Abstract
We studied the effect of HLA-C matching in 515 patients after double umbilical cord blood (dUCB) transplantation. After HLA-matching HLA-A, -B, and -DRB1 at the allele level, we scored patients according to number of donor-recipient HLA-C matches at 4 possible loci, 2 from each donor unit, at the allele level. Given a direct interaction between HLA-A, -B, and -DRB1 matching and HLA-C score, we analyzed HLA-C matching in those receiving at least one 2–4/6 HLA-matched unit (n= 389) vs. those receiving only 5–6/6 matched units (n=126). In those with at least one 2–4/6 HLA-matched unit, a better HLA-C matching score was associated with significantly lower risk of death of any cause and non-relapse mortality and better disease-free survival. There was no association with the risk of relapse, acute and chronic graft vs host disease, and hematopoietic recovery. In contrast, among patients receiving only allele-level 5–6/6 HLA-matched UCB units, HLA-C match had no demonstrable effect on any outcome. For patients receiving at least one allele-level 2–4/6 HLA-matched UCB unit, matching at HLA-C reduces non-relapse mortality and improves survival.
INTRODUCTION
One of the advantages of umbilical cord blood (UCB) grafts for allogeneic hematopoietic cell transplantation (HCT) is the less stringent requirement for donor-recipient HLA-matching as compared to unrelated adult donor (URD) HCT. Conventionally, favorable outcomes for URD HCT require allele-level matching at least at HLA-A, -B, -C, and -DRB1 (8/8 allele matching), with some centers also considering HLA-DQB1 and -DPB1. Published data demonstrate that increasing degrees of HLA mismatching of URD grafts results in higher risk of non-relapse mortality (NRM) and poorer survival.1, 2 In contrast, UCB grafts are traditionally selected when matched to the patient at HLA-A and -B at the antigen level and at -DRB1 at the allele level, while not considering HLA-C.
A recent registry-based retrospective analysis studied the effect of HLA-C matching after single UCB unit transplantation3. The key observation of that study was that in patients receiving a 5–6/6 HLA-matched unit, further matching at HLA-C also resulted in lower NRM and superior survival3. In that study most patients were children, had acute leukemia or myelodysplastic syndrome, and received a myeloablative conditioning regimen. However, in adults and larger adolescents an adequately dosed single UCB unit is often not available, necessitating consideration of a double UCB (dUCB) transplant, using two partially HLA matched units. Moreover, use of reduced intensity conditioning (RIC) is more common in adults because of co-morbidities or older age. Thus, we evaluated the effect of HLA-C matching in the setting of dUCB transplantation and RIC as well as myeloablative conditioning.
PATIENTS AND METHODS
In this retrospective study we included patients with hematological malignancies who received a dUCB transplant between 2003 and 2014 and who had HLA-C data available from the blood and marrow transplant programs at the University of Minnesota, the Massachusetts General Hospital, and the Dana Farber Cancer Institute. This retrospective study was approved by the Institutional Review Board of the participating institutions. Conditioning regimens, immune suppression, graft selection, and supportive care have been previously reported.4–7 In summary, the myeloablative regimen consisted of cyclophosphamide 60 mg/kg for 2 days, total body irradiation 1320 cGY, and fludarabine 25 mg/m2 for 3 days. There were two RIC regimens that consisted of: 1) cyclophosphamide 50 mg/kg for 1 day, fludarabine 40 mg/m2 for 5 days and total body irradiation 200 cGY with or without equine anti-thymocyte globulin (ATG) 15 mg/kg twice daily for 3 days and 2) fludarabine 30 mg/m2 for 6 days, melphalan 100 mg/kg in 1 day, and rabbit ATG 1.0–1.5 mg/kg for 4 doses on alternating days (Table 1). The immune suppression regimens were cyclosporine-A and mycophenolate mofetil (MMF), tacrolimus with sirolimus and/or MMF, sirolimus and MMF, or cyclosporine/prednisone (Table 1). Disease risk at the time of transplantation was classified into standard risk or high risk based on the ASBMT RFI 2006 risk scoring schema.8 Acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL) in first or second complete remission, chronic myeloid leukemia (CML) in first chronic phase, Hodgkin or non-Hodgkin lymphoma in complete remission or chemotherapy-sensitive partial remission, and chronic lymphocytic leukemia (CLL) in first remission were defined as standard risk; all other diseases were classified as high risk.
Table 1.
Patient, graft and transplant characteristics
| Variable | N |
|---|---|
| Number of patients | 515 |
| Median age in years (range), (IQR) | 48 (2–73), (33–59) |
| Males | 307 (60%) |
| Diagnosis | |
| Acute lymphoblastic leukemia | 90 (17%) |
| Acute myeloid leukemia | 213 (41%) |
| Chronic myeloid leukemia | 15 (3%) |
| Other Leukemia | 23 (4%) |
| Myelodysplastic syndrome | 70 (14%) |
| Hodgkin’s & Non-Hodgkin’s lymphoma | 94 (18%) |
| Other Malignancy1 | 10 (2%) |
| High risk disease | 204 (40%) |
| CMV Seropositive | 303 (59%) |
| Conditioning regimen intensity2 | |
| Myeloablative | 166 (33%) |
| Reduced intensity | 349(77%) |
| GvHD Prophylaxis3 | |
| Cyclosporine A/mycophenolate mofetil | 383 (74%) |
| Tacrolimus/sirolimus | 69 (13%) |
| Sirolimus/MMF | 39 (8%) |
| Other | 24 (4%) |
| Worst HLA Allele Match at -A, -B and -DRB1 | |
| 2/6 | 27 (5%) |
| 3/6 | 106 (21%) |
| 4/6 | 256 (50%) |
| 5/6 | 102 (20%) |
| 6/6 | 24 (5%) |
| HLA-C Matching Score | |
| 0 matches | 57 (11%) |
| 1 match | 62 (12%) |
| 2 matches | 211 (41%) |
| 3 matches | 108 (21%) |
| 4 matches | 77 (15%) |
| Gender mismatch of at least one unit | 373 (76%) |
| TNC Infused (×107/kg) Median (range), (IQR) | 4 (2–20), (3–5) |
| Variable | N |
| Year of Transplantation | |
| 2003–2005 | 127 (25%) |
| 2006–2009 | 231 (45%) |
| 2010–2014 | 157 (30%) |
| Follow-up in years Median (range), (IQR) | 5.9 (1.0–12.4), (3.2–8.2) |
Abbreviations: IQR, inter-quartile range; CMV, cytomegalovirus; GVHD, graft-vs-host disease; HLA, human leukocyte antigen; TNC, total nucleated cell dose.
