Abstract
Background
Racial and ethnic minority adults with diabetes living in under-resourced communities face multiple barriers to sustaining self-management behaviors necessary to improve diabetes outcomes. Peer support and decision support tools each have been associated with improved diabetes outcomes.
Methods
289 primarily African American adults with poor glycemic control will be recruited from the Detroit Veteran’s Administration Hospital and randomized to Technology-Enhanced Coaching (TEC) or Peer Coaching alone. Participants in both arms will be assigned a peer coach trained in autonomy-supportive approaches. Coaches are diabetes patients with prior poor glycemic control who now have good control. All participants meet face-to-face initially with their coach to review diabetes education materials and develop an action plan. Educational materials in the TEC arm are delivered via a web-based, educational tool tailored with each participant’s personalized health data (iDecide). Over the next six months, Coaches call their assigned participants once a week to provide support for weekly action steps. Data are also collected on an Observational Control group with no contact with study staff. Changes in A1c, blood pressure, other patient-centered outcomes and mediators and moderators of intervention effects will be assessed.
Discussion
Tailored e-Health tools with educational content may enhance the effectiveness of peer coaching programs to better prepare patients to set self-management goals, identify action plans, and discuss treatment options with their health care providers. The study will provide insights for scalable self-management support programs for diabetes and chronic illnesses that require high levels of sustained patient self-management.
Keywords: Diabetes, Peer-Support, Health Coaching, Decision Aid, Self-Management
Introduction
Like in many other US health systems, in spite of improvements from Veteran’s Administration (VA) health care quality initiatives, from 15–30 % of VA diabetes patients still have A1c’s of 9.0% or greater.1 Providers face barriers to initiating and intensifying medication regimens, and many patients face barriers to effective diabetes self-management. These barriers include lack of sufficient understanding of diabetes and its treatments; lack of self-confidence and/or motivation to manage diabetes well; and passivity in office visits with providers without posing their questions or articulating their concerns. In addition, many adults with diabetes lack effective support from their families and friends to help them overcome structural and other barriers to effective diabetes self-management.2 Moreover, low-income African Americans with diabetes experience a 50–100% higher burden of illness and mortality from diabetes, have worse glycemic control, and experience more barriers to diabetes self-management than non-Latino white adults.3 Although these disparities are less severe in VA than in other health systems, they are still marked.4,5 Low-income racial and ethnic minority adults report high levels of diabetes-specific emotional distress,6 and, even among Veterans, have high rates of low health literacy and numeracy.7–9 Patients who are actively involved in treatment decision-making tend to be more satisfied with their health care,10 more adherent to treatment, and have a better quality of life.11 Yet, African-American patients report receiving less information and participating less in health care clinic visits.12–14 This contributes to worse information exchange, less optimal medical decisions, and lower patient satisfaction, leading to poor medication adherence and outcomes.15,16
Higher levels of social support—especially illness-specific support—are associated with better diabetes and other illness self-management.17 Telephone based Peer support helps reduce problematic health behaviors, depression, and, in both VA and non-VA randomized controlled trials of peer health coaches or mentors, has contributed to improved diabetes outcomes.18–22 A recent VA Randomized Control Trial (RCT) led by co-investigator Judith Long found that telephone-based peer mentoring led to greater improvements in glycemic control among African American diabetic Veterans than financial incentives or usual care.5
Along with peer support, decision and educational aids ‘tailored’ based on characteristics unique to that person have been found to improve health outcomes.23,24 In three recent RCTs, diabetes medication decision support tools increased knowledge, blood sugar management, patient involvement in treatment decision-making, and decreased diabetes-related distress.25,26 Yet, many of these tools have targeted patients who have a high level of computer and health literacy. While a recent AHRQ review of 150 consumer health informatics (CHI) applications concluded that these programs engage consumers, enhance traditional clinical interventions, and improve health outcomes,27 the report identified significant knowledge gaps, including the need to evaluate CHI applications among racial and ethnic minority populations and low-literate populations. The report also emphasized the lack of CHI applications engaging nontraditional health care supporters such as peer mentors. To address these gaps in knowledge, the aim of this study is to evaluate the addition of Tailored Interactive Diabetes Medication and Self-Management Decision Support Tools into a Telephone Based Peer Coaching Program.
Research Design and Methods
Overview
We will conduct a parallel, two-armed randomized controlled trial including diabetes patients who have poor glycemic control. The trial will compare a six-month telephone-based peer mentor program in which the peer mentors do not have access to a tailored interactive computer-based tool (iDecide) versus a 6-month telephone-based peer coaching intervention with an initial face-to-face session facilitated by iDecide followed by weekly telephone contacts. We will compare changes in A1cs between these two arms, and with an observed, usual care group (Aim 1). This will enable us to examine the efficacy of peer support in this population, which differs from the populations and sites of our prior peer support interventions, and if significant differences are found to compare the effect size with those of our earlier RCTs comparing peer support with usual care comparison groups. Between the two randomized peer support and peer support + iDecide arms, we will also compare changes in blood pressure control and medication adherence as well as the key patient-centered outcomes of patients’ satisfaction and involvement with care, perceived social support, and diabetes-specific quality of life (Aim 2). Point-of-service A1cs and blood pressure will be assessed at baseline, 6 months, and 12 months. To assist in VA efforts to create a menu of options for patients, we will examine patient characteristics associated with willingness to participate and engage in the proposed intervention, as well as key mediators and moderators of intervention effects (Aim 3). The study duration will be 4 years, to allow for peer coach and patient recruitment, completion of the 6-month program, and assessment of outcomes at 6 months and at 12 months.
Many prior health services studies focus on intervention efficacy as the exclusive measure of success rather than a program’s potential for successful implementation and dissemination in ‘real-world’ clinics.28 To increase the usefulness of this study’s findings to VA and other health systems, we will use the RE-AIM framework to evaluate intervention elements of interest to clinical managers.29 The goal of RE-AIM is to broaden the focus of interventional research to include dimensions critical to an intervention’s implementation in usual care after a trial is completed. We will use mixed methods research methods30 to investigate elements important for implementation and dissemination. This approach involves the collection, analysis, and mixing of both quantitative and qualitative data. The specific design will be an “embedded” mixed methods design involving collecting qualitative data during the intervention to better understand the mechanisms influencing implementation and the outcomes. A chief characteristic of an embedded design is that the qualitative data provides a supportive role and is embedded at different phases of the trial.31 Using mixed methods, we will gather data on how peer coaches, primary care clinic staff, and patients experience the intervention and how the experiences of participants together with the results of the trial help us modify the intervention for future use. Using this approach, we hope to ensure that the intervention has the greatest possible likelihood of adoption in VA and other health systems should we find it has positive effects on processes and outcomes of care.
Study Sample and Setting
Description of Site
The John D. Dingell VAMC in Detroit serves 44,453 Veterans. In FY11, this site provided 447,409 outpatient visits and 5,055 inpatient hospitalizations. Approximately 62% of Veterans who receive their care at the Detroit VA are African American, with a large number living within Detroit. Although 19 MDs, 26 RNs, 10 LPNs and 9 MAs serve these patients in the outpatient setting, the number of diabetes patients with poor risk factor control far exceeds their capacity to provide regular, sustained care management. As of FY11, 28% or 1909 of the 8,263 Veterans with diabetes who received care at the Detroit VA had A1cs>8% over the prior year.
Peer Coach Selection, Training, and Assessment of Fidelity
If the selected peer coaches participate only for 6-month periods each, we will recruit 87 peer coaches (fewer if peer coaches choose to participate longer than 6 months). Based on the experiences of prior peer coach interventions, we anticipate that a significant number of peer mentors will choose to participate for longer than 6 months and that a number of participants who complete the intervention will be interested in serving as peer mentors themselves. All participants at intervention completion will be encouraged to be a peer mentor unless they complete in the final 6 months of the study. In our planning discussions with Detroit VA PCPs, most thought of 2–5 of their diabetes patients they believed would be good peer coaches and could recommend. Many of these had had poor glycemic control in the past but now had good control (A1c<7.5%), one of our preferred characteristics for the peer mentors. To recruit the initial cohort of peer mentors, we will first contact patients recommended by primary care providers. If we do not have sufficient numbers through that strategy, we will identify and reach out to patients who through electronic medical record review had prior poor glycemic control (A1c>8.0%) and in the last 6–12 months had A1cs<7.5%. Study participants who successfully complete the 6-month intervention and are interested in becoming peer mentors will also be actively recruited to be peer coaches.
