Abstract
The speed of stem cell differentiation has to be properly coupled with self-renewal, both under basal conditions for tissue maintenance and during regeneration for tissue repair. Using the Drosophila midgut model, we analyze at the cellular and molecular levels the differentiation program required for robust regeneration. We observe that the intestinal stem cell (ISC) and its differentiating daughter, the enteroblast (EB), form extended cell-cell contacts in regenerating intestines. The contact between progenitors is stabilized by cell adhesion molecules, and can be dynamically remodeled to elicit optimal juxtacrine Notch signaling to determine the speed of progenitor differentiation. Notably, increasing the adhesion property of progenitors by expressing Connectin is sufficient to induce rapid progenitor differentiation. We further demonstrate that JAK/STAT signaling, Sox21a and GATAe form a functional relay to orchestrate EB differentiation. Thus, our study provides new insights into the complex and sequential events that are required for rapid differentiation following stem cell division during tissue replenishment.
Author summary
Adult tissue/organ function is maintained by stem cells. Key question in stem cell biology is how the pool of stem cells can be robustly expanded yet timely contracted through differentiation according to the need of a tissue. Over the last years, the mechanisms underlying stem cell activation have been extensively studied, while the genetic control of progenitor differentiation, especially during regeneration, is still poorly understood. Using the fruit fly Drosophila midgut as model, we investigate the cellular changes and the genetic program required for efficient progenitor differentiation during intestinal regeneration. We first detect the presence of extended cell-cell contact between a stem cell and its differentiating daughter in regenerating intestine, compared to homeostatic conditions. The extended cell-cell contact is consolidated by cell adhesion molecules and enhances Notch signaling in the differentiating progenitors leading to their fast differentiation into enterocytes. We further uncover a genetic program, involving the JAK/STAT and Dpp signaling, the Sox21a and GATAe transcription factors, which acts in the differentiating progenitors to instruct their terminal differentiation. Thus, our study presents an integrated view of stem cell differentiation during tissue regeneration and the findings here are likely to apply to mammals.
Introduction
In metazoans, the digestive tract supports organismal growth and maintenance. Genetic disorders or microbial dysbiosis that prevent the digestion and absorption of nutrients are major causes of morbidity and mortality in humans. In mammals, mature intestinal cells are short-lived and constantly replaced by newborn differentiated cells. This is ensured by the existence of fast-cycling intestinal stem cells (ISCs) [1]. Although ISC division is important, failure in or improper differentiation into mature intestinal cells can equally cause a wide range of disorders that compromise organ function, such as intestinal cancer [2] and microvillus inclusion disease [3].
There is a great extent of similarity in intestinal functions and maintenance between flies and mammals [4]. Over the past decade, research has revealed the extreme plasticity of the Drosophila ISCs. For instance, stem cell activity and epithelial renewal can be adjusted in response to i) changes in nutrient availability [5–7], ii) physiological requirements for reproduction [8–10], iii) aging [11–13], iv) intestinal damage or infection [14–16], and v) body injury [17, 18]. Thus, both local and remote signals coordinate ISC activity to ensure intestinal homeostasis.
In the adult Drosophila midgut, ISCs differentiate into either polyploid absorptive enterocytes (ECs) or diploid secretory enteroendocrine cells (EEs) (Fig 1A). Recent studies indicated that EC and EE are generated through distinct mechanisms [19–21]. A post-mitotic and intermediate differentiating cell called enteroblast (EB) is differentiated into EC in a Notch-dependent manner [22, 23], while the production of EE through a so-far not molecularly characterized enteroendocrine mother cell (EMC) requires only low levels of Notch signaling [24]. ISCs and EBs (referred to as progenitor cells) reside basally next to the visceral muscles, while ECs cover the apical brush border (Fig 1B). In both flies and mammals, Notch signaling plays the same central roles in the choice of an absorptive or secretory fate in the intestinal lineages [25] [26]. Drosophila ISCs express the Notch ligand, Delta (Dl), which turns on Notch activity in its sibling cells for EB fate commitment [23, 25] (Fig 1C). Moreover, JAK/STAT signaling [14, 27], the transcription factors Escargot (Esg) [28–30], Sox21a [31, 32], GATAe [33], and Dpp signaling [34–36] have recently been shown to regulate progenitor differentiation. While stem cell proliferation has been the focus of most studies, the cellular mechanisms that mediate proper conversion of the expanded stem cell pool into mature intestinal cells especially during regeneration, are currently missing. Moreover, an integrated view of intestinal regeneration has not been established.
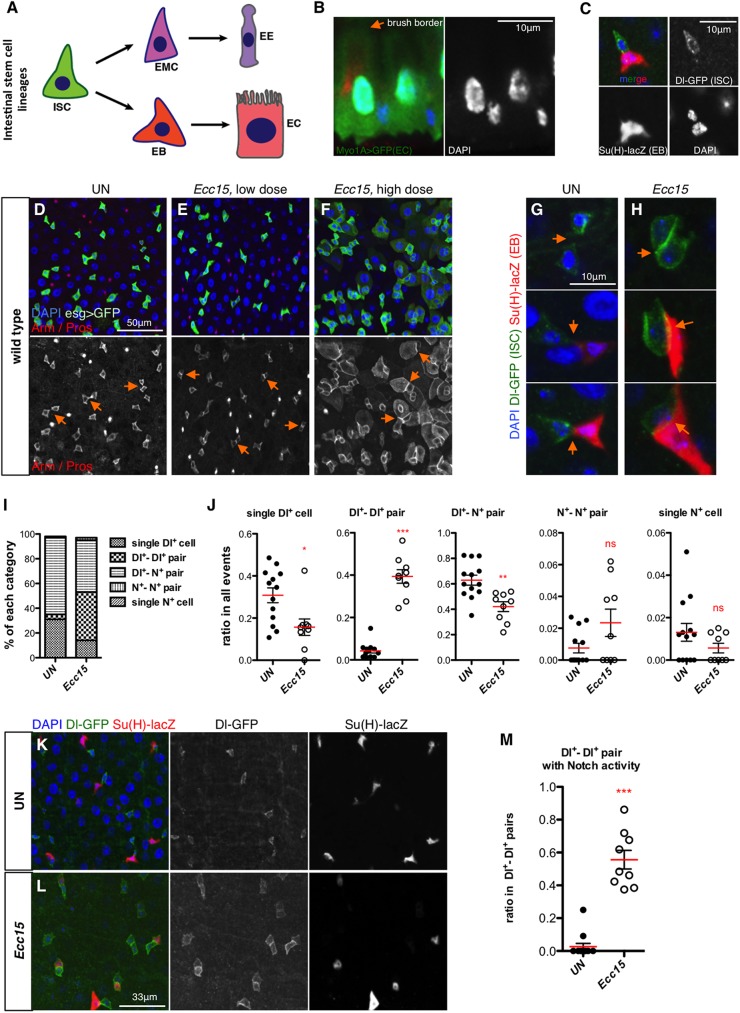
Fig 1. Increased progenitor contact is a general feature of regenerating intestine.
(A) Current model of intestinal stem cell (ISC) lineages. Cell types in the Drosophila midgut include ISC, enterocyte (EC) and its precursor enteroblast (EB), enteroendocrine cell (EE) and its precursor enteroendocrine mother cell (EMC). (B) Basal-apical (bottom to top) organization of the Drosophila midgut epithelium. ECs are visualized by Myo1A>GFP (Green) and brush border is indicated. (C) A typical ISC-EB pair in wild type midgut of an unchallenged fly. ISC is detected with Dl-GFP (green) and EB with Su(H)-lacZ (red). (D-F) Representative intestines of unchallenged (D) flies, flies orally infected with a low dose (E) or a high dose of Ecc15 (F). (G-H) Representative progenitor pairs in unchallenged control (G) and Ecc15-infected (H) intestines. Junctions between progenitor pairs are indicated with orange arrows in (D-H). (I-J) Summary of all the progenitor combinations in unchallenged control (n = 969, from 13 guts) and Ecc15-infected (n = 654, from 9 guts) intestines, and ratios of each category (including single Dl-GFP+ cell, Dl-GFP+—Dl-GFP+ pair, Dl-GFP+—Notch+ pair, Notch+—Notch+ pair and single Notch+ cell, from left to right) in all the events under both conditions. (K-L) Representative images of unchallenged control (K) and Ecc15-infected (L) intestines. Note that the increase in progenitor contact and presence of many Dl-GFP+—Dl-GFP+ pairs with one cell showing weak Notch activity are detected in (L). (M) Ratio of Dl-GFP+—Dl-GFP+ pairs with Notch activity (n = 42 in control and 259 in Ecc15 challenged guts). Each dot represents one gut.
Here, we investigate the cellular and genetic basis underlying efficient differentiation of progenitor cells during intestinal regeneration. Our data uncover that enhanced cell-cell contact between an ISC and its differentiating daughter, consolidated by cell adhesion molecules, is required for efficient Notch signaling and rapid progenitor differentiation into EC during regeneration. We further identify a regulatory cascade involving, sequentially, JAK/STAT signaling, Sox21a and GATAe, that functions in EBs and is required for rapid differentiation. Our integrated study of intestinal regeneration provides new insights into stem cell differentiation that likely apply to other systems.
Results
Increased progenitor contact is a general feature of the regenerating intestine
To understand the molecular and cellular mechanisms underlying intestinal regeneration, we analyzed the behaviors of progenitors in the gut of flies orally infected with the gram-negative bacterium Erwinia carotovora carotovora 15 (Ecc15). Oral infection with Ecc15 causes damage to the intestinal epithelium, which is quickly repaired through activating ISC proliferation and progenitor differentiation to maintain tissue integrity [15]. Unless otherwise noted, we focused our study on the anterior midgut, a region where the relatively low overall cell density allows better identification of individual cells. Interestingly, progenitor pairs with extended contact were observed in intestines following infection (Fig 1D and 1E), visualized by β-catenin staining (Armadillo (Arm) in Drosophila). Most differentiating EBs were in extended contact with at least one cell with strong esg>GFP signal (Fig 1F). Furthermore, ingestion of dextran sulfate sodium (DSS), which damages the intestinal epithelium and activates regeneration [16], also led to formation of progenitor pairs with extended cell-cell contact (S1A Fig). Since extended progenitor contact was not observed in basal homeostatic conditions, these results indicate that increased progenitor contact area is a general feature of the regenerating intestine.
Both Ecc15 infection and DSS treatment activate stem cell proliferation. To investigate if division of stem cells is required for the formation of extended progenitor contact, we used colcemid, a microtubule-depolymerizing drug, which blocks dividing cells in metaphase. The presence of colcemid suppressed the formation of extended contact that is normally induced by Ecc15 infection (S1B and S1C Fig). This suggests that during regeneration stem cells first proliferate before generating progenitors with increased cell-cell contact. However, the formation of extended cell-cell contact was not affected in Sox21a mutant gut where EB to EC differentiation is blocked (S1D and S1E Fig). Moreover, clusters of ISCs induced upon expression of a Notch RNAi using the progenitor specific driver esgTS also showed extended contact (S1F Fig). Thus, the formation of increased cell contact during regeneration appears to be a stem cell intrinsic behavior, which occurs independently of developmental signals regulating terminal differentiation.
Regenerating intestines show an increase in Dl-GFP+-Dl-GFP+ pairs with extended contact
Dl/Notch signaling plays a central role in determining the ISC and EB cell fate and is further involved in EB differentiation into EC. Since this signal transduction requires cell-cell contact between the signaling sending and receiving cells, the change in contact area likely affects Dl/Notch signaling dynamics [37]. We hypothesized that the extended progenitor contact observed in epithelial damage-induced regenerating intestines could promote efficient differentiation by enhancing Dl/Notch signaling. This led us to further analyze Notch signaling state in progenitor pairs of regenerating intestines by applying cell-type specific markers.
To unambiguously identify ISCs, we used an endogenous Dl-GFP fusion line with an ISC restricted expression [38] (Fig 1G). EBs were visualized by Su(H)-lacZ, a reporter gene of Notch activity [39, 40]. We first confirmed the increase in progenitor contact upon bacterial infection (Fig 1G and 1H). In line with previous results [5, 25, 41], ISCs in unchallenged conditions largely undergo asymmetric division, which generates another self-renewing ISC (Dl-GFP+) and a committed EB (Su(H)-lacZ+) (Fig 1G and 1I). When we quantified all progenitor combinations, including single Dl-GFP+ cells, Dl-GFP+—Dl-GFP+ pairs, Dl-GFP+—Notch+ pairs, Notch+—Notch+ pairs and single Notch+ cells, in both unchallenged and bacteria-infected (Ecc15, 12 hours post infection) intestines, we uncovered a significant increase of the Dl-GFP+—Dl-GFP+ pairs in infected guts (Fig 1I and 1J). Notably, the ratio of Dl-GFP+—Dl-GFP+ pairs increased from 5% in unchallenged intestines to around 40% in infected guts. This change was accompanied by a reduction in the proportion of single Dl-GFP+ cells in regenerating intestines, suggesting that most of them had recently divided. We also observed a drop in the ratio of Dl-GFP+—Notch+ pairs, from 62% to 42%. Collectively, these data are consistent with the notion that increased contact directly arises from newborn progenitor pairs that have just completed mitosis.
