SUMMARY
Working memory is an essential component of human cognition. Persistent activity related to working memory has been reported in many brain areas, including the inferior temporal and prefrontal cortex [1–8]. The medial temporal lobe (MTL) contains “concept cells” that respond invariantly to specific individuals or places whether presented as images, text, or speech [9, 10]. It is unknown, however, whether the MTL also participates in working memory processes. We thus sought to determine whether human MTL neurons respond to images held in working memory. We recorded from patients with chronically intractable epilepsy as they performed a task that required them to remember three or four sequentially presented pictures across a brief delay. 48% of visually selective neurons continued to carry image-specific information after image offset, but most ceased to encode previously presented images after a subsequent presentation of a different image. However, 8% of visually selective neurons encoded previously presented images during a final maintenance period, despite presentation of further images in the intervening interval. Population activity of stimulus-selective neurons predicted behavioral outcome in terms of correct and incorrect responses. These findings indicate that the MTL is part of a brain-wide network for working memory.
RESULTS
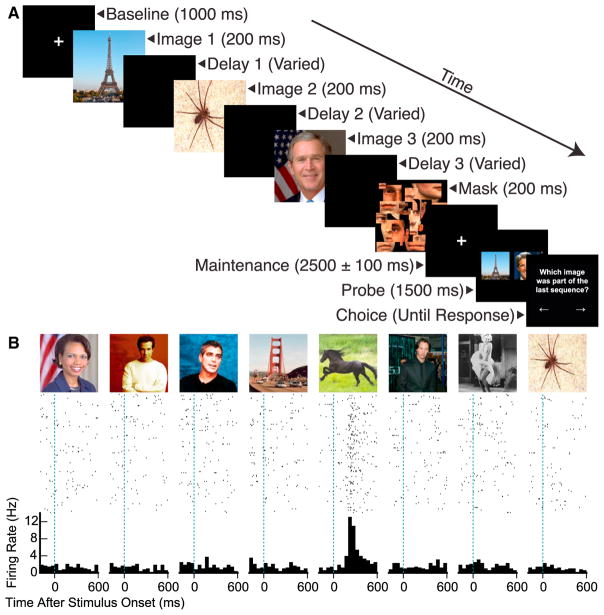
Over 41 sessions, we recorded 1,807 units (668 single units, 1,139 multi-units) from the parahippocampal cortex (PHC; 387 units), entorhinal cortex (EC; 378 units), hippocampus (518 units), and amygdala (524 units) from microwires chronically implanted in the medial temporal lobes of 18 patients undergoing treatment for pharmacologically intractable epilepsy. Subjects performed 192 or 216 trials of a modified Sternberg task, in which subjects viewed four or three pictures chosen from a pool of eight or nine, respectively, followed by a mask and a final maintenance period (Figure 1A). After this maintenance period, subjects saw two images and signaled which was present in the previous stream by a key press. The task required only that subjects remember the pictures, and not their order.
Figure 1. Experimental Design and Example Response.
(A) Behavioral task. In each trial, the subject saw a stream of four or three images, chosen from a pool of eight or nine, respectively, for 200 ms each. In three-quarters of trials, a blank screen was presented after each image, such that the inter-stimulus interval was either 200, 500, or 800 ms. In the remaining one-quarter of trials, there was no intervening blank screen. Following image presentations, subjects saw a mask followed by a fixation cross, which was presented for a minimum of 2.4 s. The fixation cross then disappeared, and subjects saw two probe pictures simultaneously, one of which had been presented in the preceding stream of images. After these probes disappeared, the subject pressed a key to signal the previously presented image.
(B) Spike rasters and peri-stimulus time histograms for a stimulus-selective single unit recorded from the right entorhinal cortex at sample presentation, including presentations at all inter-stimulus intervals. Images at the top indicate presented stimuli.
As in previous reports [9, 11–13], medial temporal lobe (MTL) neurons responded selectively to presentation of specific stimuli. 17% of units (312/1,807) were significantly modulated by the presented image (α = 0.001, permutation test of maximum Poisson likelihood ratio over time windows from 100 to 1,000 ms after stimulus onset). Figure 1B shows an example of one selective response. In line with our previous findings [13], the PHC contained a greater proportion of stimulus-selective units (25%, 96/387) compared with the EC (17%, 64/378; p = 0.009, Fisher’s exact test), the hippocampus (15%, 77/518; p = 0.0003), and the amygdala (14%, 75/524; p < 0.0001). There were no significant differences between other regions (all p > 0.3).
