Abstract
In this study we tested whether a selective reward could affect the adaptation of saccadic eye movements in monkeys. We induced the adaptation of saccades by displacing the target of a horizontal saccade vertically as the eye moved toward it, thereby creating an apparent vertical dysmetria. The repeated upward target displacement caused the originally horizontal saccade to gradually deviate upward over the course of several hundred trials. We induced this directional adaptation in both right- and leftward saccades in every experiment (n=20). However, in half of the experiments (n=10), we rewarded monkeys only when they made leftward saccades and in the other half only for rightward saccades. The reaction time of saccades in the rewarded direction was shorter and we, like others, interpreted this change as a sign of the reward’s preferential effect in that direction. Saccades in the rewarded direction showed more rapid adaptation of their directions than did saccades in the non-rewarded direction, indicating that the selective reward increased the speed of saccade adaptation. The differences in adaptation speed were reflected in changes in saccade metrics, which were usually more noticeable in the deceleration phases of saccades than in their acceleration phases. Because previous studies have shown that the oculomotor cerebellum is involved with saccade deceleration and also participates in saccade adaptation, it is possible that selective reward could influence cerebellar plasticity.
Keywords: Saccade, adaptation, reward, cerebellum
Introduction
Growth, injury and aging may cause movements to become inaccurate. When inaccuracies occur, the dysmetric movement gradually changes so it lands on target. This improvement in movement accuracy has been called motor adaptation. The characteristics of motor adaptation have been extensively studied for saccadic eye movements, which rapidly shift the direction of gaze from one object of interest to another. Saccades provide an attractive motor system model because dysmetrias can be produced behaviorally by surreptitiously displacing the target as the eye moves toward it, thereby causing the initial saccade to miss the target (McLaughlin, 1967). During many repetitions of this apparent dysmetria, the metrics of the initial saccade, e.g., amplitude, direction or both, gradually change so that the line of sight eventually lands near the displaced target. The subject, either a human or monkey, need not be explicitly encouraged to engage in this motor adaptation. Rather, it occurs automatically when the subject is following the jumping target (Frens and van Opstal, 1994; Hopp and Fuchs, 2004).
However, evidence in humans suggests that adaptation might be susceptible to some forms of motivation. Although saccade adaptation occurs without specific motivation, clinical studies suggest that general motivation does affect the adaptation speed. In patients with Parkinson’s disease, where apathy is a major non-motor symptom, saccade adaptation is slower than in age-matched healthy control subjects (MacAskill et al., 2002; Abouaf et al., 2012). Also, motivation in the form of a differential reward can affect saccade characteristics in some non-adaptation tasks. For example, if saccades made to simple target steps in one direction are rewarded more than those in another direction, saccades associated with the larger reward have both shorter reaction times and faster velocities in both monkeys and humans (Takikawa et al., 2004; Hikosaka et al., 2006; Milstein and Dorris, 2007, 2011; Collins, 2012). These studies suggest that the motivation provided by a differential reward can influence saccadic responses. Therefore, we test here whether the specific motivation of a selective reward also has an effect on saccade adaptation. In this study, we examine adaptation in the monkey to provide a potential model for future neurophysiological experiments.
To test the effect of selective motivation on saccade adaptation, we induced adaptation of both right- and leftward saccades simultaneously, but rewarded the monkeys only when they made a saccade either to the left or right. We found that saccades in the rewarded direction adapted faster than did those in the non-rewarded direction. Moreover, the differences in saccade metrics were much more likely to be reflected in their deceleration than acceleration phases. This observation is discussed in the context of the neural circuitry in the oculomotor cerebellum, which has been implicated in saccade adaptation (Catz et al., 2005; Soetedjo and Fuchs, 2006; Catz et al., 2008; Soetedjo et al., 2008b, a; Kojima et al., 2010b).