Interestingly, 57% of the Dl-GFP+—Dl-GFP+ pairs had one cell showing weak but specific Notch activity in Ecc15-infected intestines as revealed by the expression of the Su(H)-lacZ reporter (Fig 1K–1M; S2A and S2B Fig). Use of an antibody against Dl confirmed that both cells, including the one with weak Su(H)-lacZ expression, were indeed stem cells as defined by the expression of the Dl marker (S2C Fig). We further excluded the possibility of EE differentiation, since the EE marker Prospero (Pros) was never observed in such Dl-GFP+—Dl-GFP+ pairs with Notch activity (n>50) (S2D Fig). Importantly, ISCs undergoing mitosis were never found to express Su(H)-lacZ reporter (n>30) (S2E Fig), indicating that Notch activity was established in one cell of a newly formed Dl-GFP+—Dl-GFP+ pair after mitosis (S2F and S2G Fig). Although Dl-GFP+—Dl-GFP+ pairs formed during infection can arise either from single Dl-GFP+ cells or from Dl-GFP+—Notch+ progenitor pairs, these results support a symmetric expansion of the Dl+ cell pool followed by diverting a subset of them to be committed into EB and quickly differentiated, likely due to the presence of the extended cell-cell contact (Fig 2L).
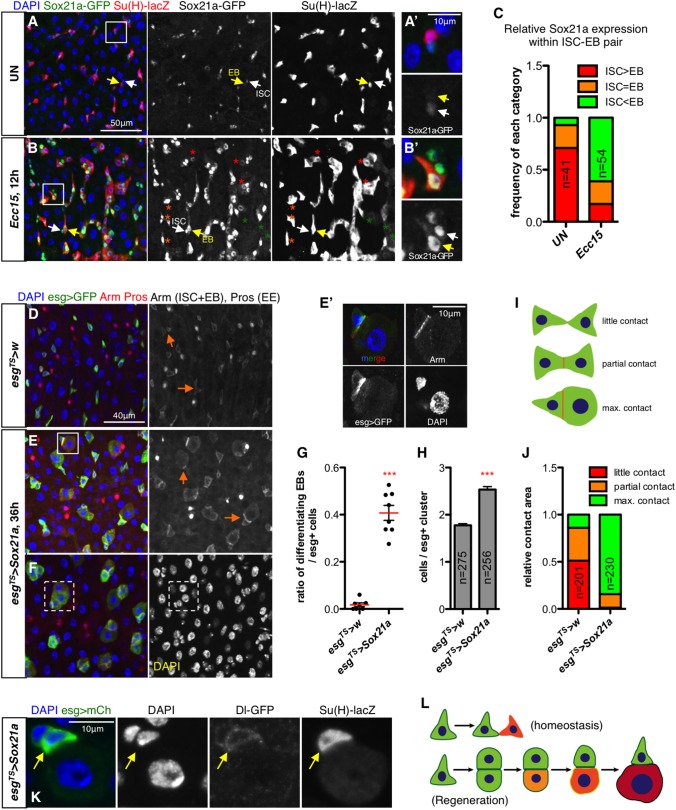
Fig 2. Sox21a regulates intestinal regeneration.
(A-B and A’-B’) Confocal images of unchallenged control (UN, A) and Ecc15-infected (B) midguts carrying Sox21a-GFP and Su(H)-lacZ. Representative ISCs (Sox21a-GFP+ Su(H)-lacZ—) are labeled with white arrows, and EBs (Su(H)-lacZ+) with yellow arrows. Some EBs are further indicated with stars of different colors in (B): Sox21a-GFPhigh Su(H)-lacZhigh (orange), Sox21a-GFPlow Su(H)-lacZhigh (red) and Sox21a-GFPnegative Su(H)-lacZlow (green). (C) Quantification of relative Sox21a-GFP intensity within individual ISC-EB pairs from intestines shown in A (n = 41 pairs, from 5 guts) and B (n = 54 pairs, from 7 guts). (D-F and E’) Representative intestines of wild-type flies (D) or flies over-expressing Sox21a in progenitors using esgTS driver for 36 hours at 29°C (E, E’ and F). Note that Armadillo (Arm) is evenly distributed along the plasma membrane in (D) but is highly enriched at the interface between progenitor cells in (E) (arrows). Prospero (Pros, red, nuclear) marks EEs. Close-up view of a progenitor pair outlined in (E) is shown in (E’). An esg>GFP+ nest containing multiple cells is outlined in (F). (G-H) Quantification of the ratio of differentiating EBs out of all the esg>GFP+ cells (G) and the average number of cells in each esg>GFP+ nest (H) in intestines of wild-type flies (esgTS>w) or flies over-expressing Sox21a in progenitors (esgTS>Sox21a) for 36 hours. (I-J) Schematic progenitor contact categories (I) and the distribution of each contact category in intestines of wild-type flies (esgTS>w) or flies over-expressing Sox21a in progenitors (esgTS>Sox21a) for 36 hours (J). Nuclei are stained by DAPI. (K) Representative image of progenitors from esgTS>Sox21a flies carrying both ISC and EB markers after 36 hours of transgene expression. Progenitors are shown with esg>mCherry (green). Yellow arrow indicates the Dl-GFP+ cell with Notch activity. (L) Schematic representation of modes of ISC division and cell fate commitment during homeostasis and regeneration. The presence of strong junction between the two progenitors promotes Notch signaling allowing rapid differentiation. Green lines indicate Dl, and red fillings denote Notch activation. Each dot represents one gut.
Sox21a is induced in intestinal progenitors during regeneration
We and others have previously shown that the transcription factor Sox21a is both necessary and sufficient for the differentiation of EB to EC [31, 32]. This was supported by the observations that ISC progenies are blocked at the EB stage in the absence of Sox21a, while overexpressing Sox21a in progenitors induced their precocious differentiation into mature EC. However, it is not yet established through which mechanisms Sox21a regulates progenitor differentiation, especially during regeneration following intestinal damage.
To further analyze the role of Sox21a in EB differentiation, we first monitored its expression levels in intestinal cells at different stages of their differentiation using a sGFP (superfolder green fluorescent protein)-tagged Sox21a transgene that is controlled by its own regulatory sequences [42]. Since it has been shown that Sox21a is expressed in both ISC and EB, cells that express Sox21a-GFP but not Su(H)-lacZ reporter are expected to be ISCs. As expected for a transcription factor, Sox21a-GFP signal was localized to the nuclei (Fig 2A and 2B). In unchallenged 5–7 day-old adults, Sox21a-GFP was expressed in both ISC and EB, with higher levels in ISC than in EB within an ISC-EB pair (Fig 2A, 2A’ and 2C). Oral infection with Ecc15 induced a marked increase of Sox21a-GFP expression in the progenitors (Fig 2B and 2B’). However, quantification of the relative intensity of Sox21a-GFP levels between cells within each ISC-EB pair indicated that regenerating intestines now expressed stronger Sox21a-GFP in the EB than in its sibling ISC (Fig 2C). Careful examination further revealed that Sox21a-GFP levels started declining in middle-sized EBs at a stage when Notch activity remained high. Moreover, Sox21a-GFP expression was totally shut down before the Notch activity reporter (Fig 2B).
Intestinal regeneration can be triggered by activating JNK or Ras/MAPK signaling in progenitors [12, 43, 44]. Interestingly, activating either pathway in progenitor cells with esg-Gal4 tub-Gal80ts for 36 hours also elevated Sox21a-GFP expression (S3A–S3E Fig), consistent with a previous observation [45]. However, akin to infection, stronger Sox21a-GFP expression was again seen in EBs, supporting the specific role of Sox21a in EB for EC differentiation. Collectively, this analysis shows that Sox21a displays a dynamic expression pattern, which coincides with the process of intestinal progenitor differentiation.
Expressing Sox21a efficiently promotes progenitor differentiation
As shown previously [31], expressing Sox21a in progenitor cells with the esgTS driver for only 36 hours led to their differentiation into EC (Fig 2D–2F). Thus, over-expressing Sox21a provides a useful framework to unravel the sequence of events underlying stem cell differentiation into mature ECs. Taking advantage of this approach, we monitored the cellular changes resulting from overexpressing Sox21a in progenitors. Cells that were precociously differentiating towards ECs (termed “differentiating EBs”) showed an increase in cell size, became polyploid and expressed Pdm1, a marker for ECs [31]. Differentiating EBs accounted for around 40% of the esg>GFP+ progenitors expressing Sox21a, while they were barely seen in midgut progenitors of wild type flies (Fig 2G). They retained a weak esg>GFP signal and were located at a similar basal position as genuine ISC/EB (S3F Fig). In addition, each esg>GFP+ nest contained a slightly increased number of cells (Fig 2F and 2H), consistent with the notion that Sox21a also promotes a low level of ISC proliferation.
In homeostatic guts, most progenitor cells have limited membrane contact (Fig 2D). In Sox21a overexpressing guts, nearly all the differentiating EBs displayed an extended plasma membrane contact, with at least one cell with a strong esg>GFP signal (Fig 2E and 2E’; S3F Fig). Increased membrane contact was also reflected by the increased GFP signal at the membrane in progenitor cells expressing the membrane-tethered mCD8::GFP (Fig 2E’). To quantify changes in membrane contact, we measured contact area relative to the size of the smaller cell in more than 200 progenitor pairs. Maximal cell-cell contact between progenitors was reached as early as 36 hours after Sox21a expression was induced (Fig 2I and 2J). Similarly to the situation observed with infection, expressing Sox21a using the progenitor driver esg-Gal4 tub-Gal80ts also induced Dl-GFP+—Dl-GFP+ pairs with one cell exhibiting Notch activity with a high frequency (Fig 2K).
Sox21a promotes progenitor differentiation by enhancing Dl/Notch signaling
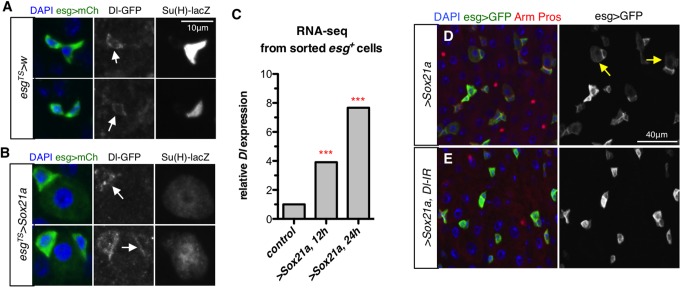
The observation that over-expressing Sox21a induces progenitors to differentiate towards ECs rather than EEs [31], suggests that Sox21a could enhance Notch activity. To test this idea, we analyzed how Sox21a impacts Notch signaling. Interestingly, Dl-GFP signal was enriched at the extended ISC-EB contact area upon Sox21a expression, in contrast to their even distribution in wild-type ISCs (Fig 3A and 3B). Furthermore, transcriptomic analysis using FACS-sorted progenitors revealed a four to seven fold increase of Dl mRNA in intestinal progenitors expressing Sox21a for only 12 or 24 hours (Fig 3C; S1 Table). The increase of Dl mRNA levels was further validated by quantitative PCR (qPCR) from dissected guts (S4 Fig). Thus, expressing Sox21a in progenitors increased Dl transcription and the amount of Dl protein at the ISC-EB interface, which are both expected to reinforce efficient Notch signal transduction. Finally, knocking down Dl with two independent RNAi lines both suppressed Sox21a-induced differentiation (Figs 3D and 3E and 4A; S5A and S5B Fig). Collectively, our data indicate that Sox21a mediates rapid differentiation at least in part by up-regulating Dl and by increasing progenitor contact, two features likely to enhance Notch signaling.
Fig 3. Sox21a promotes differentiation by enhancing the Dl/Notch signaling.