Responses Persist until Subsequent Image Presentation
Although subjects saw each image for 200 ms in all trials, we manipulated the blank period following image offset (inter-stimulus interval, ISI) so that it lasted 0, 200, 500, or 800 ms. We sought to determine whether neurons ceased to encode stimulus information shortly after the start of these blank periods or whether stimulus information persisted.
We first measured the latency and duration of the visual response for each image-selective unit by determining the contiguous time window that optimized the increase in likelihood for a Poisson model with different firing rates for different stimuli over a model assuming a constant mean rate. This procedure effectively determines the response onset and offset as the endpoints of the window maximizing the amount of stimulus information, assuming Poisson spiking (see the Supplemental Experimental Procedures). To ensure the accuracy of latencies and durations, we restricted this analysis to the 107 units that were selective when tested separately for each ISI (α = 0.05, permutation test of maximum Poisson likelihood ratio), although results below did not change substantially if all 312 visually selective units were included.
Among these 107 units there was no significant difference in latency between ISIs (F(3,309) = 0.8, p = 0.48, two-way repeated-measures ANOVA) but, as previously reported [13], latencies differed by region (F(3,103) = 14.6, p < 10−7). Average latencies were 198 ms in the PHC, 272 ms in the EC, 286 ms in the hippocampus, and 238 ms in the amygdala (Table S1). These latencies are earlier than those in our previous study [13]; the latency measure in the present study combines information across multiple trials, providing better latency estimates for weakly modulated units but potentially shifting estimates toward the earliest latency in any trial (see the Supplemental Experimental Procedures).
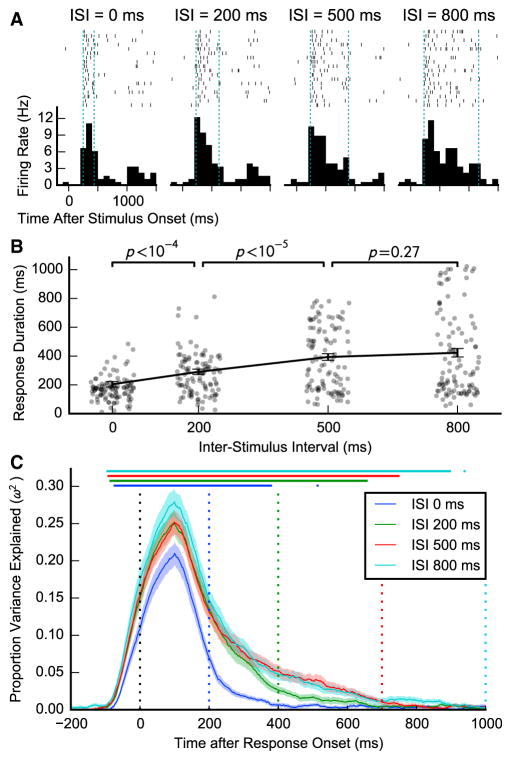
Response durations increased with ISI, suggesting that stimulus-selective activity persisted past image offset but was blocked by the presentation of the following image. A two-way ANOVA on estimated response durations revealed significant effects of both ISI (F(3,309) = 30.5, ε = 0.84, p < 10−14, two-way repeated-measures ANOVA with Greenhouse-Geisser correction) and region (F(3,103) = 6.5, p = 0.0005), but there was no significant interaction (F(9,309) = 1.3, p = 0.22). Responses were longer in the 200 ms, 500 ms, and 800 ms ISI conditions compared with the zero ISI condition (200 ms: t(106) = 4.1, p < 0.0001; 500 ms: t(106) = 7.3, p < 10−10; 800 ms: t(106) = 6.8, p < 10−9; dependent samples t test), and in the 500 ms and 800 ms conditions compared with the 200 ms condition (500 ms: t(106) = 4.9, p < 10−5; 800 ms: t(106) = 5.1, p < 10−5). There was no significant difference between the 500 ms and 800 ms ISI conditions (t(106) = 1.1, p = 0.27). Figure 2A shows a unit where response duration differed by ISI. Figure 2B shows durations by ISI for the population. Averaged across ISIs, responses were longer in the PHC and amygdala compared with the EC and hippocampus (PHC versus EC: t(63) = 4.2, p < 0.0001; PHC versus hippocampus: t(51) = 2.5, p = 0.02; amygdala versus EC: t(41) = 5.0, p < 0.0001; amygdala versus hippocampus: t(35) = 2.7, p = 0.01; unequal variance t test), but did not differ between the PHC and amygdala (t(63) = 0.05, p = 0.96) or between the EC and hippocampus (t(31) = −1.3, p = 0.22) (Table S2).
