Abstract
Spinocerebellar ataxia type 1 (SCA1) is a dominantly inherited neurodegenerative disease caused by the expansion of a polyglutamine (Q) repeat tract in the protein ataxin-1 (ATXN1). Beginning as a cerebellar ataxic disorder, SCA1 progresses to involve the cerebral cortex, hippocampus, and brainstem. Using SCA1 knock-in mice that mirror the complexity of the human disease, we report a significant decrease in the capacity of adult neuronal progenitor cells (NPCs) to proliferate. Remarkably, a decrease in NPCs proliferation can be observed in vitro, outside the degenerative milieu of surrounding neurons or glia, demonstrating that mutant ATXN1 acting cell autonomously within progenitor cells interferes with their ability to proliferate. Our findings suggest that compromised adult neurogenesis contributes to the progressive pathology of the disease particularly in areas such as the hippocampus and cerebral cortex where stem cells provide neurotropic factors and participate in adult neurogenesis. These findings not only shed light on the biology of the disease but also have therapeutic implications in any future stem cell- based clinical trials.
Keywords: Spinocerebellar ataxia type 1, SCA1, Neurogenesis, Proliferation
INTRODUCTION
Spinocerebellar ataxia type 1 (SCA1) is a dominantly inherited brain degenerative disorder caused by a CAG trinucleotide repeat expansion in the SCA1 gene. Because the expansion occurs in the coding region of the gene, this mutation results in an abnormally long glutamine (Q) tract in the encoded protein ataxin-1 (ATXN1) [1, 2]. As such, SCA1 resembles eight other neurodegenerative diseases—including Huntington’s disease and other spinocerebellar ataxic syndromes [3]. These disorders have several other features in common: they are typically adult-onset, midlife disorders; at a cellular level, they demonstrate a buildup of the culprit disease-causing protein that tends to misfold and cannot be cleared; and this despite protective cellular proteostatic defenses exemplified by chaperone-mediated ubiquitin-proteasomal and autophagic degradative pathways [4]. Indeed, converging lines of evidence suggest that disease pathology largely occurs by a gain of function mechanisms consistent with this notion of exaggerated protein accumulation. In SCA1, these functions appear to involve transcriptional misregulation and RNA metabolism, both resulting in aberrant gene expression [5–8]. These findings have begun to provide rational treatment strategies that aim to promote clearance or reverse the effects of transcriptional misregulation. Another possibility, however, is that there are defects in the normal regenerative pathways, such as those exemplified by NPC proliferation and adult neurogenesis that could also contribute to pathogenesis and might also be tackled therapeutically. In this study, we demonstrate that there is indeed a reduction in the ability of ATXN1-expressing NPCs to proliferate adding to the complexity of disease pathogenesis.
Material and Methods
Mouse Lines
Atxn1154Q/2Q mice were generated as described [9]. These mice were originally generated in C57BL/6J–129/SvEv mixed background; they have since been backcrossed for more than ten generations with C57BL/6J mice to avoid confounding effects of genetic background. All animal experiments were performed in compliance with National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and the Northwestern University Institutional Animal Care and Use Committee.
Neurosphere Cultures
Neurospheres were derived from the subgranular layer (SGL) or subventricular zone (SVZ). Subgranular layer neurospheres were isolated from hippocampi of 2-month- old mice as previously described [10]. Briefly, hippocampi of Atxn1154Q/2Q mice and their littermates were isolated, and white matter was removed. The tissue was minced into small pieces and incubated in 0.1 % trypsin for 10 min at 37 °C. Trypsin inhibitors were then added to stop the digestion, and the cell suspension was passed through a 70-μm cell strainer to remove myelin and tissue debris. The cells were then resuspended in neurosphere medium: DMEM/F12 containing L-glutamine (Gibco), penicillin/streptomycin with L-glutamine (Gibco), heparin (2 μg/mL, Sigma), N2 (Gibco), B27 (Gibco), 10 ng/mL of human recombinant bFGF (BD), 20 ng/mL of human recombinant EGF (BD), and 250 ng/mL of mouse Noggin (R&D). To selectively enrich for NPCs, we plated them on hydrophobic polystyrene plates that do not support growth of glia and neurons (at a density of 7000 cells/cm2 in 24-well plates). Note that in order to prevent premature differentiation, the neurosphere medium is serum-free; this also has the added benefit that it is not conducive to supporting the growth of glia. Neurosphere media were changed every 4–5 days, and cells were passaged after initial neurosphere formation (typically after 14 days in culture). Subventricular zone neurospheres were isolated by a similar process from brain tissue surrounding the lateral ventricles [11], although we found that it is not necessary to add mouse Noggin to the growth medium [10]. These neurospheres grow more rapidly than subgranular layer neurospheres with initial neurosphere formation at about 5–7 days in vitro.
