Abstract
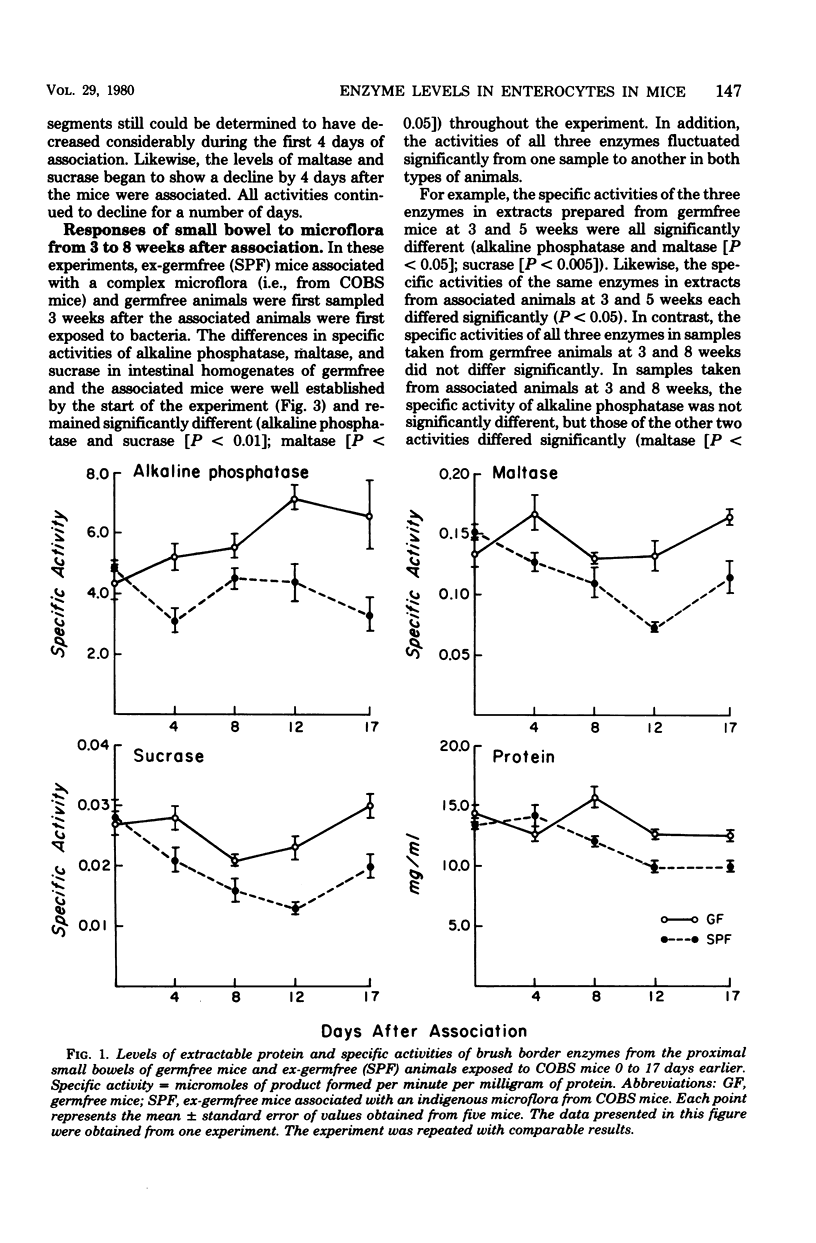
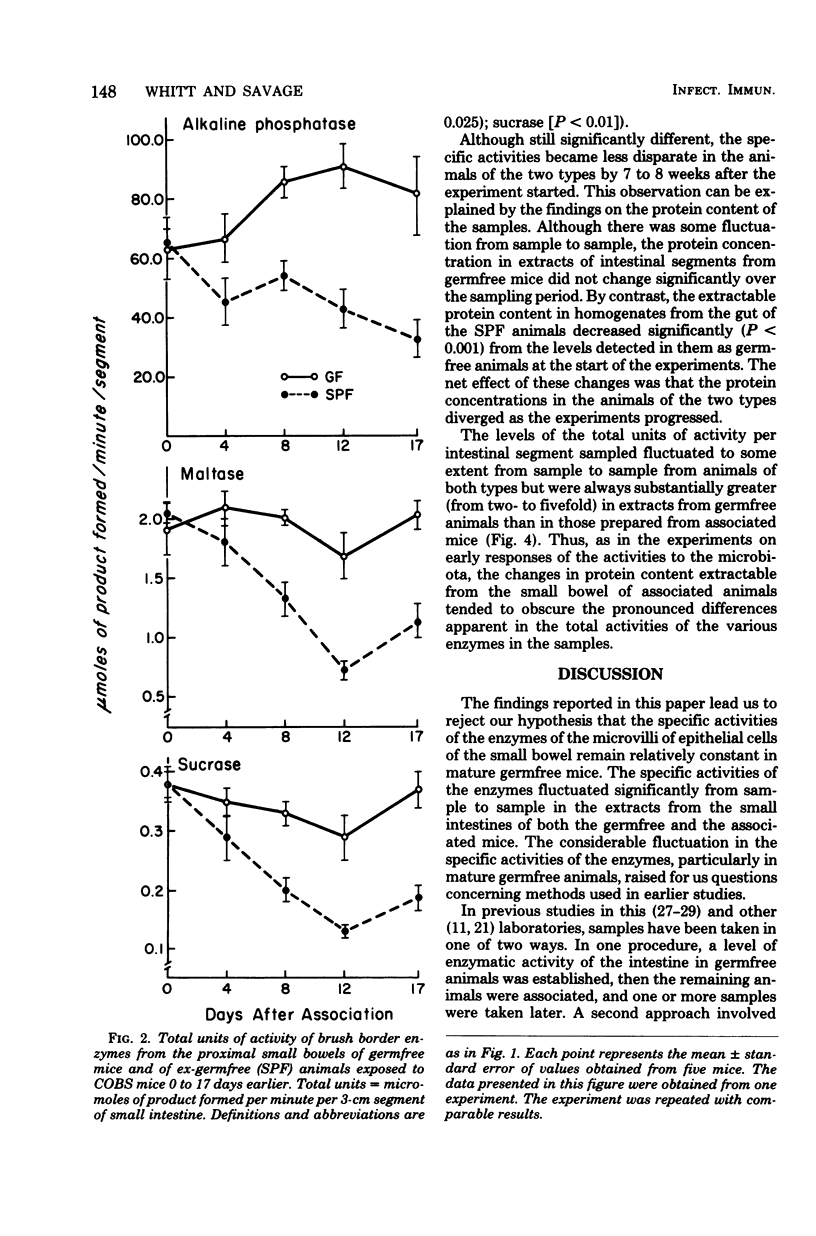
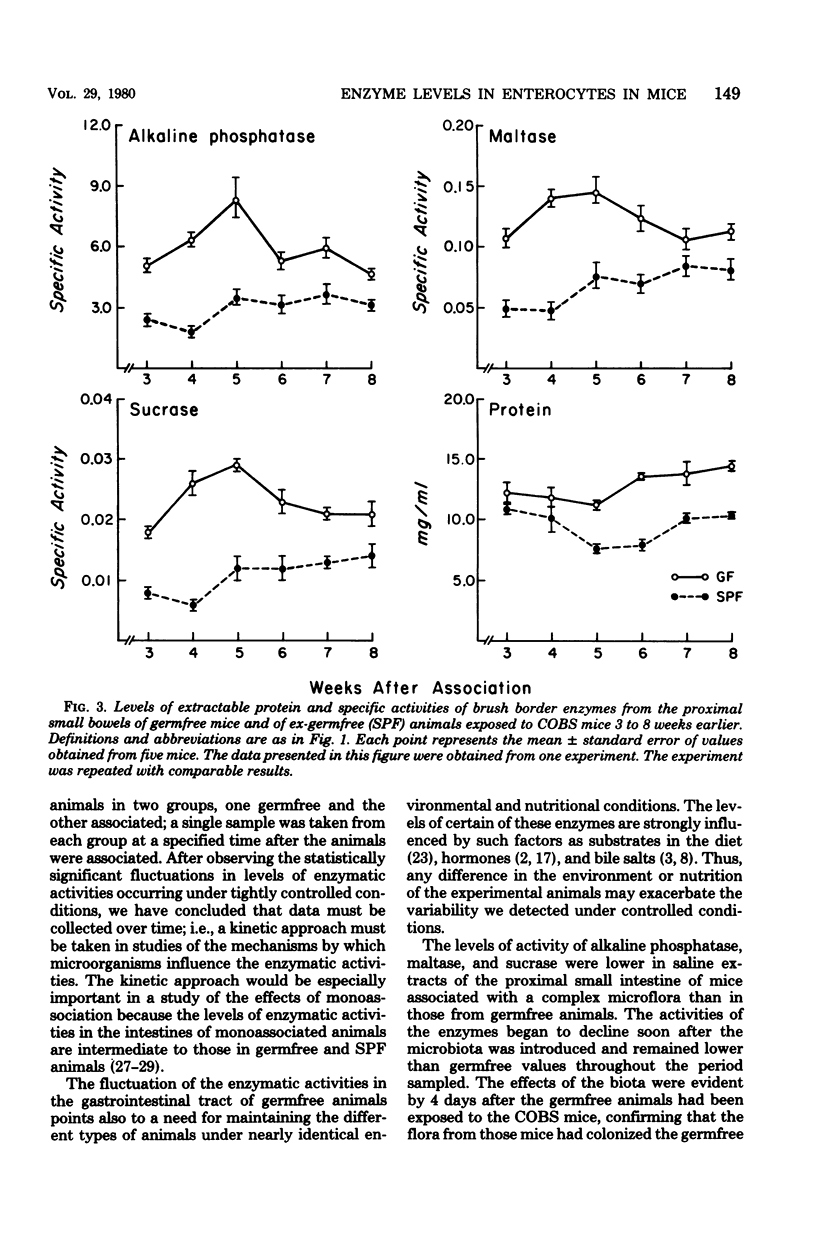
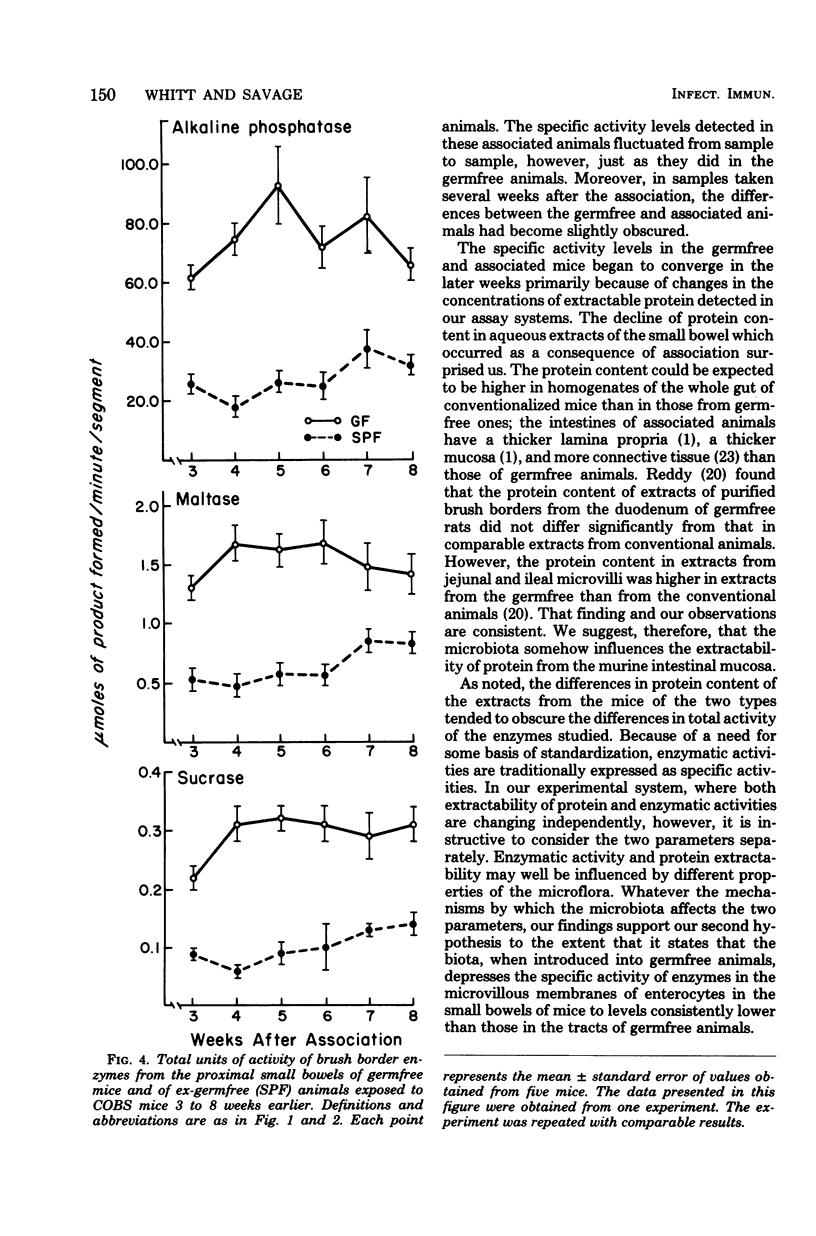
The concentration of protein and the activities of alkaline phosphatase, maltase, and sucrase were measured in saline extracts of the proximal small intestine of germfree and ex-germfree mice colonized with an indigenous microflora. The two populations of animals were maintained in plastic film isolators under tightly controlled environmental and nutritional conditions. Samples were taken at 0, 4, 8, 12, and 17 days and at 3, 4, 5, 6, 7, and 8 weeks after association. The activities were expressed as specific activities and as total units per segment of small intestine. Enzymatic activities expressed in both ways fluctuated considerably in the samples taken from one time to the next in animals of both types. The activities expressed as total units per segment of bowel of all three enzymes had decreased from levels in germfree animals by as early as 4 days after association. The total units of activity per segment of bowel tested continued to decrease for approximately 3 weeks in the associated animals to levels two- to fivefold lower than those of germfree animals. However, the specific activities of the three enzymes in the animals of the two types became less disparate at later sample times. This latter result is predictable because the concentration of protein extractable from the small intestines of the mice