Abstract
Objective
To evaluate the predictive value of the progesterone level at the beta-human chorionic gonadotropin (β-hCG) check day for ongoing pregnancy maintenance in in vitro fertilization (IVF) cycles in women with previous unexplained repeated miscarriages.
Materials and methods
One hundred and forty-eight women, with visible gestational sac after IVF, were recruited in this observational study. All subjects had unexplained recurrent miscarriages in more than two previous IVF cycles. The progesterone level at the β-hCG check day (i.e. 14 days after oocyte retrieval) was assessed. The area under the curve (AUC) of the progesterone level was evaluated to predict the ongoing pregnancy or miscarriage outcomes.
Results
The overall ongoing pregnancy rate was 60.8% (90/148). The cut-off value with β-hCG levels higher than 126.5 mIU/mL and with progesterone levels higher than 25.2 ng/mL could be the predictive factors for ongoing pregnancy maintenance (AUC = 0.788 and 0.826; sensitivity = 0.788 and 0.723; specificity = 0.689 and 0.833; P < 0.0001 and P < 0.0001, respectively). The miscarriage rates were 19.5% (15/77) in the women with β-hCG > 126.5 mIU/mL and 13.0% (10/77) in those with > 25.2 ng/mL. In the comparison of the ROC curves between both values, a similar significance was found. The subjects with β-hCG > 126.5 mIU/mL and progesterone > 25.2 ng/mL showed higher ongoing pregnancy rates [98.0% (49/50) vs. 41.8% (41/98)] than those with β-hCG ≤ 126.5 mIU/mL or progesterone ≤ 25.2 ng/mL.
Conclusions
The progesterone level at 14 days after oocyte retrieval can be a good predictive marker for ongoing pregnancy maintenance in women with repeated IVF failure with miscarriage, together with the β-hCG level. The combined cut-off value of progesterone > 25.2 ng/mL and β-hCG > 126.5 mIU/mL may suggest a good prognosis.
Introduction
The overall miscarriage rate in pregnant women after in vitro fertilization (IVF) was reported to be ~23% [1, 2]. For couples who conceive via IVF, miscarriage after confirmation of a visible intrauterine gestational sac (G-sac) can be another “hope torture” in a different way compared to infertility. Furthermore, unexplained recurrent miscarriages after IVF can be a challenging and frustrating condition for both patients and clinicians. Numerous previous studies have suggested the predictors for miscarriage after IVF, such as endometrial thickness [3] and pattern [4], anti-Müllerian hormone level [5], beta-human chorionic gonadotropin (β-hCG) [6], even previous miscarriage history [7]. However, these predictors may not be clinically valuable in terms of their sensitivity and specificity, especially at early pregnancy period, as the first checked serum β-hCG.
Progesterone is an essential hormone in the process of reproduction. It is involved in implantation and pregnancy maintenance. It has been used in IVF for luteal support after embryo transfer (ET) and early pregnancy maintenance in cases of positive β-hCG check [8]. It was suggested that progesterone plays a role in the modulation of maternal immune response [9], reduction of uterine contractility [10], and improvement of utero-placental circulation [11]. With the stimulation of hCG secreted by the placenta after implantation into the maternal side, progesterone is mainly produced by the corpus luteum in the early pregnancy period [12]. These premises implicate that not only the β-hCG level but also the maternal serum progesterone level in early pregnancy can be a predictor of maintenance of pregnancy. A recent report showed that progesterone levels on the β-hCG check day could be considered a predictor for heterotopic pregnancy after two or more embryos transferred during the IVF procedures [13]. However, there is limited knowledge on the predictive value of serum progesterone level in the early pregnancy period for miscarriage after implantation in the IVF cycles.
This study aimed to evaluate the predictive value of the progesterone level on the β-hCG check day for miscarriage after confirmation of a visible G-sac in women with miscarriage history who underwent IVF cycles.
Materials and methods
Study subjects
This prospective observational study was approved by our institutional review board with written informed consent (KUGH13147-005). The included subjects were women with visible G-sac in the uterine cavity at the 5th week of gestation after in vitro fertilization (IVF) and with history of ≥ 2 unexplained miscarriages in the previous fresh or thawed embryo transfer cycles. The exclusion criteria were as follows: old age (> 42 years); obesity (BMI > 25 kg/m2); expected low response (AMH < 0.8 ng/mL); uterine and endometrial factor infertilities; presence of male factor infertility; metabolic, hepatic, and cardiovascular disorders; and multiple pregnancies.