(A-B) ISC-EB pairs in control (esgTS>w) and Sox21a-expressing (esgTS>Sox21a) flies. ISCs are detected with Dl-GFP, EBs with Su(H)-lacZ and progenitors with esg>mCherry (green). Arrows indicate ISCs in (A) and highlight the ISC-EB contact in (B). (C) Relative Dl mRNA levels in control and Sox21a-expressing progenitors from an RNA-seq experiment using sorted esg>GFP+ cells (n = 50 midguts/group, in two biological replicates, Robinson and Smyth Exact test, ***p < 0.001). esgTS>Sox21a flies were shifted to 29°C for 12 or 24 hours to induce Sox21a expression. (D-E) Confocal images of intestines expressing Sox21a alone (D) or simultaneously with a Dl-RNAi construct (E) using esgTS for 36 hours. Arrows in (D) indicate some differentiating EBs, which are not present upon depleting Dl (E).
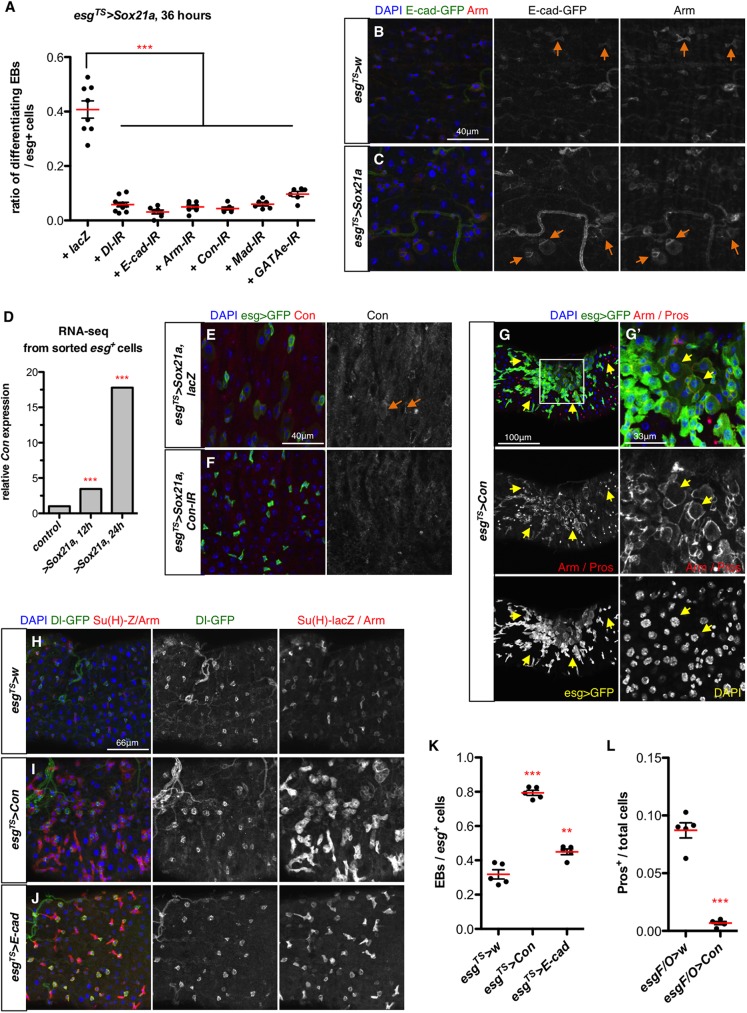
Fig 4. Cell adhesion molecules are required for Sox21a-induced differentiation.
(A) Quantification of the ratio of differentiating EBs out of all the esg>GFP+ cells in intestines of flies over-expressing Sox21a in progenitors (esgTS>Sox21a) with co-depletion of the indicated genes with RNAi for 36 hours. (B-C) Localization of E-cad (revealed with an endogenous E-cad-GFP fusion) in both control (B) and esgTS>Sox21a intestine (C). E-cad/Arm junctions are indicated by orange arrows. (D) Relative Con mRNA levels in control and Sox21a-expressing progenitors from the RNA-seq experiment described in Fig 3C. (E-F) Connectin (Con) is enriched in the progenitor contact in esgTS>Sox21a intestines (E, orange arrows) by applying an antibody against Connectin, but is not detected in esgTS>Sox21a intestines co-depleted for Connectin by RNAi (F). Progenitors (esg>GFP) and nuclei (DAPI) are shown. (G-G’) Progenitor-specific expression of Connectin using esgTS for 4 days. The region outlined in (G) is shown in (G’) with higher magnification. Note the absence of EEs (Pros+) from the region with big clusters of esg>GFP+ cells, some of which are differentiating towards EC. Some differentiating EBs are indicated with yellow arrows (G’). (H-K) representative images of midgut of control flies (H) or flies overexpressing Connectin (I) or E-cad (J) with esgTS for 4 days. ISCs express Dl-GFP (in green); EBs are marked with Su(H)-lacZ reporter (cytoplasmic, in red). Quantification of the ratio of EBs out of all the progenitors (ISC+EB) is shown in (K). (L) Quantification of the ratio of EEs (Pros+) in all the midgut cells in defined areas of the anterior midgut of flies with indicated genotype and shifted to 29°C to induce transgene expression for 7 days. Each dot represents one gut.
Cell-cell adhesion is essential for Sox21a-induced differentiation
Extended progenitor contact in regenerating guts suggested that cell adhesion molecules might be involved in rapid progenitor differentiation. E-cadherin (E-cad) forms a complex with β-catenin at adherens junctions and mediates homophilic cell-cell adhesion [46]. Using an endogenous E-cad-GFP fusion, we first confirmed that E-cad was enriched at the ISC-EB interface in Sox21a expressing intestine. Similarly, the localization of Arm was almost identical to E-Cad (Fig 4B and 4C). Simultaneous depletion of either E-cad or arm for 36 hours completely suppressed the precocious differentiation phenotype seen in intestines expressing Sox21a (esgTS>Sox21a) (Fig 4A; S5C and S5D Fig). In these intestines, progenitor pairs displayed reduced contact confirming an essential role of E-cad in the formation of extended cell-cell contact during rapid differentiation. Since E-cad can impact Wg/Wnt signaling [47], the phenotype observed was possibly due to a requirement of Wg signaling for Sox21a-induced differentiation. However, neither activating (with constitutively active β-catenin) nor blocking Wg signaling (with dominant-negative Pangolin) affected Sox21a-induced differentiation (S5H and S5I Fig). We conclude that E-cad-mediated cell-cell adhesion is required for Sox21a-induced differentiation, independently of Wg signaling, in line with a previous report [48].
Furthermore, another cell adhesion molecule, Connectin (Con), which mediates homophilic cell-cell adhesion both in vitro and in vivo [49, 50], was up-regulated in progenitor cells expressing Sox21a in our progenitor-specific transcriptomic analysis (Fig 4D; S1 Table). Using an antibody against Connectin, we confirmed its enrichment at the extended contact between progenitors in esgTS>Sox21a gut (Fig 4E; S6A Fig). Like E-cad, Connectin was also crucial for Sox21a-induced differentiation, as its depletion abolished the occurrence of differentiating EBs (Fig 4A and 4F; S5E Fig).
To determine to which extent cell-cell adhesion can contribute to differentiation, we also overexpressed Connectin in progenitors. As expected, overexpressing Connectin using esgTS altered the morphology of progenitor cells with the formation of interconnected progenitors, sometimes forming big clusters (Fig 4G; S6A–S6C Fig). These changes were also associated with an increase in ISC proliferation but not a blockage of EB differentiation (Fig 4G’; S6H Fig). Consequently, progenitor tumors were not observed in esgTS>Connectin intestines despite the presence of large esg>GFP+ clusters. Surprisingly, many progenitors in esgTS>Connectin intestines displayed the same characteristics of differentiating EBs of esgTS>Sox21a intestines, including a weak esg>GFP signal, increased cell size, and extended contact with neighboring esg>GFPstrong progenitors (Fig 4G’). Moreover, regions with clusters of esg>GFP+ cells were devoid of EEs and progenitors in such regions were differentiating towards ECs as judged from their large cell size and polyploid nuclei (Fig 4G and 4G’; S6B and S6C Fig). Further experiments indicated that Connectin overexpression in the progenitors increased the expression levels of Notch activity reporter Su(H)-lacZ (Fig 4I) and promoted EB-EC differentiation rather than EE differentiation (Fig 4H–4L; S6I and S6J Fig). Therefore, an increase in cell adhesion by over-expressing Connectin in progenitors can promote their differentiation toward ECs, by enhancing Notch activity. Nevertheless, results obtained with the esgF/O system [14] did not support an essential role of E-cad or Connectin in basal intestinal turnover (S6K–S6M Fig). Unexpectedly, knocking-down Connectin in the progenitors induced both mild stem cell proliferation and progenitor differentiation in the absence of a challenge (S6D–S6H Fig), suggesting Connectin may have other functions in the maintenance of ISC under normal conditions. We conclude that the formation of extended cell contact between progenitors through adhesion molecules is required for Sox21a-induced rapid differentiation. Importantly, we show that this process is specifically required for the rapid differentiation of progenitors but not for basal low-level intestinal turnover during homeostatic conditions. Thus, our study reveals specific mechanisms that have evolved to accelerate the differentiation program.
Sox21a acts in parallel with Notch signaling for terminal differentiation
Having analyzed the cellular changes that enhance Notch signaling activity in the ISC-EB transition during intestinal regeneration, we went on to investigate how Sox21a contributes to the processes of differentiation from EB to EC. In Drosophila, Notch deficient stem cells over-proliferate, leading to the formation of tumors composed mostly of ISCs and intermingled with EEs [22, 23, 25, 51], while the over-activation of Notch signaling drives progenitors to differentiate into ECs [22, 25]. The similarities between the function of Notch signaling and that of Sox21a in terminal differentiation led us to investigate their relationship.
While Sox21a is expressed in both ISC and EB, Notch activity is only found in EB [22, 23]. Thus, Sox21a expression in ISC is independent of Notch signaling. In addition, Notch activity was not blocked in a Sox21a mutant [31]. Several observations indicate that Sox21a and Notch signaling function interdependently for terminal differentiation. We first observed that Sox21a was required for the differentiation of progenitors into ECs upon Notch over-activation (Fig 5A and 5B), and for the formation of tumors composed of ISCs and EEs in Notch deficient clones (Fig 5C–5E). Conversely, the forced differentiation of progenitors into ECs upon expression of Sox21a was blocked in the absence of functional Notch signaling (S7A–S7D Fig). Thus, over-expression of Sox21a cannot overcome the requirement of Notch signaling for the differentiation of EB. Collectively, this led us to conclude that Sox21a and Notch signaling encompass two parallel systems that need to cooperate to ensure terminal differentiation.
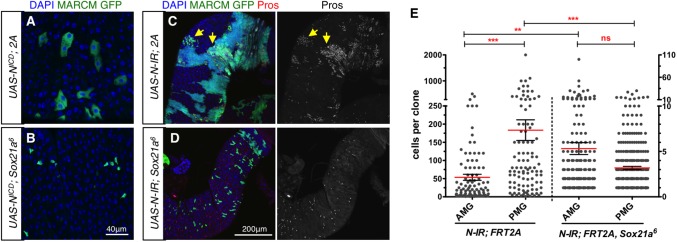
Fig 5. Sox21a acts in parallel with Notch signaling for terminal differentiation.
(A-D) Control (A and C) or Sox21a mutant (B and D) MARCM clones (positively labeled by GFP) in the posterior midgut (PMG) overexpressing UAS-NotchICD to activate Notch signaling (A and B) or over-expressing a UAS-Notch-IR construct to deplete Notch (C and D), are analyzed 7 days after clone induction (ACI). ECs are identified by their large cell size (A), and EEs express Prospero (yellow arrows in C). Different UAS-linked transgenes are combined either to a FRT2A chromosome to generate control clones (A and C) or to FRT2A, Sox21a6 chromosome to make Sox21a mutant clones (B and D). (E) Quantification of the size of Notch deficient clones (using a Notch RNAi construct) in wild type or Sox21a6 flies. Clone size was monitored in the anterior midgut (AMG) and the posterior midgut 12 days ACI at 18°C. Numbers of clones scored are 100, 104, 207 and 373, respectively from left to right, derived from 10 guts. Cell counts for Sox21a mutant clones should apply the y-axis on the right side of the figure. Note that a stronger inhibition of the Notch tumor growth by loss of Sox21a was observed in the PMG than in the AMG, agreeing with a previous finding that Sox21a is essential for ISC proliferation only in the PMG, but not in the AMG.
