Abstract
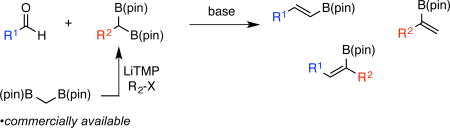
A highly stereoselective boron-Wittig reaction between stable and readily accessible 1,1-bis(pinacolboronates) and aldehydes furnishes a variety of synthetically useful di- and trisubstituted vinyl boronate esters.
Vinyl boronic esters are broadly useful functional motifs in organic synthesis.1 While they possess high chemical stability, they also participate in a variety of transformations including Suzuki-Miyaura couplings,2 Chan-Lam couplings,3 Petasis reactions,4 and Hayashi-Miyaura conjugate additions,5 amongst other transformations. While there is an abundance of reports regarding the synthesis of substituted vinyl boronates from alkynes and alkenes, access to these species from alternate, easily accessible and readily available starting materials is lacking.6 As evidenced in the Kishi synthesis of palytoxin, selective and high-yielding preparation of vinyl boronates directly from aldehydes can solve particularly challenging synthesis problems.7 Lastly, highly regio- and stereoselective syntheses of trisubstituted and 1,1-disubstituted vinyl boronates remains a significant challenge.6i,8
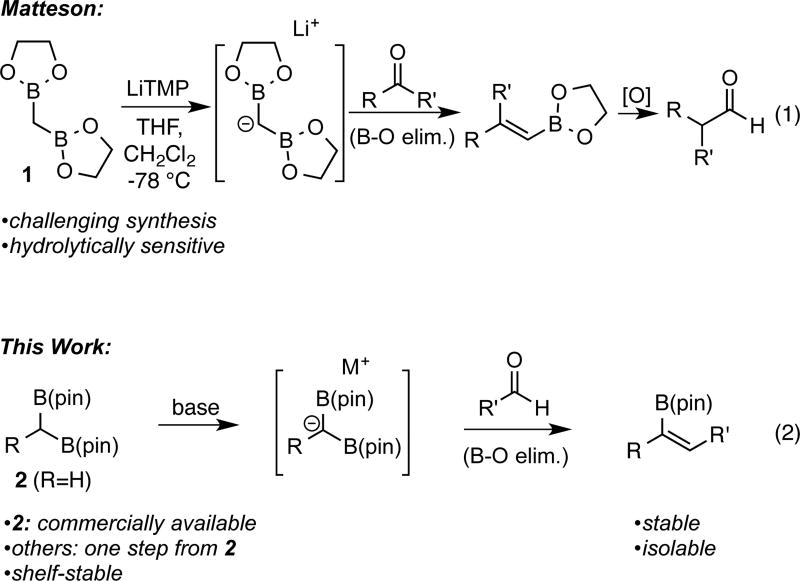
Recent efforts in our laboratory have addressed the preparation and utility of geminal bis(boronates) in organic synthesis.9 In this context, a scalable preparation of bis(boryl)methane 2 (see Scheme 1 for structure) from dibromomethane was developed and, employing alkylation reactions developed by Matteson, construction of substituted derivatives is straightforward.10 Considering the above-mentioned importance of vinyl boronates in organic synthesis, it was considered that a boron-Wittig reaction employing geminal bis(pinacolboronates) might be strategically useful. In this connection, the boron-Wittig reaction was pioneered by Pelter and Matteson in the 1970s employing alkylboranes and geminal bis(boronates), respectively.10,11,12 Most related to the present research, Matteson and coworkers examined the reaction between ethylene glycol-derived bis(boryl) methane1 and a variety of carbonyl electrophiles (Scheme 1, equation 1). While the one-pot boron-Wittig reaction/oxidation procedure to provide aldehyde homologation products was extensively studied, the reaction lacked practicality for two reasons: preparation of 1 is not straightforward, and the intermediate ethylene glycol-derived vinyl boronates were hydrolytically sensitive. Moreover, other than a singular example of a reaction between substituted 1,1-bis(boronate) esters and aldehydes,11c use of the boron-Wittig reaction to provide trisubstituted vinyl boronates has not been investigated.13 Herein, we report a highly stereoselective boron-Wittig reaction between stable and readily accessible 1,1-bis(pinacolboronates) and aldehydes to furnish a variety of synthetically useful di- and trisubstituted vinyl boronate esters (Scheme 1, equation 2). Importantly, the overall transformation represents a transition-metal-free alternative to alkyne hydroboration reactions.
