Abstract
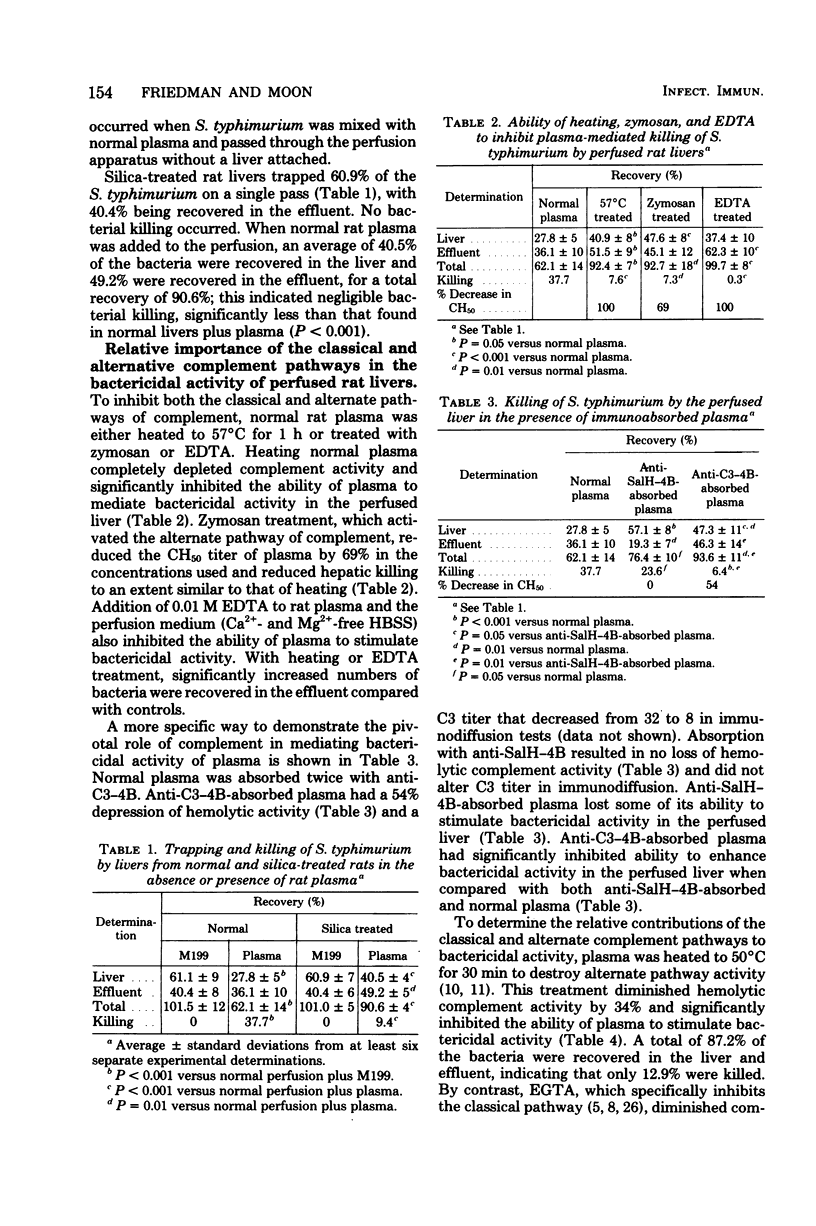
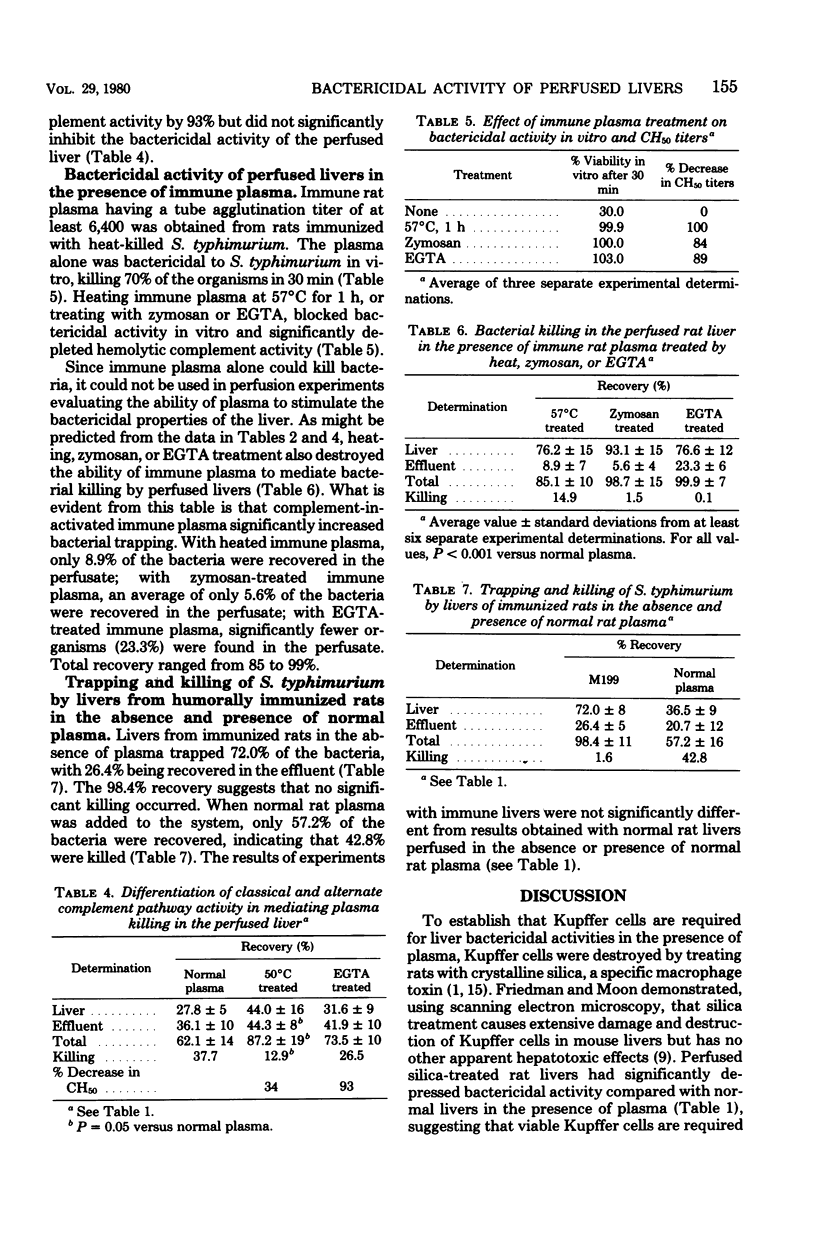
The relative roles of Kupffer cells, complement, and specific antibody in liver antimicrobial activities were investigated by using a rat liver perfusion model. Normal livers trapped an average of 60% of Salmonella typhimurium in a single pass and in the presence of plasma killed more than 60% of these organisms in 30 min. Livers depleted of Kupffer cell function by silica treatment had significantly less bactericidal ability (ca. 15%) in the presence of plasma, showing that viable Kupffer cells are required for optimal antimicrobial activity. To determine the importance of complement in Salmonella killing, plasma complement activity was inhibited by heating at 57 and 50°C, zymosan absorption, chelation with disodium ethylenediaminetetraacetate (EDTA) and depletion of rat C3 by using specific immunoabsorbent. All treatments significantly reduced bactericidal activity in the perfused liver. Chelation of plasma with EDTA had no effect, suggesting that the alternate and not the classical pathway for complement activation was involved. Immune plasma alone was bactericidal. When immune plasma was heated, zymosan absorbed, or chelated with EDTA, bactericidal activity was inhibited in the perfused liver, but bacterial trapping increased. These results suggest that complement is required for bactericidal activity in perfused livers and that specific antibody only enhances bacterial trapping.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Harington J. S., Birbeck M. An examination of the cytotoxic effects of silica on macrophages. J Exp Med. 1966 Aug 1;124(2):141–154. doi: 10.1084/jem.124.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C., Griffin F. M., Jr, Silverstein S. C. Studies of the macrophage complement receptor. Alteration of receptor function upon macrophage activation. J Exp Med. 1975 Jun 1;141(6):1278–1290. doi: 10.1084/jem.141.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre P. F., Oxman E. Phagocytosis and intracellular disposition of viable bacteria by the isolated perfused rat liver. J Reticuloendothel Soc. 1965 Nov;2(4):313–325. [PubMed] [Google Scholar]