We will hold initial trainings for new peer coaches over the course of the study period and will hold monthly meetings for peer coaches within each of the two arms to allow for discussion among peer coaches on how their calls are going and provide booster follow-up tips on communication strategies and reinforcement. Fostering a sense of community and exchange among the peer coaches themselves is an important strategy to maintain their morale and interest in continuing to serve as coaches. Research associates will make monthly calls to peer mentors who did not participate in the monthly meeting to check on how calls are going and provide support. The initial training is adapted from prior peer and community health worker trainings that we have used successfully in prior interventions.5,18,32 The training program was developed over the past six years through an iterative process involving researchers, diabetes educators, behavioral psychologists, physicians, social workers, and community representatives with diabetes from low-income minority communities. The initial training will focus on key Motivational Interviewing (MI)-based communication skills for mentors in both arms. Peer mentors in the iDecide arm will participate in an additional training session on how to navigate the iDecide tool that will be delivered on iPads. Peer coaches will also learn how to place calls using the computer-supported telephone platform. Peer mentors will receive training in and practice the following skills: 1) asking open-ended questions; 2) making reflections (content, feeling, action reflections); 3) rolling with resistance; 4) eliciting ‘change-talk’ (assessing importance and confidence, assessing goals and values, tailoring); 5) action planning (helping the participant define a key longer-term behavioral goal and specific action steps to meet this goal that they will try over the next week).
Several recent RCTs have shown that more important in developing and retaining proficiency in communication skills than a lengthy initial training is periodic follow-up reinforcement. Accordingly, the emphasis in the initial training will be to introduce basic MI-based skills with attention to strategies to continue “learning to learn” skills in booster conference call sessions—and it there is interest periodic face-to-face sessions—during the study period. The initial training will be interactive, with time to practice each of the basic skills introduced. Special focus will be placed on helping patients create action plans (described below) and on following up on how patients are doing on their action plans during the weekly phone calls. In addition, peer mentors will be asked to reflect on their own troubles with managing their DM and on the practices and behaviors that enabled them to gain control of their DM, and then discuss how they might work with others to help them incorporate these practices/behaviors into their daily lives. Role-playing activities will give peer coaches the opportunity to practice the communication and facilitation skills.
To assess fidelity and provide booster training and support, study team members trained In MI will observe a random sample of face-to-face sessions. Coaches will also complete a self-assessment checklist on a sample of their telephone coaching calls. In the arm using iDecide, the software program will enable us to evaluate approximate time each pair used to review the program, the order in which sections are covered, and the final action plans and list of questions and concerns to be discussed with health care providers. We will create aggregate summaries for each peer mentor, showing ranges and mean times for each section, whether the order of progression was logical, and compare these with benchmark figures from the use of iDecide by trained health professionals we generated from our prior work. Through this process we will assess fidelity to MI-based communication approaches in both arms and to the iDecide program in the arm using that tool. The computer telephone platform used by coaches will capture frequency and duration of calls by each peer mentor-participant dyad to evaluate intervention completion in both arms. We will use these results to refine written training materials to be included in a “Train the Trainer” manual for the translation “toolkit”. Each peer coach will be assigned 2–4 peer patients for a 6-month period. Although their role as volunteers will be emphasized, to cover any expenses they will receive a stipend for the initial training and different stipend amounts based on number of calls per month documented by the computer phone system with each of their assigned participants. We will assess how participation affects peer coaches’ own diabetes self-management attitudes, practices, and glycemic control. Thus, peer coaches will undergo informed consent and receive incentives to complete the same baseline, 6-, and 12-month assessments as other participants.
Patient Selection, Recruitment and Randomization
A rolling pool of potential recruits will be identified on a monthly basis. Patients will be identified who meet one of the following criteria within the past 12 months: (1) one hospitalization with a diabetes-related ICD-9 code; (2) two outpatient visits with a diabetes-related ICD-9 code; or (3) at least one prescription for a glucose control medication (insulin or oral agents) or monitoring supplies.33 Potential participants must also have their most recent A1c in the prior 6 months be at least 8.0% if age < 70 or at least 8.5% if age 70+). Using ICD-9 diagnostic codes, we will exclude patients if they have an active substance abuse disorder or serious psychiatric illness (PTSD, bipolar disorder, dementia, schizophrenia, or personality disorders). We will then send names of the patients to their primary care providers to identify any patients who they do not recommend inviting to participate in the program. An invitation letter will be sent to eligible patients, with a follow up call by a research associate to provide more information about the study. Patients who agree to participate will be scheduled to complete written informed consent and the baseline assessments, with coded baseline survey data entered directly onto the iPad so information for tailoring will be in the program. Randomization will be stratified by gender and whether the patient is on insulin, as gender and insulin use may moderate treatment effects. Variable block sizes will be programmed into the computer randomization and will preclude prediction of treatment assignments by study staff.
Peer Support-Alone Arm
Participants randomized to receive peer support alone (without the iDecide tool) after they complete their baseline assessments will be matched with a peer coach of the same gender, race, approximate age (+/−7 years) and whether they also use insulin to the extent possible. Matching as closely as possible on shared characteristics and self-management challenges has been found to lead to better peer relationships in prior studies. Participants in this arm will be given copies of the AHRQ consumer-focused guides, “Pills for Type 2 Diabetes” and “Insulin for Type 2 Diabetes” and encouraged to review them. The information in these two guides was used in the iDecide tool, so this group will receive the same diabetes anti-hyperglycemic medication information provided in the iDecide tool. All will receive copies of the printed guides. The coach will encourage the participant to keep a copy of the action plan and list of questions and concerns to discuss with their health care providers generated by the discussion. The peer coach will be instructed to make at least one telephone call to each peer partner each week. If the peer coach does not make an initial call within the first 10 days using the IVR system, a research associate will call them to offer support. In subsequent weeks, the peer mentor will receive outreach reminder calls from staff if they do not call one of their assigned peers in a 2 week period.
Peer Support + iDecide Arm
Participants randomized to this arm will also be matched with a peer coach based on their age, gender, race, and insulin use to the extent possible. They will be scheduled for an initial face-to-face session with their assigned mentor who will guide them through the personally tailored diabetes medication decision aid. The action plans and list of questions and concerns to discuss with their health care providers generated by the program and discussion will be available for the patient and peer coach to keep. The peer mentors will then follow up with weekly IVR-facilitated calls as above.
Observational Control Arm
Because our study is being performed in a different site than our prior interventions, we will also use an observational, usual care control arm to examine whether peer support is more effective than usual care in this population. While this arm is not randomized, it will allow us to confirm the efficacy of peer support while using few additional resources. To identify this usual care arm, we will analyze data from those who are identified as eligible for the study but who were not approached for participation. In our rolling monthly data pull, a number estimated to match the number randomized to one of the intervention groups will be set aside. This sample should approximate those who were enrolled in the trial. We will use medical record data to identify their observed A1c levels at the end of the study period and then compare the changes over the study period time with those in the intervention. We will also use the medical record data to identify key covariates, including baseline A1c, age, gender, race, insulin use and number of oral diabetes medications, and baseline use of services. More than 85% of Veterans with diabetes with at least one recorded A1c>7.5% in a 6-month period who receive care at the Detroit VA receive regular A1c testing at recommended intervals.
