Abstract
IN BRIEF This study reports performance of A1C against the oral glucose tolerance test (OGTT) in predicting prediabetes among overweight and obese African-American and Caribbean children. A retrospective chart review was completed for 230 children. Receiver operating characteristic curves were generated to find the predictive performances of different tests against the OGTT. A1C alone is a poor discriminator of prediabetes in our study population, with low sensitivity (70%) and specificity (48.8%). BMI z score, A1C, and homeostatic model assessment of insulin resistance are significant predictors of prediabetes and, when taken together, provide better discrimination for prediabetes.
Obesity is on the rise worldwide and has been described as a global pandemic (1). In 2011–2012, the prevalence of obesity was 16.9% in American youth (2). With an increasing incidence of obesity among children, health care providers must recognize and diagnose comorbidities of obesity such as diabetes and prediabetes early in their development.
Prediabetes, typically defined as blood glucose concentrations higher than normal but lower than diabetes thresholds, is a high-risk state for diabetes development. Evidence supports an association between prediabetes in childhood and development of diabetes in young adulthood (3). The prevalence of prediabetes among adolescents is 16.1% and has been increasing (4).
Although there is general consensus regarding the need for diabetes screening in high-risk children and adolescents, controversy persists regarding the most appropriate screening methodologies. In 2009, an International Expert Committee recommended using A1C as a diagnostic tool for diabetes and prediabetes (5), which was endorsed by the American Diabetes Association (ADA) in 2010 (6). Before this, blood glucose analysis was the exclusive method for diagnosing diabetes. One major limitation was that this change was based on epidemiological studies in the adult population only (7,8).
In 2017, ADA continues to recommend diabetes screening using A1C, especially in those who are overweight (BMI ≥85th percentile for age and sex) with two of the following risk factors: 1) first- or second-degree relative with type 2 diabetes, 2) minority race/ethnicity, 3) signs of insulin resistance (e.g., acanthosis nigricans) or conditions associated with insulin resistance (e.g., hypertension, dyslipidemia, polycystic ovary syndrome, small-for-gestational-age birth weight), or 4) mother with diabetes or gestational diabetes during child’s gestation (9). Pediatricians have followed this guideline by screening patients for prediabetes and diabetes using random measures such as A1C, among others.
A1C measures nonenzymatic glycosylation of hemoglobin and can be used reliably for the diagnosis of prediabetes and diabetes in adults as long as the assay is approved by the National Glycohemoglobin Standardization Program (NGSP) (www.ngsp.org), which standardizes >99% of the assays used in the United States to the Diabetes Control and Complications Trial standard. Diagnostic criteria for prediabetes include an A1C of 5.7–6.4% (39–46 mmol/mol); a fasting plasma glucose (FPG) level of 100–125 mg/dL (5.6–6.9 mmol/l), indicating impaired fasting glucose (IFG); or a 2-hour glucose level of 140–199 mg/dL (7.8–11.0 mmol/l) during a 75-g oral glucose tolerance test (OGTT), indicating impaired glucose tolerance (IGT) (9–11). According to ADA guidelines, the same criteria apply to the pediatric population (9).
The OGTT, which is considered the gold standard for diagnosing prediabetes and diabetes, is subject to limitations, including the need for patients to be fasting. A1C, a random measurement, does not require fasting and is therefore more convenient. A1C values are relatively stable after collection (12) and reflect approximately 3 months of glycemia. A1C has been shown to have less day-to-day, as well as inter- and intra-subject variability than plasma glucose concentrations (13,14). However, despite NGSP standardization, intra-subject variations in A1C results have been observed among obese youth when using two different NGSP-certified methodologies (15). Additionally, conditions involving high red blood cell turnover, including hemoglobinopathies, anemia, pregnancy, recent blood loss or transfusion, hemolysis, or erythropoietin use, interfere with the reliability of A1C as a glycemic indicator (9).
