Abstract
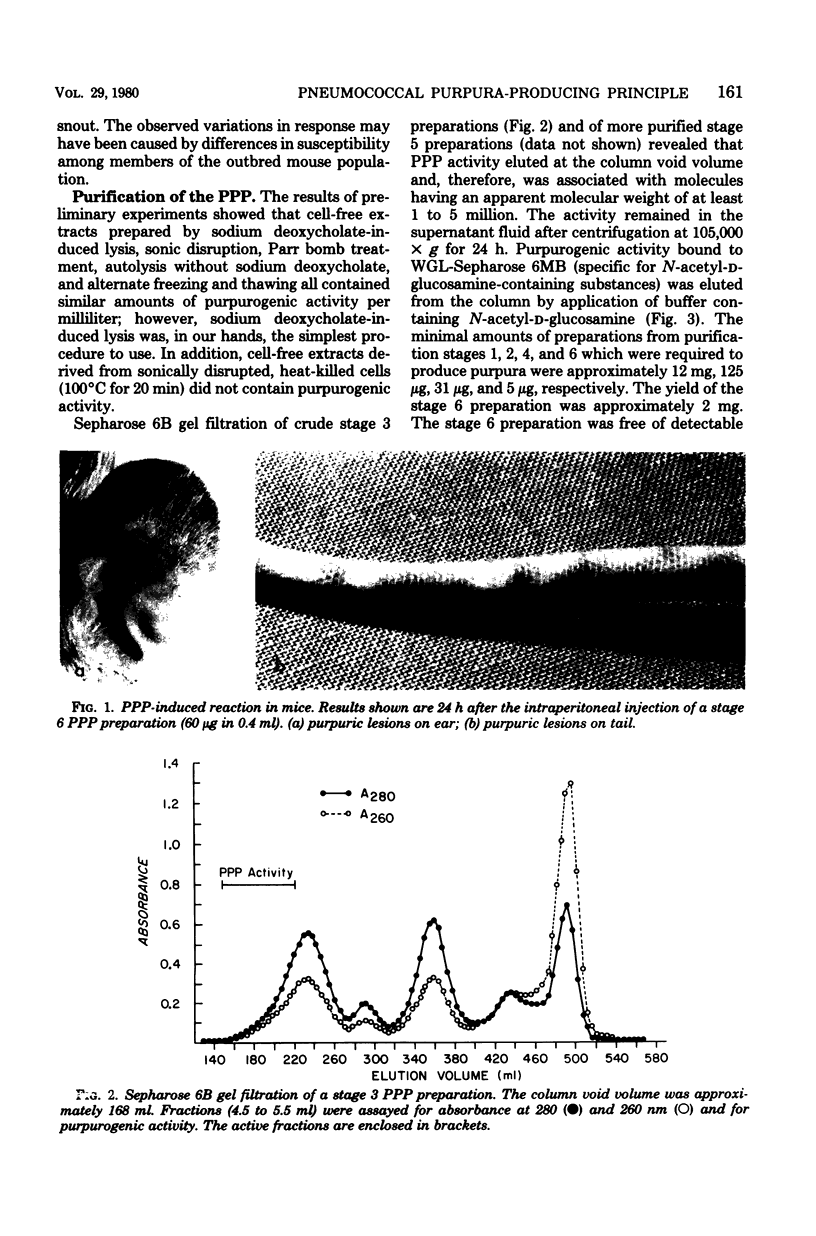
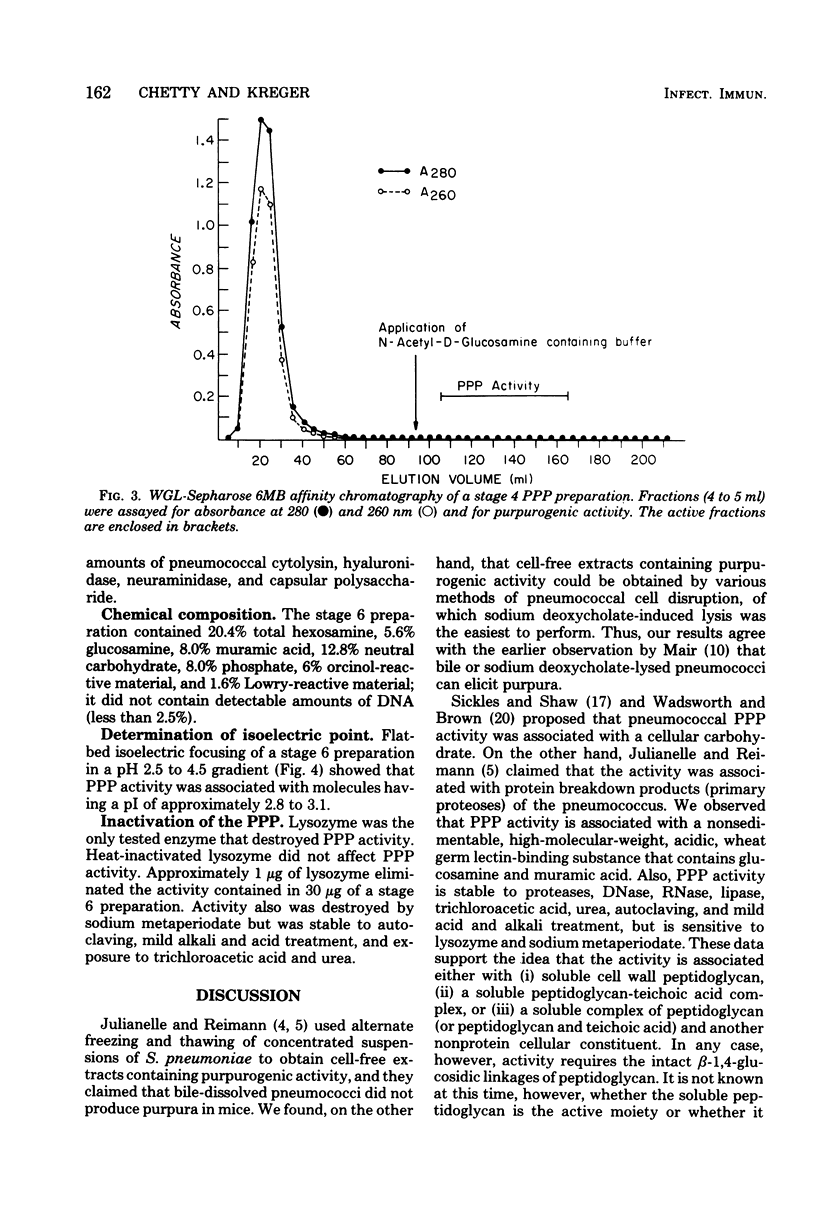
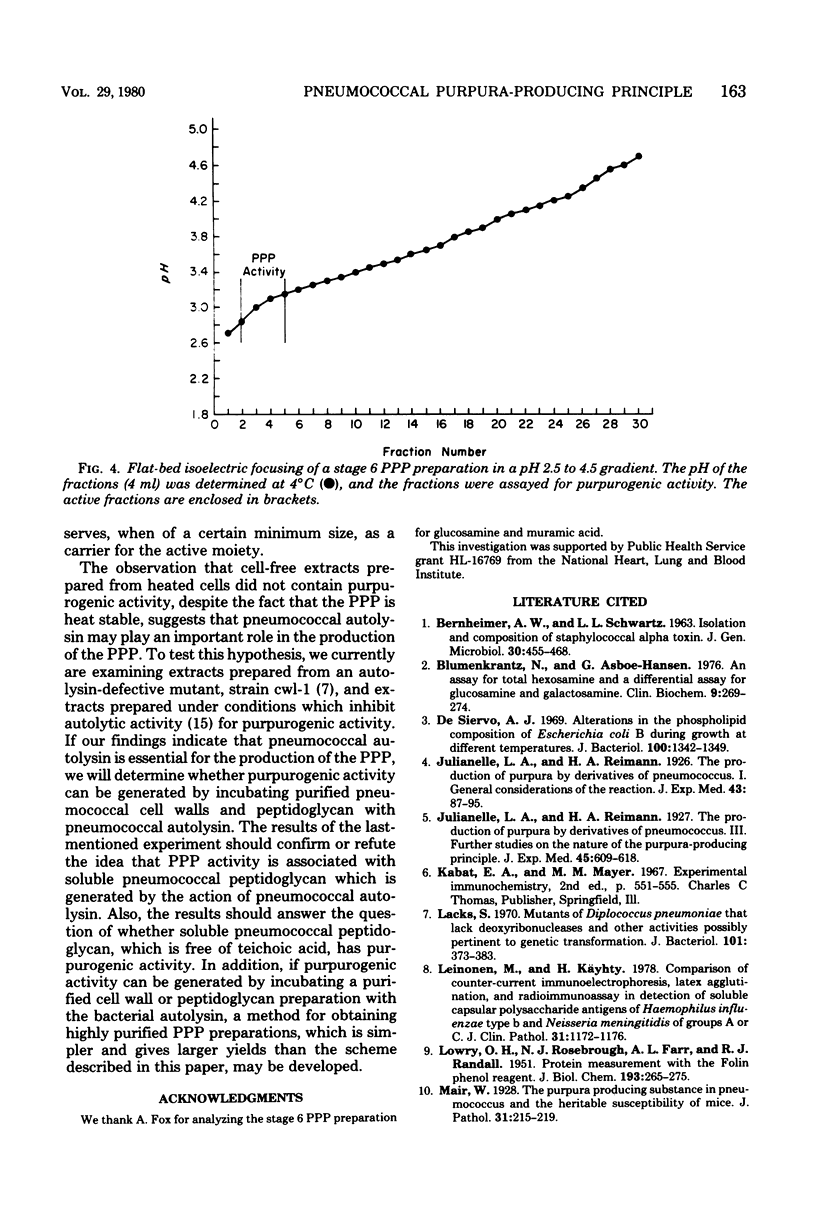
Purpura was grossly observable in albino mice 6 to 8 h after the intraperitoneal injection of sterile, deoxyribonuclease-treated, cell-free extracts prepared by sodium deoxycholate-induced lysis, sonic disruption, Parr bomb treatment, autolysis without sodium deoxycholate, or alternate freezing and thawing of washed suspensions of Streptococcus pneumoniae type I. Cell-free extracts obtained from sonically disrupted, heat-killed cells (100°C for 20 min) did not contain purpurogenic activity. The reaction was maximal at approximately 24 h postinjection, started to fade slowly after 24 to 48 h, and usually was not grossly observable by 4 to 6 days postinjection. The purpura-producing principle (PPP) in the cell-free extract was purified by sequential ammonium sulfate precipitation, protamine sulfate precipitation, Sepharose 6B gel filtration, wheat germ lectin-Sepharose 6MB affinity chromatography, ribonuclease and trypsin treatment, and a second Sepharose 6B gel filtration step. The final preparation (i) contained glucosamine (5.6%), muramic acid (8.0%), neutral carbohydrate (12.8%), phosphate (8.0%), orcinol-reactive material (6.0%), and Lowry-reactive material (1.6%), and (ii) was free of detectable amounts of deoxyribonucleic acid, capsular polysaccharide, neuraminidase, cytolysin, and hyaluronidase. The isoelectric point and molecular size of the PPP were approximately pI 3.0 