of the two types was the same at the beginning of the experiment, but by the later sampling times, the concentration of protein extractable from small bowels of ex-germfree mice was significantly lower than that from germfree mice. The fluctuations in levels of the enzymatic activities, even under controlled environmental and nutritional conditions, point to the necessity of using such conditions and a kinetic approach in studies of the effects of the microbiota on the activities of enzymes in the microvillous membranes of small bowel enterocytes. The changes in protein concentrations suggest that such activities and the amounts of protein extractable from the mucosa are influenced by different properties of the microflora. Thus, studies in which the enzymes are extracted from the entire mucosa and the activities are expressed as units per weight of extractable protein may give misleading results concerning the influence of the microbiota on the enterocyte membranes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMS G. D., BAUER H., SPRINZ H. Influence of the normal flora on mucosal morphology and cellular renewal in the ileum. A comparison of germ-free and conventional mice. Lab Invest. 1963 Mar;12:355–364. [PubMed] [Google Scholar]
- Cook R., Moog F. Influence of biliary stasis on alkaline phosphatase in the small intestine. Biochim Biophys Acta. 1970 Jul 21;215(1):220–223. doi: 10.1016/0304-4165(70)90414-9. [DOI] [PubMed] [Google Scholar]
- DAHLQVIST A., BORGSTROM B. Digestion and absorption of disaccharides in man. Biochem J. 1961 Nov;81:411–418. doi: 10.1042/bj0810411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlqvist A. Assay of intestinal disaccharidases. Anal Biochem. 1968 Jan;22(1):99–107. doi: 10.1016/0003-2697(68)90263-7. [DOI] [PubMed] [Google Scholar]
- Ekstrom K. E., Benevenga N. J., Grummer R. H. Changes in the intestinal lactase activity in the small intestine of two breeds of swine from birth to 6 weeks of age. J Nutr. 1975 Aug;105(8):1032–1038. doi: 10.1093/jn/105.8.1032. [DOI] [PubMed] [Google Scholar]
- GORDON H. A., BRUCKNER-KARDOSS E. Effect of normal microbial flora on intestinal surface area. Am J Physiol. 1961 Jul;201:175–178. doi: 10.1152/ajplegacy.1961.201.1.175. [DOI] [PubMed] [Google Scholar]
- Gracey M., Papadimitriou J., Burke V., Thomas J., Bower G. Effects on small-intestinal function and structure induced by feeding a deconjugated bile salt. Gut. 1973 Jul;14(7):519–528. doi: 10.1136/gut.14.7.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning S. J., Helman T. A., Kretchmer N. Studies on normal and precocious appearance of jejunal sucrase in suckling rats. Biol Neonate. 1975;26(3-4):249–262. doi: 10.1159/000240736. [DOI] [PubMed] [Google Scholar]
- Kawai Y., Morotomi M. Intestinal enzyme activities in germfree, conventional, and gnotobiotic rats associated with indigenous microorganisms. Infect Immun. 1978 Mar;19(3):771–778. doi: 10.1128/iai.19.3.771-778.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Chase H. P. Undernutrition and intestinal dipeptide hydrolase activity in the rat. J Nutr. 1971 Nov;101(11):1509–1514. doi: 10.1093/jn/101.11.1509. [DOI] [PubMed] [Google Scholar]
