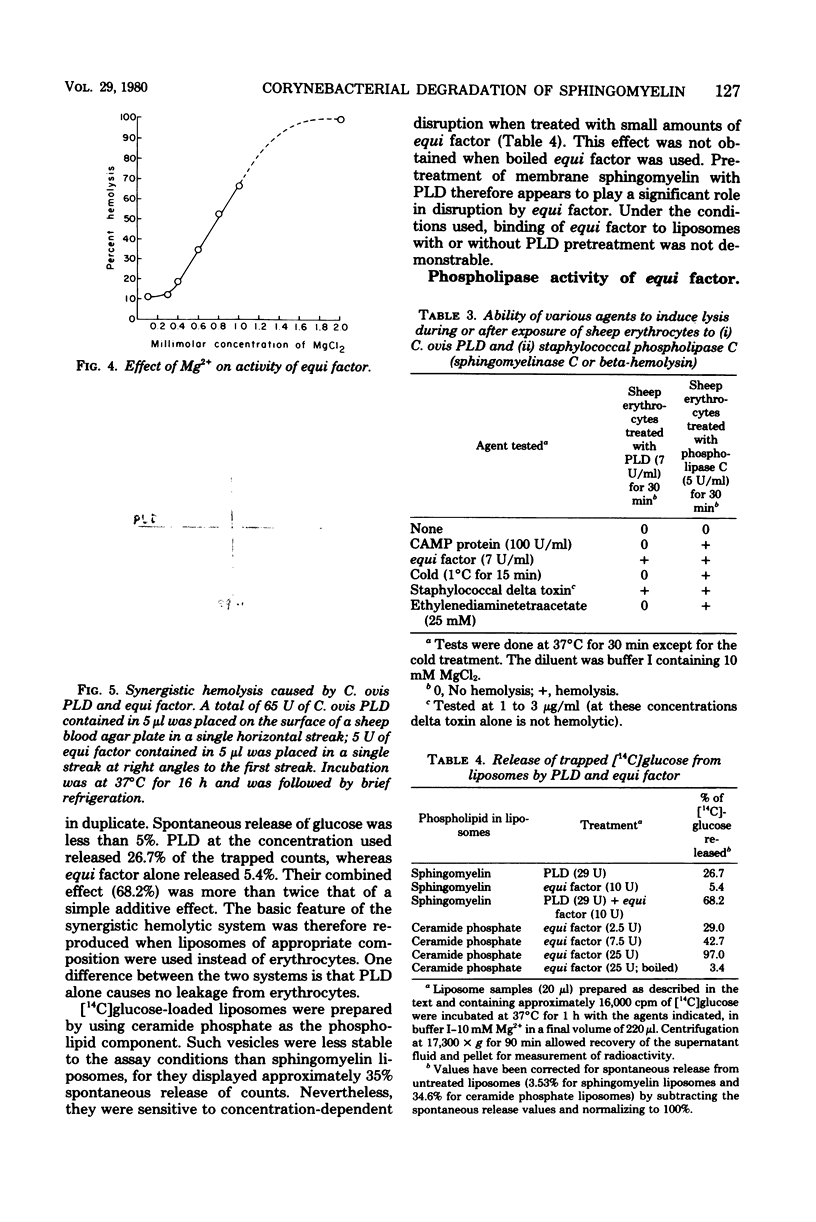
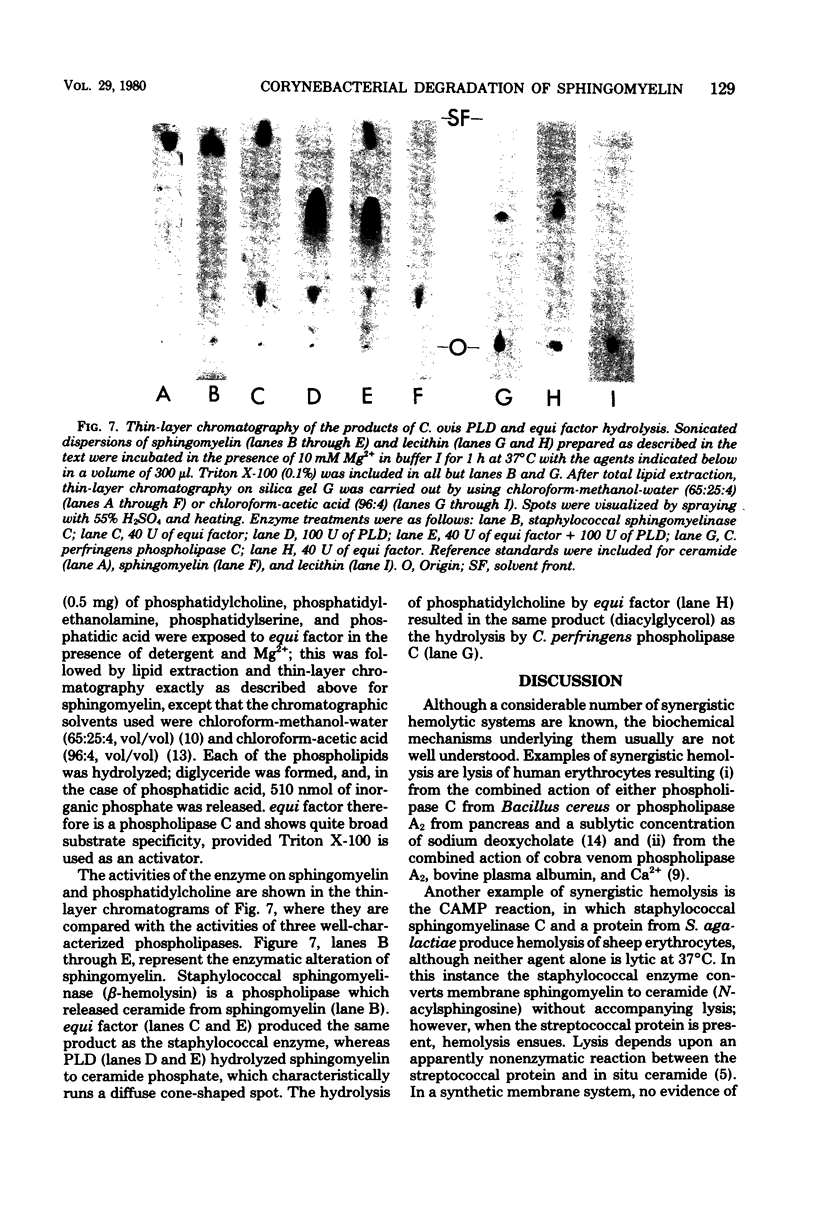
The mechanism of in vitro synergistic lysis of sheep erythrocytes by Corynebacterium ovis and Corynebacterium equi was investigated. Hemolysis required (i) the action of phospholipase D from C. ovis, (ii) the action of an extracellular protein of C. equi, and (iii) Mg2+. Maximum lysis required imposition on the system of a fourth condition (step iv), such as chilling. Steps i, ii, and iv occur sequentially and in that order. Mg2+ functions in steps i and ii. The extracellular protein C. equi was purified to homogeneity and found to be a phospholipase C capable of hydrolyzing ceramide phosphate, phosphatidic acid, and all of the isolated major phospholipids of mammalian erythrocyte membranes. The principal features of the synergistic hemolytic system could be reproduced in experiments involving liposomes containing either sphingomyelin or ceramide phosphate and trapped [14C]glucose. We inferred that sphingomyelin of sheep erythrocytes is first converted to ceramide phosphate by C. ovis phospholipase D. On the basis of results with liposomes, we propose that the ceramide phosphate is then converted to ceramide by C. equi phospholipase C. We believe that the resulting in situ ceramide then undergoes dislocation by chilling and perhaps also by virtue of an affinity between ceramide and C. equi phospholipase C. The dislocation of ceramide presumably disorganizes the lipid bilayer sufficiently to result in cell lysis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bernheimer