Scheme 1.
Previous Example and Current Goals for the Boron-Wittig Reaction.
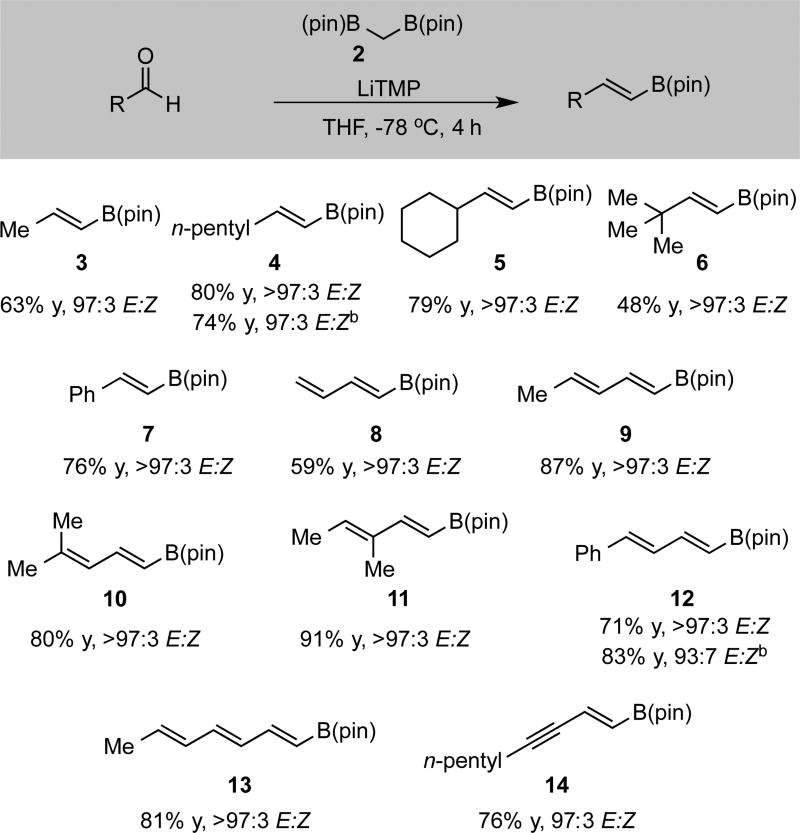
Initial studies focused on the synthesis of trans-vinyl boronate esters and utilized nonsubstituted bis(pinacolato)boryl methane 2 and lithium tetramethyl piperidide (LiTMP) as the base (Scheme 2). After deprotonation of 2 at 0 °C for 5 minutes, the mixture was cooled to −78 °C, the aldehyde added, and the reaction mixture allowed to stir 4 hours before being allowed to warm to room temperature. The reaction was then concentrated under reduced pressure and the derived vinyl boronate isolated. As depicted in Scheme 2, high yields and excellent levels of stereoselectivity were obtained with linear aldehydes (3 and 4), as well as those containing α-branches (5–7) Importantly, high levels of stereoselectivity could still be obtained even if the deprotonation and carbonyl addition were conducted at 0 °C and warmed to room temperature, thereby allowing shorter reaction times (4 and 12). Synthetically challenging dienyl and enynyl boronate esters (8–14) could also be obtained in high yield and stereoselectivity by employing corresponding unsaturated aldehydes.
Scheme 2.
Synthesis of trans-Vinylboronates via the Boron-Wittig Reaction.a
(a) See text for details. Reaction employed 1.2 equiv of 2, 1.2 equiv of LiTMP, and 1 equiv of aldehyde. Results are an average of two experiments. Yield represents isolated yield after purification by silica gel chromatography. (b) Reaction performed at 0 °C to room temperature for 1.5 hours.