Controlled ovarian stimulation (COS) protocols
COS and IVF were performed as previously reported [14, 15]. For the GnRH agonist long protocol, the GnRH agonist triptorelin (Decapeptyl®, 0.1 mg/day; Ferring, Malmo, Sweden) was started in the mid-luteal phase of the previous cycle. After pituitary down-regulation, the triptorelin dose was reduced to 0.05 mg/day, and gonadotropin (Gonal-F®; Serono, Geneva, Switzerland) was added until either the leading follicle reached a mean diameter of 18 mm or two or more follicles reached a diameter of 17 mm. Treatment with 37.5–300 IU of gonadotropin, depending on the patients’ previous or anticipated responses, was initiated on the third day of the menstrual cycle. The treatment was then individualized and adjusted in accordance with the response. For the GnRH antagonist multiple dose flexible protocol, 37.5–300 IU of gonadotropin was started on the third menstrual cycle day. The GnRH antagonist cetrorelix (Cetrotide®, 0.25 mg; Serono, Geneva, Switzerland) was added daily, started when the leading follicle reached a diameter of 14 mm, and continued until either the leading follicle reached a mean diameter of 18 mm or two or more follicles reached a diameter of 17 mm. For both protocols, recombinant hCG (Ovidrel®, Serono, Geneva, Switzerland) was administered subcutaneously 36 hours before the ultrasonography-guided oocyte retrieval. The GnRH agonist or antagonist protocol was employed in accordance with the menstrual cycle day at the patient’s visit.
In vitro fertilization (IVF)
The retrieved oocytes were cultured for 4 to 6 hours until insemination. Semen samples obtained via ejaculation on the morning of the oocyte retrieval day were liquefied at room temperature for 30 minutes and centrifuged using the SpermGrad (Vitrolife, Kungsbacka, Sweden) made of two gradients (45%/90%) at 1,500 rpm for 20 minutes. After removal of the supernatant, we layered 2 mL of the Universal IVF medium over the sperm pellet for centrifugation at 1,000 rpm for 10 minutes. After washing and conducting the swim-up procedure, only the sperm pellet in the supernatant was aspirated and used for the insemination. Fertilization was determined by the presence of 2 pronuclei (2PN) using an inverted microscope on the first day after insemination. The zygotes with 2PN were cultured individually in the microdrops of 25 μL of growth medium, G-1™ v5 (Vitrolife) overlaid with 8 mL of mineral oil (Sigma, USA) in Falcon 1007 culture dishes (Becton Dickinson Labware, Franklin Lakes, New Zealand) at 37°C under 6% CO2.
Embryo transfer (ET), luteal support and pregnancy follow-up
ET was performed 3 days after oocyte retrieval. The embryos were graded according to their morphologies and cleavage rates; they were graded from I to V based on the number and uniformity of the blastomere and percentage of fragmentation. We defined good-quality embryos as those of morphologic grades I-II/V; embryos with blastomeres of equal size with no cytoplasmic fragments or with minor cytoplasmic fragments or blebs and up to two good-quality embryos were selected and transferred into the uterus.
The luteal phase was supported with daily 8% progesterone gel (Crinone®, Serono) initially for 14 days, starting on the day of oocyte retrieval. The serum β-hCG and progesterone levels were checked on the 14th day after oocyte retrieval, and the G-sac was assessed via vaginal ultrasonography on the 21st day after oocyte retrieval. If a G-sac was visualized, the gestational age was determined as the 5th gestational week. The luteal support was continued until 8th week of gestation. For surveillance of miscarriage, all subjects were followed up for up to 18 weeks of gestation.
Statistical analysis
Data were analyzed using the Student’s t-test, Mann-Whitney U test, Chi-square test, Fisher’s exact test, and ROC curve, where appropriate. In all tests, significance was accepted for P values < .05. All data were analyzed using the Statistical Package for the Social Sciences for Windows (version 20.0, SPSS Inc.) and MedCalc (version 17.4.4, ScyMed).
Results
Baseline characteristics and overall pregnancy outcomes
In the present study, 148 women with positive β-hCG on the 14th day after oocyte retrieval were enrolled. The basal characteristics in the present study are shown in Table 1. The overall ongoing pregnancy rate over 18 weeks of gestation was 60.8% (90/148), and the miscarriage rate was 39.2% (58/148). The mean β-hCG and progesterone levels at the 14th day after oocyte retrieval were 157.3±79.5 mIU/mL and 29.9±17.4 ng/mL, respectively. There was a significant positive correlation between β-hCG and progesterone levels (rho = 0.352, P< .0001).
Table 1. Baseline characteristics of study subjects.
| Variables | mean±S.D. |
|---|---|
| Number | 148 |
| Age (years) | |
| Wife | 35.1±4.5 |
| Husband | 38.1±4.8 |
| Cause of infertility | |
| Ovulatory | 35.1% (52/148) |
| Tubal | 31.1% (46/148) |
| Unexplained | 33.8% (50/148) |
| No. of previous IVF cycles | 2.5±0.8 |
| Basal FSH (mIU/mL) | 7.2±2.3 |
| Basal AMH (ng/mL) | 1.9±1.1 |
| Protocols | |
| GnRH agonist | 58.1% (86/148) |
| GnRH antagonist | 41.9% (62/148) |
| No. of oocytes retrieved | 9.7±5.7 |
| No. of MII oocytes | 6.7±4.4 |
| No. of 2PN | 4.9±3.8 |
| Fertilization rate | 73.1% |
| No. of embryos transferred | 1.9±0.8 |
| Pregnancy outcomes | |
| Ongoing pregnancy | 60.8% (90/148) |
| Miscarriage | 39.2% (58/148) |
| β-hCG (mIU/mL) | 157.3±79.5 |
| Progesterone (ng/mL) | 29.9±17.4 |
Comparison between the ongoing pregnancy maintenance and miscarriage groups
The comparison of the variables between the ongoing pregnancy maintenance and miscarriage groups is shown in Table 2. No significant difference was found in the age of the couples, cause of infertility, number of previous IVF cycles, basal FSH and AMH levels, GnRH agonist or antagonist protocol ratio, number of oocytes retrieved, and number of embryos transferred. The ongoing pregnancy maintenance group showed higher β-hCG and progesterone levels than the miscarriage group (196.1±82.6 mIU/mL vs. 137.5±45.6 mIU/mL, P < .0001; 37.5±16.7 ng/mL vs. 18.1±10.6 ng/mL, P < .0001).