Sox21a functions downstream of the JAK/STAT pathway in EB differentiation
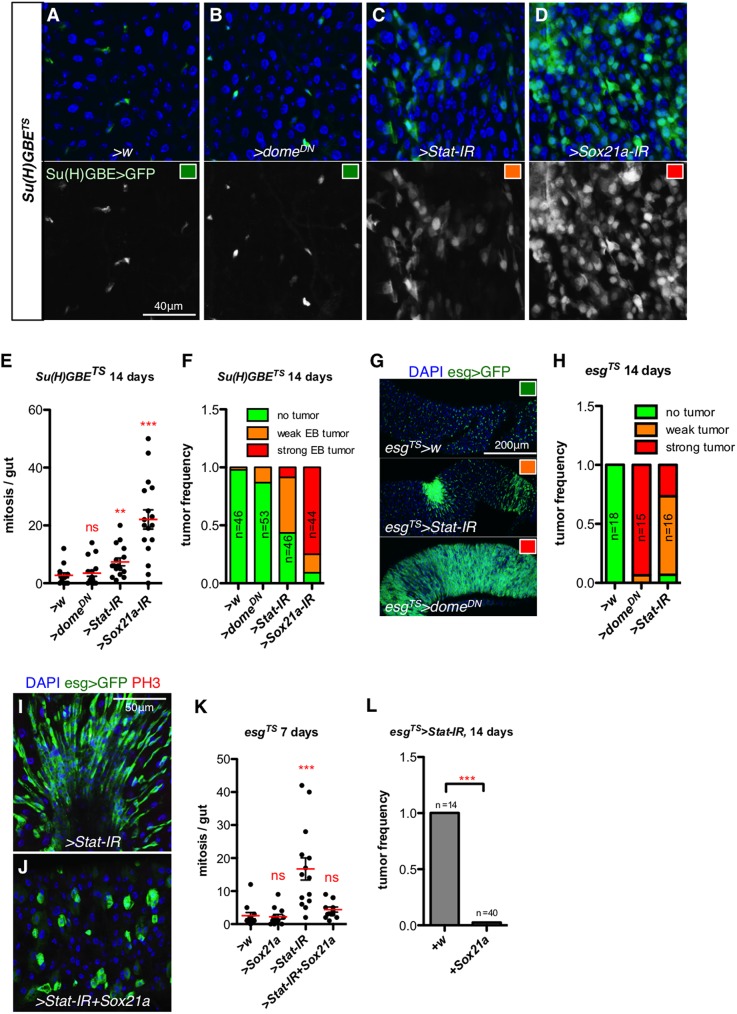
Both Sox21a and JAK/STAT have been shown to be mandatory for the EB-EC differentiation, and knockdown of either of these two factors results in the formation of EB containing tumors. Mechanistically, the formation of these tumors is caused by a feed-back amplification loop whereby the differentiation-defective EBs secrete growth factors stimulating ISC proliferation [14, 27, 31, 32]. We have previously shown that over-expression of Sox21a can partially rescue the differentiation defect caused by the loss of JAK/STAT in MARCM clones, suggesting that Sox21a functions downstream of JAK/STAT signaling in EB differentiation [31]. We further analyzed the relationship between JAK/STAT signaling and Sox21a by using this time an EB specific driver, Su(H)GBETS (Fig 6A–6D). Knocking down Sox21a specifically in EB caused the accumulation of EBs and an increase in ISC mitosis resulting in strong tumor formation, recapitulating the Sox21a mutant phenotype (Fig 6D–6F). In contrast, EB-specific depletion of Stat92E, the gene encoding the JAK/STAT transcription factor, had only mild consequences with the formation of small-sized EB tumors (Fig 6C, 6E and 6F). Unexpectedly, expressing a dominant-negative form of Domeless (DomeDN), the receptor of the JAK/STAT signaling cascade, using the same EB driver did not affect the differentiation process and did not lead to any tumor formation (Fig 6B, 6E and 6F). However, silencing the JAK/STAT pathway in both ISC and EB with esgTS, using the same UAS-DomeDN or the UAS-Stat-RNAi constructs led to the formation of massive progenitor tumors (Fig 6G and 6H). The observations that EB tumor formation requires the inactivation of JAK/STAT in both ISC and EB, and that knock-down of Sox21a only in EBs is sufficient to cause tumors, are consistent with the notion that JAK/STAT signaling is required earlier than Sox21a in the course of EB-EC differentiation. Supporting this hypothesis, we observed that expressing Sox21a could suppress tumor formation caused by loss of JAK/STAT signaling in progenitors (Fig 6I–6L), indicating that Sox21a can promote progenitor differentiation in the absence of JAK/STAT signaling. Thus, the observations that JAK/STAT signaling functions earlier than Sox21a, and that Sox21a can override the differentiation blockage caused by the loss of JAK/STAT signaling confirm and extend our previous observation based on mosaic analysis [31] that Sox21a acts downstream of JAK/STAT pathway in EB-EC differentiation.
Fig 6. Sox21a functions downstream of JAK-STAT pathway for EB differentiation.
(A-D) Representative intestines of flies with the indicated genotype observed 14 days after transgene expression using EB-specific driver Su(H)GBETS. Su(H)GBE>GFP (green) labels EBs. (E) Quantification of mitotic index in the midgut of flies after knocking down respective factors using EB-specific driver Su(H)GBETS for 14 days. (F) Quantification of tumor formation capacity in the midgut of flies shown in (A-D). The tumor-grading system is based on the degree of EB accumulation and examples are color-coded and denoted in (A-D). (G-H) Representative images of intestines with indicated genotype observed 14 days after transgene expression using the progenitor-specific driver esgTS (G) and quantification of progenitor tumor frequency (H). The tumor-grading standard is shown in (G). esg>GFP (green) labels ISC/EB. (I-J) Representative intestines of flies with the indicated genotype observed 7 days after transfer to 29°C allowing transgene expression using esgTS. (K) Quantification of mitotic index of midgut from flies with the indicated genotype. (L) Quantification of the frequency of intestinal progenitor tumors in esgTS>Stat-IR flies and esgTS>Stat-IR flies co-expressing Sox21a to induce differentiation for 14 days. Each dot represents one gut.
The Dpp signaling pathway is required in EBs for Sox21a-induced differentiation
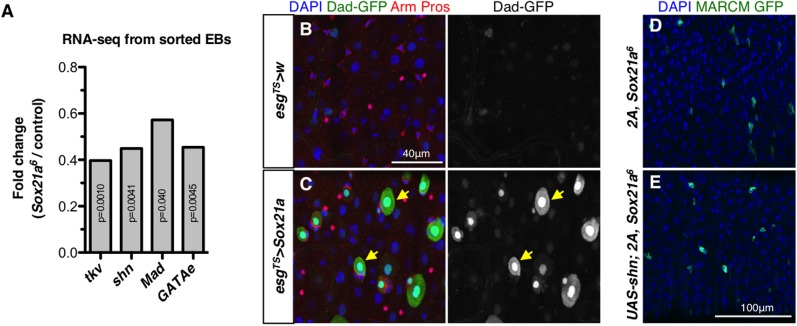
The role of the Dpp signaling pathway in EB differentiation has been controversial, with studies supporting that it is essential to this process while others suggest it is dispensable [34–36]. The observation that several genes encoding components of Dpp signaling, including the receptor thickveins (tkv), the transcription factors schnurri and Mothers against dpp (Mad), are down-regulated in Sox21a mutant EBs, supported a role of Dpp signaling in EB to EC transition (Fig 7A). We therefore explored its role in the differentiation process and its relationship with Sox21a. Interestingly, Dpp signaling was specifically induced in differentiating EBs in esgTS>Sox21a intestines as revealed by a reporter gene of Dpp signaling, Dad-GFPnls (Fig 7B and 7C). The expression of the Dad-GFPnls reporter was much stronger in differentiating EBs than in mature ECs that were already differentiated prior to the activation of Sox21a (Fig 7C), highlighting a role of Dpp signaling in the EB to EC transition. Importantly, Dpp signaling was mandatory for Sox21a-induced rapid differentiation, as depleting the key component Mad abolished progenitor differentiation of esgTS>Sox21a intestines (Fig 4A; S5F Fig). These results support a role of the Dpp pathway in EB differentiation downstream of Sox21a. Nevertheless, overexpressing the Dpp transcription factor Schnurri, which is known to promote progenitor differentiation into EC in the midgut [36], did not rescue the differentiation defect of Sox21a mutant clones (Fig 7D and 7E). We conclude that Dpp signaling is required downstream of Sox21a for rapid differentiation but is not sufficient to rescue Sox21a deficiency.
Fig 7. The Dpp signaling is required in EBs for Sox21a-induced differentiation.
(A) Fold changes of respective transcripts (thickvein (tkv), schnurri (shn), Mothers against dpp (Mad) and GATAe) in Sox21a mutant EBs compared to wild type EBs. (B-C) The level of Dpp signaling activity was detected with the Dad-GFPnls reporter (in green) in wild-type flies (B) or flies over-expressing Sox21a in progenitors for 36 hours (C). Some differentiating EBs are indicated with yellow arrows (C). Arm (in red, membrane), Pros (in red, nuclei) and DAPI staining are shown. (D-E) Sox21a mutant clones (positively labeled by GFP, D) and Sox21a mutant clones expressing the Dpp pathway transcription factor Schnurri (E) are analyzed 7 days after clone induction. Note that the blockage in differentiation of Sox21a mutant clone cells was not rescued by the shn expression.
GATAe is required in EBs for Sox21a-induced differentiation
GATAe encodes a transcription factor, which is expressed in all the cell types of the fly midgut. It has recently been shown to be important for ISC proliferation and, to a lesser extent, for EB differentiation [33]. It also has a role in ECs to maintain the regionalization of the intestine [33, 52]. Our RNA-seq experiments with FACS-sorted EBs revealed that the expression of GATAe was decreased in the absence of Sox21a (Fig 7A), suggesting that this transcription factor could contribute to the differentiation program downstream of Sox21a. We therefore investigated in further detail the role of GATAe in the differentiation process and its relationship with Sox21a.
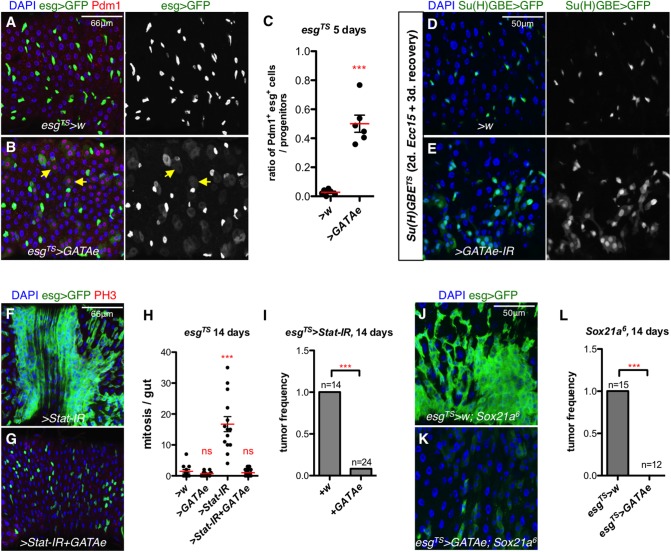
We first observed that over-expressing GATAe under the control of esgTS driver led to the precocious differentiation of progenitors into Pdm1-positive ECs (Fig 8A–8C), consistent with the notion that GATAe can promote progenitor differentiation [33]. The fast differentiation of progenitors was supported by the presence of many mature EC that still kept residual esg>GFP signal (Fig 8B), reminiscent of progenitor cells in esgTS>Sox21a intestines. Consistent with [33], loss of GATAe did not block terminal differentiation in basal conditions, since both Pdm1-positive ECs and Pros-positive EEs were found in stem cell clones deficient for GATAe (S8A and S8B Fig). Similarly, EB-specific depletion of GATAe using the EB-specific driver Su(H)GBETS did not lead to EB tumors, but rather to a slight accumulation of late-stage EBs as judged from their appearance. These results suggest that GATAe may contribute to the rate of EB differentiation. To test this idea, we analyzed the contribution of GATAe to rapid epithelial turnover induced by ingestion of bacteria. Both wild-type flies and flies with EB-specific depletion of GATAe were orally infected with Ecc15 for 2 days, a treatment that increases the pool of progenitors undergoing differentiation, and were further let to recover for 3 days. At this timepoint, the midgut of Su(H)GBETS>w control flies subjected to bacterial challenge had already returned to a homeostatic condition where only nascent EBs with small nuclei were found (Fig 8D). In sharp contrast, accumulation of EBs was observed along the midgut of Su(H)GBETS>GATAe-IR flies (Fig 8E). These EBs had a larger cell size than EBs found in wild-type flies suggesting that they were stuck in the process of differentiation. Consistent with a role of GATAe for accelerated EB differentiation, simultaneously depleting GATAe abolished progenitor differentiation in esgTS>Sox21a intestines (Fig 4A; S5G Fig). Moreover, expressing GATAe with the esgTS driver suppressed the differentiation defect and tumor formation induced by the loss of either Sox21a or Stat92E (Fig 8F–8L), revealing that GATAe acts downstream of Sox21a and JAK/STAT. Collectively, our study not only confirms that GATAe contributes to the differentiation process [33], but further reveals its critical role during regeneration as opposed to basal conditions. Our data also show that JAK/STAT-Sox21a-GATAe forms a sequential relay orchestrating the EB-EC differentiation process.