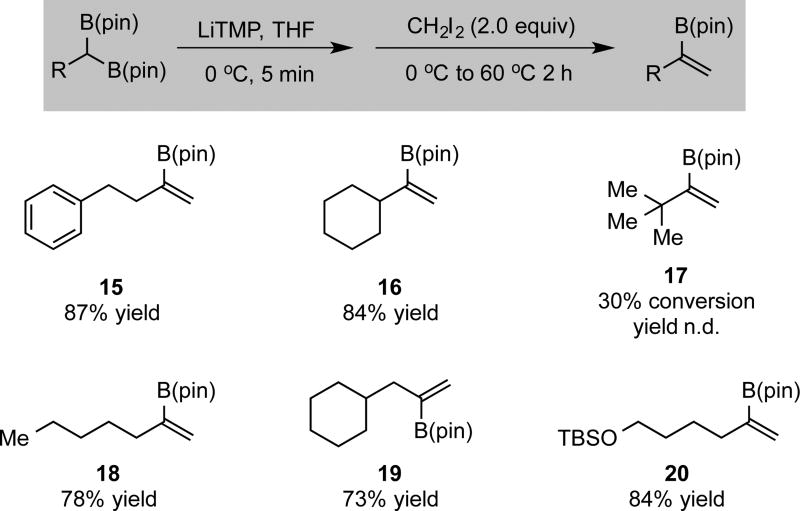
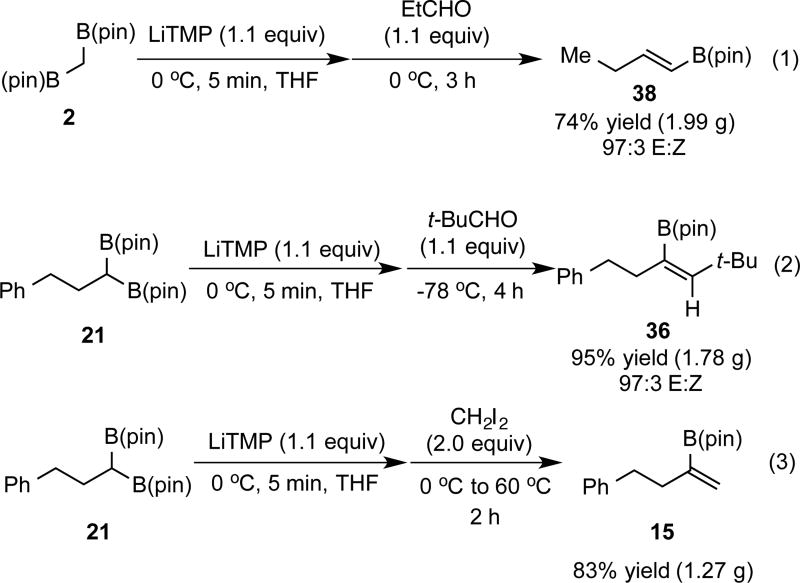
While it might be considered that construction of 1,1-disubstituted vinyl boronates could arise from the boron-Wittig reaction of substituted geminal bis(boronates) with formaldehyde, in practice this transformation was only moderately effective. Thus, reaction of lithiated bis(boronates) with formaldehyde (generated by treatment of paraformaldehyde with catalytic toluenesulfonic acid) for 4 hours at −78 °C provided moderate yields of the derived vinyl boronate (see Supporting Information). As an alternative, it was found that reaction of lithiated geminal bis(boronates) with diiodomethane for 2 hours at 60 °C provided efficient access to 1,1-disubstituted vinyl boronates. In this transformation, it is assumed that the lithiated bis(boronate) first undergoes alkylation with CH2I2 and, subsequent to nucleophilic substitution, B-I elimination results in formation of the product alkene. In this manner vinyl boronate 15 was afforded in high yield from diiodomethane and the corresponding substituted geminal bis(boronate) (Scheme 3). Additionally, α-branched vinyl boronate 16 was also furnished in good yield. Of note, this strategy provides an attractive complement to allene protoborylation and alkyne hydrometallation/borylation sequences.6i, 8d,f,h
Scheme 3.
Synthesis of 1,1-Disubstituted Vinylboronates. a
(a) Results are an average of two experiments. Isolated yields are provided.