Table 2. Comparison between ongoing pregnancy and miscarriage groups.
| Variables | Ongoing pregnancy (n = 90) | Miscarriage (n = 58) |
|---|---|---|
| Age (years) | ||
| Wife | 34.8±4.9 | 35.5±3.8 |
| Husband | 37.8±4.6 | 38.2±5.7 |
| Cause of infertility | ||
| Ovulatory | 35.6% (32/90) | 34.5% (20/58) |
| Tubal | 32.2% (29/90) | 29.3% (17/58) |
| Unexplained | 32.2% (29/90) | 36.2% (21/58) |
| No. of previous IVF cycles | 2.4±0.9 | 2.5±1.0 |
| Basal FSH (mIU/mL) | 7.2±2.6 | 7.2±3.1 |
| Basal AMH (ng/mL) | 1.9±1.5 | 1.8±0.8 |
| Protocols | ||
| GnRH agonist | 57.8% (52/90) | 58.6% (34/58) |
| GnRH antagonist | 42.2% (38/90) | 41.4% (24/58) |
| No. of oocytes retrieved | 9.9±6.1 | 9.5±5.9 |
| No. of MII oocytes | 6.8±4.1 | 6.6±3.6 |
| No. of 2PN | 4.7±3.0 | 5.0±4.2 |
| Fertilization rate | 69.2% | 75.8% |
| No. of embryos transferred | 1.9±0.9 | 1.9±1.0 |
| β-hCG (mIU/mL) | 196.1±82.6a | 137.5±45.6a |
| Progesterone (ng/mL) | 37.5±16.7b | 18.1±10.6b |
a and b P< .0001, P> .05 in the others
ROC curve of the β-hCG and progesterone levels
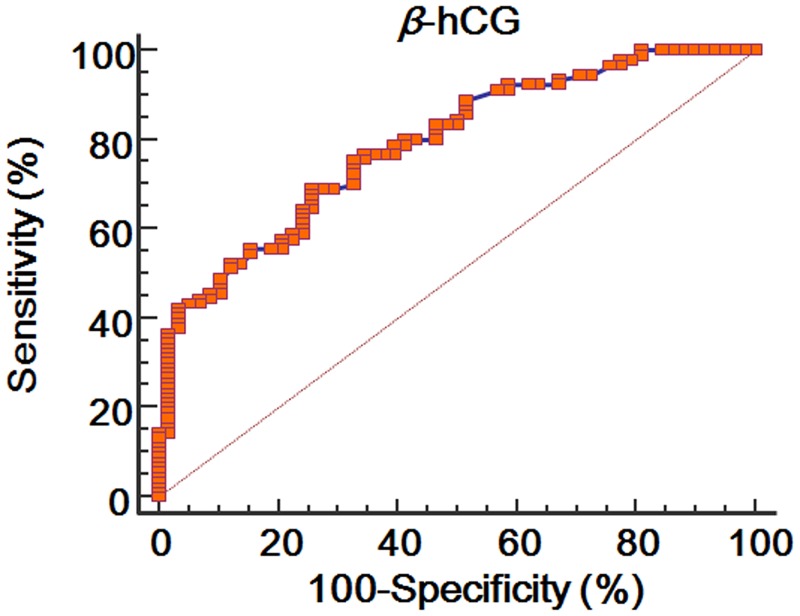
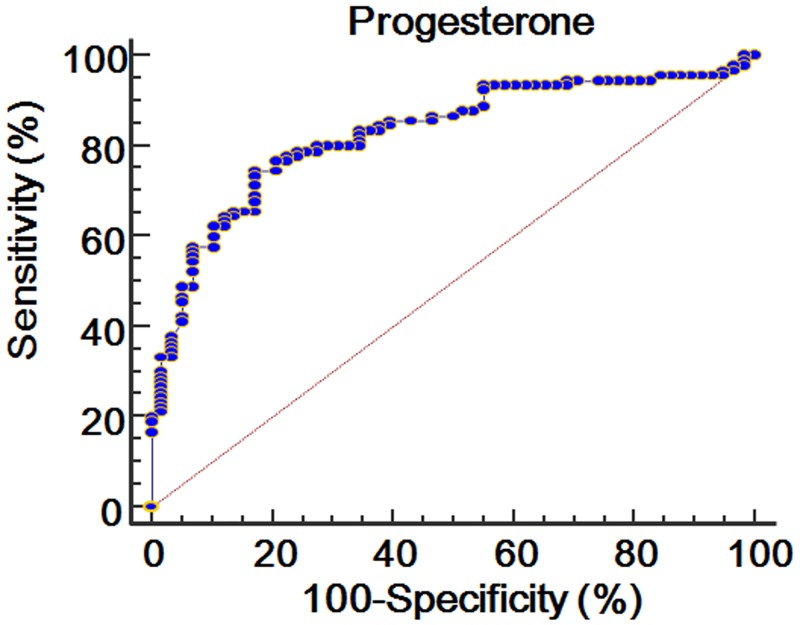
Figs 1 and 2 show the ROC curve of the β-hCG and progesterone levels for predictive maintenance of ongoing pregnancy. The area under the ROC curve (AUC) of the β-hCG level was 0.788 (standard error = 0.0369; 95% CI = 0.714 to 0.851; P < 0.0001), and its cut-off value was higher than 126.5 mIU/mL (sensitivity = 68.9%; specificity = 74.1%). The AUC of the progesterone level was 0.826 (standard error = 0.0343; 95% CI = 0.755 to 0.883; P < 0.0001), and its cut-off value was higher than 25.2 ng/mL (sensitivity = 74.4%; specificity = 82.8%).