Fig 8. GATAe is required in EBs for Sox21a-induced differentiation.
(A-B) Representative intestines of wild-type flies (A) or flies over-expressing GATAe (B) in progenitors using esgTS driver for 5 days. EC marker Pdm1 is shown in red. Note many differentiating progenitors already express Pdm1 (indicated by arrows). (C) The percentage of of Pdm1+ esg>GFP+ cells over all the esg>GFP+ progenitors in both control (esgTS>w) and GATAe overexpressing intestines (esgTS>GATAe). (D-E) Intestines of Su(H)GBETS>w (control, D) and Su(H)GBETS>GATAe-IR (E) flies which were shifted to 29°C to induce transgene expression for 3 days, then orally infected with Ecc15 for 2 days and recovered for 3 additional days. Su(H)GBE>GFP (green) labels EBs. (F-G) Representative intestines of flies with the indicated genotype observed 14 days after transfer to 29°C allowing transgene expression using esgTS. (H) Quantification of mitotic index of midgut from flies with the indicated genotype. (I) Quantification of the frequency of intestinal progenitor tumors in esgTS>Stat-IR flies and esgTS>Stat-IR flies co-expressing GATAe for 14 days. (J-K) Intestines of Sox21a mutant fly and Sox21a mutant fly carrying esgTS>GATAe transgenes, kept at 22°C for 20 days to allow the formation of progenitor tumors and then shifted to 29°C for 2 days to induce transgene expression. (L) Tumor frequency in intestines of Sox21a mutant and Sox21a mutant expressing GATAe in progenitors using esgTS for 14 days at 29°C. esg>GFP (green) labels midgut progenitors. Each dot represents one gut.
Discussion
Key questions in stem cell biology are how the pool of stem cells can be robustly expanded yet also timely contracted through differentiation to generate mature cells according to the need of a tissue, and what are the underlying mechanisms that couple stem cell proliferation and differentiation. Over the last years, the mechanisms underlying intestinal stem cell activation have been extensively studied in both flies and mammals [1, 4], while the genetic control of progenitor differentiation, especially during regeneration, has only recently begun to be understood [26, 28, 31].
The transcription factor Sox21a has recently been the focus of studies in fly intestines [31, 32, 45]. Using a Sox21a-sGFP transgene, we uncovered its dynamic expression pattern in intestinal progenitors. Higher levels of Sox21a were found in ISC during homeostatic conditions but in EB during regeneration, supporting the roles of Sox21a in both ISC maintenance and EB differentiation at different conditions. The highly dynamic expression pattern of Sox21a revealed by this sGFP-tagged transgene per se argues against accumulation and perdurance of GFP fusion protein. Indeed, immunostaining using an antibody against Sox21a also indicated stronger Sox21a expression in ISC in homeostatic condition and global activation of Sox21a in progenitors under DSS-induced regeneration [45]. However, Chen et al., (2016) suggested that Sox21a levels are always higher in EB than in ISC by applying another antibody against Sox21a. The inconsistency between these studies may have arisen from the differences in EB stages examined or the sensitivity of respective detection approaches.
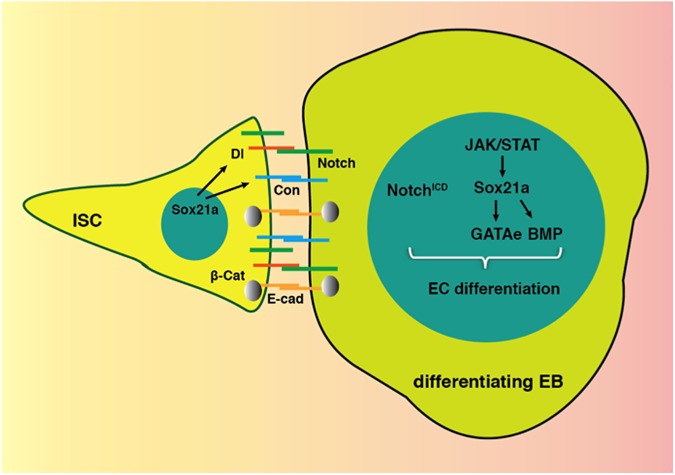
In this study, we have analyzed the cellular processes required for efficient progenitor differentiation during regeneration and uncovered three main findings revealing: i) the importance of extended contact between a stem cell and its differentiating daughter, ii) the existence of specific mechanisms allowing fast differentiation during regeneration, and iii) the characterization of a genetic program instructing the transition from EB to EC. These results together led us to propose a molecular framework underlying intestinal regeneration (Fig 9) that is discussed below step by step.
Fig 9. A genetic framework underlies rapid progenitor differentiation for tissue replenishment.
During rapid intestinal epithelial renewal, stem cells divide symmetrically with one of the two cells adopting the EB fate. Rapid differentiation involves extended cell contact maintained by the cell adhesion molecules E-cadherin (E-cad) and Connectin (Con) to enhance Notch signaling activity in EBs. A JAK/STAT-Sox21a-GATAe relay further instructs the process of EB to EC differentiation.
By studying the mechanisms of Sox21a-induced differentiation, we found that ISC establishes extended contact with its differentiating daughter within a progenitor pair. Increased interface contact was not only observed upon Sox21a expression but also during regeneration after bacterial infection and DSS-feeding. Since the presence of extended contact is rare in intestinal progenitors under homeostatic conditions, we hypothesize that extended contact between progenitors is related to increased epithelial renewal as a mechanism to elicit optimal juxtacrine Notch signaling to accelerate the speed of progenitor differentiation. The observations that down-regulation of the cell adhesion molecules E-Cadherin or Connectin suppresses rapid progenitor differentiation upon regeneration, and that overexpression of Connectin is sufficient to promote differentiation, underline the importance of increased cell-cell contact in rapid differentiation. Our study shows that one early role of Sox21a is to promote the formation of this contact zone, possibly through transcriptional regulation of Connectin. Further studies should identify the signals and pathways leading to the change of contact between progenitors to adjust the rate of differentiation.
Intestinal progenitors with extended contact in non-homeostatic midguts have been observed in some studies [14, 41], but their role and significance have not been analyzed. Previous studies have also shown that progenitor nests are outlined by E-Cadherin/β-Catenin complexes [23, 48], yet it was not known whether different degrees of progenitor contact are associated with their ISC versus EB fate. Consistent with our results, recent modeling analyses suggested a positive correlation between the contact area of progenitor pairs and the activation of Notch signaling [53, 54]. Thus, it seems that an increase in the contact area between intestinal progenitors is a hallmark of progenitors that are undergoing accelerated differentiation towards ECs. Another study by Choi et al. (2011) has suggested an inhibitory role of prolonged ISC-EB contact to restrict ISC proliferation. Collectively, these studies and our findings suggest that the strong contact between ISC and EB promotes on one hand the efficient differentiation of EBs into mature intestinal cells while on the other hand preventing stem cells from over-dividing. Thus, we hypothesize that alteration in the contact zone provides a mechanism for ensuring both the appropriate speed of differentiation and the timely resolution of stem cell proliferative capacity.
A second finding of our study consists in revealing the existence of specific mechanisms accelerating differentiation for tissue replenishment. In addition to the extended contact discussed above, we observe a difference in the pattern of ISC division between homeostatic and highly regenerative intestines. The modes of ISC division in Drosophila have been the topic of intense discussion, and the general consensus is that it is associated with an asymmetric cell fate outcome, in which one cell remains an ISC and the other engages in differentiation [5, 41, 55, 56]. In line with these previous studies, our results support the notion that asymmetric cell division is the most prevalent mode of ISC division under homeostatic conditions, where the rate of epithelial renewal is low. However, our use of ISC- and EB-specific markers shows that upon rapid regeneration an ISC divides into two cells both expressing the ISC marker Dl-GFP but with one cell showing weak Notch activity. Similarly to other Notch-mediated cell-fate decision systems [57], our study suggests that the two resulting Dl-GFP+ cells from a symmetric division stay in close contact and compete for the stem cell fate. While our study is not the first to postulate the existence of symmetric ISC division [5, 55], the use of reliable ISC- and EB-specific markers allows us to better visualize this process. Applying a dual-color lineage tracing system to unravel the final fate of respective cells in a Dl+—Dl+ pair could reinforce the existence of symmetric stem cell division. This is nevertheless technically challenging to apply here since all the current available lineage-tracing settings require a heat shock to initiate the labeling, which affects intestinal homeostasis.
Importantly, we show that the genetic program required for fast intestinal regeneration differs from the one involved in basal intestinal maintenance. Our study indicates that GATAe, Dpp signaling, and the cell adhesion molecules E-cadherin and Connectin are not critical for progenitor differentiation when the rate of epithelial renewal is low, whereas their roles become crucial upon active regeneration. We speculate that many discrepancies in the literature can be reconciled by taking into consideration that some factors are required only for rapid differentiation but not in basal conditions. For instance, the implication of Dpp signaling in differentiation has been disputed, since Zhou et al. (2015) focused on bacterial infection-induced regeneration while the other two studies dealt with basal conditions [34–36]. Our study here points to a clear role of Dpp signaling in the differentiation process upon regeneration. Therefore, better defining the genetic program that allows adjusting the speed of differentiation would be of great interest.
Cell fate determination and differentiation involve extensive changes in gene expression and possibly also gradual change of cell morphology. The EB to EC differentiation in the adult Drosophila intestine provides a model of choice to study this process. This transition includes changes in cell shape, an increase in cell size, DNA endoreplication leading to polyploidy and the activation of the set of genes required for EC function (S9A–S9C Fig). In this study, we have integrated a number of pathways (Notch, JAK/STAT and Dpp/BMP) and transcription factors (Sox21a and GATAe) into a sequential framework. We further show that Sox21a contributes to the EB-EC transition downstream of JAK/STAT but upstream of Dpp signaling and GATAe. The recurrent use of several factors, namely JAK/STAT, Sox21a and GATAe at different processes including ISC self-renewal and EB-EC differentiation is likely to be a general feature during cell fate determination, and somehow also complicates the study of differentiation. Future work should analyze how each of the factors interacts with the other in a direct or indirect manner. It would be interesting as well to further study how these factors shape intestinal regionalization as the gut exhibits conspicuous morphological changes along the length of the digestive tract [52, 58].
Several of the findings described are likely to apply to the differentiation program that takes place in mammals. Since Notch signaling plays major roles in stem cell proliferation and cell fate specification from flies to mammals [57, 59], it would be interesting to decipher whether in mammals changes in progenitor contact also impact differentiation speed and whether a specific machinery can accelerate progenitor differentiation when tissue replenishment is required.
Materials and methods
Drosophila strains
Fly strains were kept on a standard medium (maize flour, dead yeast, agar and fruit juice). esg-Gal4, tub-Gal80TS, UAS-GFP (referred to as esgTS); Su(H)GBE-Gal4, tub-Gal80TS, UAS-GFP (referred to as Su(H)GBETS); esg-Gal4, tub-Gal80TS, UAS-GFP; UAS-Flp, Act>>Gal4 (referred to as esgF/O); MARCM tester FRT2A: y,w,hsFlp; tub-Gal4, UAS-CD8::GFP; FRT2A, tub-Gal80; MARCM tester FRT82B: y,w,hsFlp, tub-Gal4, UAS-nlsGFP;;FRT82B, tub-Gal80; Su(H)-lacZ, Sox21a6, UAS-Sox21a, UAS-Sox21a-RNAi (BL53991), UAS-Stat92E-RNAi (BL31318 and 35600) and UAS-N-RNAi (VDRC100002) have been described before [31]. Dl-GFP, UAS-hep, UAS-RafACT, UAS-lacZ, UAS-NICD, UAS-armS10, UAS-PanDN, UAS-E-cad-RNAi (BL27689), UAS-Con-RNAi (BL28967), UAS-Dl-RNAi (BL28032 and 34322), UAS-arm-RNAi (BL31304 and 31305), UAS-Mad-RNAi (BL31315), UAS-GATAe-RNAi (BL 34907), UAS-Notch-RNAi (VDRC, KK) and UAS-mCherry-RNAi were obtained from Bloomington Drosophila Stock Center (BDSC). Sox21a-GFP was from VDRC stock center. UAS-shn, UAS-Stat92E and UAS-domeDN (gift from Michael Boutros), FRT82B, NeurIF65 (gift from Allison Bardin), Dad-GFPnls (gift from Fisun Hamaratoglu), UAS-Con (gift from Rob White) and FRT82B, GATAe1 (gift from Takashi Adachi-Yamada) were also used. UAS-GATAe was generated in this study.