Experimental procedures
Surgery and training
We measured eye movements in two rhesus monkeys (Macaca mulatta, male, 6.0–7.5 kg, monkeys D and A) with the electromagnetic search coil method (Robinson, 1963; Fuchs and Robinson, 1966; Judge et al., 1980). Our previous paper (Kojima et al., 2010b) describes our surgical, recording and training procedures in detail. Briefly, in an aseptic surgery we implanted each monkey with head stabilizing fixtures and an eye coil. After each monkey had recovered from the surgery, it was trained to fixate a small target spot with its eyes in a dimly lit booth, where it sat in a primate chair with its head fixed. We rewarded the monkeys with applesauce for keeping their gaze within ±2° windows of the horizontal and vertical positions of the target spot for at least 0.5 sec. Once they were trained to fixate the target spot, we trained them to make targeting saccades to a stepping spot that moved on a tangent screen within ±18° of straight-ahead. We delivered the applesauce reward (~0.16ml per drop, ~200ml/h) by a pump (masterflex tubing pump, Cole-Parmer, Vernon Hills, USA) every 2 seconds regardless of the saccade amplitude, direction or timing as long as the monkey made a saccade that landed within the ±2° window surrounding the target. The targeting saccade was required to occur within 0.6 sec of the target step and the subsequent fixation had to be maintained for 0.3 sec (“timed reward”).
The visual target for saccades was a red laser spot that was back-projected on a ground glass screen facing the monkey by a pair of X-Y mirrors attached to computer controlled galvanometers. The diameter of the red spot was ~0.4°. It took less than 1ms for the computer to start the galvanometers, and the target arrived in its new position within 6ms.
After the monkeys had learned this basic tracking task, we presented the reward only after a saccade in one horizontal direction but not in the other (“scheduled reward”). For these ~2 hour daily training sessions, we used a “scheduled reward” for the first hour and a “timed reward” for the second. The rewarded direction was reversed in the next training session. The amount of applesauce earned per hour was the same in both reward conditions (~200ml).
Behavioral paradigms
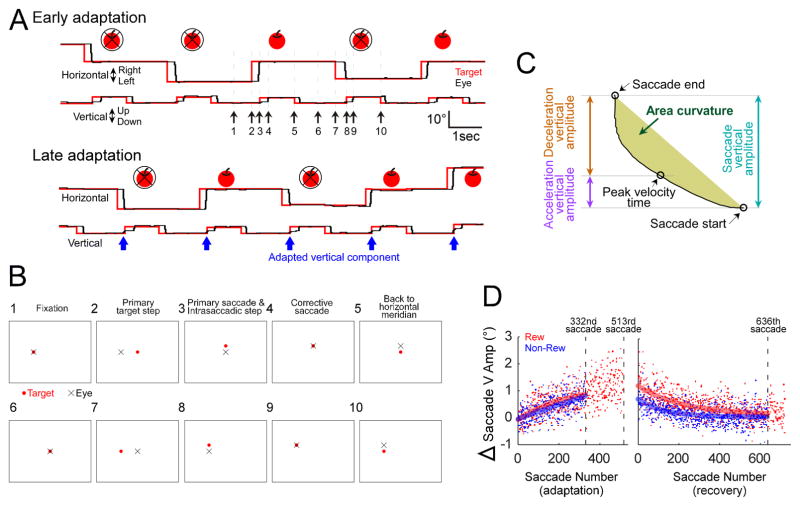
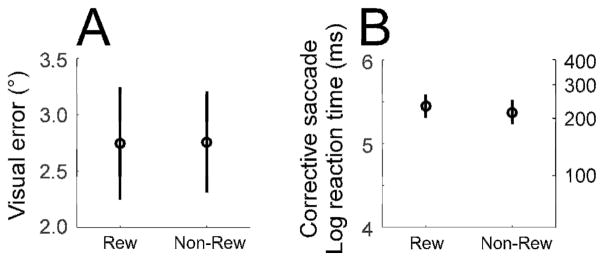
To induce adaptation, we used a cross-axis adaptation paradigm (Deubel, 1987; Frens and van Opstal, 1994; Noto et al., 1999; Chen-Harris et al., 2008) to gradually change the direction of the saccade from horizontal to slightly upward. After the monkey had fixated the target spot for 600 ms (arrow #1 in Fig. 1A, B-1), 900–1200 ms later, the spot stepped by either 10 or 12° randomly to the right or left (primary target step) (arrow #2 in Fig. 1A, B-2), but always remained within ±18° of straight-ahead. When the subsequent targeting saccade (primary saccade) had decelerated to a vector velocity ( ) of 20°/s, the target stepped a constant 3° upward from that eye position measured at that point in time so the primary saccade landed 3° below the displaced target (arrow #3 in Fig. 1A, B-3). The subject then made an upward corrective saccade to acquire the target (arrow #4 in Fig. 1A, B-4). Because the visual error that was created by the intra-saccadic step (ISS) was held constant at 3° (Robinson et al., 2003), the size of the corrective saccade also remained at about 3° during adaptation. Monkeys always made corrective saccades in response to this visual error. Across all 20 experiments, the actual median distances between the displaced target and the primary saccade’s end position in the rewarded and non-rewarded directions (2.74 vs. 2.76, respectively, with a 0.50 interquartile range) were not significantly different (Fig. 2A, Wilcoxon rank sum test, p >0.05). Also, the reaction times of the corrective saccade in the rewarded and non-rewarded directions across all experiments were not significantly different (Fig. 2B, Wilcoxon rank sum test, p >0.05) (see Data analysis below for calculation of normalization of the reaction time distribution prior to the statistical test).