and several million daltons, respectively, and the activity remained in the supernatant fluid after centrifugation for 1 day at 105,000 × g. PPP activity was destroyed by incubation with egg white lysozyme and sodium metaperiodate but was resistant to trypsin, pronase, α-amylase, deoxyribonuclease, ribonuclease, alkaline phosphatase, pancreatic lipase, 7% trichloroacetic acid, 6 M urea, autoclaving (121°C) for 30 min, and mild acid and alkali exposure. Our observations indicate that the PPP requires intact β-1,4-glucosidic linkages for activity and support the working hypothesis that activity is associated with pneumococcal peptidoglycan solubilized by the bacterium's autolysin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNHEIMER A. W., SCHWARTZ L. L. Isolation and composition of staphylococcal alpha toxin. J Gen Microbiol. 1963 Mar;30:455–468. doi: 10.1099/00221287-30-3-455. [DOI] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. An assay for total hexosamine and a differential assay for glucosamine and galactosamine. Clin Biochem. 1976 Dec;9(6):269–274. doi: 10.1016/s0009-9120(76)80075-6. [DOI] [PubMed] [Google Scholar]
- De Siervo A. J. Alterations in the phospholipid composition of Escherichia coli B during growth at different temperatures. J Bacteriol. 1969 Dec;100(3):1342–1349. doi: 10.1128/jb.100.3.1342-1349.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacks S. Mutants of Diplococcus pneumoniae that lack deoxyribonucleases and other activities possibly pertinent to genetic transformation. J Bacteriol. 