Figure 2. Activity after Stimulus Offset.
(A) Spike rasters and peri-stimulus time histograms for a single unit recorded from the right amygdala that showed increases in the duration of stimulus responses with increasing inter-stimulus interval (ISI). At ISI = 0: latency = 248 ms, duration = 188 ms; at ISI = 200 ms: latency = 230 ms, duration = 394 ms; at ISI = 500 ms: latency = 236 ms, duration = 651 ms; at ISI = 800 ms: latency = 230 ms, duration = 936 ms.
(B) Duration of image responses of 107 units with significant stimulus information computed separately for each inter-stimulus interval. Points are jittered to reveal their distribution. Error bars are ±SEM. p values indicate significance according to unequal variance t test.
(C) Debiased proportion of variance explained by image identity (partial ω2) averaged over the same 107 units shown in (A). Information was computed over all spikes in a 200 ms window centered at the corresponding time on the x axis. Colored dotted lines indicate the onset of the next picture in the corresponding ISI conditions. Bars at the top indicate significant image information at the given time point in the corresponding ISI condition (p < 0.05 corrected for all time points by permutation test of mean ω2). Error bars are ±SEM. See also Figure S1 and Tables S1 and S2.
We also investigated the persistence of responses—i.e., the extent to which neurons continued responding after image offset—in a different way, by examining the proportion of variance in firing rate explained by the stimulus in different ISI conditions (Figure 2C). We computed ω2, a debiased measure of proportion of variance explained [14], in 200 ms windows aligned to units’ latencies, averaged across the 107 units. ω2 provides an interpretable measure of information about stimulus identity without explicitly determining stimulus preferences or comparing against baseline. In the zero ISI condition, significant information was contiguously present for 379 ms after response onset (p < 0.05, permutation test of mean ω2 corrected for displayed time points). In the 200 ms ISI condition, information lasted for 656 ms after onset; in the 500 ms condition for 748 ms; and in the 800 ms condition for 895 ms. Inspecting all 312 stimulus-selective units, 48% (150) carried information from 200 to 400 ms after response onset at non-zero ISIs (α = 0.01, permutation test of Poisson likelihood ratio); 18% (55) carried information from 400 to 600 ms at ISIs over 200 ms; and 5.1% (16) carried information from 700 to 900 ms in the 800 ms ISI condition (all p < 10−6, binomial test). Computing ω2 for the interaction between stimulus and ISI revealed significant differences between 0 and 200 ms ISI conditions from 146 to 419 ms after response onset and between 200 and 500 ms conditions from 415 to 578 ms after onset (Figure S1), but not between 500 and 800 ms conditions.