Neural Stem Cell Differentiation
Singly dissociated neural stem/progenitor cells were plated on- to poly-D-lysine-coated coverslips at a cell density of 50,000 cells/well in 24-well plate in the differentiation media (DMEM/F12 with L-glutamine (Gibco), penicillin/streptomycin with L-glutamine (Gibco), supplemented with N2 (Gibco), B27 (Gibco), and 1 ng/mL of human recombinant bFGF (BD)). After 24 h, this medium was replaced with the medium without any growth factors to accelerate differentiation process. Cells were allowed to differentiate for 7 days. After 7 days, the cells were fixed with 4 % paraformaldehyde and immunostained with mouse anti-nestin (Millipore, MAB353), rabbit anti-GFAP (Dako, Z033429-2), mouse anti-β III tubulin (Millipore, AB9354), and mouse anti-O4 antibodies (Millipore, MAB345).
Single-Cell Clonogenic Assay
SVZ neurospheres were dissociated into a single-cell suspension and plated onto 96-well plates (100 cells/well). Cells were allowed to grow for 7 days with medium changes every 3–4 days. At least four mice of each genotype were used to derive neurospheres, and each cell line was plated in at least eight wells per mouse. After 1 week, newly formed secondary neurospheres were imaged with light microscope (Zeiss) and analyzed for neurosphere size with built-in Zeiss Axiovision software.
In Vitro BrdU Proliferation Assay
5′-Bromo-2′deoxyuridine (BrdU) proliferation assays were performed using a BrdU Cell Proliferation Assay Kit (Cell signaling). Briefly, singly dissociated NPCs derived from Atxn1154Q/2Q mice and their littermates were plated at a density of 20,000 cells per well in a 96-well plate. BrdU solution was added to the culture at the time of cell plating, and the cells were allowed to grow for 48 h. To assay for BrdU incorporation, the cells were fixed with fixing/denaturing solution, followed by incubating in detection antibody for 1 h at RT. The cells were washed three times with wash buffer and incubated in HRP-conjugated secondary antibody for 30 min. 3,3′, 5,5′-tetramethylbenzidine (TMB), a chromogen substrate that turns blue when oxidized from the catalytic function of HRP, was added to develop a signal, and the absorbance was read at 450 nm using dual-wavelength spectrophotometer (Tecan). Each experimental condition consists of two sample replicates derived from wild-type (N = 6) and Atxn1154Q/2Q (N = 7) mice.
BrdU Administration and Immunohistochemistry
BrdU (Sigma-Aldrich) was prepared as a 20-mg/mL solution in sterile saline and injected intraperitoneally into 10–11-week- old mice at a dose of 100 mg/kg every 12 h for three doses, and mice were sacrificed 2 days after the last injection. Brains were perfused with ice-cold PBS, fixed in 4 % paraformaldehyde overnight, and then incubated in 30 % sucrose. For immunohistochemistry of BrdU and doublecortin (DCX), 40-μm thick sections were subjected to antigen retrieval procedure as previously described [12]. Briefly, sections were incubated in deionized formamide/1XSSC solution for 2 h at 65 °C, followed by incubation in 2 N HCl for 30 min at 37 °C. Sections were neutralized with 0.1 borate buffer (pH 8.5) and washed six times, 10 min each wash, in TBS. Brain sections were blocked using 5 % normal donkey serum in 1XTBS with 0.25 % Triton X-100 (1XTBST) for 2 h at room temperature, followed by incubation of primary antibodies for 72 h in blocking solution at 4 °C. Primary antibodies used were rat anti-BrdU (1:400, Accurate Chemical) and goat anti-DCX (1:400, Santa Cruz). Secondary antibodies used were donkey anti-rat Alexa Fluor 488, donkey anti-goat Alexa Fluor 594, and donkey anti-rabbit Alexa Fluor 647 (1:250, Invitrogen). Brain sections were counterstained with DAPI (1:25,000, Invitrogen) for 5 min before mounting with polyvinyl alcohol mounting media with DABCO® (PVA-DABCO) and analyzed using confocal UV LSM 500 Zeiss microscope. Quantification of positive BrdU+ and DCX+ cells in the subgranular layer and subventricular zone was performed by stereology (StereoInvestigator 8; MBF Bioscience) or by ImageJ with cell counter plug-in, respectively, as previously described [12, 13]. At least three mice per genotype were used for the analysis with statistics performed using the Student’s t test.
Western Blot Analysis
Neurospheres were lysed in protein lysis buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 2 mM EDTA, 1 % sodium deoxycholate, 1 % NP-40, 0.2 % SDS, 100 mM PMSF, 200 mM sodium orthovanadate, and protease inhibitor cocktail). After quantifying protein concentrations by the Bradford assay, the cell lysates (containing 100 μg of proteins) were separated on a 7.5 % SDS-PAGE gel and transferred onto nitrocellulose membrane for Western blotting [14]. Primary antibodies used were mouse anti-Ataxin-1 (11NQ, NeuroMab) and mouse anti-β actin (Sigma).