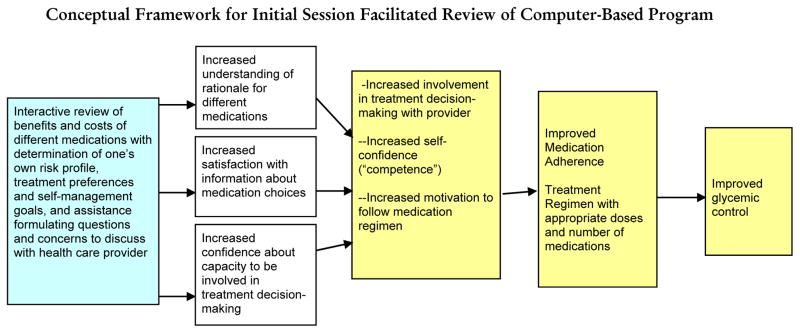
Conceptual Framework for Initial Session Facilitated Review of Computer-Based Program
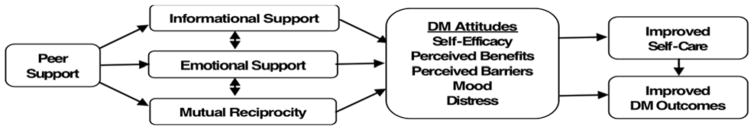
Figure 1 illustrates how we hypothesize the intervention will address key factors affecting medication adherence and intensification. Four theoretical approaches have guided development of the computer-based program and intervention approach. The tailored behavioral intervention incorporates elements of the Health Decision Model (HDM)34 and the Precaution Adoption Model (PAM)35 to emphasize clear and credible communication about the risks and saliency of high glycemic levels. Self Determination Theory (SDT) suggests the importance of encouraging patients to articulate their own values and goals (autonomy), be convinced that recommended behaviors correspond with these intrinsic values and goals (autonomous motivation), and have confidence in their ability to execute the behaviors (self-efficacy or competence). The fourth behavioral theory informing our intervention is social cognitive theory (SCT).36 SCT also emphasizes the importance of understanding the personal salience of a health decision and of developing intrinsic motivation to change behaviors. Building patients’ self-efficacy in their ability to execute specific tasks (such as medication-taking and involvement in treatment decision-making) is a fundamental aim. SCT theory has underpinned many of the successful chronic disease self-management support programs evaluated to date.37–39 These theories informed the decision tool, and the peer coaches will receive brief initial training with booster sessions in Motivational Interviewing-based communication approaches consistent with these theories. Follow-up weekly telephone contacts to provide support in the follow-up steps (“action plans”) decided upon in the initial face-to-face session from the peer coaches will further facilitate sustained improvements in self-management through the hypothesized mechanisms shown in Figure 2:
Figure 1.
Figure 2.
Mechanisms by Which Peer Support Affects Diabetes Outcomes
Outline of Decision and Self-Management Support Tool
The iDecide tool is designed to present key tailored, evidence-based information on diabetes and diabetes treatments. Importantly for a peer support intervention, all content information will be provided through the tool, with the role of the peer mentor being to assist the participant to go through the program and participate in helping the patient formulate questions and concerns to discuss with their health care provider, to set their own behavioral goals and action steps, and to help the participant practice raising the issues they plan to discuss with their health care provider. Information is personally tailored based on each participant’s: a) diabetes-related medical history and personal characteristics (e.g. age, gender, ethnicity, diabetes duration, current A1c, BP, lipid levels; current anti-hyperglycemic medications); b) diabetes-related attitudes and care practices (e.g. current diabetes care self-efficacy, self-reported medication adherence and barriers to adherence); and c) assessment of treatment preferences. Complete details have been published elsewhere.40
An outline of the iDecide tool content is shown in Table 1:
Table 1.
Outline of Tailored Medication Decision Aid
| Section | Tailored Elements |
|---|---|
| 1. Introduction | About You: Personal Health Information About Us: Description of what session will cover/how to use iPad |
| 2. What happens in your body when you have diabetes? | Animated video showing normal processes of glucose uptake and what happens with diabetes |
| 3. How to Deal with Diabetes | Animated video of ways to keep diabetes under control through being active, what you eat, and taking medications |
| 4. Your Diabetes ABCs | Explanation of importance of A1c, BP, and lipids with tailored information on participant’s levels |
| 5. How different A1c levels affect your personal risk of complications | Animated video on diabetes complications/Interactive Diabetes Risk Pictograph showing changes in lifetime risk of vision loss, kidney disease, and amputations with different A1c values A1c goal-setting |
| 6. Learning about diabetes treatments | List of participant’s current medications and doses to confirm/Animated video showing how different classes of anti-hyperglycemic medications work in the body |
| 7. Barriers Participant is Facing Taking Medications | Checklist of barriers participant identified in baseline survey/Template to brainstorm on possible strategies to address these/Information on some strategies to consider/Tailored testimonial/Choice of whether to learn about a new medication, higher dose, or address adherence barriers Prioritization of Barrier (if any) or Issue to Address |
| 8. Comparing diabetes treatments (if participant chose that) | Interactive tailored medication “Issue Cards” (blood sugar reduction, daily routine, sugar testing, low blood sugar, side-effects/problems, weight concerns, cholesterol, costs) New Medication and/or Dose Preference |
| 9. Action Planning | Interactive development of behavioral goal and action steps over next week to meet behavioral goal, Importance and confidence scales/Tailored testimonial Action Plan |
| 10. Summary | Tailored Decision Summary to print out Patient and peer mentor “to-do” lists to print out Tailored “Talk to your doctor” list of specific questions and concerns to discuss |
Action Planning
Coaches in both groups will be trained to assist participants in creating an Action Plan during the initial face-to-face visit. The Action Plan follows the I-SMART (Inspiring, Specific, Measurable, Achievable, Relevant, Time-Specific) goal-setting model that we have used in our prior diabetes self-management interventions that have improved glycemic control.18,32 Any action step that the participant deems important and feels confident to undertake can constitute the ‘action plan’. This could be talking with one’s health care provider about cost concerns or other structural barriers, resolving to ask one’s provider about a change in medications, or a step involving a way to increase physical activity, take prescribed medications, make a dietary change, or other behavioral step to improve diabetes self-management.
Weekly Follow-up Peer Coach Telephone Calls
The peer coaches will be encouraged to call each of their peer partners once a week to check in on how they are doing, ask about how their action plans are going, offer encouragement, and if necessary help their peer partners brainstorm about solutions to barriers they have been facing meeting their action steps, set a new action step to try if they fully met their prior week’s goal, or try a different action step or set a new goal. They will also ask about how the participant’s discussion with their health care provider went (if they had a visit) and provide encouragement to ask any questions and raise any concerns that they did not raise, and, if necessary, encourage the participant to practice again asking the questions or raising their concerns. If the participant missed an appointment, the coach will encourage them to make another appointment.
Because peer coaches and participants will be advised not to exchange phone numbers, peer coaches will use the computer telephone system to make all calls. To place the calls, peer coaches will dial the designated toll-free telephone number. After they are connected with the system, the coach will enter their own phone number and hear a computer voice list the first name(s) of their assigned participants. The coach will choose the participant by following the voice direction. Then the call is placed, while the coach is put on ‘hold’ briefly, hearing a message asking them to wait while the system seeks to get their partner on the telephone. If the participant does not answer the phone, there are options for the person who answers the call to designate that the peer is available and will come to the phone or that the peer is not available and cannot come to the phone. When both parties are on the line, they will then be connected.
Research staff will monitor the calling process via a password-protected Internet web site. Through the monitoring system, we will be able to identify when calls occurred, who placed each call, and how long participants talked. All calling process data will be stored in a format accessible for statistical analysis as part of our process evaluation. Though often after an initial period in our prior peer support interventions, participants and peer coaches have exchanged phone numbers, bypassing the system, peer coaches who complete 2–4 phone calls each month to a peer participant documented via the IVR system receive increased stipend amounts and thus will have an incentive to use the computer system.
Measures and Data Collection
Overview
Measurement will occur at baseline, 6 months (at the completion of the intervention), and 12 months after enrollment. At these time points participants and peer mentors will complete a written survey and assessments of A1c and blood pressure. Participants will receive $20 for each assessment they complete. To understand features of successful vs. less successful peer partnerships, we will conduct semi-structured interviews at 6 months of a purposive sample of participants and mentors in both study arms (choosing a sample with high rates of contact and a sample with low rates of contact). Using standard qualitative methods for identifying key themes in participant’s experiences with this new program, we will identify central themes from these interviews associated with the success of the peer relationships and in meeting intervention goals. Participants and peer mentors in the peer mentor +iDecide arm will be asked about their experiences with iDecide and how use of the program affected their subsequent contacts and activities (e.g., setting and meeting ‘action plan’ goals, effectively raising questions and concerns with PCPs). Quantitative information about use of the Compute telephone system will be collected via computerized records that the system maintains of all contacts with dates and time durations of all contacts as well as of attempted contacts.