Fig 1. ROC curve of β-hCG level at 14 days after oocyte retrieval for predicting ongoing pregnancy.
Fig 2. ROC curve of progesterone level at 14 days after oocyte retrieval for predicting ongoing pregnancy.
Comparison using the cut-off values of the β-hCG and progesterone levels
Using the cut-off value of the β-hCG level, the subjects with β-hCG > 126.5 mIU/mL showed higher ongoing pregnancy rates [80.5% (62/77) vs. 39.4% (28/71), P < .0001] and lower miscarriage rates [19.5% (15/77) vs. 60.6% (43/71), P < .0001] than those with β-hCG ≤ 126.5 mIU/mL, without a difference in the other variables (Table 3).
Table 3. Comparison of ongoing pregnancy outcomes according to cut-off value of β-hCG (> 126.5 ng/mL) at 14 days after oocyte retrieval.
| Variables | β-hCG > 126.5 mIU/mL (n = 77) | β-hCG ≤ 126.5 mIU/mL (n = 71) |
|---|---|---|
| Age (years) | ||
| Wife | 34.3±4.6 | 35.6±5.4 |
| Husband | 38.1±5.1 | 40.1±4.1 |
| Cause of infertility | ||
| Ovulatory | 35.1% (27/77) | 35.2% (25/71) |
| Tubal | 33.8% (26/77) | 28.2% (20/71) |
| Unexplained | 31.2% (24/77) | 36.6% (26/71) |
| No. of previous IVF cycles | 2.5±1.2 | 2.6±1.0 |
| Basal FSH (mIU/mL) | 8.0±3.0 | 8.6±4.8 |
| Basal AMH (ng/mL) | 2.0±1.2 | 1.8±0.6 |
| Protocols | ||
| GnRH agonist | 57.1% (44/77) | 59.2% (42/71) |
| GnRH antagonist | 42.9% (33/77) | 40.8% (29/71) |
| No. of oocytes retrieved | 10.1±5.7 | 9.7±7.4 |
| No. of MII oocytes | 6.7±4.7 | 6.8±5.3 |
| No. of 2PN | 4.9±3.2 | 4.8±3.9 |
| Fertilization rate | 73.2% | 70.6% |
| No. of embryos transferred | 1.8±1.0 | 1.6±0.8 |
| Pregnancy outcomes | ||
| Ongoing pregnancy | 80.5% (62/77)a | 39.4% (28/71)a |
| Miscarriage | 19.5% (15/77)a | 60.6% (43/71)a |
a P< .0001
Using the cut-off value of the progesterone level, the subjects with progesterone > 25.2 ng/mL showed higher ongoing pregnancy rates [87.0% (67/77) vs. 32.4% (23/71), P < .0001] and lower miscarriage rates [13.0% (10/77) vs. 67.6% (48/71), P < .0001] than those with progesterone ≤ 25.2 ng/mL, without a difference in the other variables (Table 4).
Table 4. Comparison of ongoing pregnancy outcomes according to cut-off value of progesterone (> 25.2 ng/mL) at 14 days after oocyte retrieval.