Myeloma (n=7), plasma cell leukemia (n=2), and renal cell carcinoma (n=1)
Myeloablative regimen consisted of cyclophosphamide 60 mg/kg for 2 days and total body irradiation 1320 cGY (n=3) and fludarabine 25 mg/m2 for 3 days (n=168). The RIC regimens were: 1) cyclophosphamide 50 mg/kg for 1 day, fludarabine 40 mg/m2 for 5 days and total body irradiation 200 cGY with (n=81) or without equine anti-thymocyte globulin 15 mg/kg twice daily for 3 days (n=152), and 2) fludarabine 30 mg/m2 for 6 days and melphalan 100 mg/kg in 1 day and rabbit anti-thymocyte globulin 1.5 mg/kg for 4 doses in alternating days (n=86).
The other immune suppression regimens were tacrolimus/mycophenolate mofetil (n=15), sirolimus/ mycophenolate mofetil (n=5), tacrolimus/sirolimus/mycophenolate mofetil (n=3), and cyclosporine/prednisone (n=4).
HLA-matching
Patients and UCB unit were HLA typed using established molecular techniques. For this analysis HLA-A, -B, -C, and -DRB1 were analyzed at the allele level. We first scored the allele level HLA-matching at HLA-A, -B, and -DRB1. We then scored the allele level matching at HLA-C considering up to 4 possible loci, 2 from each donor unit. Resulting scores ranged from 0 to 4 out of 4 possible loci (0/4, 1/4, 2/4, 3/4, and 4/4 HLA-C matches). In the group that had 2/4 HLA-C match, the score could have resulted in 1 match from each unit or both matches from one unit. However, only 8 of 211 patients (4%) had a 2/4 HLA-C match score resulting from a single unit matching at both C locus (2/2) and the other unit at none (0/2); thus, these patients were studied as a single group. The final analysis was carried out considering the degree of HLA-match of the graft pair based on the less well matched of the two donor units (worst match) and by the HLA-match of UCB unit that predominated long-term. The predominant unit was defined as the unit accounting for ≥70% of whole bone marrow or whole blood chimerism, as previously defined.9 As the analysis considering the predominant unit only studied engrafted patients (n=416), we excluded 58 who had graft failure, 17 early deaths, 3 early relapses, and 21 with no predominant unit.
Statistical considerations
Overall and disease-free survival (DFS) were estimated with Kaplan-Meier curves.10 Relapse, NRM, acute graft-versus-host disease (GVHD), chronic GVHD, and engraftment were estimated using competing risks methods.11 Cox regression analysis12 was performed to assess the independent effect of HLA-C match on OS and DFS through five years post transplantation in the presence of other factors. Fine and Gray proportional hazards regression was used to assess the independent effect of the indices on NRM, relapse, acute and chronic GVHD, and engraftment.13 Backward elimination was used to achieve a final model. Requirement for inclusion in the final model was p < 0.10 if clinically significant; however, overall HLA disparity and number of matches at HLA-C were included in all models. All regression models were stratified by center due to disparity in characteristics across centers. The OS and DFS were stratified by conditioning due to violation of the proportional hazards assumption. Also, we attempted to keep similar factors in the final models across OS, DFS, relapse, and NRM as well as between grade II–IV acute GVHD and chronic GVHD. Martingale residuals were used to test against non-proportionality.14 Logistic regression was employed to investigate the independent influence of HLA-C matching on neutrophil engraftment, excluding deaths prior to day 28 and treating late deaths as graft failures if not already identified as graft failures. SAS 9.3 (SAS Institute, Cary, NC) and R 3.0.2 were used to perform all statistical analyses.
RESULTS
EFFECT OF HLA-C MATCHING SCORE OF THE WORST HLA-MATCHED UNIT ON OUTCOMES
Demographics
Patient, dUCB graft, and transplant characteristics for the 515 HCT are summarized in Table 1. Three-quarters of patients received at least one unit that was 2–4/6 HLA-matched at -A, -B, or -DRB1 to the patient at the allele level. The HLA-C matching score was 4 in 93 (19%), 3 in 90 (18%), 2 in 196 (40%), and 0–1 in 111 (23%) patients. The median patient age was 48 years (range, 2–73). Approximately two-thirds of patients were male, had acute leukemia, were CMV seropositive, and received RIC. The majority (82%) received cyclosporine A/MMF immunosuppression.
Survival and DFS
The point estimates for survival, DFS, relapse, and NRM are summarized in Table 2. In univariate analysis the HLA-C matching score did not affect survival, DFS, or relapse. However, higher HLA-C matching score resulted in lower NRM (Figure 1A–D and Table 2). We recognized an interaction between matching at HLA-A, -B, and -DRB1 and the number of matches at HLA-C regarding survival, DFS, and NRM. Thus, in multivariate analysis for these endpoints the effect of the HLA-C matching score analysis was performed considering two groups based on matching at HLA-A, -B, and -DRB1 at the allele level: those receiving at least one 2–4/6 HLA-matched unit (n=389) versus those receiving only 5–6/6 matched units (5/6 & 5–6/6; n=126). In patients receiving at least one 2–4/6 HLA-matched unit, better matching at HLA-C was associated with significantly lower risk of death of any cause and NRM and better DFS (Table 3). There was no association with the risk of relapse. In contrast, among patients receiving only the 5–6/6 HLA-matched units, HLA-C match had no demonstrable effect on the risk of death, DFS, or NRM (Table 3). However, the relatively small number of patients resulting from the stratification limited the power to detect differences in this group. We found no effect of HLA-C matching score on the risk of relapse (Table 4). We also looked of the effect of on outcomes considering only the worst HLA-C matched of the 2 units resulting in a score 0, 1, or 2. Findings remained the same with improved DFS and NRM, but no effect on relapse for those receiving less well HLA-matched units, and no overall effect on better HLA-matched cases (Table S1 and Table S2).