Fig. 1.
Cross-axis adaptation paradigm. A, actual eye (black) and target (red) movement data from early and late in a representative adaptation. We gave a reward (apple icon) 300 ms after the corrective saccade following a saccade in one horizontal direction but not in the other. In late adaptation, a vertical component appeared (blue arrows). B, illustration of target and eye position on the screen at each event time (black arrows 1–10 in A). C, measurement of the vertical amplitude of the saccade trajectory between its start and end positions (cyan arrow). Acceleration (saccade start to peak velocity) and deceleration (peak velocity to saccade end) vertical amplitudes are shown as purple and orange arrows, respectively. The area measure of saccade curvature is indicated in olive. The vertical scale of the saccade trajectory was increased to better visualize these small components. D, change in saccade vertical amplitude as a function of saccade number with exponential fits for a representative experiment (exp #1, Table 1) in the rewarded (“Rew”, red) and non-rewarded (“Non-Rew”, blue) directions. The number of saccades in the rewarded and non-rewarded direction for the exponential fits are equal.
Fig. 2.
Visual error (A) and corrective saccade reaction time (B) across all experiments. There was no significant difference in either measure for the rewarded and non-rewarded directions. Circle and error bar are median and interquartile range, respectively.
In contrast to the conventional adaptation paradigm (McLaughlin, 1967) in which the ISS occurs at the onset of the primary saccade, we caused the ISS to occur at the end of the primary saccade (when it had decelerated to a vector velocity of 20°/s) in order to create a constant visual error when the saccade landed (Robinson et al., 2003; Zimmermann and Lappe, 2010; Kojima et al., 2015). This modified paradigm induces adaptation comparable to the conventional McLaughlin paradigm in both humans and monkeys (Robinson et al., 2003; Zimmermann and Lappe, 2010; Kojima et al., 2015). To minimize the effect, if any, of small differences in the timing of the ISS, we turned off the target during the saccade, i.e., when vector saccade velocity first exceeded and subsequently dropped below 20°/s. Eight hundred ms after the corrective saccade, the target returned to its location prior to the ISS (arrow #5 in Fig. 1A, B-5), so the target and eye always started from the horizontal meridian on each trial (arrow #6 in 1A, B-6).
We used a cross-axis adaptation because in preliminary control adaptations where selective reward was not employed, our monkeys exhibited asymmetrical amplitude adaptations, i.e., somewhat greater adaptation for right- than leftward saccades (not shown). In contrast, cross axis adaptation caused symmetrical directional adaptation for left- and rightward saccades (control experiment, see Fig. 6). We used an upward ISS because it induced a somewhat greater adaptation than did a downward ISS in our monkeys. This cross-axis paradigm, when repeated over several hundred trials, caused the originally horizontal primary saccade to acquire an upward component (Fig. 1A, bottom, blue arrows, see also Fig. 3).
Fig. 6.