- LESHER S., WALBURG H. E., Jr, SACHER G. A., Jr GENERATION CYCLE IN THE DUODENAL CRYPT CELLS OF GERM-FREE AND CONVENTIONAL MICE. Nature. 1964 May 30;202:884–886. doi: 10.1038/202884a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Malathi P., Ramaswamy K., Caspary W. F., Crane R. K. Studies on the transport of glucose from disaccharides by hamster small intestine in vitro. I. Evidence for a disaccharidase-related transport system. Biochim Biophys Acta. 1973 May 25;307(3):613–626. doi: 10.1016/0005-2736(73)90306-4. [DOI] [PubMed] [Google Scholar]
- Moog F., Grey R. D. Alkaline phosphatase isozymes in the duodenum of the mouse: attainment of pattern of spatial distribution in normal development and under the influence of cortisone or actinomycin D. Dev Biol. 1968 Nov;18(5):481–500. doi: 10.1016/0012-1606(68)90053-5. [DOI] [PubMed] [Google Scholar]
- Norman A. W., Mircheff A. K., Adams T. H., Spielvogel A. Studies on the mechanism of action of calciferol. 3. Vitamin D-mediated increase of intestinal brush order alkaline phosphatase activity. Biochim Biophys Acta. 1970 Aug 14;215(2):348–359. doi: 10.1016/0304-4165(70)90034-6. [DOI] [PubMed] [Google Scholar]
- Reddy B. S. Calcium and magnesium absorption: role of intestinal microflora. Fed Proc. 1971 Nov-Dec;30(6):1815–1821. [PubMed] [Google Scholar]