Adult studies have shown that A1C is a good predictor of diabetes-related complications (16). However, studies in children and adolescents have demonstrated that A1C has lower sensitivity and specificity than OGTT in the diagnosis of both prediabetes and diabetes (17). Nowicka et al. (18) demonstrated that, among children and adolescents, A1C values between 5.7 and 6.4% have only 47% concordance with OGTT, whereas A1C ≥6.5% have 62% concordance with OGTT. Although the authors noted that de-creasing the A1C threshold to 5.8% would improve the sensitivity and specificity of A1C for identifying type 2 diabetes, they concluded that A1C should not be used alone for diagnosing prediabetes or diabetes. Similarly, Lee et al. (19) found that the A1C cut-off value of 5.7% has only 32% sensitivity and 74% specificity for predicting dysglycemia (diabetes or prediabetes). These authors advocated using a random glucose level of 100 or 110 mg/dL or a 1-hour glucose challenge test value of 110 or 120 mg/dL in clinical practice because of the higher predictive value of these tests. In a middle-school cohort, Buse et al. (20) determined that A1C does not define the same group of youth with increased diabetes risk as is defined by IFG using the OGTT. Few studies in children have examined insulin resistance parameters and prediabetes predictors as determined by OGTT.
The aims of this study are to determine the association between A1C and prediabetes as defined by OGTT and to identify metabolic parameters and anthropometric measures that are associated with prediabetes.
Methods
An institutional review board–approved retrospective chart review was completed for children and adolescents with a BMI at or above the 85th percentile for age and sex who were seen in the pediatric endocrine service at SUNY Downstate Medical Center and Kings County Hospital Center in the past 10 years (January 2005 to August 2015). All patients had A1C and 2-h OGTT testing within 3 months of the clinic visit date. BMI percentiles and z scores were obtained based on 2000 Centers for Disease Control and Prevention growth charts (21). Patients with diabetes, anemia, or metformin use were excluded.
Study subjects were divided into two groups (prediabetes and normal) based on OGTT results. Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated using the formula (22):
FPG (mg/dL) × fasting serum insulin (mU/L)/405
Area under the curve (AUC) for glucose and insulin was calculated by trapezoid rule.
Definitions
ADA definitions for prediabetes were used. Prediabetes based on OGTT was defined as IFG (FPG 100–125 mg/dL) or IGT (OGTT 2-h glucose 140–199 mg/dL), or both. Prediabetes based on A1C was defined as an A1C value ranging from 5.7 to 6.4%. Dyslipidemia was defined as meeting one of the following criteria: triglycerides ≥100 mg/dL (0–9 years of age) or ≥130 mg/dL (10–21 years), HDL cholesterol <40 mg/dL, LDL cholesterol ≥130 mg/dL, or total cholesterol ≥ 200 mg/dL (23).
Biochemical Assays
A1C was measured by high-performance liquid chromatography using Bio-Rad Variant II Turbo 2.0 (Bio-Rad Laboratories, Hercules, Calif.) standardized per NGSP standards. Serum insulin levels were determined by electrochemiluminescence immunoassay on a Roche Modular E170 analyzer (Diamond Diagnostics, Holliston, Mass.) and on an ADVIA centaur XP system (Siemens Medical Solutions, Malvern, Pa.). Plasma glucose was determined by enzymatic UV test (hexokinase method) on a Beckman coulter analyzer (AU2700 and AU5821 systems; Beckman Coulter, Indianapolis, Ind.) and hexokinase enzymatic method (Roche modular E170).
Statistical Analysis
Comparison of the two groups was performed with χ2, Mann-Whitney U, and t tests. The χ2 test was used to determine an association between A1C and OGTT. Receiver operating characteristic (ROC) curves were generated, and the area under the ROC curve (AUC-ROC) was used to determine the performance of predictors for prediabetes. Stepwise logistic regression was used to determine predictors significantly associated with prediabetes. P values <0.05 were considered statistically significant. Continuous variables are presented as means and SD when normally distributed or medians and quartiles (25th–75th percentile) when variables are skewed.
Results
A total of 301 charts were reviewed, of which 230 met the inclusion criteria. Of the 230 subjects included in the study, 131 (57%) were female and 99 (43%) were male. A majority (83%) of subjects were of African-American or Caribbean descent. The ages of the study subjects ranged from 6 to 21 years with a mean age of 13.5 ± 2.9 years. The mean A1C of the study population was 5.7 ± 0.5%. Sixty subjects (26%) were categorized as having prediabetes by OGTT, whereas 129 (56%) had an A1C ≥5.7%. The clinical and biochemical characteristics of the study population are shown in Table 1.
TABLE 1.