Total RNA Extraction and Quantitative Real-Time RT-PCR
Total RNA was isolated from cells in culture using RNeasy Plus Mini Kit (Qiagen). RNA concentrations were determined using a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) at 260 nm/280 nm, and RNA integrity was determined by running a sample on a 1 % denaturing agarose gel. Complementary DNA (cDNA) synthesis was performed with SuperScript©III First-strand synthesis SuperMix (Invitrogen), using 1 mg of total RNA and oligo dT primers. Expression level of each gene was determined by the Realplex 2 mastercycler system (Eppendorf) employing SYBR Green PCR master mix (Applied Biosystems). Cycling conditions were as follows: 10 min at 95 °C, followed by 40 cycles at 95 °C for 15 s and 56 °C for 1 min. Samples were analyzed in duplicates, and a melting curve analysis was performed in each sample at the end of qPCR reaction. Expression levels of glyceraldehyde 3- phosphate dehydrogenase (GAPDH) were used as internal controls. Relative gene expression was determined by 2−ΔΔCt method. The threshold cycle (Ct) value was determined for target genes and the endogenous internal controls in each sample. The difference between target gene Ct and internal GAPDH control Ct was determined for each sample, resulting in the ΔCt value. The ΔCt of a calibrator wild-type sample was subtracted from each sample ΔCt to yield the ΔΔCt value. Relative fold change was calculated as 2−ΔΔCt [15, 16].
Real-Time PCR Primers
Vegfa forward: 5′-TGGTGACATGGTTAATCGGT-3′
Vegfa reverse: 5′-AGAAAGACAGAACAAAGCCAGA-3′
Gapdh forward: 5′-GTGGAGTCATACTGGAACAT GTAG-3′
Gapdh reverse: 5′-AATGGTGAAGGTCGGTGTG-3′
Statistical Analysis
Data are presented as mean ± SE. Comparison of wild-type (WT) and Atxn1154Q/2Q data were analyzed statistically by Student’s t test using Prism version 6.0 for Windows (Graphpad Software, San Diego, USA).
RESULTS
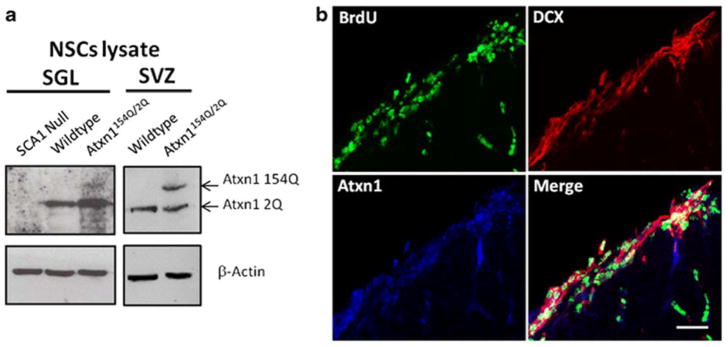
Atxn1154Q/2Q mice develop a neurodegenerative disease highly reminiscent of human SCA1. Not only they do develop the typical motor incoordination reflective of cerebellar Purkinje cell damage but they also display hippocampal cognitive deficits as exemplified by deficits in fear-conditioning and spatial memory and cortical pyramidal dysfunction as exemplified by increased tone/clasping [9]. Given the contribution of stem cells to hippocampal and cortical functioning [17, 18], we decided to test whether there are any changes in neurogenesis in the SCA1 mouse model. We focused our studies on mice around 11 weeks of age, around the time they begin to show changes in behavior by rotarod testing [19, 20]. We also observed cognitive impairment by Morris Water Maze at this age (data not shown). To investigate the role of ATXN1 in adult neurogenesis, we first tested whether neural precursor cells express ATXN1. For these experiments, we extracted and cultured NPCs as neurospheres derived from both the SVZ and SGL of adult mice—the two canonical sites of adult neurogenesis. As can be seen in Fig. 1, these neurospheres show expression of ATXN1 by Western blot analysis (Fig. 1a). Moreover, using BrdU staining—a thymidine analog taken up by actively dividing cells— and doublecortin [21, 22] to identify intermediate progenitor cells committed to the neuronal lineage, we demonstrate that these proliferating cells express ATXN1 in vivo (Fig. 1b).
Fig. 1. Neural progenitor cells express ataxin-1.