Table 2.
Point estimates of outcomes considering the HLA-C matching score
| HLA-C Score | N |
Overall Survival at 5 years |
Disease-Free Survival at 5 years |
Relapse at 5 years |
Non-Relapse Mortality at 2 years |
||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate (CI 95%) |
P-value | Estimate (CI 95%) |
P-value | Estimate (CI 95%) |
P-value | Estimate (CI 95%) |
P-value | ||
| 0/4 | 57 | 34% (21–46%) |
0.25 | 29% (18–41%) |
0.45 | 32% (19–45%) |
0.29 | 37% (24–50%) |
0.01 |
| 1/4 | 62 | 33% (21–45%) |
24% (14–36%) |
33% (20–46%) |
40% (28–53%) |
||||
| 2/4 | 211 | 37% (30–44%) |
32% (26–39%) |
27% (20–33%) |
36% (29–43%) |
||||
| 3/4 | 108 | 45% (35–54%) |
36% (27–46%) |
40% (30–50%) |
22% (14–30%) |
||||
| 4/4 | 77 | 47% (35–58%) |
38% (27–49%) |
42% (30–55%) |
19% (11–28%) |
||||
| HLA-C Score | N | Acute GVHD 100 days |
Chronic GVHD 2 years |
Neutrophil Recovery 42 days |
Platelet Recovery 6 months |
||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate (CI 95%) |
P-value | Estimate (CI 95%) |
P-value | Estimate (CI 95%) |
P-value | ||||
| 0/4 | 57 | 40% (27–54%) |
0.92 | 26% (14–38%) |
0.40 | 87% (77–94%) |
0.01 | 65% (49–80%) |
0.05 |
| 1/4 | 62 | 44% (31–56%) |
18% (8–28%) |
89% (80–96%) |
63% (48–78%) |
||||
| 2/4 | 211 | 41% (34–48% |
22% (16–28%) |
86% (81–90%) |
69% (60–77%) |
||||
| 3/4 | 108 | 40% (30–49%) |
17% (10–24%) |
89% (82–94%) |
73% (62–84%) |
||||
| 4/4 | 77 | 44% (33–56%) |
21% (12–30%) |
90% (83–96%) |
73% (59–86%) |
||||
Abbreviations: HLA, human leukocyte antigen; CI, confidence interval; GVHD, graft-vs.-host disease.
Figure 1.
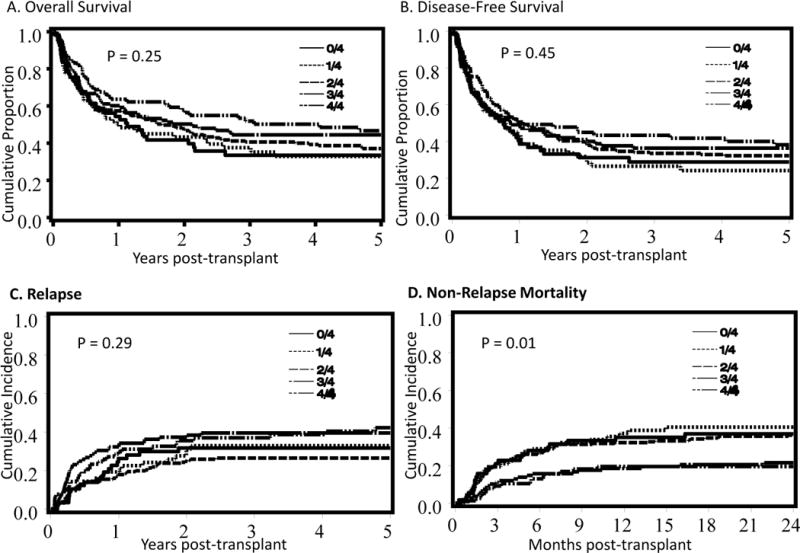
Estimates of (A) Overall survival and (B) disease-free survival, and cumulative incidence of (C) relapse and (D) non-relapse mortality according to HLA-C matching score group.
Table 3.
Multiple regression analysis of the effect of HLA-C matching score on the outcomes after dUCB transplantation in which there was an interaction with conventional HLA-matching at HLA-A,-B, and -DRB1.