Description of Measures by Aim
Aim 1: Test the effectiveness of a technology-enhanced peer coaching (TEC) program in improving glucose control relative to peer support alone
At baseline, 6 months, and 12 months, a trained staff member will measure patients’ A1c, using a Bayer DCA 2000+ analyzer, a portable analyzer producing A1c assays in approximately six minutes using finger stick blood samples. These assays have a test coefficient of variation (CV, measure of test precision) <5% as required by the National Diabetes Data Group and accurately measure A1c levels.41 The DCA 2000 is reliable, easy to use, and much less burdensome to patients than venous blood draws. Our team has familiarity with the DCA 2000 from our prior studies.18,32
For our “usual care” observed group, we will use electronic medical record data to measure A1c levels. More than 85% of diabetes patients who meet our eligibility criteria receive A1c testing at recommended intervals, which should ensure adequate numbers of patients with follow-up A1c levels in our control sample. For those with missing A1c data, we will use several approaches to examine possible biases.
Aim 2: Assess the impact of the intervention on blood pressure and medication adherence as well as on key patient-centered outcomes, including patients’ satisfaction and involvement with care, perceived social support, and diabetes-specific quality of life
These measures will be assessed at baseline, 6 months, and 12 months. We will measure blood pressure using an Omron® automatic blood pressure monitor with memory and take the average of the two readings following American Heart Association guidelines. (Ideally we would also measure lipid levels, but have not found sufficiently reliable point-of-service machines for these measurements. At baseline we will collect information on most recent LDL, HDL, and total cholesterol levels from electronic medical records. If there are no recent values, the iDecide program text in that section will emphasize the importance of having these risk factor levels checked.) The baseline and 6-month surveys will take approximately 25 minutes to complete, and the 12-month survey will take approximately 15 minutes. Measures for the key patient-centered outcomes are:
The Health-Care Climate Questionnaire (HCCQ) (twelve items)
The Health-Care Climate Questionnaire (HCCQ) (α=0.82) assesses patients’ perceptions of the degree to which they experience their health-care providers (or their peer mentor) to be autonomy supportive versus controlling in providing general health support or with respect to a specific health-care issue. It was originally validated in a study of patients visiting their primary-care physicians, was used in a published study of obese patients participating in a weight-loss program, has since been used in multiple studies of professional and lay health care supporters, and a version that includes six items measuring practical support for diabetes self-management has since been validated in two studies.42 We will ask participants to complete this about their PCP at baseline, six-months, and 12-months. Participants will complete this assessment about their peer mentor at six months.
Involvement in Care Decisions (five items)
To assess changes in how involved patients feel they are in diabetes goal and treatment decision-making with their health care providers, we will use the goal-setting subscale of the Patient Assessment of Chronic Illness Care (PACIC).43
Diabetes-Specific Social Support (twelve items)
To assess perceived diabetes-specific support we will use the Diabetes Support Scale (DSS), a 12-item scale developed to assess whether an Internet-based diabetes support group changed participants’ perceptions of social support, social network size, or actual provision of support (α> 0.90). The instrument correlates with illness intrusiveness and self-care behaviors and is responsive to intervention effects.44
Diabetes-Specific Distress (two items)
This instrument captures the patient’s perspective on the emotional burden of diabetes and its treatment.45 It has consistently high internal reliability with a Cronbach’s α of 0.90 and good convergent validity with a measure of general psychological distress (r=.63). It is also associated with changes in A1c and other outcomes over time. In a review of seven effective diabetes intervention studies, mean scores improved from baseline to follow up in all studies.46
Aim 3: Identify patient characteristics associated with engagement in the intervention and mediators and moderators of the intervention’s impact on patient outcomes
Table 2 presents the measures we will use to assess factors we hypothesize may be associated with willingness to engage in the intervention (which may also be moderators) and mediators and moderators of intervention effects as well as time points at which we will measure each of these. We include measures found to be significant mediators and moderators of effects in our P2P RCT.18,47
Table 2.
| Mediators | Time Points* | Original Source |
|---|---|---|
| Medication-taking Self-efficacy | 1, 2, 3 | Perceived Competence Scale48 |
| Autonomous Motivation | 1, 2, 3 | Treatment Self-Regulation Questionnaire (TSRQ)49 |
| Medication adherence | 1, 2, 3 | Morisky Medication Adherence Measure50 |
| Assessment of level and type of support received by Peer coach | 2, 3 | Process measures used in P2P and by Robert Wood Johnson Foundation Diabetes Initiative18 |
| Self-efficacy for shared decision-making with providers | 1, 2, 3 | Self-efficacy for Decision-making scale51 |
| Medication doses and insulin starts | 1, 2, 3 | Survey items and electronic medical records |
| Moderators/Engagement Factors | ||
| Race | 1 | 2010 US Census measures |
| Preferred and actual decision-making with providers | 1 | Preferred and Actual Participation in Decision-making Scales52 |
| Attachment Style | 1 | Relationship Questionnaire53 |
| Perceived Need for DM self-management support | 1 | Support Needs Scale from Diabetes Care Profile54 |
| Perceived Benefits and Barriers to Taking DM medications | 1 | Two subscales from instrument assessing perceived barriers to and benefits from taking diabetes medications based on health beliefs model55 |
| Health Literacy | 1 | Functional Health Literacy Screener7 |
| Numeracy | 1 | Subjective Numeracy Scale (SNS)56 |
| Food insecurity | 1 | Food Security Survey Module57 |
| Neighborhood Safety, Availability of Healthy Foods, and Walking Environment | 1 | Neighborhood Safety Measure, Availability of Healthy Foods, Walking Environment58 |
Time point 1 is baseline, 2 is at 6 months, 3 is at 12 months.
We will measure standard demographic information including age, gender, and marital status. Socio-economic status (SES) will be measured using highest grade in school completed, income, and household size. We will collect data on diabetes duration and whether all diabetes care is received at VA and distance Veteran travels to Detroit VA. We will also collect data on any adverse symptoms experienced during the study period such as hypoglycemic symptoms or episodes, although have had no hypoglycemic episodes in our prior peer support interventions. Data on diabetes-related complications and other co-morbidities will be drawn from EMRs.
Analyses
Quality Control and Data Management
To ensure consistent delivery of the intervention and uniform application of patient enrollment and data collection protocols, we will host a “kick-off” meeting in Ann Arbor during Year 1 for all investigators and research associates. We will discuss study goals, design and specific procedures for patient enrollment, survey data collection, collection of physiologic data, and documentation. After the meeting, team members will meet with clinicians to inform them about the study and solicit their support. Descriptions of the study will be produced in a variety of formats for distribution via group emails and as handouts in clinic. The Project Manager and Dr. Heisler will be in weekly contact with the Detroit site investigator and research associates to ensure that the study protocol is applied consistently. From prior studies, we are familiar with procedures for managing the electronic data within a randomized trial. Data quality issues will be discussed at weekly meetings between the Study Team, Project Manager and Dr. Heisler. A coding decisions log will be maintained so that coding will be consistent and any necessary changes to the data collection tools can be discussed and documented. All survey data collection will be completed on a computer, requiring item completion and drop down choices. All data will be checked electronically for out-of-range values and logical consistency across variables.