Dl-GFP (BL59819) encodes an endogenously GFP-tagged Dl protein resulting from recombination mediated cassette exchange of a Mi{MIC} insertion in the Dl coding intron [38]. This line is homozygous lethal. Sox21a-GFP transgenic line derives from a GFP-tagged fosmid clone containing a large genomic region including Sox21a [42]. In most cases, the driver lines (esgTS or Su(H)GBETS) were crossed to the w1118 strain, UAS-mCherry-RNAi, or UAS-lacZ, and the progenies were used as control for overexpression experiments. w1118 flies carrying one copy of esgTS (esgTS>w) were used as wild type to visualize the contact between progenitors in different conditions.
Oral infection with Ecc15 or other treatments
Erwinia carotovora carotovora15 (Ecc15) was grown in LB medium at 29°C with shaking overnight, and harvested by centrifugation at 3000g at 4°C for 30 minutes. The pellet was then suspended in the residual LB, and bacterial concentration was adjusted to OD600 = 200. Flies older than 3 days were first dry-starved in an empty tube for 2 hours, and then transferred into a classical fly food vial containing a filter paper that totally covers the food and was soaked with a solution consisting of 5% sucrose and Ecc15 at OD200 (1:1), or 6% DSS (average MW 40 kDa, sigma) treated flies were kept at 29°C until dissection. Colcemid treatment was done as reported previously [34]. 200ug/ml colcemid (Sigma) was added to 5% sucrose to pre-treat the flies for 12 hours, and then an Ecc15 infection was performed in the presence of 200ug/ml colcemid.
Immunohistochemistry and microscopy
Flies were transferred overnight into a classical fly food vial containing a filter paper soaked with a solution consisting of 5% sucrose to clean the digestive tract. Then, 10–15 intestines of mated adult females were dissected in phosphate-buffered saline (PBS), and fixed for at least one hour at room temperature in 4% paraformaldehyde (PFA) in PBS. They were subsequently rinsed in PBS+0.1% Triton X-100 (PBT), permeabilized and blocked in 2% BSA PBT for one hour, and incubated with primary antibodies in 2% BSA PBT for overnight at 4°C. After one hour of washing, secondary antibodies and DAPI were applied at room temperature for two hours.
Primary antibodies used are: mouse anti-Pros (DSHB, 1:100), mouse anti-Arm (DSHB, 1:100), mouse anti-Dl (DSHB, 1:100), mouse anti-βPS (DSHB, 1:100), mouse anti-Con (DSHB, 1:4), rabbit anti-pH3 (Millipore, 1:1000), Chicken anti-GFP (Abcam, 1:1000), rabbit anti-βGal (Cappel, 1:1000), mouse anti-βGal (Sigma, 1:1000), and Rat anti-mCherry (Life Technologies, 1:500). Alexa488-, Alexa555- or Alexa647-conjugated secondary antibodies (Life Technologies) were used. Nuclei were counterstained by DAPI (Sigma, 1:10’000). All the images were taken on a Zeiss LSM 700 confocal microscope at BIOP in EPFL. Images were processed using Image J and Adobe Photoshop software. Shown in figures are maximal intensity projections of all the confocal z stacks.
Generation of UAS-GATAe transgene
To generate the UAS-GATAe construct, the following primers (caccATGGTCTGCAAAACTATCTC and TTAGTTATTCGATGATCGCTC) were used to amplify the 2.2kb GATAe-PA coding regions from cDNA clone LD08432 purchased from DGRC. The PCR product was first cloned into pENTR-D-TOPO (Life Technologies) vector, and then swapped into pTW destination vector to make UAS-GATAe. Transgenic flies were established by standard P element-mediated germ-line transformation (BestGene Inc.). At least three independent transgenic lines were tested for expression level.
Conditional expression of UAS-linked transgenes
The TARGET system was used in combination with the indicated Gal4 drivers to conditionally express UAS-linked transgenes [60]. Flies were grown at 18°C to limit Gal4 activity. After 3–5 days at 18°C, adult flies with the appropriate genotypes were shifted to 29°C, a temperature inactivating the temperature-sensitive Gal80’s ability to suppress Gal4, and dissected after indicated time of transgene activation.
MARCM clone induction
Mosaic analysis with a repressible cell marker (MARCM) technique was used for clonal analysis [61]. For clone induction, 3-5-day-old flies with the appropriate genotypes were heat-shocked for 30 min at 37.5°C in a water bath. The flies were immediately transferred into a new tube and kept at 25°C or indicated temperature until dissection. Overexpression experiments were performed by combining the UAS-linked transgenes with the FRT2A, the FRT2A, Sox21a6, or the FRT82B, NeurIF65 chromosome. Note that UAS-linked transgenes were only expressed in the clones indicated by the presence of GFP.
FACS for RNA-seq and RNA-seq data analysis
esgTS virgin females were crossed to either w1118 as control or UAS-Sox21a for overexresspion at 18°C. Eclosed flies were maintained at 18°C for 5–7 days. Around 50 flies for each biological replicate were dissected in ice-cold 1xPBS made with DEPC-treated water under a dry-ice chilled dissecting microscope, within a one-hour time frame. Proventriculus, hindgut and midgut/hindgut junction were removed to collect only midgut esg>GFP positive cells. Two biological replicates were performed, and the activation of Sox21a expression was done by shifting esgTS>Sox21a flies to 29°C for 12 hours and 24 hours, respectively. Cell dissociation, FACS sorting, total RNA isolation and mRNA amplification were performed as described [31]. RNA-seq was performed on a Hi-Seq2000 (Illumina) with 100 nt single-end sequencing, and sequencing data was analyzed as described before [31]. Sequencing data will be deposited in public database prior to publication.
qRT-PCR analysis of gene expression
Total RNA was extracted from dissected whole guts (12–15 guts per sample) using Trizol and cDNA was synthesized using the PrimeScript RT reagent Kit (TaKaRa). 0.5μg total RNA was used for reverse transcription with oligo dT, and the 1st strand cDNA was diluted 10–20 times with water to be further used in real time PCR. Real time PCR was performed in triplicate for each sample using SYBR Green (Roche) on a LightCycler 480 System (Roche). Expression values were calculated using the ΔΔCt method and relative expression was normalized to Act5C. Results are shown as mean ± SEM of at least 3 independent biological samples. Statistical analysis was performed in Prism Software using the unpaired t test. Primers used for qPCR are as follows.
Dl: 5’TGTGAACATGGACATTGCGA3’ and 5’GTCTGTGGTTGGTGCAGTAG3’
Sox21a: 5’GGACAGAAGCGTCCATTCAT3’ and 5’TGACTTGTTGAGCGTCTTGG3’
Act5C: 5’CAGAGCAAGCGTGGTATCCT3’ and 5’GGTGTGGTGCCAGATCTTCT3’
Statistical analysis
All analyses were done with GraphPad Prism. Unpaired t test were used unless otherwise noted. p values are indicated by *p < 0.05, **p < 0.01, ***p < 0.001, ns: p > 0.05. Shown are means and SEM. Data are representative of at least three experiments. Each dot represents one gut except Fig 5E. Sample size is also indicated in the figures.
Supporting information
(A) Representative intestine of flies orally treated with 6% DSS for 20 hours. Note the presence of progenitor pairs with strong cell-cell contact as revealed by Arm staining (indicated by orange arrows). Pros (red, nuclei) marks EE. (B-C) Representative intestines of flies orally treated with 200ug/ml colcemid for 12 hours and then further challenged with Ecc15 for 10hours (C) and unchallenged control (B). Note that colcemid feeding arrests stem cells in metaphase and this treatment inhibits the formation of increased cell junction that is normally induced by Ecc15 infection. Cells in metaphase were marked with an antibody against Phospho-Histone H3 (PH3, a mitotic marker, in white). (D-E) Two representative images of midgut from Sox21a6 mutant flies orally infected with Ecc15 for 10 hours. Long junctions between progenitors (indicated with orange arrows and marked by Arm staining) form normally. (F) Representative image of midgut from esgTS>Notch-IR flies shifted to 29°C for 4 days to induce Notch tumor formation. Note the presence of long junctions (shown with esg>mCD8::GFP) between ISCs within each cluster.
(TIF)
(A-G) Representative images of progenitor cells from intestines of flies orally infected with Ecc15. ISCs are marked with Dl-GFP (green) and EBs with Su(H)-lacZ (red). Other markers (βPS, Dl, Prospero or PH3) are shown in gray. βPS (beta-integrin) highlights the basal extracellular matrix, Prospero marks EEs and PH3 is a mitotic marker. Images in (A-B) are sagittal views of the intestinal epithelium and others (C-G) show frontal plane. Note that the two cells in the ISC pair in (A) are both basally localized, but one of the two ISCs expresses weak Notch reporter (red). In comparison, progenitors with strong Notch activity exhibit more apical localization (B). A mitotic cell is shown in (E). (F and G) show ISC pairs that have just derived from an ISC division, and one cell within the ISC-ISC pair in (G) expresses weak Su(H)-lacZ.
(TIF)
(A-D) Sox21a expression in control flies (A) and flies over-expressing hep (hemipterous, encoding the JNK kinase), B) or RafACT (C-D) in intestinal progenitors using esgTS for 36 hours. Sox21a expression is monitored by the Sox21a-GFP transgene (green). Mitotic cells were marked with an antibody against Phospho-Histone H3 (PH3, a mitotic marker, in red). Note that the higher levels of Sox21a reporter expression are found in nuclei of large size (indicated with yellow arrows in B-D) within progenitor cells, presumably differentiating EBs. Strikingly, expression of RafACT generated giant progenitors with nuclei of larger size than that of surrounding enterocytes, in line with the potent growth-promoting function of Ras/MAPK signaling. (E) Quantification of mitotic index in the midgut of flies with the indicated genotype. (F) Sagittal view of the midgut progenitors expressing Sox21a for 36 hours. Progenitors (marked by esg>GFP), E-Cadherin junction (marked by Arm) and nuclei (DAPI) are shown. In each case, esg>GFPweak cells maintain a strong contact with another more basally localized esg>GFPstrong cell, as revealed by increased Arm staining in the junction. Each dot represents one gut.
(TIF)
qPCR measurement of mRNA levels of Sox21a and Dl in dissected midgut of flies with indicated genotypes after activation of transgene expression for 36 hours. Expression is normalized to Act5C.
(TIF)
(A-I) Representative intestines of esgTS>Sox21a flies co-expressing various transgenes (as indicated) for 36 hours. Progenitor cells are shown separately on the right panel (revealed by esg>GFP). Left panel shows the merge of DAPI (blue), esg>GFP (green) and Arm (red) channels. Prospero (EE marker) is also shown in a subset of images (A, B, D, F, G and I). Full quantification of the differentiation phenotype is shown in Fig 4A.
(TIF)
(A) Immunostaining of midgut progenitors overexpressing Connectin (esgTS>Connectin) with an anti-Connectin antibody. Note that Connectin is localized to the membrane junctions between progenitors. (B-C) Control intestine (B) and progenitor-specific expression of Connectin (C) using esgTS for 4 days. Note the absence of EEs (Pros+) from the region with big cluster of esg>GFP+ cells. esg>GFP (in green), Arm (in red, membrane), Pros (in red, nuclei, indicated with yellow arrows) and DAPI staining are shown. (D-G) Midguts of flies with indicated genotype shifted to 29°C for 4 days and then either challenged with Ecc15 for 12hours (F-G) or unchallenged (UN, D-E). Orange arrows indicate differentiating EBs. Mitotic cells are marked with PH3 in red. (H) Quantification of mitotic index in the midgut of flies with the indicated genotype 4 days after transgene expression. Each dot represents one gut. (I-J) Midgut turnover revealed by the esgF/O system with control (I) or Connectin (J) overexpression for 7 days at 29°C. Note that the number of Pros (in red, nuclei, indicated with yellow arrows) expressing cells is largely reduced in (J). (K-M) basal-level midgut turnover revealed by the esgF/O system with control (K), E-cad (L) or Connectin (M) knockdown for 2 weeks at 29°C.