Characteristics of the Study Population (n = 230)
| Mean ± SD | Range | |
|---|---|---|
| Age (years) | 13.53 ± 2.88 | 6.17–21.42 |
| BMI (kg/m2) | 34.44 ± 7.75 | 20.47–60.3 |
| BMI z score | 2.30 ± 0.40 | 1.10–3.39 |
| A1C (%) | 5.70 ± 0.51 | 4.1–8.0 |
| Glucose (mg/dL) | ||
| Fasting | 91.97 ± 13.56 | 70.0–194.0 |
| 1-h | 124.65 ± 32.71 | 59.0–262.0 |
| 2-h | 113.36 ± 30.81 | 46.0–299.0 |
| Insulin (mU/L) | ||
| Fasting | 28.15 ± 20.15 | 1.2–132.70 |
| 1-h | 160.97 ± 114.73 | 24.0–652.0 |
| 2-h | 162.97 ± 140.19 | 11.58–886.6 |
| HOMA-IR | 6.4 ± 5.0 | 0.29–33.91 |
| Total cholesterol (mg/dL) | 155.9 ± 31.26 | 78.0–248.0 |
| Triglycerides (mg/dL) | 87.78 ± 39.69 | 30.0–234.0 |
| HDL cholesterol (mg/dL) | 43.57 ± 10.74 | 21.0–96.0 |
| LDL cholesterol (mg/dL) | 93.47 ± 27.57 | 30.3–174.1 |
| n | % | |
| Sex | 131 female/99 male | 56.96/43.04 |
| Prediabetes (OGTT) | 60 | 26 |
| Prediabetes (A1C) | 129 | 56 |
| Subjects with acanthosis nigricans | 195 | 84.78 |
| Subjects with dyslipidemia | 102 | 46.6 |
| Subjects with family history of diabetes | 153 | 68.3 |
To convert to SI units: A1C (mmol/mol) = 10.93 × A1C (%) – 23.5; glucose (mmol/L) = glucose (mg/dL) × 0.0555; insulin (pmol/L) = insulin (mU/L) × 6.945; cholesterol (mmol/L) = cholesterol (mg/dL) × 0.0259; triglyceride (mmol/L) = triglyceride (mg/dL) × 0.0113; HDL (mmol/L) = HDL (mg/dL) × 0.0259; LDL (mmol/L) = LDL (mg/dL) × 0.0259.
Mean A1C was higher in the group with prediabetes than the group with normal OGTT results (5.89 ± 0.46 vs. 5.64 ± 0.47%, P = 0.0005). The prediabetic group also had higher AUC glucose, HOMA-IR, and 2-h insulin levels on OGTT. The two groups were not statistically different with respect to BMI z score, lipid profile, AUC insulin, or fasting insulin levels. Subjects in both groups were of similar ages. The comparison of the two groups is shown in Table 2. No significant associations were found between prediabetes and sex, dyslipidemia, acanthosis nigricans, or family history of diabetes, as shown in Table 3.
TABLE 2.
Comparison of Normal OGTT to Prediabetic OGTT Group
| Normal OGTT (n = 170) | Prediabetes OGTT (n = 60) | P | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (years) | 13.33 | 2.90 | 14.07 | 2.80 | 0.0889 |
| BMI z score | 2.33 | 0.41 | 2.22 | 0.38 | 0.055 |
| A1C (%) | 5.64 | 0.47 | 5.89 | 0.46 | 0.0005 |
| LDL cholesterol (mg/dL) | 95.41 | 27.78 | 87.32 | 26.22 | 0.65 |
| Median | 25th–75th Percentile | Median | 25th–75th Percentile | P | |
|---|---|---|---|---|---|
| AUC glucose | 104.25 | 95.5–111.7 | 130.38 | 114.9–155 | <0.0001 |
| AUC insulin | 99.59 | 68.0–144.8 | 117.93 | 73.3–198.9 | 0.0956 |
| HOMA-IR | 4.60 | 3.17–6.79 | 5.66 | 4.02–10.52 | 0.013 |
| Insulin (mU/L) | |||||
| Fasting | 21.8 | 14.76–32.0 | 25.90 | 16.7–39.3 | 0.10 |
| 1-h | 129.85 | 77.7–195.6 | 138.0 | 75.5–261.7 | 0.45 |
| 2-h | 106.90 | 66.8–197.9 | 176.90 | 97.95–257.60 | 0.016 |
| Glucose (mg/dL) | |||||
| Fasting | 89.0 | 83.0–92.0 | 100 | 93.5–106.5 | <0.001 |
| 1-h | 115.0 | 97.0–135.0 | 146.0 | 119.0–175.0 | <0.001 |
| 2-h | 102.0 | 93.0–113.0 | 140.5 | 119.5–157.0 | <0.001 |
| Total cholesterol (mg/dL) | 156.0 | 139–180.0 | 148.5 | 130.0–175.0 | 0.17 |
| Triglycerides (mg/dL) | 79.0 | 58.0–103.0 | 83.0 | 55.0–115.0 | 0.55 |
| HDL cholesterol (mg/dL) | 43.55 | 36.0–50.2 | 42.10 | 36.6–47.2 | 0.60 |
To convert to SI units: A1C (mmol/mol) = 10.93 × A1C (%) – 23.5; glucose (mmol/L) = glucose (mg/dL) × 0.0555; insulin (pmol/L) = insulin (mU/L) × 6.945; cholesterol (mmol/L) = cholesterol (mg/dL) × 0.0259; triglyceride (mmol/L) = triglyceride (mg/dL) × 0.0113; HDL (mmol/L) = HDL (mg/dL) × 0.0259; LDL (mmol/L) = LDL (mg/dL) × 0.0259. Boldface P values indicate statistical significance.