Unit of Analysis and Sample Size Calculation
Patients within pairs (peer-peer coach dyads) will be the primary unit of analysis for the study. Thus, we calculated the sample size to adjust for the likely correlation between members of the pairs (intraclass correlation (ICC), or rho) in the intervention group. Accordingly, we calculated the sample size to provide 80% power to detect a difference between experimental groups of 0.5% in A1c with an alpha of .05, two-tailed.59 We conducted the power analysis using the methods of Cohen and, based on the within-pair ICC of our prior reciprocal peer support intervention, adjusted for a within-pair intraclass correlation of 0.03 as implemented in STATA 12 software.60 We estimated the standard deviation of a decline in A1c in this population (1.45) using data from our prior randomized controlled trials.5,18 To provide the needed power, 126 subjects will be needed in each group (after attrition). To conservatively allow for up to 15% attrition, a rate higher than occurred in any of our prior VA diabetes RCTs, we will recruit 145 patients for each arm, for a total of 290 patients. The estimate of the standard error of the change in BP was estimated from a database of actual BPs obtained in routine clinical practice for 24,000 patients with diabetes and hypertension in one large service network in the VA over a 24-month period in FY 2004–2005. It depends on both the variation in BP change at the person level and variability within person between measurements. We estimated the standard error for the change in blood pressure as 17 mmHg. Our target sample size will provide 80% power to detect a difference between experimental groups of 6 mmHg and differences between each of our key patient-centered outcome measures.
Approach to All Analyses
We will follow international guidelines for analysis and reporting of clinical trials.59 In the first phase (data verification), we examined the distribution of all study variables to assess extreme values, missing data, variances, possible coding errors, skewness, and type of distribution. We have now examined baseline data for clinically important differences across the two study groups for potential prognostic indicators, such as patients’ age, race, comorbidities, and baseline use of services.(See Table 3 below) Differences between experimental arms in baseline characteristics will be included as covariates in analyses comparing outcomes.
Table 3.
| Characteristic | Control mean (SD) |
Intervention mean (SD) |
Observational Control mean (SD) |
p-value | |
|---|---|---|---|---|---|
| N | 144 | 146 | 180 | ||
| Age | 62.1 (10.5) | 64.3 (9.7) | 65.0 (8.4) | 0.025 | |
| Male | 142 (98.6%) | 141 (96.6%) | 175 (97.2%) | 0.53 | |
| African American | 87 (60.4%) | 90 (61.6%) | 0.83 | ||
| Latino | 2 (1.4%) | 7 (4.8%) | 0.099 | ||
| Work Status | Employed | 39 (27.1%) | 35 (24.5%) | 0.84 | |
| Not Employed | 26 (18.1%) | 23 (16.1%) | |||
| Retired | 69 (47.9%) | 72 (50.3%) | |||
| Disabled | 10 (6.9%) | 13 (9.1%) | |||
| Education Level | < HS | 8 (5.6%) | 4 (2.7%) | 0.45 | |
| HS Grad | 42 (29.2%) | 36 (24.7%) | |||
| Some Tech or vocational | 10 (6.9%) | 13 (8.9%) | |||
| Some college or more | 84 (58.3%) | 93 (63.7%) | |||
| Number of People in Household | 1 | 37 (25.7%) | 50 (34.2%) | 0.14 | |
| 2 | 59 (41.0%) | 62 (42.5%) | |||
| 3–5 | 40 (27.8%) | 31 (21.2%) | |||
| 6 or more | 8 (5.6%) | 3 (2.1%) | |||
| Income | $0– $15,000 | 31 (21.5%) | 30 (20.7%) | 0.93 | |
| $16,000–$30,000 | 37 (25.7%) | 44 (30.3%) | |||
| $31,000– $55,000 | 31 (21.5%) | 28 (19.3%) | |||
| $56,000 and above | 23 (16.0%) | 23 (15.9%) | |||
| Prefer not to disclose | 22 (15.3%) | 20 (13.8%) | |||
| # of years with diabetes | 15.3 (9.9) | 15.0 (10.2) | 0.82 | ||
| Self-Rated Health Fair or Poor | 64 (44.4%) | 65 (44.5%) | 0.99 | ||
| Baseline A1c | 9.6 (1.5) | 9.5 (1.5) | 9.2 (1.5) | 0.14 | |
| Baseline Total Cholesterol | 164.6 (51.3) | 164.2 (47.5) | 159.4 (40.0) | 0.51 | |
| Baseline HDL | 41.9 (13.4) | 45.1 (12.8) | 41.3 (10.7) | 0.016 | |
| Baseline LDL | 83.7 (35.9) | 87.1 (35.4) | 85.9 (34.0) | 0.71 | |
| On statin | 99 (68.8%) | 88 (60.3%) | 113 (62.8%) | 0.30 | |
| On insulin | 88 (61.1%) | 79 (54.1%) | 104 (57.8%) | 0.48 | |
| # Oral Anti hyperglycemic meds | 1.0 (0.8) | 1.0 (0.8) | 0.9 (0.8) | 0.81 |
In the second phase of our outcomes analyses, we will evaluate possible bivariate associations between patients’ experimental condition and the outcomes, as well as between each covariate and the outcomes, prior to fitting multivariable models. This will be done to determine unadjusted measures of effect, assess possible confounders, and anticipate any collinearity in subsequent analyses. In the final phase we will fit multivariable models to identify main effects.
Analysis Plans by Aim
Aim 1: Test the effectiveness of a technology-enhanced peer coaching (TEC) program in improving glucose control relative to peer support alone
Our past experience in similar patient populations suggests that the primary endpoint from the trial (change in A1c) will be close to normally distributed. To assess the primary endpoint for Specific Aim 1 (change in mean HbA1c from baseline to 6 months), we will use a general linear mixed regression model:
where i represents the patient, l represents the intervention, j is the pair-group, βl are parameters estimated from the data, Xil is the value of the lth fixed effect (peer support versus usual care) for the ith patient, bj are parameters estimated from the data, Zij is the value of the jth random effect (pairs) for the ith patient, and εi is the residual error.61 We anticipate that members of peer coach-peer partner pairs in both arms, because of their interactions with each other, might show a positive intraclass correlation (ICC), a component of the variance attributable to the group. As recommended for group-randomized trials, the mixed model analyses will thus address potentially inflated type I errors that could occur if such clustering were not taken into account.62 If patients in either arm drop out of the study or request reassignment to another peer coach, they will be analyzed according to their initial pairing in an intent-to-treat analysis.
After unadjusted changes in A1c are determined, further analyses using mixed-model ANCOVA will adjust for confounding effects of any variables that differed substantially between treatment arms. Both unadjusted and adjusted means with 95% confidence intervals will be reported for both arms. While the primary endpoint is the mean difference between baseline and 6-month A1c concentrations, subsequent analyses will be conducted to determine whether the intervention additionally affects the difference between baseline and 12-month A1c (i.e., the sustainability of any treatment effects) using a repeated-measures mixed model ANCOVA.
We will follow the same approach to comparing the observed usual care group with the peer support alone group. However, there is a higher likelihood of imbalances as the usual care group is observational, although they are drawn from the same eligibility pool as the participants. Therefore, we will include several key covariates in the analysis, including baseline A1c, age, gender, and race. We will also examine the data for differences in other potential prognostic indicators, such as baseline medication or service use, and include these variables in the regression if necessary. We will also need to examine whether biases may be caused by missing A1c data. Although the very high level of A1c testing in VHA is likely to lead to few missing data points, we will conduct sensitivity analyses using two approaches. In the first, we will assume that those with missing A1c levels at the end of the study had no improvement in their A1cs. In the second, we will use multiple imputation methods to fill in missing A1c values.
Aim 2: Assess the impact of the intervention on blood pressure and medication adherence as well as on key patient-centered outcomes, including patients’ satisfaction and involvement with care, perceived social support, and diabetes-specific quality of life
For assessment of changes in systolic blood pressure and for the self-reported outcomes, we will use mixed effects models (similar to those in Aims 1) for continuous outcomes, and generalized estimating equations (GEE) for ordinal outcomes with clustering.63
Aim 3: Identify patient characteristics associated with engagement in the intervention and mediators and moderators of the intervention’s impact on patient outcomes
For these analyses, we will use multivariate modeling and path analyses.64 Many of these outcomes will be measured using Likert scales. Thus, we will begin these analyses by developing contingency tables for ordered categorical data. We will then use generalized estimating equations (GEE), which are appropriate for modeling ordinal outcomes with correlated data.63
We will use both quantitative and qualitative methods. We will compare patient characteristics and attitudes of participants and those not willing to participate in the study. Eligible refusers will be asked whether they would consent to a brief survey in which we will record information helpful in assessing the intervention’s reach, such as diabetes distress, perceived need for support, interpersonal attachment styles, reasons for not enrolling, and existing sources of social support.