(TIF)
(A-B) Images of intestines from fly co-expressing Sox21a and Notch-RNAi using esgTS for 36 hours. Progenitors are shown with both esg>GFP and Arm. Likely due to the relative efficiency between Sox21a overexpression and Notch depletion, half number of the intestines (n = 28) develop ISC tumors (as shown in A), and half possess both small ISC tumors and differentiating EBs (as shown in B). (C-D) A NeurIF65 mutant MARCM clone (C) and a NeurIF65 mutant clone co-expressing Sox21a (D) are analyzed 14 days after clone induction. Neuralized (Neur) encodes for an E3 ubiquitin ligase that is essential for Notch signaling. Note that ISC tumors produced in NeurIF65 mutant clones are not suppressed by the co-expression of Sox21a, indicating that functional Notch signaling is a prerequisite for Sox21a-induced EC differentiation.
(TIF)
(A-B) Wild type MARCM clones (positively labeled by GFP, A) and clones mutant for GATAe (B) are analyzed 4 days after clone induction. EEs express Prospero (nuclei), and ECs are marked by Pdm1. Note that EC or EE differentiation was not blocked in GATAe mutant clone.
(TIF)
(A-B) Representative images of control (A) and Ecc15-infected (B) intestines. EBs are visualized by the expression of Su(H)GBE>GFP (green). Nuclei are stained by DAPI. (C) Representative EBs in the course of maturation toward EC, redrawn from (B). In the absence of challenge, nascent EBs from 5–7 day-old adults exhibits a small size (A). Oral ingestion of Ecc15 causes damage to the intestinal epithelium and quickly triggers ISC activity for regeneration. Under this condition, all the intermediate states between a nascent EB and a mature EC become detectable (C).
(TIF)
The datasets were generated from FACS-sorted progenitors of flies overexpressing Sox21a with esgTS for 12 or 24 hours, and compared to control (esgTS>w). Only differentially expressed genes (12h vs Control, and 24h vs Control) with p value less than 0.05 are listed. Expression value, fold change, p value, gene name and GO terms are included.
(XLS)
Acknowledgments
We thank Drs. Claudine Neyen and Yu Cai for comments on the manuscript; Toshiyuki Harumoto for discussion; Takashi Adachi-Yamada, Fisun Hamaratoglu, Rob While, Michael Boutros, Allison Bardin, BDSC, VDRC for fly stocks; DSHB for antibodies; FCCF and BIOP platforms at EPFL, the Lausanne Genomic Technologies Facility for technical help.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This project was supported by the SNSF grant 3100A0-12079/1 to BL. ZZ was supported by a Marie-Curie IEF fellowship (gutENCODE). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nature reviews Molecular cell biology. 2014;15(1):19–33. Epub 2013/12/12. doi: 10.1038/nrm3721 . [DOI] [PubMed] [Google Scholar]
- 2.Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP, et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes & development. 2004;18(12):1385–90. doi: 10.1101/gad.287404 ; PubMed Central PMCID: PMCPMC423189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muller T, Hess MW, Schiefermeier N, Pfaller K, Ebner HL, Heinz-Erian P, et al. MYO5B mutations cause microvillus inclusion disease and disrupt epithelial cell polarity. Nature genetics. 2008;40(10):1163–5. doi: 10.1038/ng.225 . [DOI] [PubMed] [Google Scholar]
- 4.Jiang H, Edgar BA. Intestinal stem cell function in Drosophila and mice. Current opinion in genetics & development. 2012;22(4):354–60. Epub 2012/05/23. doi: 10.1016/j.gde.2012.04.002 ; PubMed Central PMCID: PMC3426656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Brien LE, Soliman SS, Li X, Bilder D. Altered modes of stem cell division drive adaptive intestinal growth. Cell. 2011;147(3):603–14. Epub 2011/11/01. doi: 10.1016/j.cell.2011.08.048 ; PubMed Central PMCID: PMC3246009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng H, Gerencser AA, Jasper H. Signal integration by Ca(2+) regulates intestinal stem-cell activity. Nature. 2015;528(7581):212–7. Epub 2015/12/04. doi: 10.1038/nature16170 ; PubMed Central PMCID: PMC4669953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi NH, Lucchetta E, Ohlstein B. Nonautonomous regulation of Drosophila midgut stem cell proliferation by the insulin-signaling pathway. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(46):18702–7. doi: 10.1073/pnas.1109348108 ; PubMed Central PMCID: PMCPMC3219098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regan JC, Khericha M, Dobson AJ, Bolukbasi E, Rattanavirotkul N, Partridge L. Sex difference in pathology of the ageing gut mediates the greater response of female lifespan to dietary restriction. eLife. 2016;5 doi: 10.7554/eLife.10956 ; PubMed Central PMCID: PMCPMC4805549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudry B, Khadayate S, Miguel-Aliaga I. The sexual identity of adult intestinal stem cells controls organ size and plasticity. Nature. 2016;530(7590):344–8. doi: 10.1038/nature16953 ; PubMed Central PMCID: PMCPMC4800002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reiff T, Jacobson J, Cognigni P, Antonello Z, Ballesta E, Tan KJ, et al. Endocrine remodelling of the adult intestine sustains reproduction in Drosophila. eLife. 2015;4:e06930 doi: 10.7554/eLife.06930 ; PubMed Central PMCID: PMCPMC4515472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi NH, Kim JG, Yang DJ, Kim YS, Yoo MA. Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging cell. 2008;7(3):318–34. doi: 10.1111/j.1474-9726.2008.00380.x ; PubMed Central PMCID: PMCPMC2408640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biteau B, Hochmuth CE, Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell stem cell. 2008;3(4):442–55. Epub 2008/10/23. doi: 10.1016/j.stem.2008.07.024 ; PubMed Central PMCID: PMC3225008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H, Zheng X, Zheng Y. Age-associated loss of lamin-B leads to systemic inflammation and gut hyperplasia. Cell. 2014;159(4):829–43. doi: 10.1016/j.cell.2014.10.028 ; PubMed Central PMCID: PMCPMC4243052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137(7):1343–55. Epub 2009/07/01. doi: 10.1016/j.cell.2009.05.014 ; PubMed Central PMCID: PMC2753793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell host & microbe. 2009;5(2):200–11. Epub 2009/02/17. doi: 10.1016/j.chom.2009.01.003 . [DOI] [PubMed] [Google Scholar]
- 16.Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell stem cell. 2009;4(1):49–61. Epub 2009/01/09. doi: 10.1016/j.stem.2008.10.016 ; PubMed Central PMCID: PMC2659574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakrabarti S, Dudzic JP, Li X, Collas EJ, Boquete JP, Lemaitre B. Remote Control of Intestinal Stem Cell Activity by Haemocytes in Drosophila. PLoS genetics. 2016;12(5):e1006089 doi: 10.1371/journal.pgen.1006089 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takeishi A, Kuranaga E, Tonoki A, Misaki K, Yonemura S, Kanuka H, et al. Homeostatic epithelial renewal in the gut is required for dampening a fatal systemic wound response in Drosophila. Cell reports. 2013;3(3):919–30. doi: 10.1016/j.celrep.2013.02.022 . [DOI] [PubMed] [Google Scholar]
- 19.Beehler-Evans R, Micchelli CA. Generation of enteroendocrine cell diversity in midgut stem cell lineages. Development. 2015;142(4):654–64. doi: 10.1242/dev.114959 ; PubMed Central PMCID: PMCPMC4325375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biteau B, Jasper H. Slit/Robo signaling regulates cell fate decisions in the intestinal stem cell lineage of Drosophila. Cell reports. 2014;7(6):1867–75. doi: 10.1016/j.celrep.2014.05.024 ; PubMed Central PMCID: PMCPMC4086754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng X, Hou SX. Enteroendocrine cells are generated from stem cells through a distinct progenitor in the adult Drosophila posterior midgut. Development. 2015;142(4):644–53. Epub 2015/02/12. doi: 10.1242/dev.113357 ; PubMed Central PMCID: PMC4325374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439(7075):475–9. Epub 2005/12/13. doi: 10.1038/nature04371 . [DOI] [PubMed] [Google Scholar]
- 23.Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439(7075):470–4. Epub 2005/12/13. doi: 10.1038/nature04333 . [DOI] [PubMed] [Google Scholar]
- 24.Guo Z, Lucchetta E, Rafel N, Ohlstein B. Maintenance of the adult Drosophila intestine: all roads lead to homeostasis. Current opinion in genetics & development. 2016;40:81–6. doi: 10.1016/j.gde.2016.06.009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315(5814):988–92. Epub 2007/02/17. doi: 10.1126/science.1136606 . [DOI] [PubMed] [Google Scholar]
- 26.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–60. doi: 10.1146/annurev.physiol.010908.163145 . [DOI] [PubMed] [Google Scholar]
- 27.Beebe K, Lee WC, Micchelli CA. JAK/STAT signaling coordinates stem cell proliferation and multilineage differentiation in the Drosophila intestinal stem cell lineage. Developmental biology. 2010;338(1):28–37. Epub 2009/11/10. doi: 10.1016/j.ydbio.2009.10.045 . [DOI] [PubMed] [Google Scholar]
- 28.Korzelius J, Naumann SK, Loza-Coll MA, Chan JS, Dutta D, Oberheim J, et al. Escargot maintains stemness and suppresses differentiation in Drosophila intestinal stem cells. The EMBO journal. 2014;33(24):2967–82. Epub 2014/10/10. doi: 10.15252/embj.201489072 ; PubMed Central PMCID: PMC4282643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antonello ZA, Reiff T, Ballesta-Illan E, Dominguez M. Robust intestinal homeostasis relies on cellular plasticity in enteroblasts mediated by miR-8-Escargot switch. The EMBO journal. 2015;34(15):2025–41. Epub 2015/06/17. doi: 10.15252/embj.201591517 ; PubMed Central PMCID: PMC4551350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loza-Coll MA, Southall TD, Sandall SL, Brand AH, Jones DL. Regulation of Drosophila intestinal stem cell maintenance and differentiation by the transcription factor Escargot. The EMBO journal. 2014;33(24):2983–96. doi: 10.15252/embj.201489050 ; PubMed Central PMCID: PMCPMC4282644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhai Z, Kondo S, Ha N, Boquete JP, Brunner M, Ueda R, et al. Accumulation of differentiating intestinal stem cell progenies drives tumorigenesis. Nature communications. 2015;6:10219 Epub 2015/12/23. doi: 10.1038/ncomms10219 ; PubMed Central PMCID: PMC4703904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J, Xu N, Huang H, Cai T, Xi R. A feedback amplification loop between stem cells and their progeny promotes tissue regeneration and tumorigenesis. eLife. 2016;5 doi: 10.7554/eLife.14330 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okumura T, Takeda K, Kuchiki M, Akaishi M, Taniguchi K, Adachi-Yamada T. GATAe regulates intestinal stem cell maintenance and differentiation in Drosophila adult midgut. Developmental biology. 2016;410(1):24–35. doi: 10.1016/j.ydbio.2015.12.017 . [DOI] [PubMed] [Google Scholar]
- 34.Guo Z, Driver I, Ohlstein B. Injury-induced BMP signaling negatively regulates Drosophila midgut homeostasis. The Journal of cell biology. 2013;201(6):945–61. doi: 10.1083/jcb.201302049 ; PubMed Central PMCID: PMCPMC3678160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian A, Jiang J. Intestinal epithelium-derived BMP controls stem cell self-renewal in Drosophila adult midgut. eLife. 2014;3:e01857 Epub 2014/03/13. doi: 10.7554/eLife.01857 ; PubMed Central PMCID: PMC3948108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou J, Florescu S, Boettcher AL, Luo L, Dutta D, Kerr G, et al. Dpp/Gbb signaling is required for normal intestinal regeneration during infection. Developmental biology. 2015;399(2):189–203. Epub 2015/01/03. doi: 10.1016/j.ydbio.2014.12.017 . [DOI] [PubMed] [Google Scholar]
- 37.Bray SJ. Notch signalling in context. Nature reviews Molecular cell biology. 2016;17(11):722–35. doi: 10.1038/nrm.2016.94 . [DOI] [PubMed] [Google Scholar]
- 38.Nagarkar-Jaiswal S, Lee PT, Campbell ME, Chen K, Anguiano-Zarate S, Gutierrez MC, et al. A library of MiMICs allows tagging of genes and reversible, spatial and temporal knockdown of proteins in Drosophila. eLife. 2015;4 Epub 2015/04/01. doi: 10.7554/eLife.05338 ; PubMed Central PMCID: PMC4379497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeng X, Chauhan C, Hou SX. Characterization of midgut stem cell- and enteroblast-specific Gal4 lines in drosophila. Genesis. 2010;48(10):607–11. Epub 2010/08/04. doi: 10.1002/dvg.20661 ; PubMed Central PMCID: PMC2958251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furriols M, Bray S. Dissecting the mechanisms of suppressor of hairless function. Developmental biology. 2000;227(2):520–32. Epub 2000/11/10. doi: 10.1006/dbio.2000.9923 . [DOI] [PubMed] [Google Scholar]