TABLE 3.
Associations Between Prediabetes and Categorical Variables
| χ2 | P | |
|---|---|---|
| A1C | 6.38 | 0.0115 |
| Sex | 2.14 | 0.143 |
| Dyslipidemia | 0.0469 | 0.828 |
| Family history of diabetes | 0.7355 | 0.391 |
| Acanthosis nigricans | 0.61 | 0.434 |
Of the 230 subjects, 18 met the OGTT prediabetes definition only, 87 met the A1C prediabetes definition only, 42 met both the definitions, and 83 had normal values for both OGTT and A1C. In comparing prediabetes detected by A1C criteria to that detected by OGTT criteria, a significant association was found between the two tests (χ2 = 6.38, P = 0.0115) (Table 3).
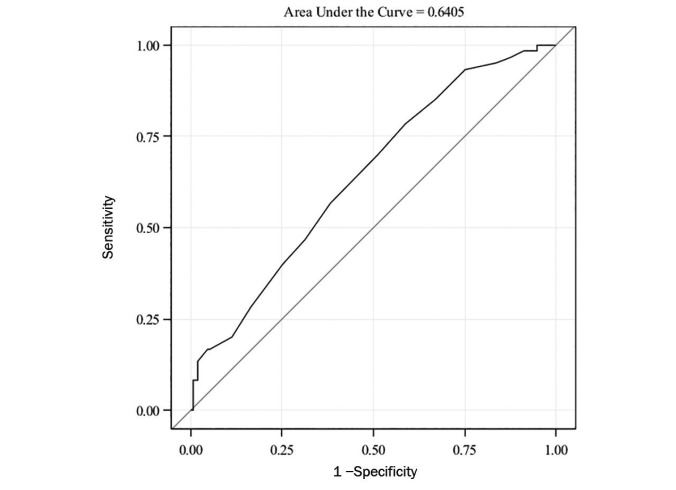
The ROC curve for A1C to detect prediabetes is shown in Figure 1. The AUC-ROC was small (0.64, 95% CI 0.56–0.72), which indicates that A1C performance is poor in detecting prediabetes with respect to OGTT. The A1C cut-off of 5.7% had an estimated sensitivity of 70% (95% CI 58–82%) and specificity of 48% (95% CI 41–56%) in detecting prediabetes by OGTT. The sensitivities and specificities at each A1C value from 5.7 to 6.4% are shown in Table 4.
FIGURE 1.
ROC curve for A1C in predicting prediabetes (OR 3.1, 95% CI 1.6–6.2, P = 0.001).
TABLE 4.