a Western blot analysis of lysates of neurospheres derived from SGL (left) and SVZ (right panel) demonstrate expression of ataxin-1 (Atxn1) in 2-month-old wild-type and mutant Atxn1154Q/2Q mice. NPCs isolated from Ataxin1 knockout mice (SCA1 Null) serve as a control. b Immunofluorescence of subventricular zone (SVZ) of the lateral ventricles with antibodies against Atxn1, BrdU, a marker of proliferation, and doublecortin (DCX), a marker of immature neurons. Scale bar = 25 μm
SCA1 Mice Show Decreased Adult Neurogenesis
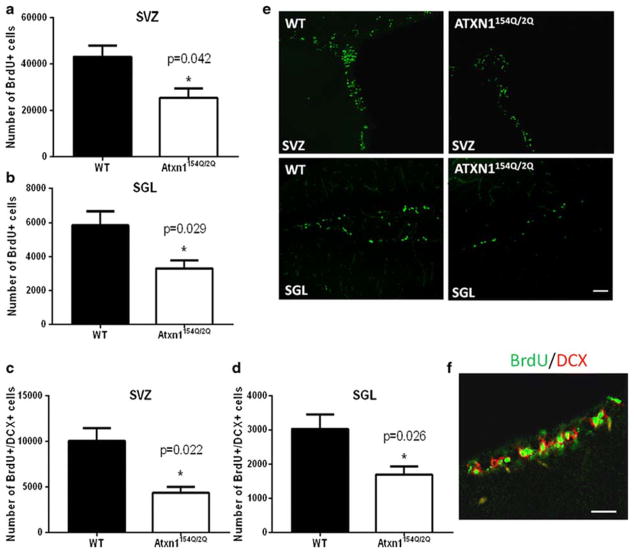
To determine whether mutant ATXN1[154Q] in Atxn1154Q/2Q mice alters neurogenesis, we examined the proliferative capacity of the stem cell niche in subventricular zone and subgranular layer of these mice. Once again, using BrdU as a way to identify actively dividing cells, we found that there is a significant decrease in the number of BrdU-positive/doublecortin-positive co-staining cells in both the subgranular zone of the dentate gyrus of SCA1 mice and the subventricular zone (compared to control wild-type littermates; Fig. 2a–f), suggesting that adult neurogenesis is significantly impaired in SCA1 mouse model.
Fig. 2. Adult neurogenesis is impaired in SCA1 mice.
The number of proliferating (BrdU positive) cells is reduced in 11-week-old Atxn1154Q/2Q mice compared to the age matched wild-type littermates in a the subventricular zone (SVZ) (*p = 0.042, Student’s t test) and b subgranular layer (SGL) (*p = 0.029, Student’s t test). To ensure countingof newly born neurons, as opposed to all proliferating cells, we also performed staining with doublecortin (DCX) that stains newly born immature neurons. The number of cells that co-stained positive for DCX and BrdU are graphed in c SVZ (*p = 0.022, Student’s t test) and d SGL (*p = 0.026, Student’s t test). e Representative confocal images of BrdU staining of SVZ and SGZ in Atxn1154Q/2Q mice and their wild-type littermates. f Representative confocal images of a section of the SGZ of a wild-type mouse to show NPCs as defined as co-staining with BrdU and DCX in SGZ. All data are presented as mean ± SEM. Scale bar = 20 μm
Decreased Neurogenesis in SCA1 Is Cell Autonomous
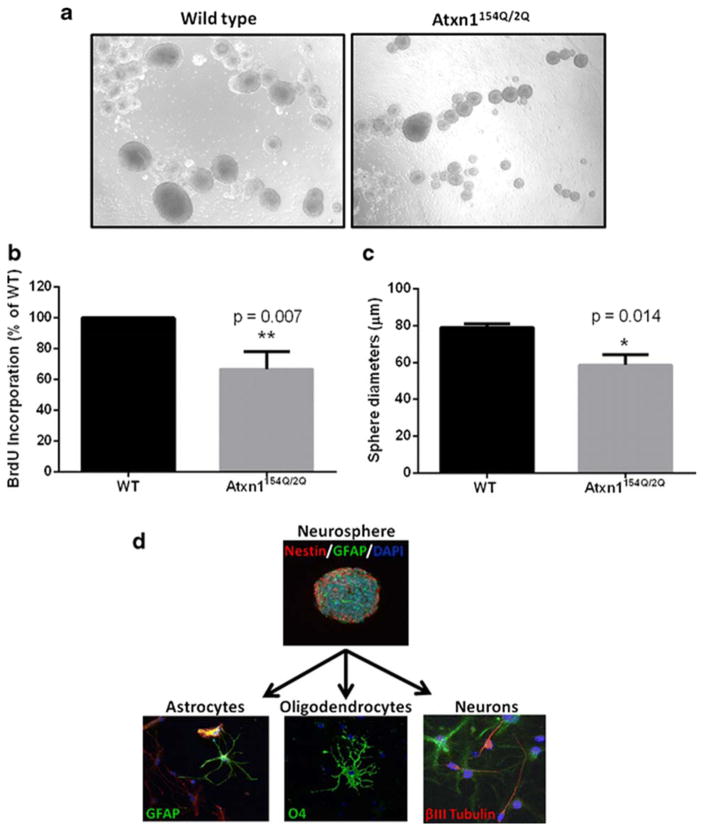
A priori, it is possible that the decreased adult neurogenesis is caused by a decrease in the proliferative capacity of SCA1 knock-in NPCs as a result of a degenerative milieu caused by degenerating neurons and/or glia; but it is also possible that SCA1 progenitor cells differ intrinsically in their ability to proliferate. To examine whether the observed decrease in adult neurogenesis in SCA1 is caused by stem cell intrinsic processes, we isolated NPCs from the subventricular zone, an especially abundant source of stem cells in the adult brain. These cells grow as neurospheres when they proliferate in culture (Fig. 3a). We detected a decreased ability of neurospheres from SCA1 knock-in mice to proliferate compared to WT (Fig. 3a–c) as seen by the size of the neurospheres and the ability to incorporate BrdU. Since these cells have been removed from their native environment of neurons and glia, these results suggest that mutant ATXN1[154Q] expression in neural progenitor cells is capable of causing intrinsic, cell autonomous proliferation deficits. We should also point out that although Atxn1154Q/2Q neurospheres show decreased proliferative ability, their ability to differentiate into multiple cell lineages does not appear to be affected as they are able to differentiate into neurons, astrocytes, and oligodendrocytes (as shown by immunocytochemistry with cell-specific markers βIII tubulin, Glial fibrillary acidic protein (GFAP), and Oligodendrocyte marker 4 (O4), respectively; Fig. 3d).