A. W., Avigad L. S., Kim K. S. Staphylococcal sphingomyelinase (beta-hemolysin). Ann N Y Acad Sci. 1974 Jul 31;236(0):292–306. doi: 10.1111/j.1749-6632.1974.tb41499.x. [DOI] [PubMed] [Google Scholar]
- Bernheimer A. W., Avigad L. S. Properties of a toxin from the sea anemone Stoichacis helianthus, including specific binding to sphingomyelin. Proc Natl Acad Sci U S A. 1976 Feb;73(2):467–471. doi: 10.1073/pnas.73.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer A. W., Linder R., Avigad L. S. Nature and mechanism of action of the CAMP protein of group B streptococci. Infect Immun. 1979 Mar;23(3):838–844. doi: 10.1128/iai.23.3.838-844.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRASER G. Haemolytic activity of Corynebacterium ovis. Nature. 1961 Jan 21;189:246–246. doi: 10.1038/189246a0. [DOI] [PubMed] [Google Scholar]
- FRASER G. THE EFFECT ON ANIMAL ERYTHROCYTES OF COMBINATIONS OF DIFFUSIBLE SUBSTANCES PRODUCED BY BACTERIA. J Pathol Bacteriol. 1964 Jul;88:43–53. doi: 10.1002/path.1700880105. [DOI] [PubMed] [Google Scholar]
- Gul S., Smith A. D. Haemolysis of intact human erythrocytes by purified cobra venom phospholipase A2 in the presence of albumin and Ca2+. Biochim Biophys Acta. 1974 Nov 15;367(3):271–281. doi: 10.1016/0005-2736(74)90084-4. [DOI] [PubMed] [Google Scholar]