| Variables | Progesterone > 25.2 ng/mL (n = 77) | Progesterone ≤ 25.2 ng/mL (n = 71) |
|---|---|---|
| Age (years) | ||
| Wife | 34.6±4.8 | 35.3±5.2 |
| Husband | 38.9±5.3 | 39.8±3.9 |
| Cause of infertility | ||
| Ovulatory | 33.8% (26/77) | 36.6% (26/71) |
| Tubal | 32.5% (25/77) | 29.6% (21/71) |
| Unexplained | 33.8% (26/77) | 33.8% (24/71) |
| No. of previous IVF cycles | 2.4±1.8 | 2.7±0.3 |
| Basal FSH (mIU/mL) | 7.7±4.2 | 8.1±2.4 |
| Basal AMH (ng/mL) | 1.9±1.3 | 2.0±0.6 |
| Protocols | ||
| GnRH agonist | 59.7% (46/77) | 62.0% (44/71) |
| GnRH antagonist | 40.3% (31/77) | 38.0% (27/71) |
| No. of oocytes retrieved | 9.9±6.1 | 10.0±6.5 |
| No. of MII oocytes | 6.8±4.6 | 7.1±5.1 |
| No. of 2PN | 4.8±3.1 | 5.1±3.5 |
| Fertilization rate | 70.6% | 71.8% |
| No. of embryos transferred | 1.9±0.8 | 1.9±0.9 |
| Pregnancy outcomes | ||
| Ongoing pregnancy | 87.0% (67/77)a | 32.4% (23/71)a |
| Miscarriage | 13.0% (10/77)a | 67.6% (48/71)a |
aP< .0001
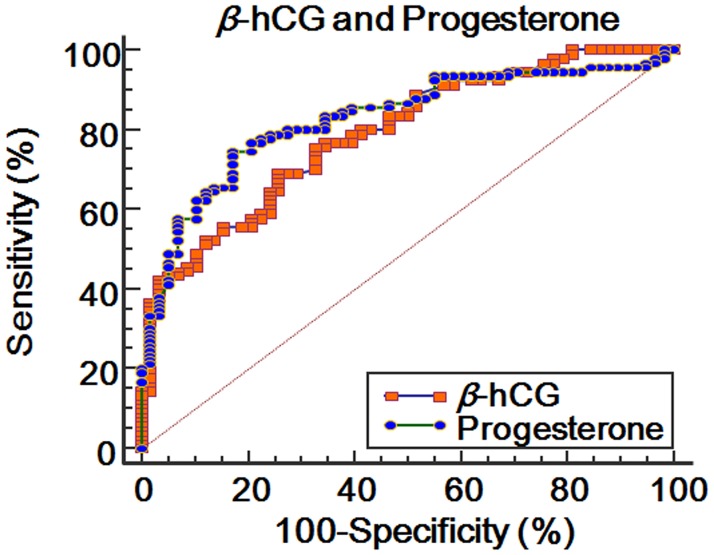
In the comparison between the subjects with β-hCG > 126.5 mIU/mL and those with progesterone > 25.2 ng/mL, there was no significant difference in the variables (Table 5). In the comparison of the ROC curves between both values, a similar significance was found (difference between the areas = 0.0378; standard error = 0.0500; 95% CI = -0.0602 to 0.136; P = 0.4594) (Fig 3).
Table 5. Comparison of pregnancy outcomes between IVF patients with β-hCG > 126.5 mIU/mL and progesterone > 25.2 ng/mL at 14 days after oocyte retrieval.
| Variables | β-hCG > 126.5 mIU/mL (n = 77) | Progesterone > 25.2 ng/mL (n = 77) |
|---|---|---|
| Age (years) | ||
| Wife | 34.3±4.6 | 34.6±4.8 |
| Husband | 38.1±5.1 | 38.9±5.3 |
| Cause of infertility | ||
| Ovulatory | 35.1% (27/77) | 33.8% (26/77) |
| Tubal | 33.8% (26/77) | 32.5% (25/77) |
| Unexplained | 31.2% (24/77) | 33.8% (26/77) |
| No. of previous IVF cycles | 2.5±1.2 | 2.4±1.8 |
| Basal FSH (mIU/mL) | 8.0±3.0 | 7.7±4.2 |
| Basal AMH (ng/mL) | 2.0±1.2 | 1.9±1.3 |
| Protocols | ||
| GnRH agonist | 57.1% (44/77) | 59.7% (46/77) |
| GnRH antagonist | 42.9% (33/77) | 40.3% (31/77) |
| No. of oocytes retrieved | 10.1±5.7 | 9.9±6.1 |
| No. of MII oocytes | 6.7±4.7 | 6.8±4.6 |
| No. of 2PN | 4.9±3.2 | 4.8±3.1 |
| Fertilization rate | 73.2% | 70.6% |
| No. of embryos transferred | 1.8±1.0 | 1.9±0.8 |
| Pregnancy outcomes | ||
| Ongoing pregnancy | 80.5% (62/77) | 87.0% (67/77) |
| Miscarriage | 19.5% (15/77) | 13.0% (10/77) |
P> .05 in all variables
Fig 3. Comparison of ROC curves between of β-hCG and progesterone levels at 14 days after oocyte retrieval for predicting ongoing pregnancy.
Significance of the combined cut-off values of the β-hCG and progesterone levels
Table 6 shows the comparison of the ongoing pregnancy outcomes according to the combined cut-off values of the β-hCG and progesterone levels. The subjects with β-hCG > 126.5 mIU/mL and progesterone > 25.2 ng/mL showed higher ongoing pregnancy rates [98.0% (49/50) vs. 41.8% (41/98)] than those with β-hCG ≤ 126.5 mIU/mL or progesterone ≤ 25.2 ng/mL.