Fig. 3. Neurospheres derived from SCA1 mice exhibit impaired cell proliferation.
Neurospheres were isolated from subventricular zones of 2-month-old mice and grown in vitro. a Representative light microscopy images of neurospheres derived from Atxn1154Q/2Q mice and their wild-type littermates. b An in vitro BrdU incorporation assay was performed to assess the proliferative ability of neurospheres (**p = 0.007, Student’s t test). c Single-cell clonogenic assay examining the size of sphere formation, another indicator of cell proliferation (*p = 0.014, Student’s t test). All data are presented as mean ± SEM. d Differentiation potential of neurospheres derived from Atxn1154Q/2Q mice into neurons, astrocytes, and oligodendrocytes
VEGF Is Decreased in Atxn1154Q/2Q Neurospheres
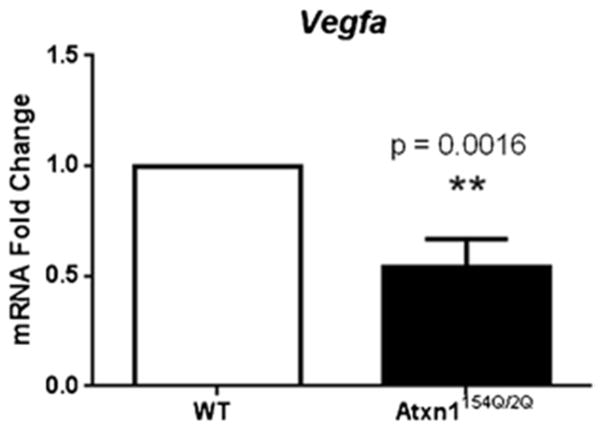
The experiments above demonstrate that neurospheres derived from SCA1 knock-in mice proliferate less. Given the importance of neurotrophic factors, such as BDNF, FGF-2, EGF, IGF-1, and VEGF to adult neurogenesis [23–30], we decided to test whether neurospheres display a reduction in their levels. We decided to focus on VEGF because we have recently shown that ATXN1 acting within neurons directly inhibits VEGF transcription leading to a decrease in VEGF levels in the cerebella of Atxn1154Q/2Q mice. To evaluate whether a similar phenomenon might also be at play in stem and neural precursor cells, we tested for a reduction of VEGF messenger RNA (mRNA) in neurospheres. As seen in Fig. 4, we do indeed see a significant reduction of VEGF mRNA levels in the neurospheres isolated from the hippocampi of Atxn1154Q/2Q mice (compared to controls; Fig. 4).
Fig. 4. VEGF-A expression is down-regulated in SCA1-derived neurospheres.
Quantitative real-time PCR analysis shows significant down-regulation of Vegfa mRNA levels in Atxn1154Q/2Q-derived neurospheres (**p = 0.0016, Student’s t test). Data are presented as mean ± SEM
DISCUSSION
In this report, we have studied the neural progenitor cell population in the context of SCA1. Using SCA1 knock-in mice which express mutant ATXN1 under its endogenous promoter, we have found that there is a significant reduction in the ability of NPCs to proliferate both in vivo and in vitro. In this regard, SCA1 joins a growing list of neurodegenerative diseases that include Alzheimer’s disease [11, 31–33], Parkinson’s disease [34–37], Huntington’s disease [38–41], and ALS [42, 43] where mouse models of neurodegeneration have revealed deleterious effects of degeneration on stem cell proliferation. The mechanisms behind impairment of adult neurogenesis in these neurodegenerative diseases are still inconclusive. However, like in many of these disorders, our results demonstrate that ATXN1 acts in progenitor cells in a cell-autonomous fashion to cause a reduction in the proliferation of neural progenitor cells. It is possible that in vivo, there also might be contributing non-cell autonomous signals that inhibit proliferation in response to other pathological processes in the SCA1 brain.
There are several implications of our work. The most obvious is that a reduction in proliferation of NPCs will hamper their ability to replenish dying or degenerating neurons. Given the proximity of neurogenesis to the hippocampus and cerebral cortices, it is likely that this reduction of proliferation in the subgranular layer and subventricular zone will have the most deleterious impact on the functions subserved by these areas of the brain—including memory, cognitive deficits, and dysexecutive symptoms [44, 45]. But we cannot rule out the possible that NPCs work at a distance to influence the health of the central nervous system including the more distant brainstem and cerebellum. Indeed, the reduction in VEGF levels that we observe could also contribute to neurodegeneration via a reduction in its paracrine effects: in particular its ability to support neuronal health via its neurotrophic effects and its ability to support the microvasculature via its angiogenic properties.