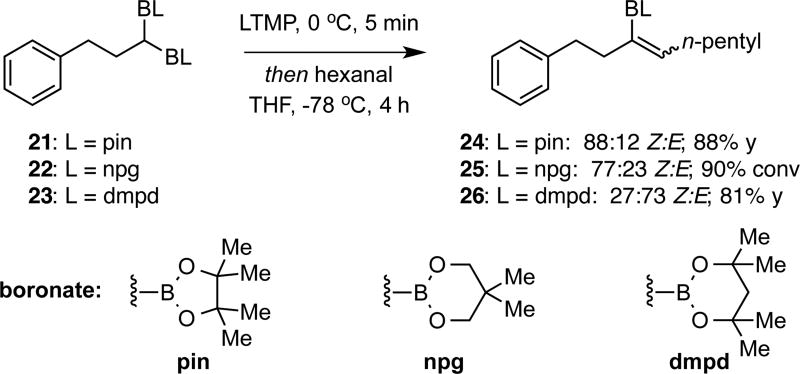
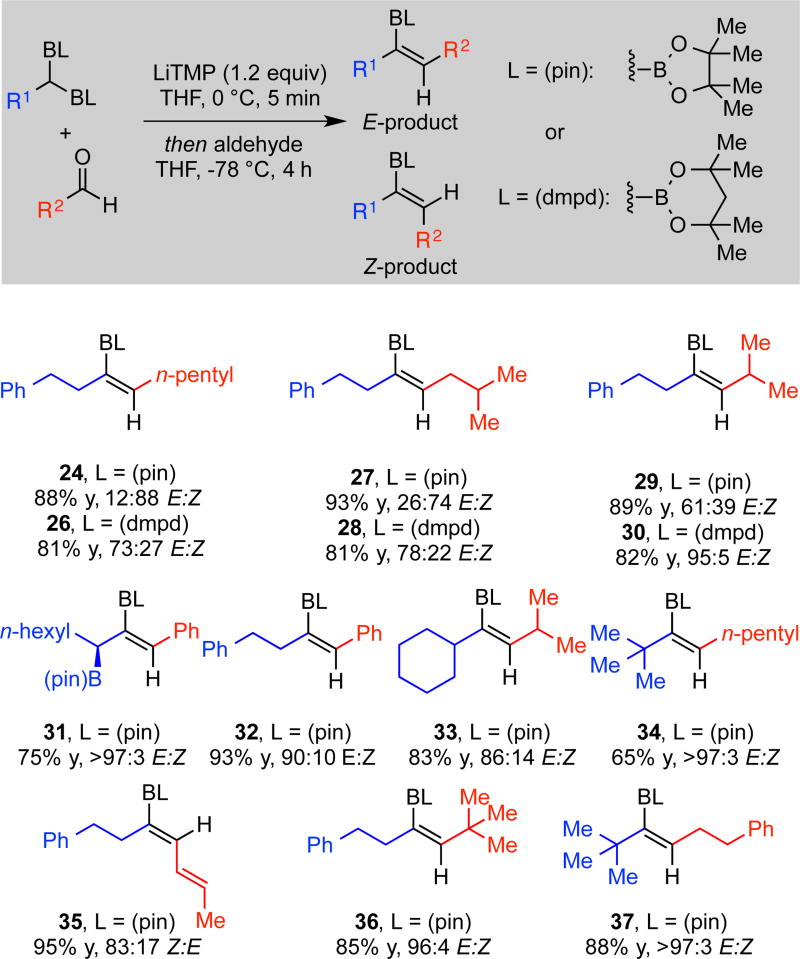
Despite the importance of trisubstituted vinyl boronates, production of these motifs by methods such as internal alkyne hydroboration can suffer from low regioselectivity.6c,14 Furthermore, methods for the trans-selective construction of trisubstituted vinyl boronates are limited.15 To address these challenges, we considered the boron-Wittig reaction between geminal bis(boronates) and aldehydes. In a preliminary experiment, pinacol-derived geminal bis(boronate) 21 was deprotonated at 0 °C with LiTMP in THF and then treated with n-hexanal. After 4 h of reaction at −78 °C, the derived Z trisubstituted vinyl boronate 24 was produced in high yield and in a synthetically useful diastereomer ratio (Scheme 4). While addition of Lewis acids or use of alternate solvents did not alter selectivity in a useful manner (see Supporting Information for this data), the use of alternate boronic esters was observed to alter stereoselection dramatically. With the neopentylglycolato (npg) derivative, the trisubstituted vinyl boronate was produced in a 77:23 Z:E ratio, while the dimethylpentanediolato (dmpd) derivative furnished the E isomer selectively (27:73 Z:E).