| Match at HLA-A,B and DRB1 |
HLA 2–4/6 | HLA 5–6/6 | ||||
|---|---|---|---|---|---|---|
| N | HR (95% CI) | P-value | N | HR (95% CI) | P-value | |
| OVERALL SURVIVAL & | ||||||
| HLA-C match score | ||||||
| 4/4* | 29 | 1.0 (ref) | 48 | 1.0 (ref) | ||
| 3/4 | 77 | 2.4 (1.1–5.1) | 0.03 | 31 | 0.9 (0.5–1.6) | 0.63 |
| 2/4 | 171 | 2.9 (1.4–5.9) | <0.01 | 40 | 0.8 (0.4–1.4) | 0.41 |
| 1/4 | 59 | 2.8 (1.3–6.1) | 0.01 | 7** | 0.7 (0.2–2.5) | 0.57 |
| 0/4 | 53 | 3.3 (1.5–7.1) | <0.01 | |||
| Worst HLA-A,-B and -DRB1 Match | ||||||
| 6/6* | 24 | 1.0 (ref) | ||||
| 5/6 | 102 | 1.1 (0.6–2.1) | 0.85 | |||
| 4/6* | 256 | 1.0 (ref) | ||||
| 3/6 | 106 | 1.1 (0.8–1.4) | 0.81 | |||
| 2/6 | 27 | 0.8 (0.5–1.4) | 0.47 | |||
| Age | ||||||
| <18 | 26 | 1.0 (ref) | 21 | 1.0 (ref) | ||
| 18–34 | 77 | 1.6 (0.8–3.3) | 0.22 | 20 | 0.5 (0.2–1.3) | 0.15 |
| 35+ | 286 | 2.8 (1.4–5.8) | 0.01 | 85 | 0.3 (0.1–0.7) | 0.01 |
| Disease Risk | ||||||
| Standard risk* | 227 | 1.0 (ref) | 84 | 1.0 (ref) | ||
| High risk | 162 | 1.6 (1.2–2.1) | <0.01 | 42 | 1.1 (0.6–1.8) | 0.74 |
| Patient CMV | ||||||
| Negative* | 158 | 1.0 (ref) | NA | |||
| Positive | 231 | 1.6 (1.2–2.2) | <0.01 | NA | ||
| DISEASE-FREE SURVIVAL & | ||||||
| HLA-C match score | ||||||
| 4/4* | 29 | 1.0 (ref) | 48 | 1.0 (ref) | ||
| 3/4 | 77 | 1.8 (1.0–3.3) | 0.07 | 31 | 0.9 (0.5–1.6) | 0.67 |
| 2/4 | 171 | 1.9 (1.1–3.4) | 0.03 | 40 | 0.8 (0.4–1.5) | 0.47 |
| 1/4 | 59 | 2.1 (1.1–3.9) | 0.02 | 7** | 0.5 (0.1–1.8) | 0.30 |
| 0/4 | 53 | 2.2 (1.2–4.2) | 0.02 | |||
| Worst HLA-A,-B and -DRB1 Match | ||||||
| 6/6* | 24 | 1.0 (ref) | ||||
| 5/6 | 102 | 0.9 (0.7–1.7) | 0.79 | |||
| 4/6* | 256 | 1.0 (ref) | ||||
| 3/6 | 106 | 1.0 (0.8–1.4) | 0.83 | |||
| 2/6 | 27 | 0.8 (0.5–1.4) | 0.52 | |||
| Age | ||||||
| <18 | 26 | 1.0 (ref) | 21 | 1.0 (ref) | ||
| 18–34 | 77 | 1.8 (0.9–3.6) | 0.12 | 20 | 0.6 (0.3–1.5) | 0.32 |
| 35+ | 286 | 2.7 (1.4 (5.5) | <0.01 | 85 | 0.3 (0.1–0.8) | 0.01 |
| Disease Risk | ||||||
| Standard risk* | 227 | 1.0 (ref) | 84 | 1.0 (ref) | ||
| High risk | 162 | 1.5 (1.2–2.0) | <0.01 | 42 | 1.2 (0.8–2.1) | 0.40 |
| Patient CMV | ||||||
| Negative* | 158 | 1.0 (ref) | NS | NS | NS | |
| Positive | 231 | 1.5 (1.2–2.0) | <0.01 | NS | NS | NS |
| aGVHD | ||||||
| No | 235 | 1.0 (ref) | 1.0 (ref) | |||
| Yes | 154 | 0.6 (0.5–0.8) | <0.01 | 0.9 (0.6–1.5) | 0.79 | |
| Non-Relapse Mortality£ | ||||||
| HLA-C match score | ||||||
| 4/4* | 29 | 1.0 (ref) | 48 | 1.0 (ref) | ||
| 3/4 | 77 | 3.8 (0.8–16.6) | 0.08 | 31 | 0.5 (0.2–1.6) | 0.24 |
| 2/4 | 171 | 6.3 (1.5–26.7) | 0.01 | 40 | 1.3 (0.5–3.2) | 0.53 |
| 1/4 | 59 | 6.2 (1.4–26.7) | 0.01 | 7** | 1.1 (0.2–5.6) | 0.95 |
| 0/4 | 53 | 6.5 (1.5–28.2) | 0.01 | |||
| Worst HLA-A,-B and -DRB1 Match | ||||||
| 6/6* | 24 | 1.0 (ref) | ||||
| 5/6 | 102 | 1.1 (0.4–3.4) | 0.82 | |||
| 4/6* | 256 | 1.0 (ref) | ||||
| 3/6 | 106 | 0.8 (0.5–1.3) | 0.46 | |||
| 2/6 | 27 | 0.9 (0.5–2.0) | 0.89 | |||
| Age | ||||||
| <18 | 26 | 1.0 (ref) | NS | NS | NS | |
| 18–34 | 77 | 1.6 (0.7–3.6) | 0.22 | NS | NS | NS |
| 35+ | 286 | 2.1 (1.0–4.3) | 0.05 | NS | NS | NS |
| Disease Risk | ||||||
| Standard risk* | 227 | 1.0 (ref) | 84 | 1.0 (ref) | ||
| High risk | 162 | 2.1 (1.4–3.0) | <0.01 | 42 | 1.7 (0.8–3.7) | 0.15 |
| Patient CMV | ||||||
| Negative* | 158 | 1.0 (ref) | NS | NS | NS | |
| Positive | 231 | 1.6 (1.1–2.3) | 0.02 | NS | NS | NS |
| Conditioning | ||||||
| MA* | 123 | 1.0 (ref) | 54 | 1.0 (ref) | ||
| RIC | 266 | 0.3 (0.2–0.5) | <0.01 | 72 | 0.4 (0.2–1.0) | 0.05 |
Abbreviations: HLA, human leukocyte antigen; dUCB, double umbilical cord blood; N, number of patients; CI, confidence interval; CMV, cytomegalovirus; MA, myeloablative conditioning; RIC, reduced intensity conditioning; GVHD, graft-vs-host disease.