Table 6. Comparison of pregnancy maintenance outcomes according to combined cut-off value of β-hCG (> 126.5 mIU/mL) and progesterone (> 25.2 ng/mL) at 14 days after oocyte retrieval.
| β-hCG > 126.5 mIU/mL and progesterone > 25.2 ng/mL (n = 50) | β-hCG ≤ 126.5 mIU/mL or progesterone ≤ 25.2 ng/mL (n = 98) | |
|---|---|---|
| Ongoing pregnancy | 98.0% (49/50)a | 41.8% (41/98)a |
| Miscarriage | 2.0% (1/50)a | 58.2% (57/98)a |
P< .0001 by Fisher’s exact test
Discussion
Study subjects and their overall pregnancy outcomes
The β-hCG level could be a predictive factor for ongoing pregnancy in IVF cycles. However, the specificity using only the β-hCG level was not sufficient for predicting ongoing pregnancy. The present study aimed to evaluate the predictive value of the progesterone level at the β-hCG check day (15 days after oocyte retrieval) for miscarriage in women with unexplained repeated miscarriages in previous IVF cycles and with visible G-sacs. Based on our results, the progesterone level combined with the β-hCG level can be suggested as a valuable predictor for ongoing pregnancy maintenance over 18 weeks of gestational age in women with visible G-sac after IVF cycles and with unexplained repeated miscarriages in previous IVF cycles.
In the overall pregnancy outcomes, the ongoing pregnancy maintenance rate over 18 weeks was 60.8% in the subjects with unexplained repeated miscarriages in previous IVF cycles, which was lower than the overall ongoing pregnancy maintenance rate in the whole group of patients with visible G-sac in our center (85.5%); such a finding is consistent with those of previous reports [1, 2]. The subjects showed a mean β-hCG level of 157.3±79.5 mIU/mL and a mean progesterone level of 29.9±17.4 ng/mL 14 days after oocyte retrieval, which is similar with those of previous reports [16–18]. In the comparison of the β-hCG and progesterone levels, significantly higher levels were shown in the ongoing pregnancy maintenance group than in the miscarriage group, which is also consistent with those of previous studies [16–18]. A previous study revealed that patients with repeated miscarriages showed significantly decreased hCG and progesterone secretion compared to healthy controls [19].
Cut-off values of the β-hCG and progesterone levels
Maternal serum progesterone level may indicate the corpus luteal responsiveness to hCG secreted from the placenta in the early pregnancy period. Owing to the importance of progesterone support in the early pregnancy period, maintenance of pregnancy may depend on the production of progesterone from the corpus luteum in spite of exogenous luteal support. In the ROC curve analysis, not only the serum β-hCG level but also the progesterone level was found to have a good predictive value for ongoing pregnancy maintenance or miscarriage. Although without a significant difference, the cut-off value > 25.2 ng/mL of the progesterone level at 14 days after oocyte retrieval showed a higher AUC, sensitivity, and specificity than the cut-off value > 126.5 mIU/mL of the β-hCG level for predicting ongoing pregnancy maintenance or miscarriage. While the β-hCG level could predict 80.5% of ongoing pregnancy maintenance up to 18 weeks of gestational age using the cut-off value > 126.5 mIU/mL, the progesterone level could predict 87.0% of ongoing pregnancy maintenance using the cut-off value > 25.2 ng/mL. As shown in Table 6, the subjects with combined cut-off values β-hCG > 126.5 mIU/mL and progesterone > 25.2 ng/mL showed a 98.0% ongoing pregnancy maintenance rate.
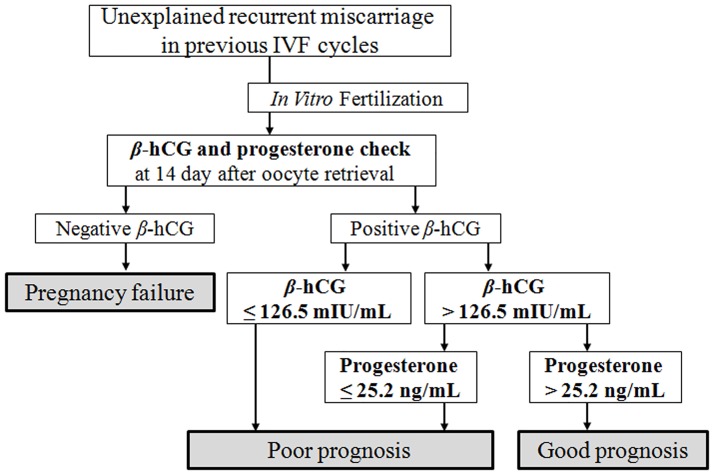
To date, few studies reported the progesterone level at the β-hCG check day as a predictive marker for ongoing pregnancy maintenance. A recent study, which used earlier β-hCG serum samples than in the present study (obtained 7 days after D3 ET), suggested a predictive value of ongoing pregnancy maintenance consistent with our results [20]. Another report showed that serum progesterone levels > 17 ng/mL at 7 days after ET could be a good predictor of IVF cycle outcomes via an ROC analysis [21]. Our present results suggest that the progesterone level at 14 days after oocyte retrieval might be a good prognostic marker for ongoing pregnancy maintenance and could be more useful when combined with the β-hCG level. We suggest a clinical strategy for repeated miscarriages after IVF as shown in Fig 4. Although the management for poor prognosis has been a matter of debate, particularly in repeated miscarriages after IVF, some reports suggested increasing the dose of luteal progesterone support. Although not restricted to IVF cycles, a recent meta-analysis suggested that supplementation with progestogens may reduce the incidence of recurrent miscarriages and seems to be safe for the fetuses in women with a history of recurrent miscarriage [22].