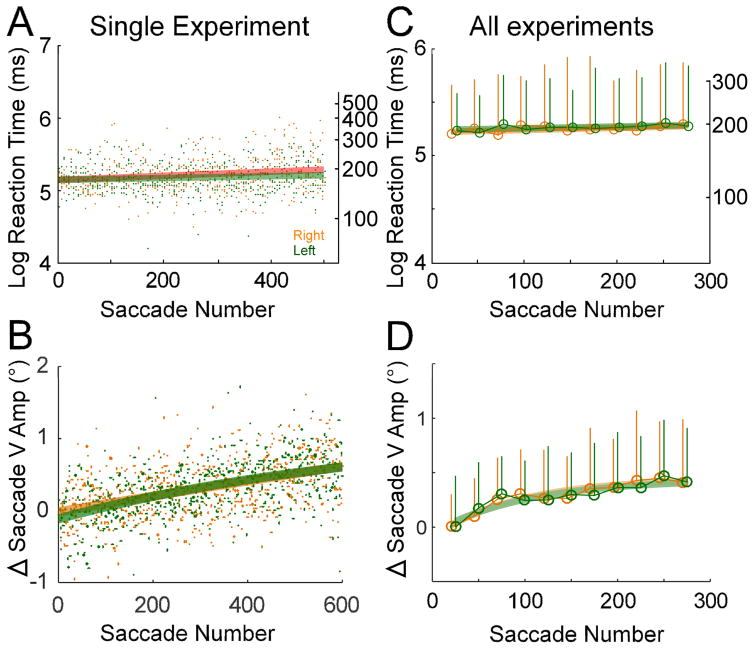
Control experiments in which an equal reward was delivered for rightward (orange data) and leftward (green data) saccades during adaptation. In the exemplar experiment (A, B), data from individual saccades (dots) during adaptation are fit with a linear regression for log reaction times or an exponential for the change in saccade vertical amplitude. Neither the log reaction times nor the change in saccade vertical amplitude were significantly different for saccades to the right and left (F-test, p >0.05). C, D, population average of all 3 control experiments for every 25-saccade bin (open circles) vs. saccade trial number. Again, log reaction time (C) and change in saccade vertical amplitude (D) were fit with linear and exponential regressions, respectively. Error bars indicate 1SD. Neither the average saccade reaction time nor the average change in saccade vertical amplitude differed for saccades to the right and left (F-test, p >0.05).
Fig. 3.
Average eye position traces for the first and last 50 saccades of an exemplar adaptation session (Exp #3). Horizontal (top) and vertical (bottom) component eye positions for the rewarded (A) and non-rewarded (B) directions, respectively. Positive eye position deflections indicate right or up. Average (solid line) ± SD (broken lines) for the first (yellow and green, A and B) and last (red and blue, A and B) 50 saccades aligned on saccade onset. C, comparison of eye positions of the last 50 saccades for the rewarded (red) and non-rewarded (blue) directions. Non-rewarded position traces are plotted with a reversed sign to superimpose data from the two reward conditions.
To make some saccades potentially more rewarding than others, we gave the applesauce reward at the end of a trial, i.e., 300 ms after the corrective saccade following a primary saccade in one horizontal direction but not in the other (Fig. 1A, apple icon). We did not deliver the reward until the end of a trial so that the sequence of events in a trial, i.e., primary target step, primary saccade and ISS, and corrective saccade was always the same whether the direction was rewarded or not. To make sure the reward was effective from the beginning of adaptation, we began the directional reward schedule before we started the adaptation and began adaptation only after the reaction time for saccades in the rewarded and non-rewarded directions had become significantly different (after ~15 min and ~200 saccades) (“scheduled reward”). We performed 10 experiments each in which either rightward or leftward saccades were rewarded (Table 1).
Table 1.
Summary of the experimental conditions (monkey, reward direction, number of saccades) and the results of statistical tests (Reaction Time, Change in saccade vertical amplitude and Change in deceleration, acceleration vertical amplitudes, and reaction time of corrective saccade) for all 20 experiments. “*” indicates a significant measure (F-test, p <0.05), “ns” indicates not significant (F-test, p >0.05).