Maintenance Period Activity
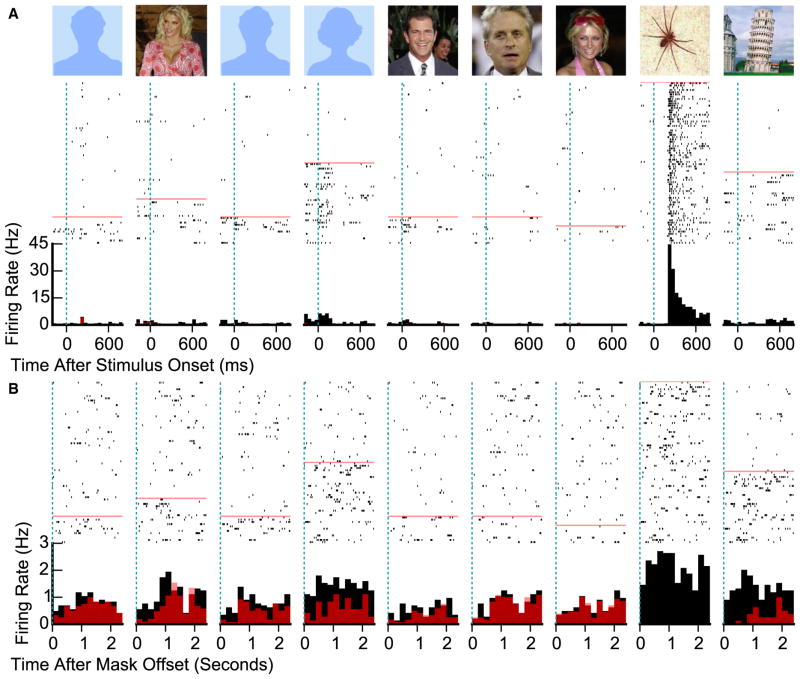
Although the blank screen between image presentations is comparable to the “delay period” in delayed match-to-sample studies (e.g., [2, 3]), our task also included a 2.4 s final maintenance period during which the subject had to hold all previously presented images in working memory. For each visually selective unit, we modeled spiking activity from 300 to 2,400 ms after the start of the maintenance period as the sum of unknown coefficients reflecting contributions of maintained stimuli. 8% of visually selective units (24/312) were modulated by stimuli held in working memory (α = 0.01, permutation test of F statistic), a substantially greater proportion than the 1% expected by chance (p < 10−13, binomial test). Of these units, 9 were in the PHC, 3 were in the EC, 3 were in the hippocampus, and 9 were in the amygdala (all p < 0.05, binomial test). Figure 3 shows one example unit; Figure S2 shows 15 additional units. Performing the same analysis over the 1,495 non-stimulus-selective units revealed only 8 significant units (p = 0.98, binomial test). Table S3 shows proportions of visually selective and maintenance-selective units separately for each subject. On average, previously presented images explained 1.1% of variance in spiking in visually selective units (ω2; bootstrap 95% confidence interval [CI] 0.6%–1.6%).
Figure 3. Maintenance Period Activity.
Spike rasters and peri-stimulus time histograms for a single unit recorded from the right amygdala that encoded a previously presented image during the maintenance period. Images at the top indicate presented stimuli, with photos of lab personnel and photos provided by the patient replaced with blue placeholders. A red line separates trials where the preferred stimulus (the spider) was not presented (top) from trials where it was (bottom). Black histograms indicate average firing rate over all trials; red histograms indicate average firing rate after removing trials that included the preferred stimulus. Responses following image presentation (A) and responses during the maintenance period (B) are shown. See also Figures S2 and S3 and Table S3.
Units selective at both presentation and maintenance generally showed similar stimulus preferences during both periods. 71% of units (17/24) were most strongly modulated during maintenance in trials where subjects were maintaining the image that was the preferred stimulus at presentation, i.e., the picture producing the greatest change in firing rate relative to baseline (chance: 12%; p < 10−10, Poisson binomial test). The average correlation between firing rate at sample presentation (between computed response onset and offset) and the maintenance period was 0.74 (inverse-Fisher-transformed mean of Fisher-transformed Pearson correlations; p < 10−7, permutation test; Figure S3A). Modulation at presentation was greater for maintenance-selective units than other visually selective units (median absolute change in spikes fired to preferred stimulus versus baseline of 1.6 versus 0.9, p = 0.02, Mann-Whitney U test), but many units were strongly modulated at presentation but non-selective during maintenance (Figure S3B).
Although only a small proportion of units showed significant stimulus-specific modulation during the maintenance period when tested individually, weaker effects were present in the remaining visually selective units as well. We determined units’ preferred stimuli on every fourth trial, and used remaining trials to compare maintenance period activity between trials when the preferred stimulus was presented and trials when it was not. For each unit, we computed maintenance period modulation as the difference in mean firing rate between these groups in units of baseline SD, adjusted for the sign of modulation by the preferred stimulus at presentation. Averaged over all visually selective units, modulation was significantly greater than zero, indicating that neurons were modulated during the maintenance period when subjects maintained their preferred stimulus in working memory (modulation = 0.09, t(311) = 6.4, p < 10−9). Modulation was significant even in visually selective units without significant maintenance period selectivity when tested individually (modulation = 0.05, t(287) = 4.9, p < 10−5). Thus, this population was also modulated during maintenance. No effect was present in the non-visually selective units (modulation = −0.002, t(1487) = −0.6, p = 0.53).