Reference group
Combined 0 and 1 HLA-C matching score
Overall survival and disease-free survival among 4/6 +4–6/6 were stratified by transplant center and intensity of the conditioning regimen, while for 5/6+-5–6/6 these outcomes were stratified by transplant center.
Stratified by transplant center
Table 4.
Multivariate regression analysis of the effect of HLA-C matching score after dUCB transplantation in endpoints in which there was no interaction with conventional HLA-matching at HLA-A,-B, and -DRB1.
| N | HR (95% Confidence interval) | P-value | |
|---|---|---|---|
| RELAPSE£ | |||
| HLA-C match score | |||
| 4/4* | 77 | 1.0 | |
| 3/4 | 108 | 1.0 (0.6–1.6) | 0.92 |
| 2/4 | 211 | 0.7 (0.4–1.1) | 0.15 |
| 1/4 | 62 | 1.0 (0.5–1.7) | 0.87 |
| 0/4 | 57 | 0.9 (0.5–1.7) | 0.80 |
| Worst HLA Match −A, -B, and −DRB1 | |||
| 6/6* | 24 | 1.0 | |
| 5/6 | 102 | 0.5 (0.3–1.1) | 0.09 |
| 4/6 | 256 | 0.6 (0.3–1.2) | 0.16 |
| 3/6 | 106 | 0.6 (0.3–1.3) | 0.22 |
| 2/6 | 27 | 0.5 (0.2–1.5) | 0.24 |
| Disease Risk | |||
| Standard risk* | 311 | 1.0 | |
| High risk | 204 | 1.0 (0.7–1.4) | 0.89 |
| Conditioning | |||
| MAC* | 166 | 1.0 | |
| RIC | 349 | 3.0 (1.9–4.7) | <0.01 |
| Grade II–IV aGVHD | |||
| No* | 305 | 1.0 | |
| Yes | 210 | 0.6 (0.4–0.9) | 0.01 |
| Grade II–IV Acute GVHD£ | |||
| HLA-C match score | |||
| 4/4* | 77 | 1.0 | |
| 3/4 | 108 | 0.9 (0.6–1.5) | 0.68 |
| 2/4 | 211 | 0.9 (0.6–1.4) | 0.70 |
| 1/4 | 62 | 1.0 (0.6–1.8) | 0.94 |
| 0/4 | 57 | 0.8 (0.5–1.5) | 0.54 |
| Worst HLA Match -A, -B, and -DRB1 | |||
| 6/6* | 24 | 1.0 | |
| 5/6 | 102 | 1.1 (0.5–2.2) | 0.81 |
| 4/6 | 256 | 0.8 (0.4–1.7) | 0.65 |
| 3/6 | 106 | 1.4 (0.7–2.9) | 0.38 |
| 2/6 | 27 | 1.1 (0.5–2.6) | 0.80 |
| Conditioning | |||
| MAC* | 166 | 1.0 | |
| RIC | 349 | 0.6 (0.5–0.8) | <0.01 |
| Chronic GVHD£ | |||
| HLA-C match score | |||
| 4/4* | 77 | 1.0 | |
| 3/4 | 108 | 1.0 (0.5–1.9) | 0.89 |
| 2/4 | 211 | 1.3 (0.7–2.5) | 0.44 |
| 1/4 | 62 | 1.0 (0.4–2.4) | 0.95 |
| 0/4 | 57 | 1.5 (0.7–3.5) | 0.31 |
| Worst HLA Match -A, -B, and -DRB1 | |||
| 6/6* | 24 | 1.0 | |
| 5/6 | 102 | 0.5 (0.2–1.1) | 0.09 |
| 4/6 | 256 | 0.5 (0.2–1.1) | 0.09 |
| 3/6 | 106 | 0.6 (0.2–1.5) | 0.30 |
| 2/6 | 27 | 0.5 (0.2–1.7) | 0.29 |
| Conditioning | |||
| MAC* | 166 | 1.0 | |
| RIC | 349 | 0.9 (0.6–1.4) | 0.68 |
| Neutrophil Engraftment by Day +42£ | |||
| HLA-C match score | |||
| 4/4* | 77 | 1.0 | |
| 3/4 | 108 | 0.8 (0.5–1.1) | 0.13 |
| 2/4 | 211 | 0.8 (0.6–1.1) | 0.15 |
| 1/4 | 62 | 1.0 (0.7–1.5) | 0.90 |
| 0/4 | 57 | 0.9 (0.6–1.3) | 0.52 |
| Worst HLA Match -A, -B, and -DRB1 | |||
| 6/6* | 24 | 1.0 | |
| 5/6 | 102 | 1.0 (0.6–1.6) | 0.93 |
| 4/6 | 256 | 0.9 (0.5–1.4) | 0.53 |
| 3/6 | 106 | 0.7 (0.4–1.2) | 0.21 |
| 2/6 | 27 | 0.7 (0.4–1.3) | 0.23 |
| Conditioning | |||
| MAC* | 166 | 1.0 | |
| RIC | 349 | 1.9 (1.5–2.4) | <0.01 |
| Age | |||
| <18* | 47 | 1.0 | |
| 18–34 | 97 | 0.7 (0.5–1.0) | 0.08 |
| 35+ | 371 | 0.9 (0.6–1.2) | 0.42 |
| CMV serostatus | |||
| Negative* | 212 | 1.0 | |
| Positive | 303 | 0.8 (0.7–1.0) | 0.06 |
| Platelet Engraft£ | |||
| HLA-C match score | |||
| 4/4* | 77 | 1.0 | |
| 3/4 | 108 | 1.0 (0.6–1.4) | 0.81 |
| 2/4 | 211 | 0.8 (0.6–1.2) | 0.36 |
| 1/4 | 62 | 0.8 (0.5–1.3) | 0.43 |
| 0/4 | 57 | 0.7 (0.5–1.2) | 0.19 |
| Worst HLA Match -A, -B, and -DRB1 | |||
| 6/6* | 24 | 1.0 | |
| 5/6 | 102 | 0.9 (0.5–1.7) | 0.92 |
| 4/6 | 256 | 0.8 (0.4–1.4) | 0.78 |
| 3/6 | 106 | 0.8 (0.4–1.5) | 0.53 |
| 2/6 | 27 | 0.7 (0.3–1.5) | 0.32 |
| Conditioning | |||
| MAC* | 166 | 1.0 | |
| RIC | 349 | 1.7 (1.3–2.1) | <0.01 |
| CMV serostatus | |||
| Negative* | 212 | 1.0 | |
| Positive | 303 | 0.8 (0.7–1.0) | 0.05 |
Abbreviations: HLA, human leukocyte antigen; dUCB, double umbilical cord blood; N, number of patients; CI, confidence interval; GVHD, graft-vs-host disease.