| Fig.# | exp# | monkey | reward direction (Right/ Left) | n (saccade)
|
Reaction Time | Saccade V amp Change | Dcc V amp Change | Acc V amp Change | Corr.Sac. ReactionTime (mean)
|
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rew | Non Rew | Rew | Non Rew | ||||||||
|
| |||||||||||
| 1 | 1 | D | R | 513 | 332 | * | * | * | ns | 223 | 215 |
| 2 | D | L | 457 | 340 | * | ns | ns | ns | 228 | 211 | |
| 3,4A,B,8,9A,B | 3 | D | R | 577 | 436 | * | * | * | ns | 223 | 213 |
| 4 | D | L | 469 | 355 | * | * | * | ns | 209 | 191 | |
| 5 | D | R | 538 | 395 | * | * | * | ns | 210 | 195 | |
| 6 | D | L | 457 | 362 | * | * | * | ns | 203 | 192 | |
| 7 | D | R | 551 | 397 | * | * | * | * | 223 | 202 | |
| 8 | D | L | 459 | 357 | * | ns | ns | ns | 215 | 192 | |
| 9 | D | R | 777 | 503 | * | * | * | * | 190 | 180 | |
| 10 | D | L | 549 | 439 | * | * | * | ns | 186 | 174 | |
| 11 | D | R | 760 | 487 | * | ns | ns | ns | 191 | 175 | |
| 12 | A | L | 540 | 345 | * | * | * | ns | 289 | 275 | |
| 13 | A | R | 577 | 457 | * | * | * | ns | 246 | 238 | |
| 14 | A | L | 703 | 378 | * | * | * | * | 279 | 239 | |
| 15 | A | R | 532 | 439 | * | * | * | * | 253 | 259 | |
| 16 | A | L | 485 | 281 | * | * | * | ns | 278 | 242 | |
| 4C,D,9C,D | 17 | A | R | 386 | 326 | * | * | * | ns | 270 | 273 |
| 18 | A | L | 472 | 335 | * | ns | ns | ns | 283 | 255 | |
| 19 | A | R | 480 | 382 | * | * | * | ns | 257 | 248 | |
| 20 | A | L | 609 | 379 | * | * | * | ns | 293 | 267 | |
|
| |||||||||||
| Population | * | * | * | ns | ns | ||||||
After ~45 minutes of adaptation, we discontinued the ISS, so the target stepped only 10 or 12° randomly to the right or left and monitored the recovery of the adapted saccade vertical amplitude and the reaction time. We rewarded the monkey after every 2 seconds of accurate tracking regardless of the saccade amplitude, direction or timing (“timed reward”). This recovery session lasted ~1 hour after which the saccade vertical amplitude for both directions had returned to the pre-adaptation values and the reaction time of saccades in both directions had become similar.
As a control experiment, we tested the effect of delivering the same reward for both left- and rightward saccades during adaptation (see Fig. 6). The amount of applesauce earned per an hour was the same in this control experiment (~200ml/h). Under these conditions, there was no difference in the reaction time and the course of adaptation for left- and rightward saccades.
Data analysis
We digitized eye and target position signals at 1 kHz using Power 1401 data acquisition/controller hardware (Power 1401, CED, Cambridge, UK) and saved these data to a hard disk for later analysis. During the experiment, we used a custom program running in Spike2 (CED, Cambridge, UK) both to control the behavior using the Power 1401 and to calculate and display some features of the data on-line, e.g., saccade direction and reaction time.
The saved data were analyzed using custom programs that also ran in Spike2. We used a vector velocity criterion of 75°/s to identify each primary saccade that occurred within 50 to 800 ms after the initial target step. Saccade onset and end were then taken as the times when the vector eye velocity exceeded and then fell below 20°/s, respectively. The programs also calculated the saccade metrics, including accelerations and decelerations, and reaction time, as well as target amplitude. These attributes along with eye and target positions were exported to MATLAB (Mathworks, Natick, USA) to analyze the various features of a saccade. We eliminated those saccade trials (1) whose initial horizontal and vertical eye positions differed by ≥5° from the coordinates of the target position at the time of the initial 10 or 12° step (~2% of all trials each in the rewarded and non-rewarded directions), (2) those that were interrupted by a large blink (revealed as a vertical transient intra-saccadic position pulse of ≥5°) (~1% of trials each in the rewarded and non-rewarded directions), and (3) those that differed in direction from the primary horizontal target vector by ≥50° or in amplitude from the primary horizontal target step amplitude by ≥5° (~0.1% of trials in the rewarded direction and ~22% in the non-rewarded direction). In total, these criteria eliminated ~3% of the trials in the rewarded direction but ~25% in the non-rewarded direction. For ~ 88% of those trials eliminated in the non-rewarded direction, saccades had been made in the direction opposite to the target step (the target was turned off for these misdirected saccades).