Maintenance period activity reflected previously presented images regardless of their positions in the stream. We observed significant maintenance period modulation whether the preferred stimulus was presented third to last (modulation = 0.07, t(311) = 4.7, p < 10−5), second to last (modulation = 0.08, t(311) = 5.3, p < 10−6), or last in the trial (modulation = 0.11, t(311) = 5.3, p < 10−6). Although modulation was numerically greater when the preferred stimulus was presented later in the stream, the effect of position was not significant (F(2,622) = 2.5, ε = 0.86, p = 0.09, one-way repeated-measures ANOVA with Greenhouse-Geisser correction; Figure S3C).
Relationship between Neural Activity and Behavior
Modulation by the preferred stimulus was stronger during initial stimulus presentation, the inter-stimulus interval, and the final maintenance period in trials when subjects correctly remembered the stimulus than when they did not. To inspect presentation and maintenance activity, we examined trials in which a given unit’s preferred stimulus was shown both as a sample image and at probe presentation, and compared firing rates in trials when subjects correctly selected the image to trials when subjects incorrectly selected the alternative image. Across the 274 visually selective units with at least one such incorrect trial, we recorded a median of 19 correct and 3 incorrect trials. To inspect activity during the ISI, we limited our analysis to trials with an ISI of 500 ms or longer, leaving 186 units with at least one correct and incorrect trial where the preferred stimulus was presented and probed, with a median of 9 correct and 2 incorrect trials. An insignificant proportion of units responded significantly differently between correct and incorrect trials, but significant effects are not expected at the unit level for these trial counts with small population-level effects (see the Supplemental Experimental Procedures). We thus examined the mean modulation across units, computed as the difference in firing rate between the relevant correct and incorrect trials in units of baseline SD, adjusted for the sign of the modulation by the preferred stimulus at presentation.
Across the population, the mean modulation was significantly greater than zero for the initial response (from 0 to 200 ms after response latency; modulation = 0.35, t(273) = 2.4, p = 0.01), for activity during the ISI (from 300 to 700 ms after response onset, or 100 to 500 ms after image offset + response latency; modulation = 0.26, t(185) = 2.4, p = 0.02), and during the maintenance period (modulation = 0.12, t(273) = 3.5, p = 0.0005). Among units with at least one error trial, maintenance period activity was linked to behavior in both the 20 units with significant maintenance period selectivity (t(19) = 3.6, p = 0.002) and the remaining 254 units (t(253) = 2.9, p = 0.004). Thus, even units that did not show significant maintenance period selectivity when tested individually were linked to behavior when tested together.
DISCUSSION
The role of the MTL in working memory processes has proved controversial [15–19]. Although MTL lesion patients are unimpaired in most tests of working memory [20–23], many patients with prefrontal lesions are equally unimpaired in such tests [24, 25], despite electrophysiological evidence for persistent activity [1, 5, 26]. Deficits are present in MTL lesion patients with more difficult working memory tasks [27–31], but these deficits have been attributed to the use of long-term memory by controls [19, 32, 33]. Thus, the involvement of the MTL in working memory remains unclear from lesion studies. fMRI results have proved equally difficult to interpret. Whereas some studies have reported activation of the MTL in the delay period of working memory tasks, others have not [19]. In any case, increased activity during the delay compared to baseline could reflect incidental task-related modulation rather than stimulus-specific representations. Some evidence for stimulus-specific activity comes from a study reporting greater delay period activation in the parahippocampal place area (PPA) when holding a scene versus a house in working memory [34], although another study reported differential PPA activation to scenes only in a delayed paired-associate task, and not in a delayed match-to-sample task [35].
Our study directly measured the activity of MTL neurons with stimulus-specific visual responses during working memory maintenance, in a task with no long-term memory demand. Activity reflected previously presented images even after presentation of additional images and a mask. Our results thus support the view of working memory as a distributed process [8], present in a variety of cortical and subcortical structures, including the MTL. Moreover, modulation was linked to behavior, indicating that the stimulus-selective persistent activity we report most likely arises from interactions between other components of this brain-wide network for working memory and the MTL, and may even have some causal role in task performance.