These studies should be considered in conjunction with our previous more comprehensive studies on the role of VEGF in SCA1 neurodegeneration [20]. We have already shown that a reduction in VEGF from neurons deleteriously affects neurons and the microvasculature. Moreover, replenishing VEGF either genetically or pharmacologically ameliorates the SCA1 cerebellar behavioral and pathological phenotype. Our current studies add a further level of complexity. Given that proliferation of NPCs is reduced, we anticipate several potential avenues for cross talk between neurons, NPC cells, and the microvasculature that could feed forward into a vicious cycle of neurodegeneration. For instance, the reduction of VEGF from NPCs and neurons could adversely affect the other, while the reduction in the microvasculature could further cause a reduction in the proliferation of NPCs that tend to proliferate around microvasculature niches and hamper the ability of degenerating neurons to keep up with their metabolic demands [46]. Indeed, the robust beneficial effects of VEGF are likely because it addresses the reduction of VEGF regardless of origin with beneficial effects on neurons, the proliferating cell population and the microvasculature. It is important also to note the potential contribution of glia to cellular cross talk in the disease [47]. Although not the focus of this study, stem cells not only produce neurons but also produce astrocytes and oligodendrocytes [48]. Thus, a reduction in astrocytes stemming from reduced proliferation of NPCs could translate into a reduction in the normal housekeeping functions of astrocytes such as the removal of glutamate and contribution to potassium homeostasis and their secretion of neurotrophic factors/cytokines that could further contribute to neurodegeneration. Likewise, glial pathology or inflammation could also deleteriously affect the health of the neurovascular stem cell niche.
From a therapeutic standpoint, our results add to the growing body of work that ameliorating the number of stem cells either by harnessing the endogenous stem cells or giving them exogenously might prove to be beneficial. Indeed, a couple of recent studies in mice involving external delivery of neuronal stem cells [49] and mesenchymal stem cells [50] has been shown to be beneficial. Our results would suggest that when designing stem cell-based therapies particularly with respect to autologous stem cell replacement, the optimal solution would be first to correct the polyglutamine expansion so that the stem cells can proliferate and behave closer to wild type in their ability to express neurotropic factors such as VEGF. In the past, this would have been difficult—but now with gene editing techniques such as CRISPR/Cas9, such reengineering could be readily incorporated into clinical trials moving forward.
Acknowledgments
We thank the members of the Opal lab for their intellectual input. We thank Jessica Huang for her help with the histopathology and mouse genotyping. MC was supported by startup funds for the Institute for the Translational Neuroscience and Minnesota Medical Foundation, while PO received grant support from the US National Institutes of Health (1R01 NS062051 and 1R01NS082351).
Footnotes
Compliance with Ethical Standards All animal experiments were performed in compliance with National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and the Northwestern University Institutional Animal Care and Use Committee.
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Orr H, Chung M-y, Banfi S, Kwiatkowski TJ, Jr, Servadio A, Beaudet AL, et al. Expansion of an unstable trinucleotide (CAG) repeat in spinocerebellar ataxia type 1. Nat Genet. 1993;4:221–6. doi: 10.1038/ng0793-221. [DOI] [PubMed] [Google Scholar]
- 2.Opal P, Zoghbi HY. Diseases of the nervous system. Diseases of the Nervous System. 2002;II:1880–95. [Google Scholar]
- 3.Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Annu Rev Neurosci. 2007;30:575–621. doi: 10.1146/annurev.neuro.29.051605.113042. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Li H, Li XJ. Intracellular degradation of misfolded proteins in polyglutamine neurodegenerative diseases. Brain Res Rev. 2008;59(1):245–52. doi: 10.1016/j.brainresrev.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin X, Antalffy B, Kang D, Orr HT, Zoghbi HY. Polyglutamine expansion down-regulates specific neuronal genes before pathologic changes in SCA1. Nat Neurosci. 2000;3(2):157–63. doi: 10.1038/72101. [DOI] [PubMed] [Google Scholar]
- 6.Serra HG, Byam CE, Lande JD, Tousey SK, Zoghbi HY, Orr HT. Gene profiling links SCA1 pathophysiology to glutamate signaling in Purkinje cells of transgenic mice. Hum Mol Genet. 2004;13(20):2535–43. doi: 10.1093/hmg/ddh268. [DOI] [PubMed] [Google Scholar]
- 7.Riley BE, Orr HT. Polyglutamine neurodegenerative diseases and regulation of transcription: assembling the puzzle. Genes Dev. 2006;20(16):2183–92. doi: 10.1101/gad.1436506. [DOI] [PubMed] [Google Scholar]