To document the adaptation, we measured the vertical amplitude of each saccade (Fig. 1C, cyan) (Noto et al., 1999). We also determined the change of the vertical amplitude during the saccade’s acceleration and deceleration phases separately (Fig. 1C, purple and orange, respectively). Acceleration occurs between saccade start and peak vector velocity and deceleration between peak vector velocity and saccade end. Because some of the previous studies have used the change in angle (Deubel, 1987; Frens and van Opstal, 1994; Chen-Harris et al., 2008) instead of vertical amplitude as a measure of cross-axis adaptation, we evaluated it as well. We have compared the population data from the two measures and they lead to the same conclusion. This is not surprising because the difference in vertical amplitude between a 10 and 12° saccade for a 5° change of oblique angle is only 0.18° ((12*tan5°)−(10*tan5°)). Because we randomly mixed 10 and 12° primary target steps and the visual error was always 3° for both primary target steps, we will present only the vertical amplitude in this study.
Cross-axis adaptation alters not only the vertical component but also the trajectory of the saccade, i.e., it becomes curved (Chen-Harris et al., 2008). Therefore, as a second measure of adaptation, we also documented the area of curvature by measuring the area between the saccade trajectory and the line connecting the beginning and end of the saccade (Fig. 1C) (Ludwig and Gilchrist, 2002; Van der Stigchel et al., 2006).
To document the course of adaptation and recovery, we plotted the change in the saccade vertical amplitude as a function of saccade number (Fig. 1D). The change in vertical amplitude is calculated by subtracting the average vertical amplitude of the first 25 saccades of adaptation from the vertical amplitude of each saccade during adaptation. Because there are more invalid trials in the non-rewarded direction, the number of saccades in that direction (332 in Fig. 1D) was smaller than in the rewarded direction (513 in Fig. 1D) (see Table 1 for each experiment). To compare the effects of reward, we analyzed the same number of saccades for both directions (from #1 to #332 during adaptation, from #1 to #636 during recovery in Fig. 1D). We calculated the natural logarithm of the reaction times, e.g., Fig. 4A, because their distribution usually was not Gaussian but had a long tail.
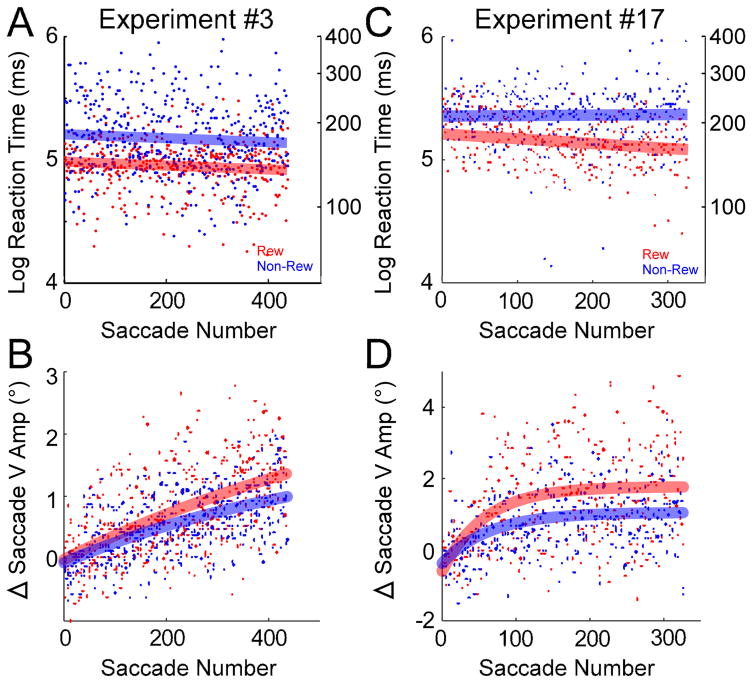
Fig. 4.
Reaction times and saccade vertical amplitude during adaptation in the rewarded (red) and non-rewarded (blue) directions for experiments #3 (A, B) and #17 (C, D) in Table 1. In each panel, a dot represents data from a single saccade trial. Log reaction times are fitted with lines; changes in saccade vertical amplitude are fitted with exponentials. Rewarded saccades (red data) showed significantly shorter reaction times (A, C) than did non-rewarded saccades (blue data) (F-test, p <0.05). Rewarded saccades showed significantly faster adaptation (B, D) than did non-rewarded saccades (F-test, p <0.05).