- 41.Goulas S, Conder R, Knoblich JA. The Par complex and integrins direct asymmetric cell division in adult intestinal stem cells. Cell stem cell. 2012;11(4):529–40. Epub 2012/10/09. doi: 10.1016/j.stem.2012.06.017 ; PubMed Central PMCID: PMC3465556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarov M, Barz C, Jambor H, Hein MY, Schmied C, Suchold D, et al. A genome-wide resource for the analysis of protein localisation in Drosophila. eLife. 2016;5 doi: 10.7554/eLife.12068 ; PubMed Central PMCID: PMCPMC4805545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buchon N, Broderick NA, Chakrabarti S, Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes & development. 2009;23(19):2333–44. Epub 2009/10/03. doi: 10.1101/gad.1827009 ; PubMed Central PMCID: PMC2758745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang H, Grenley MO, Bravo MJ, Blumhagen RZ, Edgar BA. EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell stem cell. 2011;8(1):84–95. Epub 2010/12/21. doi: 10.1016/j.stem.2010.11.026 ; PubMed Central PMCID: PMC3021119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meng FW, Biteau B. A Sox Transcription Factor Is a Critical Regulator of Adult Stem Cell Proliferation in the Drosophila Intestine. Cell reports. 2015;13(5):906–14. doi: 10.1016/j.celrep.2015.09.061 . [DOI] [PubMed] [Google Scholar]
- 46.van Roy F, Berx G. The cell-cell adhesion molecule E-cadherin. Cell Mol Life Sci. 2008;65(23):3756–88. doi: 10.1007/s00018-008-8281-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeanes A, Gottardi CJ, Yap AS. Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene. 2008;27(55):6920–9. doi: 10.1038/onc.2008.343 ; PubMed Central PMCID: PMCPMC2745643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maeda K, Takemura M, Umemori M, Adachi-Yamada T. E-cadherin prolongs the moment for interaction between intestinal stem cell and its progenitor cell to ensure Notch signaling in adult Drosophila midgut. Genes to cells: devoted to molecular & cellular mechanisms. 2008;13(12):1219–27. doi: 10.1111/j.1365-2443.2008.01239.x . [DOI] [PubMed] [Google Scholar]
- 49.Nose A, Mahajan VB, Goodman CS. Connectin: a homophilic cell adhesion molecule expressed on a subset of muscles and the motoneurons that innervate them in Drosophila. Cell. 1992;70(4):553–67. . [DOI] [PubMed] [Google Scholar]
- 50.Raghavan S, White RA. Connectin mediates adhesion in Drosophila. Neuron. 1997;18(6):873–80. . [DOI] [PubMed] [Google Scholar]
- 51.Patel PH, Dutta D, Edgar BA. Niche appropriation by Drosophila intestinal stem cell tumours. Nature cell biology. 2015;17(9):1182–92. Epub 2015/08/04. doi: 10.1038/ncb3214 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buchon N, Osman D, David FP, Fang HY, Boquete JP, Deplancke B, et al. Morphological and molecular characterization of adult midgut compartmentalization in Drosophila. Cell reports. 2013;3(5):1725–38. Epub 2013/05/07. doi: 10.1016/j.celrep.2013.04.001 . [DOI] [PubMed] [Google Scholar]
- 53.Guisoni N, Martinez-Corral R, Garcia-Ojalvo J, de Navascues J. Diversity of fate outcomes in cell pairs under lateral inhibition. Development. 2017;144(7):1177–86. doi: 10.1242/dev.137950 . [DOI] [PubMed] [Google Scholar]
- 54.Shaya O, Binshtok U, Hersch M, Rivkin D, Weinreb S, Amir-Zilberstein L, et al. Cell-Cell Contact Area Affects Notch Signaling and Notch-Dependent Patterning. Developmental cell. 2017;40(5):505–11 e6. doi: 10.1016/j.devcel.2017.02.009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Navascues J, Perdigoto CN, Bian Y, Schneider MH, Bardin AJ, Martinez-Arias A, et al. Drosophila midgut homeostasis involves neutral competition between symmetrically dividing intestinal stem cells. The EMBO journal. 2012;31(11):2473–85. doi: 10.1038/emboj.2012.106 ; PubMed Central PMCID: PMCPMC3365418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Montagne C, Gonzalez-Gaitan M. Sara endosomes and the asymmetric division of intestinal stem cells. Development. 2014;141(10):2014–23. doi: 10.1242/dev.104240 . [DOI] [PubMed] [Google Scholar]
- 57.Perdigoto CN, Bardin AJ. Sending the right signal: Notch and stem cells. Biochimica et biophysica acta. 2013;1830(2):2307–22. doi: 10.1016/j.bbagen.2012.08.009 . [DOI] [PubMed] [Google Scholar]
- 58.Marianes A, Spradling AC. Physiological and stem cell compartmentalization within the Drosophila midgut. eLife. 2013;2:e00886 doi: 10.7554/eLife.00886 ; PubMed Central PMCID: PMCPMC3755342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koch U, Lehal R, Radtke F. Stem cells living with a Notch. Development. 2013;140(4):689–704. doi: 10.1242/dev.080614 . [DOI] [PubMed] [Google Scholar]
- 60.McGuire SE, Mao Z, Davis RL. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Science's STKE: signal transduction knowledge environment. 2004;2004(220):pl6 Epub 2004/02/19. doi: 10.1126/stke.2202004pl6 . [DOI] [PubMed] [Google Scholar]
- 61.Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends in neurosciences. 2001;24(5):251–4. Epub 2001/04/20. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Representative intestine of flies orally treated with 6% DSS for 20 hours. Note the presence of progenitor pairs with strong cell-cell contact as revealed by Arm staining (indicated by orange arrows). Pros (red, nuclei) marks EE. (B-C) Representative intestines of flies orally treated with 200ug/ml colcemid for 12 hours and then further challenged with Ecc15 for 10hours (C) and unchallenged control (B). Note that colcemid feeding arrests stem cells in metaphase and this treatment inhibits the formation of increased cell junction that is normally induced by Ecc15 infection. Cells in metaphase were marked with an antibody against Phospho-Histone H3 (PH3, a mitotic marker, in white). (D-E) Two representative images of midgut from Sox21a6 mutant flies orally infected with Ecc15 for 10 hours. Long junctions between progenitors (indicated with orange arrows and marked by Arm staining) form normally. (F) Representative image of midgut from esgTS>Notch-IR flies shifted to 29°C for 4 days to induce Notch tumor formation. Note the presence of long junctions (shown with esg>mCD8::GFP) between ISCs within each cluster.
(TIF)
(A-G) Representative images of progenitor cells from intestines of flies orally infected with Ecc15. ISCs are marked with Dl-GFP (green) and EBs with Su(H)-lacZ (red). Other markers (βPS, Dl, Prospero or PH3) are shown in gray. βPS (beta-integrin) highlights the basal extracellular matrix, Prospero marks EEs and PH3 is a mitotic marker. Images in (A-B) are sagittal views of the intestinal epithelium and others (C-G) show frontal plane. Note that the two cells in the ISC pair in (A) are both basally localized, but one of the two ISCs expresses weak Notch reporter (red). In comparison, progenitors with strong Notch activity exhibit more apical localization (B). A mitotic cell is shown in (E). (F and G) show ISC pairs that have just derived from an ISC division, and one cell within the ISC-ISC pair in (G) expresses weak Su(H)-lacZ.
(TIF)
(A-D) Sox21a expression in control flies (A) and flies over-expressing hep (hemipterous, encoding the JNK kinase), B) or RafACT (C-D) in intestinal progenitors using esgTS for 36 hours. Sox21a expression is monitored by the Sox21a-GFP transgene (green). Mitotic cells were marked with an antibody against Phospho-Histone H3 (PH3, a mitotic marker, in red). Note that the higher levels of Sox21a reporter expression are found in nuclei of large size (indicated with yellow arrows in B-D) within progenitor cells, presumably differentiating EBs. Strikingly, expression of RafACT generated giant progenitors with nuclei of larger size than that of surrounding enterocytes, in line with the potent growth-promoting function of Ras/MAPK signaling. (E) Quantification of mitotic index in the midgut of flies with the indicated genotype. (F) Sagittal view of the midgut progenitors expressing Sox21a for 36 hours. Progenitors (marked by esg>GFP), E-Cadherin junction (marked by Arm) and nuclei (DAPI) are shown. In each case, esg>GFPweak cells maintain a strong contact with another more basally localized esg>GFPstrong cell, as revealed by increased Arm staining in the junction. Each dot represents one gut.
(TIF)
qPCR measurement of mRNA levels of Sox21a and Dl in dissected midgut of flies with indicated genotypes after activation of transgene expression for 36 hours. Expression is normalized to Act5C.
(TIF)
(A-I) Representative intestines of esgTS>Sox21a flies co-expressing various transgenes (as indicated) for 36 hours. Progenitor cells are shown separately on the right panel (revealed by esg>GFP). Left panel shows the merge of DAPI (blue), esg>GFP (green) and Arm (red) channels. Prospero (EE marker) is also shown in a subset of images (A, B, D, F, G and I). Full quantification of the differentiation phenotype is shown in Fig 4A.
(TIF)
(A) Immunostaining of midgut progenitors overexpressing Connectin (esgTS>Connectin) with an anti-Connectin antibody. Note that Connectin is localized to the membrane junctions between progenitors. (B-C) Control intestine (B) and progenitor-specific expression of Connectin (C) using esgTS for 4 days. Note the absence of EEs (Pros+) from the region with big cluster of esg>GFP+ cells. esg>GFP (in green), Arm (in red, membrane), Pros (in red, nuclei, indicated with yellow arrows) and DAPI staining are shown. (D-G) Midguts of flies with indicated genotype shifted to 29°C for 4 days and then either challenged with Ecc15 for 12hours (F-G) or unchallenged (UN, D-E). Orange arrows indicate differentiating EBs. Mitotic cells are marked with PH3 in red. (H) Quantification of mitotic index in the midgut of flies with the indicated genotype 4 days after transgene expression. Each dot represents one gut. (I-J) Midgut turnover revealed by the esgF/O system with control (I) or Connectin (J) overexpression for 7 days at 29°C. Note that the number of Pros (in red, nuclei, indicated with yellow arrows) expressing cells is largely reduced in (J). (K-M) basal-level midgut turnover revealed by the esgF/O system with control (K), E-cad (L) or Connectin (M) knockdown for 2 weeks at 29°C.
(TIF)
(A-B) Images of intestines from fly co-expressing Sox21a and Notch-RNAi using esgTS for 36 hours. Progenitors are shown with both esg>GFP and Arm. Likely due to the relative efficiency between Sox21a overexpression and Notch depletion, half number of the intestines (n = 28) develop ISC tumors (as shown in A), and half possess both small ISC tumors and differentiating EBs (as shown in B). (C-D) A NeurIF65 mutant MARCM clone (C) and a NeurIF65 mutant clone co-expressing Sox21a (D) are analyzed 14 days after clone induction. Neuralized (Neur) encodes for an E3 ubiquitin ligase that is essential for Notch signaling. Note that ISC tumors produced in NeurIF65 mutant clones are not suppressed by the co-expression of Sox21a, indicating that functional Notch signaling is a prerequisite for Sox21a-induced EC differentiation.
(TIF)
(A-B) Wild type MARCM clones (positively labeled by GFP, A) and clones mutant for GATAe (B) are analyzed 4 days after clone induction. EEs express Prospero (nuclei), and ECs are marked by Pdm1. Note that EC or EE differentiation was not blocked in GATAe mutant clone.
(TIF)
(A-B) Representative images of control (A) and Ecc15-infected (B) intestines. EBs are visualized by the expression of Su(H)GBE>GFP (green). Nuclei are stained by DAPI. (C) Representative EBs in the course of maturation toward EC, redrawn from (B). In the absence of challenge, nascent EBs from 5–7 day-old adults exhibits a small size (A). Oral ingestion of Ecc15 causes damage to the intestinal epithelium and quickly triggers ISC activity for regeneration. Under this condition, all the intermediate states between a nascent EB and a mature EC become detectable (C).
(TIF)
The datasets were generated from FACS-sorted progenitors of flies overexpressing Sox21a with esgTS for 12 or 24 hours, and compared to control (esgTS>w). Only differentially expressed genes (12h vs Control, and 24h vs Control) with p value less than 0.05 are listed. Expression value, fold change, p value, gene name and GO terms are included.
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.