Our findings have implications for claims of “activity-silent” working memory [36, 37]. The stimulus-selective persistent activity we observed is weak and unlikely to be measurable with non-invasive techniques, but it is nonetheless highly significant. It is thus possible that, in reports of activity-silent working memory, maintenance is subserved by similarly weak activity that is invisible to the techniques used but sufficient for behavior. Manipulations such as the transcranial magnetic stimulation used in [37] could increase the strength of preexisting persistent activity, rather than reactivating a truly latent representation. Further studies are necessary to determine whether working memory is ever truly silent, or whether it is present but beyond the detection threshold of non-invasive techniques. Moreover, our finding that maintenance period modulation is higher in correct trials than incorrect trials suggests that whatever mechanisms give rise to this activity are causally involved in the task. Thus, if short-term synaptic plasticity is important to execution of this working memory task [36, 38], it must either produce persistent activity or act in concert with it to produce behavior.
The effects we observe in the human MTL resemble previous reports in macaque inferior temporal cortex (IT). Most animal studies of working memory have required maintenance of only a single, unmasked stimulus. Although some IT neurons show stimulus-selective persistent activity for up to 10 s after sample offset [2, 3] or even after the trial is complete [39], activity in the majority of neurons rapidly decays to baseline [6, 7]. We find similar effects in the MTL. However, in the IT, presentation of an occluding stimulus weakens persistent activity [7], and presentation of intervening non-match stimuli eradicates it entirely [4]. We observed stimulus-specific persistent activity in the MTL in the final maintenance period of our task, but this activity was significant in only 8% of visually selective units. Our results resemble a report of stimulus-specific activity in 6% of macaque entorhinal neurons after presentation of intervening non-match stimuli [40]. By contrast, in one of few animal studies of multiple-item working memory, 43% of neurons in macaque lateral prefrontal cortex (lPFC) showed activity related to the first image after presentation of a second image [26]. However, these lPFC neurons had strikingly different stimulus selectivity between image presentation and the second delay, whereas MTL neurons preferred the same images at presentation and maintenance.
In summary, we found that the human MTL represents stimuli held in working memory. In many neurons, information decayed slowly after image offset and dissipated shortly after the next image presentation. A small subpopulation carried information even after presentation of additional images and a mask. Population activity of stimulus-selective neurons during both encoding and the maintenance period predicted behavioral outcome in terms of correct and incorrect responses. The presence of weak but behaviorally relevant stimulus-selective persistent activity in the MTL indicates that it is part of a brain-wide network subserving working memory.
Supplementary Material
Highlights.
MTL neuronal responses decay after stimulus offset and are blocked by subsequent stimuli
MTL delay period activity reflects the contents of working memory
Activity of MTL neurons predicts successful working memory performance
The MTL is part of a brain-wide network for working memory
Acknowledgments
We thank all patients for their participation; M. Cerf, A. Kraskov, M. Ison, K. Laird, A. Postolova, N. Parikshak, and V. Isiaka for help with recordings; E. Behnke and T. Fields for technical support; and B. Samimizad for assistance with spike sorting. Research was supported by the Volkswagen Foundation (Lichtenberg Program), German Research Council (DFG MO930/4-1 and SFB 1089), US National Institute of Neurological Disorders and Stroke, G. Har-old and Leila Y. Mathers Foundation, Gimbel Discovery Fund, Dana Foundation, and Human Frontiers Science Program. Each subject provided informed written consent. All studies conformed to the guidelines of the Medical Institutional Review Board of UCLA and the Institutional Review Board of Caltech.
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, three figures, and three tables and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2017.02.013.
AUTHOR CONTRIBUTIONS
F.M., R.Q.Q., C.K., and I.F. designed the study. I.F. performed the surgeries. F.M. implemented the paradigm and collected the data. S.K. and F.M. analyzed the data. S.K. wrote the paper. All authors discussed the results and commented on the manuscript.