Scheme 4.
Development of Boron-Wittig Conditions With 1,1-Bis(boronates) and Aldehydes.a
To survey the utility of the boron-Wittig reaction for the construction of trisubstituted vinyl boronates, a number of linear and α-branched aldehydes were reacted with geminal bis(boronates) 21 or 23. As observed in Scheme 5, trisubstituted vinyl boronates are furnished in moderate to high diastereoselectivity and good yield. Of note, both the cis- and the trans- isomer of trisubstituted vinyl boronate 27/28 can be afforded in moderate stereoselectivity simply by varying the ligand on the boron. Cyclohexyl and tert-butyl substituted pinacol-derived geminal bis(boronates) produce sterically hindered vinyl boronates with high stereoselectivity (33 and 34). Notably, these products would be difficult to access via alternative methods. Also of note, a synthetically challenging Z-trisubstituted dienylboronate was accessed in excellent yield and good selectivity through the reaction of an α,β-unsaturated aldehyde (35). Further, enantiomerically enriched tris(boronates)16 can be converted to bis(boronates) with complete conservation of enantiomeric purity and in excellent diastereoselectivity (31). Lastly, a trend emerges and should be noted: the E isomer is favored when either large boronate substituents (R1) or large aldehyde substituents (R2) are employed (29, 31, 33, 34, 36, 37) and this selectivity can be enhanced with the dmpd ligand (30). In contrast, when both a small aldehyde (i.e., linear alkyl R2) and a small boronate is employed, the Z product stereochemistry is favored (24, 35). Although stereoselectivity is only moderate for construction of some trisubstituted vinyl boronates, the lack of regioselectivity that can plague other methods is avoided through the boron-Wittig reaction. For example, through appropriate reagent choice, the boron-Wittig reaction provides access to both regioisomers 36 and 37 in high yield and with excellent diastereoselectivity.
Scheme 5.
Scope of Boron-Wittig Reaction for Trisubstituted Vinyl Boronates.a
(a) Results are an average of two experiments. Yield represents isolated of purified material.
Considering that the boron-Wittig reaction employs commercially available and easily accessible reagents and does not require the use of expensive transition-metals, large-scale operations are likely to be of interest. To study this feature, 1,2-disubstituted (38), trisubstituted (36), and 1,1-disubstituted (15) vinyl boronates were targeted (Scheme 6) and it was found that they can be accessed efficiently in high yields, even on gram scale.
Scheme 6.
Synthesis of 1,1-Disubstituted Vinylboronates.
In conclusion, a boron-Wittig reaction that employs readily available geminal bis(boronates) has been described. The overall transformation represents a simple and efficient method for the synthesis of synthetically challenging vinylboronates that are often less accessible by other routes. Furthermore, the described method is transition-metal free and utilizes readily available or easily accessible starting materials, and is therefore appealing for large-scale operations.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (GM 059417). J.R.C. was supported by a LaMattina Graduate Fellowship.