Fig 4. Suggested predictive value of β-hCG and progesterone levels during the follow-up of pregnant IVF cases with previous unexplained recurrent miscarriages.
Points to consider for interpretation of present data
First, the results of the present study were retrieved from the subjects who underwent IVF cycles using the ovarian gonadotropin stimulation protocol, which was different from the general pregnancy cases of unstimulated ovaries and corpus luteum. A previous study revealed that gonadotropin promotes progesterone synthesis and output from the granulosa cells of the ovaries without luteinization in gonadotropin-stimulated IVF cycles, which was different from the natural cycle [23]. Second, the subjects in this study were unique in terms of their repeated miscarriages after pregnancy in the previous IVF cycles. Thus, there may be concerns in the generalization of these results to all IVF cycles, particularly when applied to the first IVF cycle. However, this study is the first study on the progesterone level at the β-hCG check day in IVF patients with previous repeated miscarriages. Lastly, the cost-effectiveness with additional progesterone samples at the β-hCG check day in IVF procedures should be analyzed based on the hormonal control of endometrium [24] in the future.
Conclusions
Our study showed that the progesterone level 14 days after oocyte retrieval can be a good predictive marker for ongoing pregnancy maintenance in women with repeated IVF failure, together with the β-hCG level. The combined cut-off value of progesterone > 25.2 ng/mL and β-hCG > 126.5 mIU/mL may suggest a good prognosis.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the Ministry of Science, ICT and Future Planning (2016R1E1A1A01943455 and 2016R1D1A1A02937287). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Levi Setti PE, Moioli M, Smeraldi A, Cesaratto E, Menduni F, Livio S, et al. Obstetric outcome and incidence of congenital anomalies in 2351 IVF/ICSI babies. J Assist Reprod Genet. 2016;33(6):711–7. doi: 10.1007/s10815-016-0714-4 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.La Sala GB, Nicoli A, Villani MT, Gallinelli A, Nucera G, Blickstein I. Spontaneous embryonic loss rates in twin and singleton pregnancies after transfer of top- versus intermediate-quality embryos. Fertil Steril. 2005;84(6):1602–5. doi: 10.1016/j.fertnstert.2005.05.045 . [DOI] [PubMed] [Google Scholar]
- 3.Yuan X, Saravelos SH, Wang Q, Xu Y, Li TC, Zhou C. Endometrial thickness as a predictor of pregnancy outcomes in 10787 fresh IVF-ICSI cycles. Reprod Biomed Online. 2016;33(2):197–205. doi: 10.1016/j.rbmo.2016.05.002 . [DOI] [PubMed] [Google Scholar]
- 4.Chen SL, Wu FR, Luo C, Chen X, Shi XY, Zheng HY, et al. Combined analysis of endometrial thickness and pattern in predicting outcome of in vitro fertilization and embryo transfer: a retrospective cohort study. Reproductive biology and endocrinology: RB&E. 2010;8:30 Epub 2010/03/26. doi: 10.1186/1477-7827-8-30 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koshy AK, Gudi A, Shah A, Bhide P, Timms P, Homburg R. Pregnancy prognosis in women with anti-Mullerian hormone below the tenth percentile. Gynecol Endocrinol. 2013;29(7):662–5. doi: 10.3109/09513590.2013.797395 . [DOI] [PubMed] [Google Scholar]
- 6.Carmona F, Balasch J, Creus M, Fabregues F, Casamitjana R, Civico S, et al. Early hormonal markers of pregnancy outcome after in vitro fertilization and embryo transfer. J Assist Reprod Genet. 2003;20(12):521–6. doi: 10.1023/B:JARG.0000013654.85531.ac ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haas J, Lerner-Geva L, Yerushalmi GM, Maman E, Yinon Y, Baum M, et al. Previous abortion is a positive predictor for ongoing pregnancy in the next cycle in women with repeated IVF failures. Reprod Biomed Online. 2012;25(4):339–44. doi: 10.1016/j.rbmo.2012.06.020 . [DOI] [PubMed] [Google Scholar]
- 8.Kim YJ, Ku SY, Jee BC, Suh CS, Kim SH, Choi YM, et al. Increased responsiveness may be associated with tripronuclear embryos in both conventional in vitro fertilization and intracytoplasmic sperm injection cycles using gonadotropin-releasing hormone agonist long protocols: a self-matched observational study. J Obstet Gynaecol Res. 2011;37(1):37–44. doi: 10.1111/j.1447-0756.2010.01313.x . [DOI] [PubMed] [Google Scholar]
- 9.Canellada A, Alvarez I, Berod L, Gentile T. Estrogen and progesterone regulate the IL-6 signal transduction pathway in antibody secreting cells. J Steroid Biochem Mol Biol. 2008;111(3–5):255–61. doi: 10.1016/j.jsbmb.2008.06.009 . [DOI] [PubMed] [Google Scholar]