Reference group
Stratified by transplant center
Graft-vs.-Host Disease
The cumulative incidences of grade II–IV acute (Figure 2A) and chronic GVHD (Figure 2B) were not influenced by HLA-C matching score (Table 2) even when adjusting for other factors in multivariate analysis (Table 4).
Figure 2.
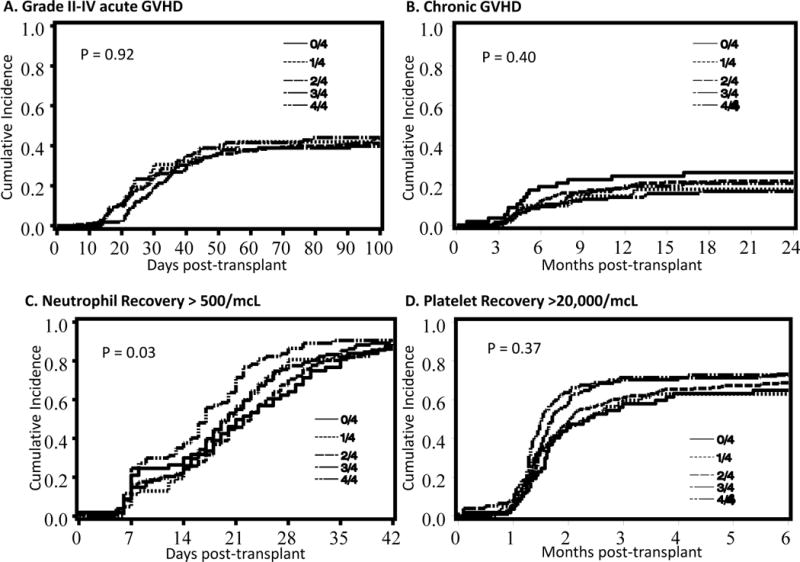
Cumulative incidences of (A) grade II–IV acute graft-vs-host disease, (B) chronic graft-vs-host disease, (C) neutrophil recovery > 500/mcL and (D) plaleted recovery > 20,000/mcL according to HLA-C matching score group.
Hematopoietic recovery
We found that patients who had a higher HLA-C matching score had improved neutrophil recovery by day +42 and platelet recovery at 6 months (Table 2). However, after adjusting for matching at HLA-A, -B, and -DRB1, conditioning regimen, age of the patient, and CMV serostatus, there was no independent effect of the HLA-C matching score on hematopoietic recovery (Table 4)
EFFECT OF THE HLA-C MATCHING SCORE OF THE PREDOMINANT UNIT ON OUTCOMES
In dUCB transplantation, a single unit will provide long-term hematopoiesis in most patients defined as the predominat unit at day +100. We therefore studied the effect of the HLA-C matching score of the predominant unit on outcomes. This patient subset (n=416) excluded those with graft failure, dual donor chimera (both donor present), and early deaths. The median age was 47 years (range, 2–73), and the majority were male (60%), had acute leukemia (61%), and received CSA/MMF immune suppression (77%). Many also had high-risk disease (36%) and received myeloablative conditioning (32%). Considering the allele level HLA-matching at HLA-A, -B, and -DRB1, the predominant unit was 2/6 in 15 (4%), 3/6 in 33 (8%), 4/6 in 184 (44%), 5/6 in 123 (30%), and 6/6 in 31 (7%). The HLA-C matching score of the predominant unit was zero in 46 (11%), 1 in 50 (12%), 2 in 169 (41%, and 4 in 88 (21%). We observed no independent effect of the HLA-C matching score of the predominant unit on DFS, relapse, or NRM (Table 5).
Table 5.
Multivariate analysis of the effect of HLA-C matching score after dUCB transplantation considering the predominant cord blood unit.