Footnotes
ASSOCIATED CONTENT
Experimental procedures, full spectroscopic data for new compounds and chiral separations. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
References
- 1.(a) Ramachandran PV, Brown HC. Organoboranes for Synthesis; ACS Symposium Series 783. American Chemical Society; Washington, DC: 2001. Recent Advances in Borane Chemistry. [Google Scholar]; (b) Matteson DS. Tetrahedron. 1989;45:1859. [Google Scholar]; (c) Brown HC, Campbell JB., Jr Aldrichim. Acta. 1981;14:3. [Google Scholar]; (d) Hall DG, editor. Boronic Acids: Preparation Applications in Organic Synthesis and Medicine. 2. Wiley-VCH; Weinheim, Germany: 2011. [Google Scholar]
- 2.(a) Miyaura N, Suzuki A. Chem. Rev. 1995;95:2457. [Google Scholar]; (b) Suzuki A. J. Organomet. Chem. 1999;576:147. [Google Scholar]; (c) Lennox AJ, Lloyd-Jones GC. Chem. Soc. Rev. 2014;43:412. doi: 10.1039/c3cs60197h. [DOI] [PubMed] [Google Scholar]
- 3.(a) Shade RE, Hyde AM, Olsen J-C, Merlic CA. J. Am. Chem. Soc. 2010;132:1202. doi: 10.1021/ja907982w. [DOI] [PubMed] [Google Scholar]; (b) Winternheimer DJ, Merlic CA. Org. Lett. 2010;12:2508. doi: 10.1021/ol100707s. [DOI] [PubMed] [Google Scholar]
- 4.(a) Candeias NR, Montalbano F, Cal PMSD, Gois PMP. Chem. Rev. 2010;110:6169. doi: 10.1021/cr100108k. [DOI] [PubMed] [Google Scholar]; (b) de Graaff C, Ruijter E, Orru RVA. Chem. Soc. Rev. 2012;41:3969. doi: 10.1039/c2cs15361k. [DOI] [PubMed] [Google Scholar]
- 5.(a) Takaya Y, Ogasawara M, Hayashi T. Tetrahedron Lett. 1998;39:8479. [Google Scholar]; (b) Takaya Y, Senda T, Kurushima H, Ogasawara M, Hayashi T. Tetrahedron: Asymmetry. 1999;10:4047. [Google Scholar]
- 6.For selected references to the synthesis of: trans-vinyl boronates from terminal alkynes: Brown HC, Gupta SK. J. Am. Chem. Soc. 1975;97:5249.Lane CF, Kabalka GW. Tetrahedron. 1976;32:981.Tucker CE, Davidson J, Knochel P. J. Org. Chem. 1992;57:3482.Pereira S, Srebnik M. Organometallics. 1995;14:3127.cis-vinyl boronates from terminal alkynes: Ohmura T, Yamamoto Y, Miyaura N. J. Am. Chem. Soc. 2000;122:4990.Molander GA, Ellis NM. J. Org. Chem. 2008;73:6841. doi: 10.1021/jo801191v.Mirzayans PM, Pouwer RH, Williams CM, Bernhardt PV. Tetrahedron. 2009;65:8297.Gunanathan C, Hölscher M, Pan F, Leitner W. J. Am. Chem. Soc. 2012;134:14349. doi: 10.1021/ja307233p.Jang H, Zhugralin AR, Lee Y, Hoveyda AH. J. Am. Chem. Soc. 2011;133:7859. doi: 10.1021/ja2007643.Yoshida H, Kageyuki I, Takaki K. Org. Lett. 2014;16:3512. doi: 10.1021/ol501465x.Barbeyron R, Benedetti E, Cossy J, Vasseur JJ, Arseniyadis S, Smietana M. Tetrahedron. 2014;70:8431.From alkenes: Blackwell HE, O’Leary DJ, Chatterjee AK, Washenfelder RA, Bussmann DA, Grubbs RH. J. Am. Chem. Soc. 2000;122:58.Morrill C, Grubbs RH. J. Org. Chem. 2003;68:6031. doi: 10.1021/jo0345345.Morrill C, Funk TW, Grubbs RH. Tetrahedron Lett. 2004;45:7733.Funk TW, Efskind J, Grubbs RH. Org. Lett. 2005;7:187. doi: 10.1021/ol047929z.McNulty L, Kohlbacher K, Borin K, Dodd B, Bishop J, Fuller L, Wright Z. J. Org. Chem. 2010;75:6001. doi: 10.1021/jo1003423.Hemelaere R, Carreaux F, Carboni B. J. Org. Chem. 2013;78:6786. doi: 10.1021/jo400872x.Kiesewetter ET, O’Brien RV, Yu EC, Meek SJ, Schrock RR, Hoveyda AH. J. Am. Chem. Soc. 2013;135:6026. doi: 10.1021/ja403188t.