We will model independent associations and pathways linking intervention exposure to outcomes using nested multivariate regression. Subsequent nested models will introduce potential mediators, and we will evaluate changes in the magnitude of the relationship between experimental condition and outcomes before and after the covariates are introduced. Analyses of potential moderators will use standard approaches to evaluate potential interactions between these covariates and patients’ experimental condition.64 Independent variables and moderators will be centered before testing interactions, so that multicollinearity between first order and higher-order terms will be minimized. Statistically significant interactions will be interpreted by plotting regression lines for high and low values of the moderator variable. Stata routines greatly facilitate the plotting of these relationships.65
To gain more in-depth understanding of factors associated with level of engagement in the intervention and with outcomes, along with examining baseline correlates of different levels of engagement using quantitative approaches with the baseline survey and clinical data, we will conduct and analyze semi-structured interviews conducted at the 6-month assessment. The use of the computer telephone platform will enable us to categorize patient-mentor dyads into different levels of frequency of contacts and duration of contacts (“engagement”). The system records data on dates of all completed telephone contacts and duration of each telephone call into a data set in separate data fields. It is thus easy to examine the ‘dose’ of intervention engagement of each dyad, depending on how frequently and how long they spoke together. We will conduct semi-structured interviews with purposive samples of peer mentors and participants with different levels of intervention engagement (low, medium, and high). We will perform a thematic analysis of the interview data with participants with different levels of engagement in the intervention (in both arms). Our overall approach to thematic analysis will be what Miller and Crabtree refer to as the “Editing Analysis Style,” which contains both deductive and inductive elements. Following this approach, two investigators will independently read interview transcripts, break down patient interview responses into individual segments that express a single idea or theme (e.g., particular ways respondents found the telephone calls useful or not useful) and label these phrases with appropriate codes. An iterative process will be used to compare results until agreement is reached on the categories and criteria for inclusion.66 We will seek to examine in more depth factors contributing both to successful and unsuccessful peer mentor-partner matches.
Approach to Missing Data
Clinical trial analyses often are limited to patients with complete data. However, even in cases with low rates of attrition such as our prior VA diabetes RCTs, this strategy may yield overly optimistic effect size estimates. Problems adhering to the protocol or worse health status often are associated with missing data. Although we will conduct an initial analysis using only observed data, we will conduct a second analysis that imputes missing data. We will impute missing data using the method described by Lavori, Dawson, and Shera.67 This uses logistic regression to model patients’ likelihood of having outcome data and define strata within which outcome values are missing at random. We will then stratify patients according to these propensities and randomly sample from the observed outcome distribution and impute these values for missing data within each stratum. When data are missing for items within scales, we will use recommended imputation procedures rather than deleting patients list-wise from the analysis.68 In addition, we will compare dropout rates in each arm, using the chi-square tests and compare subjects with complete follow-up to those with missing data with respect to observed baseline characteristics. Finally, we will repeat analyses assuming that all subjects with incomplete data had no improvements in their baseline A1cs as a conservative approach.
Limitations of Study Design
This study design seeks to address the principal aim of determining whether adding a tailored, interactive tool to a peer mentor intervention improves diabetes clinical outcomes significantly more than a peer mentor intervention alone. Because this will be the only difference between our two study arms, this design addresses a key limitation of many diabetes self-management interventions that are often multi-factorial and thus make it impossible to separate out the independent effects of the different intervention components. Because developing web-based, tailored tools is an expensive enterprise, it is important to ensure that an investment in such e-health tools indeed provides a significant additional clinical benefit to patients. However, our study design still faces two principal limitations. First, the ideal study design would be a 4-armed randomized trial with one arm receiving usual care and another arm receiving an iDecide session alone as well as the two arms we include in our study. However, our main study aim is to determine the incremental benefit of adding a tailored tool to an intervention that we had in prior RCTs found to lead to A1c improvements of approximately 1% lower in A1c percentage points than nurse care management and usual care. Accordingly, we powered this study to detect a 0.6% difference in A1c between our two study arms. A 0.6% difference is a clinically significant difference, but requires a considerably larger sample size than detecting a 1% difference. A 4-armed RCT would require an even larger sample and would be beyond available resources. Moreover, since our secondary question is whether we can replicate our prior RCT findings of significantly greater A1c lowering through peer support than through usual care, we believe we can adequately compare differences in A1c over the study period between our intervention arms and an observational cohort who do not receive the intervention. A second limitation is that our proactive identification of patients to recruit requires that patients have had an assessment of A1c within our enrollment period of 24 months. Thus, vulnerable patients who have not had a VA provider appointment with an A1c level drawn over the 24 months during which eligible A1cs will be determined will not be captured. Such patients who fall out of clinical care for extended periods are often among the highest risk patients.
Another potential limitation of all randomized controlled trials face is missing data. We have described how we will address missing data analytically. However, because the principal cause of missing data is dropout, we have instituted a number of measures that have successfully worked in our prior VA RCTs to prevent differential dropouts among participants in the study arms: 1. Participants in both arms will be receiving equivalent amounts of attention as both arms will receive peer mentor calls; 2. We will emphasize to any participants who no longer want to receive peer mentor calls that they will still receive incentive payments for completing follow-up assessments at 6 and 12 months, and prioritize assessments if they are only willing to complete some components of the assessments (e.g., just checking their A1c level if they are only willing to complete one thing, and ordering survey questions in order of priority if they only want to answer some survey questions; 3. We will send personalized mail reminders and telephone participants for their follow-up assessments, with at least 6 attempts made at different times to reach each participant; 4. We will fill in missing A1c and BP values with values from the electronic medical record if they are within +/− 30 days of their scheduled assessment.
Trial Status
Recruitment began in September 2014 and was completed September 2016. The 6 month intervention period will end in March 2017 at which time analysis of 6 month outcomes will begin.
Conclusion
Because diabetes is so prevalent and the self-management behaviors necessary for successful glucose control are so challenging, finding effective interventions is a critical public health issue. Providers and patients face numerous barriers and new resources must be found. This protocol details the design, delivery and analysis plan of an RCT aimed at testing an innovative program coupling peer support with decision support tools, interventions that have both been associated with improved outcomes for diabetes patients.
Acknowledgments
Funding: This work was supported by the Veteran’s Administration Health Services Research and Development: grant number IIR 12–412
Footnotes
Trial Registration: The ClinicalTrials.gov registration number is NCT01855399.
IRB Approval: November 14, 2013; Central Veteran’s Administration IRB # 13-35
References
- 1.Department of Veterans Affairs. [Accessed June 29, 2011];VHA Office of Quality and Performance Report. http://vaww.pdw.med.va.gov/MeasureMaster/MMReport.asp.