Sensitivity and Specificity of A1C Cut-Offs for Prediabetes
| A1C Cut-Off Value (%) | Sensitivity (%) | Specificity (%) |
|---|---|---|
| 5.7 | 70.0 | 48.82 |
| 5.8 | 56.67 | 61.76 |
| 5.9 | 46.67 | 68.82 |
| 6.0 | 40.0 | 74.71 |
| 6.1 | 28.33 | 83.53 |
| 6.2 | 20.0 | 88.82 |
| 6.3 | 16.67 | 94.71 |
| 6.4 | 16.67 | 95.29 |
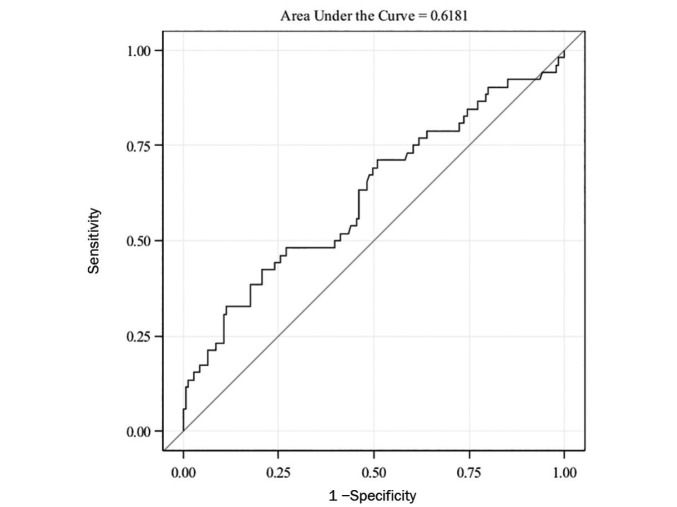
The AUC-ROC for HOMA-IR alone was also small (0.61, 95% CI 0.52–0.71) (Figure 2).
FIGURE 2.
ROC curve for HOMA-IR in predicting prediabetes (OR 1.117, 95% CI 1.043–1.196, P = 0.002).
Stepwise regression analysis was performed with the following variables: BMI z score, A1C, HOMA-IR, family history of diabetes, dyslipidemia, and presence of acanthosis nigricans. Only BMI z score, A1C, and HOMA-IR were found to be significantly associated with prediabetes after adjusting for age and sex. Table 5 shows the estimated odds ratios (ORs) for each of the significant predictors.
TABLE 5.
Predictors of Prediabetes From Stepwise Logistic Regression Analysis
| OR | 95% CI | P | |
|---|---|---|---|
| Age | 1.117 | 0.974–1.282 | 0.1126 |
| Sex (female vs. male) | 1.984 | 0.929–4.238 | 0.0767 |
| BMI z score | 0.389 | 0.156–0.966 | 0.0419 |
| A1C | 5.898 | 2.346–14.827 | 0.0002 |
| HOMA-IR | 1.135 | 1.050–1.228 | 0.0015 |
Boldface P values indicate statistical significance.
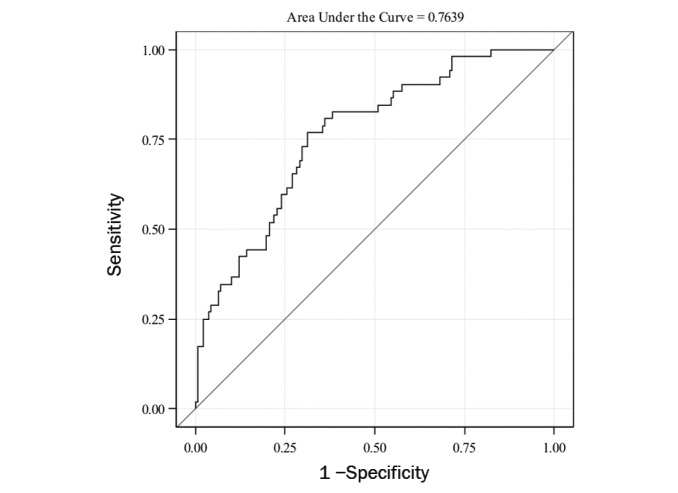
The ROC curve for predicting prediabetes using the three significant predictors obtained from stepwise regression (BMI z score, A1C, and HOMA-IR) had better performance with an AUC-ROC of 0.78 (95% CI 0.71–0.85) (Figure 3). The AUC-ROC for this model was significantly higher than for the model with A1C alone (P = 0.03) or HOMA-IR alone (P = 0.003).
FIGURE 3.
ROC curve for BMI z score, A1C, and HOMA-IR together in predicting prediabetes.
Discussion
This is one of the few studies evaluating A1C as a diagnostic tool for prediabetes in the pediatric population. We found that A1C alone is a poor discriminator of prediabetes in overweight and obese children of African-American and Caribbean descent.
The prevalence of prediabetes based on IFG or IGT in our study population was 26%. This result is consistent with other studies reporting a 12.3–28.0% prevalence of IFG or IGT among U.S. overweight/obese children and adolescents of different ethnicities (24–26).
As shown in previous studies, subjects with prediabetes had higher HOMA-IR (27) and higher 2-h plasma insulin levels on OGTT (24), indicating a higher degree of insulin resistance, which is a well-known precursor of type 2 diabetes (28). This again speaks to the fact that prediabetes is a high-risk state for development of diabetes, and medical attention should be given to individuals with prediabetes who have signs and symptoms of insulin resistance, even at a young age.