- Reddy B. S., Wostmann B. S. Intestinal disaccharidase activities in the growing germfree and conventional rats. Arch Biochem Biophys. 1966 Mar;113(3):609–616. doi: 10.1016/0003-9861(66)90238-4. [DOI] [PubMed] [Google Scholar]
- Siddons R. C., Coates M. E. The influence of the intestinal microflora on disaccharidase activities in the chick. Br J Nutr. 1972 Jan;27(1):101–112. doi: 10.1079/bjn19720074. [DOI] [PubMed] [Google Scholar]
- TREXLER P. C., REYNOLDS L. I. Flexible film apparatus for the rearing and use of germfree animals. Appl Microbiol. 1957 Nov;5(6):406–412. doi: 10.1128/am.5.6.406-412.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolton D. P., Savage D. C. Influence of certain indigenous gastrointestinal microorganisms on duodenal alkaline phosphatase in mice. Appl Environ Microbiol. 1976 Jun;31(6):880–888. doi: 10.1128/aem.31.6.880-888.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolton D. P., Savage D. C. Influence of the indigenous gastrointestinal microbial flora on duodenal Mg2+ -dependent and (Na+ + K+) -stimulated adenosine triphosphatase activities in mice. Infect Immun. 1976 Apr;13(4):1193–1198. doi: 10.1128/iai.13.4.1193-1198.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolton D. P., Stanley C., Savage D. C. Influence of the indigenous gastrointestinal microbial flora on duodenal alkaline phosphatase activity in mice. Infect Immun. 1971 Jun;3(6):768–773. doi: 10.1128/iai.3.6.768-773.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]