- 7.Armstrong RW, Beau J-M, Cheon SH, Christ WJ, Fujioka H, Ham W-H, Hawkins LD, Jin H, Kang SH, Kishi Y, Martinelli MJ, McWhorter WW, Jr, Mizuno M, Nakata M, Stutz AE, Talamas FX, Taniguchi M, Tino JA, Ueda K, Uenishi J, White JB, Yonaga M. J. Am. Chem. Soc. 1989;111:7525. [Google Scholar]
- 8.(a) Takahashi K, Ishiyama T, Miyaura N. J. Organomet. Chem. 2001;47:625. [Google Scholar]; (b) Takagi J, Takahashi K, Ishiyama T, Miyaura N. J. Am. Chem. Soc. 2002;124:8001. doi: 10.1021/ja0202255. [DOI] [PubMed] [Google Scholar]; (c) Moran WJ, Morken JP. Org. Lett. 2006;8:2413. doi: 10.1021/ol060735u. [DOI] [PubMed] [Google Scholar]; (d) Gao F, Hoveyda AH. J. Am. Chem. Soc. 2010;132:10961. doi: 10.1021/ja104896b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Meng F, Jung B, Haeffner F, Hoveyda AH. Org. Lett. 2013;15:1414. doi: 10.1021/ol4004178. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Guan W, Michael AK, McIntosh ML, Koren-Selfridge L, Scott JP, Clark TB. J. Org. Chem. 2014;79:7199. doi: 10.1021/jo500773t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Jang H, Jung B, Hoveyda AH. Org. Lett. 2014;16:4658. doi: 10.1021/ol5022417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Sun C, Potter B, Morken JP. J. Am. Chem. Soc. 2014;136:6534. doi: 10.1021/ja500029w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hong K, Liu X, Morken JP. J. Am. Chem. Soc. 2014;136:10581. doi: 10.1021/ja505455z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Potter B, Szymaniak AA, Edelstein EK, Morken JP. J. Am. Chem. Soc. 2014;136:17918. doi: 10.1021/ja510266x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matteson DS, Moody RJ. Organometallics. 1982;1:20. [Google Scholar]
- 11.(a) Matteson DS, Moody RJ, Jesthi PK. J. Am. Chem. Soc. 1975;97:5608. [Google Scholar]; (b) Matteson DS, Jesthi PK. J. Organomet. Chem. 1976;110:25. [Google Scholar]; (c) Matteson DS, Moody RJ. J. Am. Chem. Soc. 1977;99:3196. [Google Scholar]; (d) Pelter A, Buss D, Colclough E, Singaram B. Tetrahedron. 1993;49:7077. [Google Scholar]
- 12.For chromium-promoted coversion of aldehydes to vinyl boronates, see: Takai K, Shinomiya N, Kaihara H, Yoshida N, Moriwake T, Utimoto K. Synlett. 1995:963.
- 13.The stereoselective synthesis of tetrasubstituted alkenyl boronates from phenyl ketones was reported. However, reactions of aldehydes were not investigated: Endo K, Hirokami M, Shibata T. J. Org. Chem. 2010;75:3469. doi: 10.1021/jo1003407.Endo K, Sakamoto A, Ohkubo T, Shibata T. Chem Lett. 2011;40:1440.
- 14.(a) Brown HC, Gupta SK. J. Am. Chem. Soc. 1972;94:4370. [Google Scholar]; (b) He X, Hartwig JF. J. Am. Chem. Soc. 1996;118:1696. [Google Scholar]; (c) Konno T, Chae J, Tanaka T, Ishihara T, Yamanaka H. Chem. Commun. 2004:690. doi: 10.1039/b316065c. [DOI] [PubMed] [Google Scholar]; (d) Semba K, Fujihara T, Terao J, Tsuji Y. Chem. Eur. J. 2012;18:4179. doi: 10.1002/chem.201103612. [DOI] [PubMed] [Google Scholar]
- 15.Sundararaju B, Fürstner A. Angew. Chem. Int. Ed. 2013;52:14050. doi: 10.1002/anie.201307584. [DOI] [PubMed] [Google Scholar]
- 16.Coombs JR, Zhang L, Morken JP. J. Am. Chem. Soc. 2014;136:16140. doi: 10.1021/ja510081r. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.