- 8.Cvetanovic M, Kular RK, Opal P. LANP mediates neuritic pathology in Spinocerebellar ataxia type 1. Neurobiol Dis. 2012;48(3):526–32. doi: 10.1016/j.nbd.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watase K, Weeber EJ, Xu B, Antalffy B, Yuva-Paylor L, Hashimoto K, et al. A long CAG repeat in the mouse Sca1 locus replicates SCA1 features and reveals the impact of protein solubility on selective neurodegeneration. Neuron. 2002;34(6):905–19. doi: 10.1016/s0896-6273(02)00733-x. [DOI] [PubMed] [Google Scholar]
- 10.Bonaguidi MA, Peng CY, McGuire T, Falciglia G, Gobeske KT, Czeisler C, et al. Noggin expands neural stem cells in the adult hippocampus. J Neurosci Off J Soc Neurosci. 2008;28(37):9194–204. doi: 10.1523/JNEUROSCI.3314-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demars M, Hu YS, Gadadhar A, Lazarov O. Impaired neurogenesis is an early event in the etiology of familial Alzheimer’s disease in transgenic mice. J Neurosci Res. 2010;88(10):2103–17. doi: 10.1002/jnr.22387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu YS, Xu P, Pigino G, Brady ST, Larson J, Lazarov O. Complex environment experience rescues impaired neurogenesis, enhances synaptic plasticity, and attenuates neuropathology in familial Alzheimer’s disease-linked APPswe/PS1DeltaE9 mice. FASEB J Off Publ Fed Am Soc Exp Biol. 2010;24(6):1667–81. doi: 10.1096/fj.09-136945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyers EA, Gobeske KT, Bond AM, Jarrett JC, Peng CY, Kessler JA. Increased bone morphogenetic protein signaling contributes to age-related declines in neurogenesis and cognition. Neurobiol Aging. 2016;38:164–75. doi: 10.1016/j.neurobiolaging.2015.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venkatraman A, Hu YS, Didonna A, Cvetanovic M, Krbanjevic A, Bilesimo P, et al. The histone deacetylase HDAC3 is essential for Purkinje cell function, potentially complicating the use of HDAC inhibitors in SCA1. Hum Mol Genet. 2014;23(14):3733–45. doi: 10.1093/hmg/ddu081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cvetanovic M, Rooney RJ, Garcia JJ, Toporovskaya N, Zoghbi HY, Opal P. The role of LANP and ataxin 1 in E4F-mediated transcriptional repression. EMBO Rep. 2007;8(7):671–7. doi: 10.1038/sj.embor.7400983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu YS, Long N, Pigino G, Brady ST, Lazarov O. Molecular mechanisms of environmental enrichment: impairments in Akt/GSK3beta, neurotrophin-3 and CREB signaling. PLoS One. 2013;8(5):e64460. doi: 10.1371/journal.pone.0064460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitamura T, Inokuchi K. Role of adult neurogenesis in hippocampal- cortical memory consolidation. Mol Brain. 2014;7:13. doi: 10.1186/1756-6606-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–60. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 19.Watase K, Gatchel JR, Sun Y, Emamian E, Atkinson R, Richman R, et al. Lithium therapy improves neurological function and hippocampal dendritic arborization in a spinocerebellar ataxia type 1 mouse model. PLoS Med. 2007;4(5):e182. doi: 10.1371/journal.pmed.0040182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cvetanovic M, Patel JM, Marti HH, Kini AR, Opal P. Vascular endothelial growth factor ameliorates the ataxic phenotype in a mouse model of spinocerebellar ataxia type 1. Nat Med. 2011;17(11):1445–7. doi: 10.1038/nm.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, et al. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21(1):1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- 22.Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467(1):1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- 23.Faigle R, Song H. Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim Biophys Acta. 2013;1830(2):2435–48. doi: 10.1016/j.bbagen.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99(18):11946–50. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cattaneo E, McKay R. Proliferation and differentiation of neuronal stem cells regulated by nerve growth factor. Nature. 1990;347(6295):762–5. doi: 10.1038/347762a0. [DOI] [PubMed] [Google Scholar]
- 26.Kirschenbaum B, Goldman SA. Brain-derived neurotrophic factor promotes the survival of neurons arising from the adult rat forebrain subependymal zone. Proc Natl Acad Sci U S A. 1995;92(1):210–4. doi: 10.1073/pnas.92.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maric D, Fiorio Pla A, Chang YH, Barker JL. Self-renewing and differentiating properties of cortical neural stem cells are selectively regulated by basic fibroblast growth factor (FGF) signaling via specific FGF receptors. J Neurosci Off J Soc Neurosci. 2007;27(8):1836–52. doi: 10.1523/JNEUROSCI.5141-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci Off J Soc Neurosci. 1999;19(19):8487–97. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erlandsson A, Brannvall K, Gustafsdottir S, Westermark B, Forsberg-Nilsson K. Autocrine/paracrine platelet-derived growth factor regulates proliferation of neural progenitor cells. Cancer Res. 2006;66(16):8042–8. doi: 10.1158/0008-5472.CAN-06-0900. [DOI] [PubMed] [Google Scholar]