The presence of acanthosis nigricans on physical examination has been suggested to be a marker of hyperinsulinemia and insulin resistance (29–31). However, other studies have found no or minimal association between acanthosis nigricans and insulin levels or insulin sensitivity after adjusting for age and adiposity in overweight children of different ethnicities (32,33). African Americans, Native Americans, and Hispanics have higher rates of acanthosis nigricans compared to whites and Asians (29,34). A majority of subjects in our study (85%) had acanthosis nigricans on physical examination, which was not a significant predictor of prediabetes. The clinical use of acanthosis nigricans as an indicator of hyperinsulinemia is not conclusive.
Although the prediabetic group had higher A1C levels and A1C is strongly associated with OGTT results, when the A1C cut-off of ≥5.7% was used to detect prediabetes on OGTT, A1C performance was poor. The AUC-ROC of A1C for detecting prediabetes was low at 0.64, similar to findings of previously reported studies. Nowicka et al. (18) obtained an AUC-ROC of 0.60 (95% CI 0.56–0.65) for A1C performance with respect to IGT in children and adolescents, and Lee et al. (19) reported an A1C AUC-ROC of 0.54 (95% CI 0.47–0.61) for predicting dysglycemia (prediabetes or diabetes) in adolescents (10–17 years of age).
A1C had poor sensitivity over a range of cut-off values for predicting prediabetes among children and adolescents in our study, which is similar to the results obtained from the National Health and Nutrition Examination Survey sample cohort from 1999 to 2006 (17). The optimal A1C cut-off for detecting prediabetes in our study population was 5.7%, which had a relatively high sensitivity (70%) but low specificity (48%). The sensitivity of a cut-off value of 5.7% to detect prediabetes was higher in our study than has been reported earlier in children (17,19). This could be attributed to differences in the ethnic make-up of our study population compared to others. Non-Hispanic black adults and children are known to have higher A1C values than Mexican Americans and non-Hispanic whites (35,36).
A1C, HOMA-IR, and BMI z score were the strongest predictors of prediabetes in our study subjects, after adjusting for age and sex. Both higher HOMA-IR and higher A1C levels increased the odds of having prediabetes. Although BMI z scores were not significantly different between the prediabetic and normal OGTT groups, our results showed that the higher BMI z score (OR = 0.39) decreased the odds of having prediabetes, which is contrary to what we expected. This unusual finding may be the result of exclusion of subjects with diabetes, who are more likely to have a higher BMI z score than subjects with prediabetes. All three of these predictors, when taken into account together, provided better discrimination for prediabetes than A1C or HOMA-IR alone. Thus, A1C can be used as a clinical tool to predict prediabetes in children when it is taken into account with other clinical predictors of prediabetes. HOMA-IR, as a measure of insulin resistance, is used for research purposes and is not used to diagnose insulin resistance because of a lack of standardization of insulin assays (37). At this time, we do not advocate using HOMA-IR for predicting prediabetes in obese children.
Our study has limitations. It is not possible to completely exclude selection bias in this retrospective study of obese children with a high average A1C of 5.7%, which is already in the prediabetes range, given that OGTT usually was ordered when A1C was elevated or when clinically indicated. In some cases, both A1C measurement and OGTT were done as part of the initial evaluation regardless of previous laboratory values, whereas in other cases, patients were referred to the endocrine clinic for previously elevated A1C and thus the OGTT was ordered because of the previously measured abnormal A1C. This might have caused a possible selection or referral bias resulting in an average A1C in the prediabetes range (5.7 ± 0.5%).
Additionally, the subjects in this study had a homogenous background, so care must be taken in generalizing the results to a wider patient population. However, this is one of the few studies comparing A1C to OGTT in an ethnic minority pediatric population.
We also acknowledge the limitations of FPG and 2-h glucose testing in identifying prediabetes and diabetes because of the poor concordance (38) and lack of reproducibility of these tests (39,40). However, these were the gold standard tests for diagnosis of diabetes and prediabetes before 2009, and no superior markers have been proposed.
Finally, this was a cross-sectional study, so future longitudinal studies in children are needed to define A1C cut-offs that predict long-term morbidity.