1970 Feb;101(2):373–383. doi: 10.1128/jb.101.2.373-383.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinonen M., Käyhty H. Comparison of counter-current immunoelectrophoresis, latex agglutination, and radioimmunoassay in detection of soluble capsular polysaccharide antigens of Haemophilus influenzae type b and Neisseria meningitidis of groups A or C. J Clin Pathol. 1978 Dec;31(12):1172–1176. doi: 10.1136/jcp.31.12.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman M., Southwick M. A. Studies on Respiratory Diseases: The Pathology of Pneumococcus Infections in Mice. J Bacteriol. 1930 May;19(5):363–374. doi: 10.1128/jb.19.5.363-374.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronda-Lain C., Lopez R., Tapia A., Tomasz A. Role of the pneumococcal autolysin (murein hydrolase) in the release of progeny bacteriophage and in the bacteriophage-induced lysis of the host cells. J Virol. 1977 Jan;21(1):366–374. doi: 10.1128/jvi.21.1.366-374.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPIRO R. G. PERIODATE OXIDATION OF THE GLYCOPROTEIN FETUIN. J Biol Chem. 1964 Feb;239:567–573. [PubMed] [Google Scholar]
- STELLWAGEN E., SCHACHMAN H. K. The dissociation and reconstitution of aldolase. Biochemistry. 1962 Nov;1:1056–1069. doi: 10.1021/bi00912a016. [DOI] [PubMed] [Google Scholar]
- Stahl W. L., O'Toole R. D. Pneumococcal neuraminidase: purification and properties. Biochim Biophys Acta. 1972 May 12;268(2):480–487. doi: 10.1016/0005-2744(72)90343-9. [DOI] [PubMed] [Google Scholar]