- 30.Arsenijevic Y, Weiss S, Schneider B, Aebischer P. Insulin-like growth factor-I is necessary for neural stem cell proliferation and demonstrates distinct actions of epidermal growth factor and fibroblast growth factor-2. J Neurosci Off J Soc Neurosci. 2001;21(18):7194–202. doi: 10.1523/JNEUROSCI.21-18-07194.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verret L, Jankowsky JL, Xu GM, Borchelt DR, Rampon C. Alzheimer’s-type amyloidosis in transgenic mice impairs survival of newborn neurons derived from adult hippocampal neurogenesis. J Neurosci Off J Soc Neurosci. 2007;27(25):6771–80. doi: 10.1523/JNEUROSCI.5564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang R, Dineley KT, Sweatt JD, Zheng H. Presenilin 1 familial Alzheimer’s disease mutation leads to defective associative learning and impaired adult neurogenesis. Neuroscience. 2004;126(2):305–12. doi: 10.1016/j.neuroscience.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 33.Wen PH, Hof PR, Chen X, Gluck K, Austin G, Younkin SG, et al. The presenilin-1 familial Alzheimer disease mutant P117L impairs neurogenesis in the hippocampus of adult mice. Exp Neurol. 2004;188(2):224–37. doi: 10.1016/j.expneurol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Kohl Z, Winner B, Ubhi K, Rockenstein E, Mante M, Munch M, et al. Fluoxetine rescues impaired hippocampal neurogenesis in a transgenic A53T synuclein mouse model. Eur J Neurosci. 2012;35(1):10–9. doi: 10.1111/j.1460-9568.2011.07933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winner B, Melrose HL, Zhao C, Hinkle KM, Yue M, Kent C, et al. Adult neurogenesis and neurite outgrowth are impaired in LRRK2 G2019S mice. Neurobiol Dis. 2011;41(3):706–16. doi: 10.1016/j.nbd.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winner B, Rockenstein E, Lie DC, Aigner R, Mante M, Bogdahn U, et al. Mutant alpha-synuclein exacerbates age-related decrease of neurogenesis. Neurobiol Aging. 2008;29(6):913–25. doi: 10.1016/j.neurobiolaging.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, et al. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7(7):726–35. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- 38.Gil JM, Mohapel P, Araujo IM, Popovic N, Li JY, Brundin P, et al. Reduced hippocampal neurogenesis in R6/2 transgenic Huntington’s disease mice. Neurobiol Dis. 2005;20(3):744–51. doi: 10.1016/j.nbd.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 39.Kohl Z, Regensburger M, Aigner R, Kandasamy M, Winner B, Aigner L, et al. Impaired adult olfactory bulb neurogenesis in the R6/2 mouse model of Huntington’s disease. BMC Neurosci. 2010;11:114. doi: 10.1186/1471-2202-11-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simpson JM, Gil-Mohapel J, Pouladi MA, Ghilan M, Xie Y, Hayden MR, et al. Altered adult hippocampal neurogenesis in the YAC128 transgenic mouse model of Huntington disease. Neurobiol Dis. 2011;41(2):249–60. doi: 10.1016/j.nbd.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 41.Phillips W, Morton AJ, Barker RA. Abnormalities of neurogenesis in the R6/2 mouse model of Huntington’s disease are attributable to the in vivo microenvironment. J Neurosci Off J Soc Neurosci. 2005;25(50):11564–76. doi: 10.1523/JNEUROSCI.3796-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Z, Martin LJ. The adult neural stem and progenitor cell niche is altered in amyotrophic lateral sclerosis mouse brain. J Comp Neurol. 2006;497(3):468–88. doi: 10.1002/cne.21012. [DOI] [PubMed] [Google Scholar]
- 43.DiFebo F, Curti D, Botti F, Biella G, Bigini P, Mennini T, et al. Neural precursors (NPCs) from adult L967Q mice display early commitment to Bin vitro^ neuronal differentiation and hyperexcitability. Exp Neurol. 2012;236(2):307–18. doi: 10.1016/j.expneurol.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 44.Donato SD, Mariotti C, Taroni F. Spinocerebellar ataxia type 1. Handb Clin Neurol. 2012;103:399–421. doi: 10.1016/B978-0-444-51892-7.00025-5. [DOI] [PubMed] [Google Scholar]
- 45.Cho KO, Lybrand ZR, Ito N, Brulet R, Tafacory F, Zhang L, et al. Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nat Commun. 2015;6:6606. doi: 10.1038/ncomms7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science (New York, NY) 2004;304(5675):1338–40. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 47.Cvetanovic M, Ingram M, Orr H, Opal P. Early activation of microglia and astrocytes in mouse models of spinocerebellar ataxia type 1. Neuroscience. 2015;289:289–99. doi: 10.1016/j.neuroscience.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2(4):287–93. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- 49.Chintawar S, Hourez R, Ravella A, Gall D, Orduz D, Rai M, et al. Grafting neural precursor cells promotes functional recovery in an SCA1 mouse model. J Neurosci Off J Soc Neurosci. 2009;29(42):13126–35. doi: 10.1523/JNEUROSCI.0647-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsuura S, Shuvaev AN, Iizuka A, Nakamura K, Hirai H. Mesenchymal stem cells ameliorate cerebellar pathology in a mouse model of spinocerebellar ataxia type 1. Cerebellum (Lond, Engl) 2014;13(3):323–30. doi: 10.1007/s12311-013-0536-1. [DOI] [PubMed] [Google Scholar]