A1C measurement has several advantages over other diagnostic methods, including that it is easier to obtain than an OGTT or measurement of fasting serum markers. To identify more children at risk of developing diabetes, a random A1C can serve as a useful screening tool. However, A1C measurement alone should be used with caution in children and adolescents because of its low sensitivity. One may feel reassured mistakenly with a normal A1C result. A complete clinical picture, including physical examination findings, family history, and other laboratory parameters, should be taken into account with the A1C value when making determinations about risk of diabetes and prediabetes. Repeating measurement of A1C may improve its sensitivity. More studies are needed to validate reliable markers of prediabetes and diabetes in children.
Conclusion
A1C is a readily available screening tool for prediabetes and diabetes, but a normal A1C result should be interpreted with caution because of the low sensitivity of this test. Additional testing, including repeat A1C measurement and/or OGTT, may be useful. Relying on a one-time normal A1C value may result in missed or delayed diagnosis of prediabetes in children and adolescents. Current markers used in children for prediabetes screening are not perfect, and further studies are needed. Early identification of children with prediabetes can help direct necessary interventions toward those at highest risk for developing diabetes in the future.
Acknowledgment
An oral presentation of this study was given at the June 2016 ADA Scientific Sessions in New Orleans, La.
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
References
- 1.Swinburn BA, Sacks G, Hall KD, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet 2011;378:804–814 [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Flegal KM. Prevalence of obesity in the United States. JAMA 2014;312:189–190 [DOI] [PubMed] [Google Scholar]
- 3.Nguyen QM, Srinivasan SR, Xu JH, Chen W, Berenson GS. Fasting plasma glucose levels within the normoglycemic range in childhood as a predictor of prediabetes and type 2 diabetes in adulthood: the Bogalusa Heart Study. Arch Pediatr Adolesc Med 2010;164:124–128 [DOI] [PubMed] [Google Scholar]
- 4.Li C, Ford ES, Zhao G, Mokdad AH. Prevalence of pre-diabetes and its association with clustering of cardiometabolic risk factors and hyperinsulinemia among U.S. adolescents: National Health and Nutrition Examination Survey 2005–2006. Diabetes Care 2009;32:342–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Expert Committee International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Diabetes Association Standards of medical care in diabetes—2010. Diabetes Care 2010;33(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rohlfing CL, Little RR, Wiedmeyer HM, et al. Use of GHb (HbA1c) in screening for undiagnosed diabetes in the U.S. population. Diabetes Care 2000;23:187–191 [DOI] [PubMed] [Google Scholar]
- 8.Buell C, Kermah D, Davidson MB. Utility of A1C for diabetes screening in the 1999 2004 NHANES population. Diabetes Care 2007;30:2233–2235 [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association Classification and diagnosis of diabetes. In Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(Suppl. 1):S11–S2427979889 [Google Scholar]
- 10.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 11.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2003;26(Suppl. 1):S5–S20 [DOI] [PubMed] [Google Scholar]
- 12.Little RR, Rohlfing CL, Tennill AL, Connolly S, Hanson S. Effects of sample storage conditions on glycated hemoglobin measurement: evaluation of five different high performance liquid chromatography methods. Diabetes Technol Ther 2007;9:36–42 [DOI] [PubMed] [Google Scholar]
- 13.Lippi G, Targher G. A laboratory standpoint on the role of hemoglobin A1c for the diagnosis of diabetes in childhood: more doubts than certainties? Pediatr Diabetes 2011;12:183–186 [DOI] [PubMed] [Google Scholar]
- 14.Rohlfing C, Wiedmeyer HM, Little R, et al. Biological variation of glycohemoglobin. Clin Chem 2002;48:1116–1118 [PubMed] [Google Scholar]