References
- 1.Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;173:652–654. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- 2.Fuster JM, Jervey JP. Inferotemporal neurons distinguish and retain behaviorally relevant features of visual stimuli. Science. 1981;212:952–955. doi: 10.1126/science.7233192. [DOI] [PubMed] [Google Scholar]
- 3.Miyashita Y, Chang HS. Neuronal correlate of pictorial short-term memory in the primate temporal cortex. Nature. 1988;331:68–70. doi: 10.1038/331068a0. [DOI] [PubMed] [Google Scholar]
- 4.Miller EK, Li L, Desimone R. Activity of neurons in anterior inferior temporal cortex during a short-term memory task. J Neurosci. 1993;13:1460–1478. doi: 10.1523/JNEUROSCI.13-04-01460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci. 1996;16:5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freedman DJ, Riesenhuber M, Poggio T, Miller EK. A comparison of primate prefrontal and inferior temporal cortices during visual categorization. J Neurosci. 2003;23:5235–5246. doi: 10.1523/JNEUROSCI.23-12-05235.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woloszyn L, Sheinberg DL. Neural dynamics in inferior temporal cortex during a visual working memory task. J Neurosci. 2009;29:5494–5507. doi: 10.1523/JNEUROSCI.5785-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christophel TB, Klink PC, Spitzer B, Roelfsema PR, Haynes JD. The distributed nature of working memory. Trends Cogn Sci. 2017;21:111–124. doi: 10.1016/j.tics.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Quiroga RQ, Reddy L, Kreiman G, Koch C, Fried I. Invariant visual representation by single neurons in the human brain. Nature. 2005;435:1102–1107. doi: 10.1038/nature03687. [DOI] [PubMed] [Google Scholar]
- 10.Quian Quiroga R, Kraskov A, Koch C, Fried I. Explicit encoding of multimodal percepts by single neurons in the human brain. Curr Biol. 2009;19:1308–1313. doi: 10.1016/j.cub.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fried I, MacDonald KA, Wilson CL. Single neuron activity in human hippocampus and amygdala during recognition of faces and objects. Neuron. 1997;18:753–765. doi: 10.1016/s0896-6273(00)80315-3. [DOI] [PubMed] [Google Scholar]
- 12.Kreiman G, Koch C, Fried I. Category-specific visual responses of single neurons in the human medial temporal lobe. Nat Neurosci. 2000;3:946–953. doi: 10.1038/78868. [DOI] [PubMed] [Google Scholar]
- 13.Mormann F, Kornblith S, Quiroga RQ, Kraskov A, Cerf M, Fried I, Koch C. Latency and selectivity of single neurons indicate hierarchical processing in the human medial temporal lobe. J Neurosci. 2008;28:8865–8872. doi: 10.1523/JNEUROSCI.1640-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olejnik S, Algina J. Measures of effect size for comparative studies: applications, interpretations, and limitations. Contemp Educ Psychol. 2000;25:241–286. doi: 10.1006/ceps.2000.1040. [DOI] [PubMed] [Google Scholar]
- 15.Ranganath C, Blumenfeld RS. Doubts about double dissociations between short- and long-term memory. Trends Cogn Sci. 2005;9:374–380. doi: 10.1016/j.tics.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Jonides J, Lewis RL, Nee DE, Lustig CA, Berman MG, Moore KS. The mind and brain of short-term memory. Annu Rev Psychol. 2008;59:193–224. doi: 10.1146/annurev.psych.59.103006.093615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baddeley A, Allen R, Vargha-Khadem F. Is the hippocampus necessary for visual and verbal binding in working memory? Neuropsychologia. 2010;48:1089–1095. doi: 10.1016/j.neuropsychologia.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Graham KS, Barense MD, Lee ACH. Going beyond LTM in the MTL: a synthesis of neuropsychological and neuroimaging findings on the role of the medial temporal lobe in memory and perception. Neuropsychologia. 2010;48:831–853. doi: 10.1016/j.neuropsychologia.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Jeneson A, Squire LR. Working memory, long-term memory, and medial temporal lobe function. Learn Mem. 2011;19:15–25. doi: 10.1101/lm.024018.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Penfield W, Milner B. Memory deficit produced by bilateral lesions in the hippocampal zone. AMA Arch Neurol Psychiatry. 1958;79:475–497. doi: 10.1001/archneurpsyc.1958.02340050003001. [DOI] [PubMed] [Google Scholar]
- 21.Drachman DA, Arbit J. Memory and the hippocampal complex. II Is memory a multiple process? Arch Neurol. 1966;15:52–61. doi: 10.1001/archneur.1966.00470130056005. [DOI] [PubMed] [Google Scholar]