- 2.Gallant MP. The influence of social support on chronic illness self-management: a review and directions for research. Health Educ Behav. 2003;30(2):170–195. doi: 10.1177/1090198102251030. [DOI] [PubMed] [Google Scholar]
- 3.CDC. Health Disparities Experienced by black or African Americans - United States. 2005 [PubMed] [Google Scholar]
- 4.Heisler M, Smith DM, Hayward RA, Krein SL, Kerr EA. Racial disparities in diabetes care processes, outcomes, and treatment intensity. Med Care. 2003;41(11):1221–1232. doi: 10.1097/01.MLR.0000093421.64618.9C. [DOI] [PubMed] [Google Scholar]
- 5.Long JA, Jahnle EC, Richardson DM, Loewenstein G, Volpp KG. Peer mentoring and financial incentives to improve glucose control in African American veterans: a randomized trial. Ann Intern Med. 2012;156(6):416–424. doi: 10.1059/0003-4819-156-6-201203200-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spencer MS, Kieffer EC, Sinco B, Palmisano G, Guzman JR, James SA, Graddy-Dansby G, Two Feathers J, Heisler M. Diabetes-Specific Emotional Distress among African Americans and Hispanics with Type 2 Diabetes. J Health Care Poor Underserved. 2006;17(2):88–106. doi: 10.1353/hpu.2006.0095. [DOI] [PubMed] [Google Scholar]
- 7.Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36(8):588–594. [PubMed] [Google Scholar]
- 8.Osborn CY, Cavanaugh K, Wallston KA, White RO, Rothman RL. Diabetes numeracy: an overlooked factor in understanding racial disparities in glycemic control. Diabetes Care. 2009;32(9):1614–1619. doi: 10.2337/dc09-0425. 2732142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schillinger D, Barton LR, Karter AJ, Wang F, Adler N. Does literacy mediate the relationship between education and health outcomes? A study of a low-income population with diabetes. Public Health Rep. 2006;121(3):245–254. doi: 10.1177/003335490612100305. 1525295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golin C, DiMatteo MR, Duan N, Leake B, Gelberg L. Impoverished diabetic patients whose doctors facilitate their participation in medical decision making are more satisfied with their care. J Gen Intern Med. 2002;17(11):866–875. doi: 10.1046/j.1525-1497.2002.20120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosen P, Anell A, Hjortsberg C. Patient views on choice and participation in primary health care. Health Policy. 2001;55(2):121–128. doi: 10.1016/s0168-8510(00)00122-6. [DOI] [PubMed] [Google Scholar]
- 12.Cooper LA, Roter DL, Johnson RL, Ford DE, Steinwachs DM. Patient-centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med. 2003;139:907–953. doi: 10.7326/0003-4819-139-11-200312020-00009. [DOI] [PubMed] [Google Scholar]
- 13.Frosch DL, May SG, Rendle KA, Tietbohl C, Elwyn G. Authoritarian physicians and patients’ fear of being labeled ‘difficult’ among key obstacles to shared decision making. Health Aff (Millwood) 2012;31(5):1030–1038. doi: 10.1377/hlthaff.2011.0576. [DOI] [PubMed] [Google Scholar]
- 14.Peek ME, Quinn MT, Gorawara-Bhat R, Odoms-Young A, Wilson SC, Chin MH. How is shared decision-making defined among African-Americans with diabetes? Patient Educ Couns. 2008;72(3):450–458. doi: 10.1016/j.pec.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heisler M, Bouknight RR, Hayward RA, Smith DM, Kerr EA. The relative importance of physician communication, participatory decision making, and patient understanding in diabetes self-management. J Gen Intern Med. 2002;17(4):243–252. doi: 10.1046/j.1525-1497.2002.10905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piette JD, Schillinger D, Potter MB, Heisler M. Dimensions of patient-provider communication and diabetes self-care in an ethnically diverse population. J Gen Intern Med. 2003;18(8):624–633. doi: 10.1046/j.1525-1497.2003.31968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glasgow RE, Toobert DJ. Social environment and regimen adherence among type II diabetic patients. Diabetes Care. 1988;11(5):377–386. doi: 10.2337/diacare.11.5.377. [DOI] [PubMed] [Google Scholar]
- 18.Heisler M, Vijan S, Makki F, Piette JD. Diabetes control with reciprocal peer support versus nurse care management: a randomized trial. Ann Intern Med. 2010;153(8):507–515. doi: 10.7326/0003-4819-153-8-201010190-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghorob A, Vivas MM, De Vore D, Ngo V, Bodenheimer T, Chen E, Thom DH. The effectiveness of peer health coaching in improving glycemic control among low-income patients with diabetes: protocol for a randomized controlled trial. BMC public health. 2011;11:208. doi: 10.1186/1471-2458-11-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thom D, Bodenheimer T, Chen E, Hessler D, De Vore D, Ghorob A. Peer health coaching improves glycemic in low-income patients with diabetes: a randomized controlled trial. doi: 10.1370/afm.1443. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dennis CL. Peer support within a health care context: a concept analysis. International journal of nursing studies. 2003;40(3):321–332. doi: 10.1016/s0020-7489(02)00092-5. [DOI] [PubMed] [Google Scholar]
- 22.Colon Y. Telephone support groups: a nontraditional approach to reaching underserved cancer patients. Cancer Pract. 1996;4(3):156–159. [PubMed] [Google Scholar]
- 23.Hawkins RP, Kreuter M, Resnicow K, Fishbein M, Dijkstra A. Understanding tailoring in communicating about health. Health Educ Res. 2008;23(3):454–466. doi: 10.1093/her/cyn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chua HF, Liberzon I, Welsh RC, Strecher VJ. Neural correlates of message tailoring and self-relatedness in smoking cessation programming. Biol Psychiatry. 2009;65(2):165–168. doi: 10.1016/j.biopsych.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mullan RJ, Montori VM, Shah ND, Christianson TJ, Bryant SC, Guyatt GH, Perestelo-Perez LI, Stroebel RJ, Yawn BP, Yapuncich V, Breslin MA, Pencille L, Smith SA. The diabetes mellitus medication choice decision aid: a randomized trial. Arch Intern Med. 2009;169(17):1560–1568. doi: 10.1001/archinternmed.2009.293. [DOI] [PubMed] [Google Scholar]
- 26.Wallace AS, Seligman HK, Davis TC, Schillinger D, Arnold CL, Bryant-Shilliday B, Freburger JK, DeWalt DA. Literacy-appropriate educational materials and brief counseling improve diabetes self-management. Patient Educ Couns. 2009;75(3):328–333. doi: 10.1016/j.pec.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibbons MC, Wilson RF, Samal L, Lehmann CU, Dickersin K, Lehmann HP, Aboumatar H, Finkelstein J, Shelton E, Sharma R, Bass EB. AHRQ. 2009. Impact of consumer health informatics applications. Evidence report/Technology assessment No. 188. [PMC free article] [PubMed] [Google Scholar]
- 28.Shojania KG, Ranji SR, McDonald KM, Grimshaw JM, Sundaram V, Rushakoff RJ, Owens DK. Effects of Quality Improvement Strategies for Type 2 Diabetes on Glycemic Control: A Meta-Regression Analysis. JAMA. 2006;296(4):427–440. doi: 10.1001/jama.296.4.427. [DOI] [PubMed] [Google Scholar]
- 29.Glasgow RE, McKay HG, Piette JD, Reynolds KD. The RE-AIM framework for evaluating interventions: what can it tell us about approaches to chronic illness management? Patient Ed Counsel. 2001;44(2):119–127. doi: 10.1016/s0738-3991(00)00186-5. [DOI] [PubMed] [Google Scholar]
- 30.Creswell J. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches. Thousand Oaks, CA: Sage Publications; 2003. [Google Scholar]
- 31.Donovan J, Mills N, Smith M, Brindle L, Jacoby A, Peters T, Frankel S, Neal D, Hamdy F. Quality improvement report: Improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (prostate testing for cancer and treatment) study. Commentary: presenting unbiased information to patients can be difficult. BMJ. 2002;325(7367):766–770. doi: 10.1136/bmj.325.7367.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spencer MS, Rosland AM, Kieffer EC, Sinco BR, Valerio M, Palmisano G, Anderson M, Guzman JR, Heisler M. Effectiveness of a community health worker intervention among African American and Latino adults with type 2 diabetes: a randomized controlled trial. Am J Public Health. 2011;101(12):2253–2260. doi: 10.2105/AJPH.2010.300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krein SL, Klamerus ML, Vijan S, Lee JL, Fitzgerald JT, Pawlow A, Reeves P, Hayward RA. Case management for patients with poorly controlled diabetes: a randomized trial. Amer J Med. 2004;116(11):732–739. doi: 10.1016/j.amjmed.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 34.Becker MH, Janz NK. The Health Belief Model Applied to Understanding Diabetes Regimen Compliance. Diabetes Educ. 1985:41–47. [Google Scholar]