- Des Prez R. M., Bryan C. S., Hawiger J., Colley D. G. Function of the classical and alternate pathways of human complement in serum treated with ethylene glycol tetraacetic acid and MgCl2-ethylene glycol tetraacetic acid. Infect Immun. 1975 Jun;11(6):1235–1243. doi: 10.1128/iai.11.6.1235-1243.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F. Properdin: initiation of alternative complement pathway. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3220–3224. doi: 10.1073/pnas.72.8.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine D. P., Marney S. R., Jr, Colley D. G., Sergent J. S., Des Prez R. M. C3 shunt activation in human serum chelated with EGTA. J Immunol. 1972 Oct;109(4):807–809. [PubMed] [Google Scholar]
- Friedman R. L., Moon R. J. Hepatic clearance of Salmonella typhimurium in silica-treated mice. Infect Immun. 1977 Jun;16(3):1005–1012. doi: 10.1128/iai.16.3.1005-1012.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigli I., Ruddy S., Austen K. F. The stoichiometric measurement of the serum inhibition of the first component of complement by the inhibition of immune hemolysis. J Immunol. 1968 Jun;100(6):1154–1164. [PubMed] [Google Scholar]
- Goodkofsky I., Lepow I. H. Functional relationship of factor B in the properdin system to C3 proactivator of human serum. J Immunol. 1971 Oct;107(4):1200–1204. [PubMed] [Google Scholar]
- HOWARD J. G., WARDLAW A. C. The opsonic effect of normal serum on the uptake of bacteria by the reticulo-endothelial system; perfusion studies with isolated rat liver. Immunology. 1958 Oct;1(4):338–352. [PMC free article] [PubMed] [Google Scholar]
- Huber H., Douglas S. D., Fudenberg H. H. The IgG receptor: an immunological marker for the characterization of mononuclear cells. Immunology. 1969 Jul;17(1):7–21. [PMC free article] [PubMed] [Google Scholar]
- KESSEL R. W., MONACO L., MARCHISIO M. A. THE SPECIFICITY OF THE CYTOTOXIC ACTION OF SILICA--A STUDY IN VITRO. Br J Exp Pathol. 1963 Aug;44:351–364. [PMC free article] [PubMed] [Google Scholar]
- Mantovani B., Rabinovitch M., Nussenzweig V. Phagocytosis of immune complexes by macrophages. Different roles of the macrophage receptor sites for complement (C3) and for immunoglobulin (IgG). J Exp Med. 1972 Apr 1;135(4):780–792. doi: 10.1084/jem.135.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon R. J., Vrable R. A., Broka J. A. In situ separation of bacterial trapping and killing functions of the perfused liver. Infect Immun. 