- 10.Patil AS, Swamy GK, Murtha AP, Heine RP, Zheng X, Grotegut CA. Progesterone Metabolites Produced by Cytochrome P450 3A Modulate Uterine Contractility in a Murine Model. Reprod Sci. 2015;22(12):1577–86. doi: 10.1177/1933719115589414 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dang Y, Li W, Tran V, Khalil RA. EMMPRIN-mediated induction of uterine and vascular matrix metalloproteinases during pregnancy and in response to estrogen and progesterone. Biochem Pharmacol. 2013;86(6):734–47. doi: 10.1016/j.bcp.2013.06.030 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi Y, Wilson K, Hannon PR, Rosewell KL, Brannstrom M, Akin JW, et al. Coordinated Regulation among Progesterone, Prostaglandins, and EGF-like Factors in Human Ovulatory Follicles. J Clin Endocrinol Metab. 2017. doi: 10.1210/jc.2016-3153 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu M, Zhang X, Geng L, Xia M, Zhai J, Zhang W, et al. Risk Factors and Early Predictors for Heterotopic Pregnancy after In Vitro Fertilization. PloS one. 2015;10(10):e0139146 Epub 2015/10/29. doi: 10.1371/journal.pone.0139146 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim YJ, Ku SY, Jee BC, Suh CS, Kim SH, Choi YM, et al. A comparative study on the outcomes of in vitro fertilization between women with polycystic ovary syndrome and those with sonographic polycystic ovary-only in GnRH antagonist cycles. Arch Gynecol Obstet. 2010;282(2):199–205. doi: 10.1007/s00404-010-1401-9 . [DOI] [PubMed] [Google Scholar]
- 15.Ku SY, Suh CS, Kim SH, Choi YM, Kim JG, Moon SY. A pilot study of the use of low dose human menopausal gonadotropin in ovulation induction. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2003;109(1):55–9. [DOI] [PubMed] [Google Scholar]
- 16.Sung N, Kwak-Kim J, Koo HS, Yang KM. Serum hCG-beta levels of postovulatory day 12 and 14 with the sequential application of hCG-beta fold change significantly increased predictability of pregnancy outcome after IVF-ET cycle. J Assist Reprod Genet. 2016;33(9):1185–94. doi: 10.1007/s10815-016-0744-y ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanbo TG, Eskild A. Maternal hCG concentrations in early IVF pregnancies: associations with number of cells in the Day 2 embryo and oocytes retrieved. Human reproduction (Oxford, England). 2015;30(12):2758–63. doi: 10.1093/humrep/dev269 . [DOI] [PubMed] [Google Scholar]
- 18.Kim JH, Shin MS, Yi G, Jee BC, Lee JR, Suh CS, et al. Serum biomarkers for predicting pregnancy outcome in women undergoing IVF: human chorionic gonadotropin, progesterone, and inhibin A level at 11 days post-ET. Clin Exp Reprod Med. 2012;39(1):28–32. doi: 10.5653/cerm.2012.39.1.28 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Schonfeldt V, Rogenhofer N, Ruf K, Thaler CJ, Jeschke U. Sera of patients with recurrent miscarriages containing anti-trophoblast antibodies (ATAB) reduce hCG and progesterone production in trophoblast cells in vitro. J Reprod Immunol. 2016;117:52–6. doi: 10.1016/j.jri.2016.07.005 . [DOI] [PubMed] [Google Scholar]
- 20.Wang Q, Zhang R, Jia M, Luo L, Ding C, Li TC, et al. Serum human chorionic gonadotropin measured 7 days following day 3 embryo transfer might predict pregnancy outcome in IVF. Gynecol Endocrinol. 2017;33(1):62–6. doi: 10.1080/09513590.2016.1203894 . [DOI] [PubMed] [Google Scholar]
- 21.Aslih N, Ellenbogen A, Shavit T, Michaeli M, Yakobi D, Shalom-Paz E. Can we alter pregnancy outcome by adjusting progesterone treatment at mid-luteal phase: a randomized controlled trial. Gynecol Endocrinol. 2017:1–5. doi: 10.1080/09513590.2017.1298742 . [DOI] [PubMed] [Google Scholar]
- 22.Saccone G, Schoen C, Franasiak JM, Scott RT Jr., Berghella V. Supplementation with progestogens in the first trimester of pregnancy to prevent miscarriage in women with unexplained recurrent miscarriage: a systematic review and meta-analysis of randomized, controlled trials. Fertil Steril. 2017;107(2):430–8 e3. doi: 10.1016/j.fertnstert.2016.10.031 . [DOI] [PubMed] [Google Scholar]
- 23.Oktem O, Akin N, Bildik G, Yakin K, Alper E, Balaban B, et al. FSH Stimulation promotes progesterone synthesis and output from human granulosa cells without luteinization. Human reproduction (Oxford, England). 2017;32(3):643–52. doi: 10.1093/humrep/dex010 . [DOI] [PubMed] [Google Scholar]
- 24.Ku SY, Choi YM, Suh CS, Kim SH, Kim JG, Moon SY, et al. Effect of gonadotropins on human endometrial stromal cell proliferation in vitro. Archives of gynecology and obstetrics. 2002;266(4):223–8. doi: 10.1007/s00404-002-0292-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.