| N | HR (95% Confidence interval) | P-value | |
|---|---|---|---|
| Disease-Free Survival | |||
| HLA-C match score | |||
| 2/2* | 117 | 1.0 | |
| 1/2 | 232 | 1.0 (0.8–1.4) | 0.83 |
| 0/2 | 67 | 1.1 (0.8–1.7) | 0.52 |
| HLA Match -A, -B, and -DRB1 | |||
| 5–6/6* | 154 | 1.0 | |
| 4/6 | 184 | 0.9 (0.6–1.3) | 0.72 |
| 2–3/6 | 78 | 1.0 (0.7–1.5) | 0.91 |
| Risk | |||
| Standard* | 268 | 1.0 | |
| High | 148 | 1.3 (1.0–1.7) | 0.08 |
| CMV | |||
| No* | 175 | 1.0 | |
| Yes | 241 | 1.4 (1.1–1.8) | 0.01 |
| Relapse | |||
| HLA-C match score | |||
| 2/2* | 117 | 1.0 | |
| ½ | 232 | 0.9 (0.6–1.3) | 0.44 |
| 0/2 | 67 | 1.0 (0.6–1.7) | 0.93 |
| HLA Match -A, -B, and -DRB1 | |||
| 5–6/6* | 154 | 1.0 | |
| 4/6 | 184 | 0.9 (0.6–1.4) | 0.60 |
| 2–3/6 | 78 | 0.9 (0.5–1.6) | 0.73 |
| Conditioning | |||
| MA* | 136 | 1.0 | |
| RIC | 280 | 2.6 (1.6–4.2) | <0.01 |
| Grade II–IV AGVHD | |||
| No* | 217 | 1.0 | |
| Yes | 199 | 0.7 (0.5–1.0) | 0.06 |
| Non-Relapse Mortality | |||
| HLA-C match score | |||
| 2/2* | 117 | 1.0 | |
| 1/2 | 232 | 1.4 (0.8–2.3) | 0.20 |
| 0/2 | 67 | 1.4 (0.7–2.7) | 0.31 |
| HLA Match -A, -B, and -DRB1 | |||
| 5–6/6* | 154 | 1.0 | |
| 4/6 | 184 | 0.9 (0.6–1.5) | 0.72 |
| 2–3/6 | 78 | 1.2 (0.7–2.0) | 0.61 |
| Conditioning | |||
| MA* | 136 | 1.0 | |
| RIC | 280 | 0.5 (0.3–0.7) | <0.01 |
| Disease Risk | |||
| Standard | 268 | 1.0 | |
| High | 148 | 1.7 (1.1–2.5) | 0.01 |
| CMV | |||
| Negative* | 175 | 1.0 | |
| Positive | 241 | 1.5 (1.0–2.2) | 0.07 |
HLA, human leukocyte antigen; dUCB, double umbilical cord blood; N, number of patients; CI, confidence interval; GVHD, graft-vs-host disease; CMV cytomegalovirus.
Reference group
Stratified by transplant center
DISCUSSION
Studies on the effect of HLA-related factors in dUCB transplantation represent a unique challenge as both donor units may contribute to outcomes, but only one predominates in the long-term9. In many studies, the effect of HLA match on transplant outcomes have often focused on the HLA match of the worst matched of the two UCB units. Studies on the effect of killer immunoglobulin-like receptor (KIR)-matching15, 16, donor specific HLA antibodies17–19, and allele level HLA-matching20, 21 have yielded conflicting results, in particular when comparing data from the single and double UCB transplantation settings. In this study, we considered allele level matching at HLA-A, -B and -DRB1 of the less well matched of the two UCB units composing the graft and separately scored HLA-C matching. Our report contrasts to a previously reported study in single UCB HCT that considered class I HLA loci at the antigen level.3
Not unexpectedly, we found an interaction between at HLA-A, -B and -DRB1 and HLA-C matching score on the effect on survival, DFS, and NRM endpoints. Our main finding in this stratified analysis was that for patients receiving a less well HLA-matched dUCB graft with at least one of the units being 2–4/6 matched to the patient at HLA-A, -B and -DRB1, a higher HLA-C matching score was associated with improved survival, DFS, and reduced NRM. Notably, there were no differences in outcomes for 2/6 vs 3/6 vs. 4/6 HLA-matched patients. We speculate that this may have resulted from relative small number of patients or that in the contxt of significant mismatch, any improvement in HLA-matching is beneficial. In contrast, the HLA-C matching score had no effect on these endpoints in patients receiving only 5–6/6 HLA-matched units. However, the smaller number of patients in the better HLA matched group that resulted from the need of stratification reduced our power and may have obscured any differences in outcomes. Notably, this observation differs from the study of HLA-C matching in single UCB transplantation3 where HLA-C had favorably influenced the outcomes of better HLA-matched HCT (5–6/6), but had no effect in recipients of a 4/6 HLA-matched unit.3 The discrepancy between these two studies may, at least in part, be explained by differences in patient and transplant characteristics. In the current study, we had an older population and a higher proportion receiving RIC.
In a subgroup analysis, we studied the effect of the HLA-C matching score of the long-term predominant unit, but found no independent effect on DFS, relapse, or NRM. This observation contrasts with our findings for the whole group of patients where the HLA-C score influenced DFS and NRM. While it would have been interesting to have demonstrated that the HLA-C matching score of the long-term predominant UCB unit also predicted long term outcomes, this analytical approach has significant limitations. We speculate that the discrepancy noted above resulted from bias introduced by excluding patients who did not engraft and those who had early deaths, two events that are important determinants of the risk of NRM and consequently DFS. Moreover, as our ability to predict the winning UCB unit remains limited, any effect of HLA-C score of the predominant unit would be difficult to translate into clinical practice.
Consistent with single UCB data,3 we observed no independent effect of the number of matches in HLA-C on the risk of GVHD and relapse. There was also no independent effect on hematopoietic recovery. These observations contrast with those in URD bone marrow and peripheral blood grafts where antigen, but not allele, mismatches at HLA-C led to higher risk of GVHD and resulted in higher NRM1, 2. The greater immune tolerance even after partially matched UCB transplantation results in a relative low risk of GVHD, but a preserved graft-vs-malignancy effect.
The practical implication of our data is that for dUCB transplantation patients receiving at least one 2–4/6 HLA allele level-matched unit, additional HLA-C antigen-level matching improves survival and reduces NRM and should be pursued whenever possible. However, in patients receiving only well matched units (≥5/6) as part of a double UCB graft, further matching at HLA-C offers no additional benefit.
Supplementary Material
Table S1. Multiple Regression Analysis of the effect of HLA-C matching score on the outcomes after dUCB transplantation in endpoints that there was an interaction with conventional HLA-matching at -A,-B, and -DRB1 (OS & NRM).
Table S2. Multivariate analysis of the effect of HLA-C after dUCB transplantation in endpoints that there was NO interaction with conventional HLA-matching at -A,-B, and -DRB1 (Relapse).
Key Points.
In patients receiving at least one 2–4/6 HLA-matched UCB unit, additional matching at HLA-C reduces non-relapse mortality and improves disease-free and overall survival
In patients receiving 5–6/6 HLA-matched UCB units, further matching at HLA-C offers no additional benefit.