1975 Aug;12(2):411–418. doi: 10.1128/iai.12.2.411-418.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munthe-Kaas A. C., Berg T., Seglen P. O., Seljelid R. Mass isolation and culture of rat kupffer cells. J Exp Med. 1975 Jan 1;141(1):1–10. doi: 10.1084/jem.141.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munthe-Kaas A. C., Kaplan G., Seljelid R. On the mechanism of internalization of opsonized particles by rat Kupffer cells in vitro. Exp Cell Res. 1976 Nov;103(1):201–212. doi: 10.1016/0014-4827(76)90256-1. [DOI] [PubMed] [Google Scholar]
- Munthe-Kaas A. C. Phagocytosis in rat Kupffer cells in vitro. Exp Cell Res. 1976 May;99(2):319–327. doi: 10.1016/0014-4827(76)90589-9. [DOI] [PubMed] [Google Scholar]
- Reynolds H. Y., Atkinson J. P., Newball H. H., Frank M. M. Receptors for immunoglobulin and complement on human alveolar macrophages. J Immunol. 1975 Jun;114(6):1813–1819. [PubMed] [Google Scholar]
- Sawyer R. T., Moon R. J., Beneke E. S. Hepatic clearance of Candida albicans in rats. Infect Immun. 1976 Dec;14(6):1348–1355. doi: 10.1128/iai.14.6.1348-1355.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber A. D., Frank M. M. Role of antibody and complement in the immune clearance and destruction of erythrocytes. II. Molecular nature of IgG and IgM complement-fixing sites and effects of their interaction with serum. J Clin Invest. 1972 Mar;51(3):583–589. doi: 10.1172/JCI106847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeoka A. O., Hall R. T., Hemming V. G., Allred C. D., Hill H. R. Role of antibody and complement in opsonization of group B streptococci. Infect Immun. 1978 Jul;21(1):34–40. doi: 10.1128/iai.21.1.34-40.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Pike M. C. Interaction of complex polysaccharides with the complement system: effect of calcium depletion on terminal component consumption. Infect Immun. 1975 Feb;11(2):273–279. doi: 10.1128/iai.11.2.273-279.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARDLAW A. C., HOWARD J. G. A comparative survey of the phagocytosis of different species of bacteria by Kupffer cells; perfusion studies with the isolated rat liver. Br J Exp Pathol. 1959 Apr;40(2):113–117. [PMC free article] [PubMed] [Google Scholar]