- 15.Chan CL, McFann K, Newnes L, Nadeau KJ, Zeitler PS, Kelsey M. Hemoglobin A1c assay variations and implications for diabetes screening in obese youth. Pediatr Diabetes 2014;15:557–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JM, Wu EL, Tarini B, Herman WH, Yoon E. Diagnosis of diabetes using hemoglobin A1c: should recommendations in adults be extrapolated to adolescents? J Pediatr 2011;158:947–952.e1–e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nowicka P, Santoro N, Liu H, et al. Utility of hemoglobin A(1c) for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes Care 2011;34:1306–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JM, Gebremariam A, Wu EL, LaRose J, Gurney JG. Evaluation of nonfasting tests to screen for childhood and adolescent dysglycemia. Diabetes Care 2011;34:2597–2602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buse JB, Kaufman FR, Linder B, et al. Diabetes screening with hemoglobin A(1c) versus fasting plasma glucose in a multiethnic middle-school cohort. Diabetes Care 2013;36:429–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11 2002. May(246):1–190 [PubMed] [Google Scholar]
- 22.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 23.Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart , Lung, and Blood Institute. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: summary report. Pediatrics 2011;128(Suppl. 5):S213–S256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinha R, Fisch G, Teague B, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med 2002;346:802–810 [DOI] [PubMed] [Google Scholar]
- 25.Goran MI, Bergman RN, Avila Q, et al. Impaired glucose tolerance and reduced beta-cell function in overweight Latino children with a positive family history for type 2 diabetes. J Clin Endocrinol Metab 2004;89:207–212 [DOI] [PubMed] [Google Scholar]
- 26.Cruz ML, Shaibi GQ, Weigensberg MJ, Spruijt-Metz D, Ball GD, Goran MI. Pediatric obesity and insulin resistance: chronic disease risk and implications for treatment and prevention beyond body weight modification. Annu Rev Nutr 2005;25:435–468 [DOI] [PubMed] [Google Scholar]
- 27.Yeckel CW, Weiss R, Dziura J, et al. Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. J Clin Endocrinol Metab 2004;89:1096–1101 [DOI] [PubMed] [Google Scholar]
- 28.Lillioja S, Mott DM, Spraul M, et al. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus: prospective studies of Pima Indians. N Engl J Med 1993;329:1988–1992 [DOI] [PubMed] [Google Scholar]
- 29.Stuart CA, Smith MM, Gilkison CR, Shaheb S, Stahn RM. Acanthosis nigricans among Native Americans: an indicator of high diabetes risk. Am J Public Health 1994;84:1839–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hud JA Jr, Cohen JB, Wagner JM, Cruz PD Jr. Prevalence and significance of acanthosis nigricans in an adult obese population. Arch Dermatol 1992;128:941–944 [PubMed] [Google Scholar]
- 31.Mukhtar Q, Cleverley G, Voorhees RE, McGrath JW. Prevalence of acanthosis nigricans and its association with hyperinsulinemia in New Mexico adolescents. J Adolesc Health 2001;28:372–376 [DOI] [PubMed] [Google Scholar]
- 32.Nguyen TT, Keil MF, Russell DL, et al. Relation of acanthosis nigricans to hyperinsulinemia and insulin sensitivity in overweight African American and white children. J Pediatr 2001;138:474–480 [DOI] [PubMed] [Google Scholar]
- 33.Kobaissi HA, Weigensberg MJ, Ball GD, Cruz ML, Shaibi GQ, Goran MI. Relation between acanthosis nigricans and insulin sensitivity in overweight Hispanic children at risk for type 2 diabetes. Diabetes Care 2004;27:1412–1416 [DOI] [PubMed] [Google Scholar]
- 34.Bent KN, Shuster GF 3rd, Hurley JS, Frye D, Loflin P, Brubaker C. Acanthosis nigricans as an early clinical proxy marker of increased risk of type II diabetes. Public Health Nurs 1998;15:415–421 [DOI] [PubMed] [Google Scholar]
- 35.Saaddine JB, Fagot-Campagna A, Rolka D, et al. Distribution of HbA(1c) levels for children and young adults in the U.S.: Third National Health and Nutrition Examination Survey. Diabetes Care 2002;25:1326–1330 [DOI] [PubMed] [Google Scholar]
- 36.Eldeirawi K, Lipton RB. Predictors of hemoglobin A1c in a national sample of nondiabetic children: the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol 2003;157:624–632 [DOI] [PubMed] [Google Scholar]
- 37.Stern SE, Williams K, Ferrannini E, DeFronzo RA, Bogardus C, Stern MP. Identification of individuals with insulin resistance using routine clinical measurements. Diabetes 2005;54:333–339 [DOI] [PubMed] [Google Scholar]
- 38.Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med 2002;19:708–723 [DOI] [PubMed] [Google Scholar]
- 39.Libman IM, Barinas-Mitchell E, Bartucci A, Robertson R, Arslanian S. Reproducibility of the oral glucose tolerance test in overweight children. J Clin Endocrinol Metab 2008;93:4231–4237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mooy JM, Grootenhuis PA, de Vries H, et al. Intra-individual variation of glucose, specific insulin and proinsulin concentrations measured by two oral glucose tolerance tests in a general Caucasian population: the Hoorn Study. Diabetologia 1996;39:298–305 [DOI] [PubMed] [Google Scholar]