- 22.Baddeley AD, Warrington EK. Amnesia and the distinction between long- and short-term memory. J Verbal Learn Verbal Behav. 1970;9:176–189. [Google Scholar]
- 23.Cave CB, Squire LR. Intact verbal and nonverbal short-term memory following damage to the human hippocampus. Hippocampus. 1992;2:151–163. doi: 10.1002/hipo.450020207. [DOI] [PubMed] [Google Scholar]
- 24.D’Esposito M, Postle BR. The dependence of span and delayed-response performance on prefrontal cortex. Neuropsychologia. 1999;37:1303–1315. doi: 10.1016/s0028-3932(99)00021-4. [DOI] [PubMed] [Google Scholar]
- 25.Müller NG, Machado L, Knight RT. Contributions of subregions of the prefrontal cortex to working memory: evidence from brain lesions in humans. J Cogn Neurosci. 2002;14:673–686. doi: 10.1162/08989290260138582. [DOI] [PubMed] [Google Scholar]
- 26.Warden MR, Miller EK. The representation of multiple objects in prefrontal neuronal delay activity. Cereb Cortex. 2007;17(Suppl 1):i41–i50. doi: 10.1093/cercor/bhm070. [DOI] [PubMed] [Google Scholar]
- 27.Hannula DE, Tranel D, Cohen NJ. The long and the short of it: relational memory impairments in amnesia, even at short lags. J Neurosci. 2006;26:8352–8359. doi: 10.1523/JNEUROSCI.5222-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olson IR, Page K, Moore KS, Chatterjee A, Verfaellie M. Working memory for conjunctions relies on the medial temporal lobe. J Neurosci. 2006;26:4596–4601. doi: 10.1523/JNEUROSCI.1923-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olson IR, Moore KS, Stark M, Chatterjee A. Visual working memory is impaired when the medial temporal lobe is damaged. J Cogn Neurosci. 2006;18:1087–1097. doi: 10.1162/jocn.2006.18.7.1087. [DOI] [PubMed] [Google Scholar]
- 30.Nichols EA, Kao YC, Verfaellie M, Gabrieli JDE. Working memory and long-term memory for faces: evidence from fMRI and global amnesia for involvement of the medial temporal lobes. Hippocampus. 2006;16:604–616. doi: 10.1002/hipo.20190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ezzyat Y, Olson IR. The medial temporal lobe and visual working memory: comparisons across tasks, delays, and visual similarity. Cogn Affect Behav Neurosci. 2008;8:32–40. doi: 10.3758/cabn.8.1.32. [DOI] [PubMed] [Google Scholar]
- 32.Shrager Y, Levy DA, Hopkins RO, Squire LR. Working memory and the organization of brain systems. J Neurosci. 2008;28:4818–4822. doi: 10.1523/JNEUROSCI.0710-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeneson A, Wixted JT, Hopkins RO, Squire LR. Visual working memory capacity and the medial temporal lobe. J Neurosci. 2012;32:3584–3589. doi: 10.1523/JNEUROSCI.6444-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ranganath C, DeGutis J, D’Esposito M. Category-specific modulation of inferior temporal activity during working memory encoding and maintenance. Brain Res Cogn Brain Res. 2004;20:37–45. doi: 10.1016/j.cogbrainres.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 35.Ranganath C, Cohen MX, Dam C, D’Esposito M. Inferior temporal, prefrontal, and hippocampal contributions to visual working memory maintenance and associative memory retrieval. J Neurosci. 2004;24:3917–3925. doi: 10.1523/JNEUROSCI.5053-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stokes MG. ‘Activity-silent’ working memory in prefrontal cortex: a dynamic coding framework. Trends Cogn Sci. 2015;19:394–405. doi: 10.1016/j.tics.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rose NS, LaRocque JJ, Riggall AC, Gosseries O, Starrett MJ, Meyering EE, Postle BR. Reactivation of latent working memories with transcranial magnetic stimulation. Science. 2016;354:1136–1139. doi: 10.1126/science.aah7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mongillo G, Barak O, Tsodyks M. Synaptic theory of working memory. Science. 2008;319:1543–1546. doi: 10.1126/science.1150769. [DOI] [PubMed] [Google Scholar]
- 39.Yakovlev V, Fusi S, Berman E, Zohary E. Inter-trial neuronal activity in inferior temporal cortex: a putative vehicle to generate long-term visual associations. Nat Neurosci. 1998;1:310–317. doi: 10.1038/1131. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki WA, Miller EK, Desimone R. Object and place memory in the macaque entorhinal cortex. J Neurophysiol. 1997;78:1062–1081. doi: 10.1152/jn.1997.78.2.1062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.