Acknowledgments
The authors acknowledge, the faculty, advanced practice providers, inpatient unit and clinic nurses, pharmacists and social workers of the Blood and Marrow Transplant Programs who cared for the patients at the University of Minnesota Medical Center, Dana Farber Cancer Institute, and Massachusetts General Hospital.
This work was supported in part by grants from the National Cancer Institute P01 CA65493 (C.G.B, T.E.D., M.R.V., J.E.W.), Leukemia and Lymphoma Society Scholar in Clinical Research Award, grant CDP-2417-11 (C.G.B.), and National Heart, Lung and Blood Institute U54 HL081030-32 (CC and KKB).
Footnotes
CONFLICT OF INTEREST
There are no relevant conflicts of interest to disclose.
References
- 1.Lee SJ, Klein J, Haagenson M, Baxter-Lowe LA, Confer DL, Eapen M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110(13):4576–83. doi: 10.1182/blood-2007-06-097386. Epub 2007/09/06. [DOI] [PubMed] [Google Scholar]
- 2.Woolfrey A, Klein JP, Haagenson M, Spellman S, Petersdorf E, Oudshoorn M, et al. HLA-C antigen mismatch is associated with worse outcome in unrelated donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(6):885–92. doi: 10.1016/j.bbmt.2010.09.012. Epub 2010/09/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eapen M, Klein JP, Sanz GF, Spellman S, Ruggeri A, Anasetti C, et al. Effect of donor-recipient HLA matching at HLA A, B, C, and DRB1 on outcomes after umbilical-cord blood transplantation for leukaemia and myelodysplastic syndrome: a retrospective analysis. Lancet Oncol. 2011;12(13):1214–21. doi: 10.1016/S1470-2045(11)70260-1. Epub 2011/10/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballen KK, Spitzer TR, Yeap BY, McAfee S, Dey BR, Attar E, et al. Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biol Blood Marrow Transplant. 2007;13(1):82–9. doi: 10.1016/j.bbmt.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, McGlave PB, Miller JS, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105(3):1343–7. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 6.Brunstein CG, Barker JN, Weisdorf DJ, DeFor TE, Miller JS, Blazar BR, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110(8):3064–70. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cutler C, Stevenson K, Kim HT, Brown J, McDonough S, Herrera M, et al. Double umbilical cord blood transplantation with reduced intensity conditioning and sirolimus-based GVHD prophylaxis. Bone Marrow Transplant. 2011;46(5):659–67. doi: 10.1038/bmt.2010.192. Epub 2010/08/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ASBMT. American Society for Blood and Marrow Transplantation RFI 2006. 2006 Available from: http://www.asbmt.org.
- 9.Ramirez P, Wagner JE, Defor TE, Blazar BR, Verneris MR, Miller JS, et al. Factors predicting single-unit predominance after double umbilical cord blood transplantation. Bone Marrow Transplant. 2012;47(6):799–803. doi: 10.1038/bmt.2011.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 11.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16:901–10. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 12.Cox DR. Regression Models and Life Tables. Journal of the Royal Statisitical Society. 1972;B34:187–220. [Google Scholar]
- 13.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 14.Therneau TM, Grambsch PM. Martingale-based residuals for survival models. Biometrika. 1990;77(1):1478–60. [Google Scholar]
- 15.Brunstein CG, Wagner JE, Weisdorf DJ, Cooley S, Noreen H, Barker JN, et al. Negative effect of KIR alloreactivity in recipients of umbilical cord blood transplant depends on transplantation conditioning intensity. Blood. 2009;113(22):5628–34. doi: 10.1182/blood-2008-12-197467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willemze R, Rodrigues CA, Labopin M, Sanz G, Michel G, Socie G, et al. KIR-ligand incompatibility in the graft-versus-host direction improves outcomes after umbilical cord blood transplantation for acute leukemia. Leukemia. 2009;23(3):492–500. doi: 10.1038/leu.2008.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunstein CG, Noreen H, DeFor TE, Maurer D, Miller JS, Wagner JE. Anti-HLA antibodies in double umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2011;17(11):1704–8. doi: 10.1016/j.bbmt.2011.04.013. Epub 2011/05/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cutler C, Kim HT, Sun L, Sese D, Glotzbecker B, Armand P, et al. Donor-specific anti-HLA antibodies predict outcome in double umbilical cord blood transplantation. Blood. 2011;118(25):6691–7. doi: 10.1182/blood-2011-05-355263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takanashi M, Atsuta Y, Fujiwara K, Kodo H, Kai S, Sato H, et al. The impact of anti-HLA antibodies on unrelated cord blood transplantations. Blood. 2010;116(15):2839–46. doi: 10.1182/blood-2009-10-249219. [DOI] [PubMed] [Google Scholar]
- 20.Eapen M, Klein JP, Ruggeri A, Spellman S, Lee SJ, Anasetti C, et al. Impact of allele-level HLA matching on outcomes after myeloablative single unit umbilical cord blood transplantation for hematologic malignancy. Blood. 2014;123(1):133–40. doi: 10.1182/blood-2013-05-506253. Epub 2013/10/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunstein CG, Pettersdorf E, Defor TE, Noreen H, Maurer D, MacMillan ML, et al. Impact of Allele Level HLA Mismatch on Outcomes in Recipients of Double Umbilical Cord Blood Transplantation. Biol Blood Marrow Transplant. 2015 doi: 10.1016/j.bbmt.2015.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Multiple Regression Analysis of the effect of HLA-C matching score on the outcomes after dUCB transplantation in endpoints that there was an interaction with conventional HLA-matching at -A,-B, and -DRB1 (OS & NRM).
Table S2. Multivariate analysis of the effect of HLA-C after dUCB transplantation in endpoints that there was NO interaction with conventional HLA-matching at -A,-B, and -DRB1 (Relapse).