- 35.Weinstein ND. The precaution adoption process. Health Psychol. 1988;7(4):355–386. doi: 10.1037//0278-6133.7.4.355. [DOI] [PubMed] [Google Scholar]
- 36.Bandura A. Social Foundations of Thought and Action. A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall; 1986. [Google Scholar]
- 37.Chodosh J, Morton SC, Mojica W, Maglione M, Suttorp MJ, Hilton L, Rhodes S, Shekelle P. Meta-analysis: chronic disease self-management programs for older adults. Ann Intern Med. 2005;143(6):427–438. doi: 10.7326/0003-4819-143-6-200509200-00007. [DOI] [PubMed] [Google Scholar]
- 38.Lorig KR, Ritter P, Stewart AL, Sobel DS, Brown BW, Jr, Bandura A, Gonzalez VM, Laurent DD, Holman HR. Chronic disease self-management program: 2-year health status and health care utilization outcomes. Med Care. 2001;39(11):1217–1223. doi: 10.1097/00005650-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Lorig KR, Ritter PL, Laurent DD, Plant K. Internet-based chronic disease self-management: a randomized trial. Med Care. 2006;44(11):964–971. doi: 10.1097/01.mlr.0000233678.80203.c1. [DOI] [PubMed] [Google Scholar]
- 40.Henderson VA, Barr KL, An LC, Guajardo C, Newhouse W, Mase R, Heisler M. Community-based participatory research and user-centered design in a diabetes medication information and decision tool. Prog Community Health Partnersh. 2013;7(2):171–184. doi: 10.1353/cpr.2013.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arsie MP, Marchioro L, Lapolla A, Giacchetto GF, Bordin MR, Rizzotti P, Fedele D. Evaluation of diagnostic reliability of DCA 2000 for rapid and simple monitoring of HbA1c. Acta Diabetol. 2000;37(1):1–7. doi: 10.1007/s005920070028. [DOI] [PubMed] [Google Scholar]
- 42.Gensichen J, Von Korff M, Rutter CM, Seelig MD, Ludman EJ, Lin EH, Ciechanowski P, Young BA, Wagner EH, Katon WJ. Physician support for diabetes patients and clinical outcomes. BMC public health. 2009;9:367. doi: 10.1186/1471-2458-9-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glasgow RE, Whitesides H, Nelson CC, King DK. Use of the Patient Assessment of Chronic Illness Care (PACIC) with diabetic patients: relationship to patient characteristics, receipt of care, and self-management. Diabetes Care. 2005;28(11):2655–2661. doi: 10.2337/diacare.28.11.2655. [DOI] [PubMed] [Google Scholar]
- 44.Barrera M, Jr, Glasgow RE, McKay HG, Boles SM, Feil EG. Do Internet-based support interventions change perceptions of social support?: An experimental trial of approaches for supporting diabetes self-management. Amer J Community Psychol. 2002;30(5):637–654. doi: 10.1023/A:1016369114780. [DOI] [PubMed] [Google Scholar]
- 45.Fisher L, Glasgow RE, Mullan JT, Skaff MM, Polonsky WH. Development of a brief diabetes distress screening instrument. Ann Fam Med. 2008;6(3):246–252. doi: 10.1370/afm.842. 2384991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welch G, Weinger K, Anderson B, Polonsky WH. Responsiveness of the Problem Areas In Diabetes (PAID) questionnaire. Diabet Med. 2003;20(1):69–72. doi: 10.1046/j.1464-5491.2003.00832.x. [DOI] [PubMed] [Google Scholar]
- 47.Piette JD, Resnicow K, Choi H, Heisler M. A Diabetes Peer Support Intervention That Improved Glycemic Control: Mediators and Moderators of Intervention Effectiveness. 2012 doi: 10.1177/1742395313476522. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams GC, McGregor HA, King D, Nelson CC, Glasgow RE. Variation in perceived competence, glycemic control, and patient satisfaction: relationship to autonomy support from physicians. Patient Educ Couns. 2005;57(1):39–45. doi: 10.1016/j.pec.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Williams GC, Freedman ZR, Deci EL. Supporting autonomy to motivate patients with diabetes for glucose control. Diabetes Care. 1998;21(10):1644–1651. doi: 10.2337/diacare.21.10.1644. [DOI] [PubMed] [Google Scholar]
- 50.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich) 2008;10(5):348–354. doi: 10.1111/j.1751-7176.2008.07572.x. 2562622. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Lorig A. Outcome Measures for Health Education and Other Health Care Interventions. London: Sage; 1996. [Google Scholar]
- 52.Degner LF, Sloan JA. Decision making during serious illness: what role do patients really want to play? J Clin Epidemiol. 1992;45(9):941–950. doi: 10.1016/0895-4356(92)90110-9. [DOI] [PubMed] [Google Scholar]
- 53.Ciechanowski P, Russo J, Katon W, Von Korff M, Ludman E, Lin E, Simon G, Bush T. Influence of patient attachment style on self-care and outcomes in diabetes. Psychosom Med. 2004;66(5):720–728. doi: 10.1097/01.psy.0000138125.59122.23. [DOI] [PubMed] [Google Scholar]
- 54.Fitzgerald JT, Anderson RM, Gruppen LD, Davis WK, Aman LC, Jacober SJ, Grunberger G. The reliability of the Diabetes Care Profile for African Americans. Eval Health Prof. 1998;21(1):52–65. doi: 10.1177/016327879802100103. [DOI] [PubMed] [Google Scholar]
- 55.Chao J, Nau DP, Aikens JE, Taylor SD. The mediating role of health beliefs in the relationship between depressive symptoms and medication adherence in persons with diabetes. Res Social Adm Pharm. 2005;1(4):508–525. doi: 10.1016/j.sapharm.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 56.Fagerlin A, Zikmund-Fisher BJ, Ubel PA, Jankovic A, Derry HA, Smith DM. Measuring numeracy without a math test: development of the Subjective Numeracy Scale. Med Decis Making. 2007;27(5):672–680. doi: 10.1177/0272989X07304449. [DOI] [PubMed] [Google Scholar]
- 57.USDA Economic Research Service. [Accessed November 26, 2012];U.S. Household Food Security Survey Module: Six-Item Short Form. 2012 http://www.ers.usda.gov/datafiles/Food_Security_in_the_United_States/Food_Security_Survey_Modules/short2012.pdf.
- 58.Mujahid MS, Diez Roux AV, Morenoff JD, Raghunathan T. Assessing the measurement properties of neighborhood scales: from psychometrics to ecometrics. Am J Epidemiol. 2007;165(8):858–867. doi: 10.1093/aje/kwm040. [DOI] [PubMed] [Google Scholar]
- 59.Campbell M, Grimshaw J, Steen N. Sample size calculations for cluster randomised trials. Changing Professional Practice in Europe Group (EU BIOMED II Concerted Action) J Health Serv Res Policy. 2000;5(1):12–16. doi: 10.1177/135581960000500105. [DOI] [PubMed] [Google Scholar]
- 60.StataCorp LP. Stata 12 User’s Manual. College Town, TX: Stata Corporation; 2011. [Google Scholar]
- 61.Roberts C, Roberts SA. Design and analysis of clinical trials with clustering effects due to treatment. Clin Trials. 2005;2(2):152–162. doi: 10.1191/1740774505cn076oa. [DOI] [PubMed] [Google Scholar]
- 62.Krein SL, Hofer TP, Kerr EA, Hayward RA. Whom should we profile? Examining diabetes care practice variation among primary care providers, provider groups, and health care facilities. Health Serv Res. 2002;37(5):1159–1180. doi: 10.1111/1475-6773.01102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heagerty PJ, Zeger SL. Marginal regression models for clustered ordinal measurements. J Amer Statist Assoc. 1996;91(435):1024–1036. [Google Scholar]
- 64.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7(1):83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tomz M, Wittenberg J, King G. CLARIFY: Software for Interpreting and Presenting Statistical Results. Version 2.1. Stanford University, University of Wisconsin, and Harvard University; 2003. [Google Scholar]
- 66.Mason J. Qualitative Researching. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- 67.Lavori PW, Dawson R, Shera D. A multiple imputation strategy for clinical trials with truncation of patient data. Stat Med. 1995;14(17):1913–1925. doi: 10.1002/sim.4780141707. [DOI] [PubMed] [Google Scholar]
- 68.Little RJ, Rubin DB. The analysis of social science data with missing values. Sociologic Methods and Research. 1989;18:292–326. [Google Scholar]