- Kreger A. S., Kim K. S., Zaboretzky F., Bernheimer A. W. Purification and properties of staphylococcal delta hemolysin. Infect Immun. 1971 Mar;3(3):449–465. doi: 10.1128/iai.3.3.449-465.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder R., Bernheimer A. W. Effect on sphingomyelin-containing liposomes of phospholipase D from Corynebacterium ovis and the cytolysin from Stoichactis helianthus. Biochim Biophys Acta. 1978 Aug 25;530(2):236–246. doi: 10.1016/0005-2760(78)90009-7. [DOI] [PubMed] [Google Scholar]
- Roelofsen B., Zwaal R. F., Comfurius P., Woodward C. B., van Deenen L. L. Action of pure phospholipase A 2 and phospholipase C on human erythrocytes and ghosts. Biochim Biophys Acta. 1971 Sep 14;241(3):925–929. doi: 10.1016/0005-2736(71)90024-1. [DOI] [PubMed] [Google Scholar]
- Rogolsky M. Nonenteric toxins of Staphylococcus aureus. Microbiol Rev. 1979 Sep;43(3):320–360. doi: 10.1128/mr.43.3.320-360.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucek A., Michalec C., Soucková A. Identification and characterization of a new enzyme of the group "phospholipase D" isolated from Corynebacterium ovis. Biochim Biophys Acta. 1971 Jan 13;227(1):116–128. doi: 10.1016/0005-2744(71)90173-2. [DOI] [PubMed] [Google Scholar]
- Soucek A., Soucková A. Toxicity of bacterial sphingomyelinases D. J Hyg Epidemiol Microbiol Immunol. 1974;18(3):327–335. [PubMed] [Google Scholar]
- Vesterberg O., Wadström T., Vesterberg K., Svensson H., Malmgren B. Studies on extracellular PROTEINS FROM Staphylococcus aureus. I. Separation and characterization of enzymes and toxins by isoelectric focusing. Biochim Biophys Acta. 1967 Apr 11;133(3):435–445. doi: 10.1016/0005-2795(67)90547-8. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]