To produce a population average across all 20 experiments, we plotted the average vertical amplitude change over each successive bin of 25 saccades as a function of saccade number, e.g., Fig. 5. For each bin of 25 saccades, we calculated the mean and standard deviation of the saccadic reaction time, saccade vertical amplitude, deceleration of vertical amplitude, acceleration of vertical amplitude and the area of curvature during adaptation. In the same way, we determined the saccade vertical amplitude during recovery.
Fig. 5.
Average reaction times and change in saccade vertical amplitudes during adaptation for all 20 experiments. A, average of log reaction time for successive 25-saccade bins (open circles) vs. saccade trial number fit with linear regressions. Error bars indicate 1SD. Reaction times across the population were significantly shorter for saccades in the rewarded direction (F-test, p <0.05). In this and all similar subsequent figures, the red and blue lines are shifted slightly left and right, respectively, to allow viewing of the SDs. B, population average of the change in saccade vertical amplitude for every 25-saccade bin (open circles) vs. saccade trial number fit with exponentials. Error bars indicate 1SD. Saccades in the rewarded direction adapted significantly faster than in the non-rewarded direction (F-test, p <0.05). The last 4 red circles represent additional bins in the rewarded direction that were not included in the fits (see text).
Statistical test
To document the selective effect of the reward, we fitted the natural logarithm of the reaction time vs. saccade number in the rewarded and non-rewarded directions with a linear regression (Eq. 1).
| (Eq. 1) |
To document the selective effect of reward on the changes of the vertical amplitude of the overall saccade, saccade deceleration and acceleration and curvature vs. trial number, we fit each relation with an exponential function (Eq. 2), e.g., Fig. 1D.
| (Eq. 2) |
We tested whether the difference in reward scheduling produced different fits by means of an overall F-test for regression. Briefly, the null hypothesis is that one relationship fits all data points from the rewarded and non-rewarded data sets. The alternative hypothesis is that the fits are different. The F-ratio is the ratio between the percent difference of the sum-of-squares of errors from the null hypothesis and the sum of the two sums-of-squares of errors from the two alternative hypotheses fits (reward vs. combined data points and non-reward vs. combined data points) and the percent difference in their degree of freedoms. If the null hypothesis is true, the F-ratio is 1.0. We then computed a p value from the F distribution (Motulsky and Christopoulos, 2004).
When p <0.05, we consider that the data in the rewarded and non-rewarded direction were significantly different. In all 20 experiments, the linear fit for the reaction time vs. saccade number of rewarded saccades lay below that of non-rewarded saccades (e.g., Fig. 4A), indicating that the reaction time of rewarded saccades was significantly shorter than that of non-rewarded saccades (p <0.05) (Table 1, “*” in column “Reaction Time” for each experiment and population).
These experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals (2011) and exceeded the minimal requirements recommended by the Institute of Laboratory Animal Resources and the Association for Assessment and Accreditation of Laboratory Animal Care International. All the procedures were evaluated and approved by the local Animal Care and Use Committee of the University of Washington.
Results
Effect of selective reward on the course of adaptation
Fig. 3 shows the eye position traces of horizontal and vertical saccade components for a representative experiment. In both the rewarded and non-rewarded directions, the average traces of the horizontal component of the first and last 50 saccades overlapped in (Fig. 3A, B, top panel). In contrast, the vertical component of the last 50 saccades was greater than that of the first 50 saccades in both directions (Fig. 3A, B, bottom panel). However, this increase in the vertical component was greater in the rewarded direction than non-rewarded direction (Fig. 3C bottom panel).
Fig. 4 shows the course of vertical amplitude change throughout adaptation. The representative experiment in Fig. 3 is shown in Fig. 4A, B and another exemplar experiment from the other monkey is shown in Fig. 4C, D. The log reaction time and the change in saccade vertical amplitude as a function of saccade number are fit with linear and exponential functions, respectively. In both experiments, saccadic reaction time in the rewarded direction was significantly shorter than in the non-rewarded direction (p <0.05, Fig. 4A, C; Table 1, “*” in column “Reaction Time”). Also, the change in saccade vertical amplitude in the rewarded direction occurred significantly faster than in the non-rewarded direction (p <0.05, Fig. 4B, D; Table 1, “*” in column “Saccade V amp Change”).
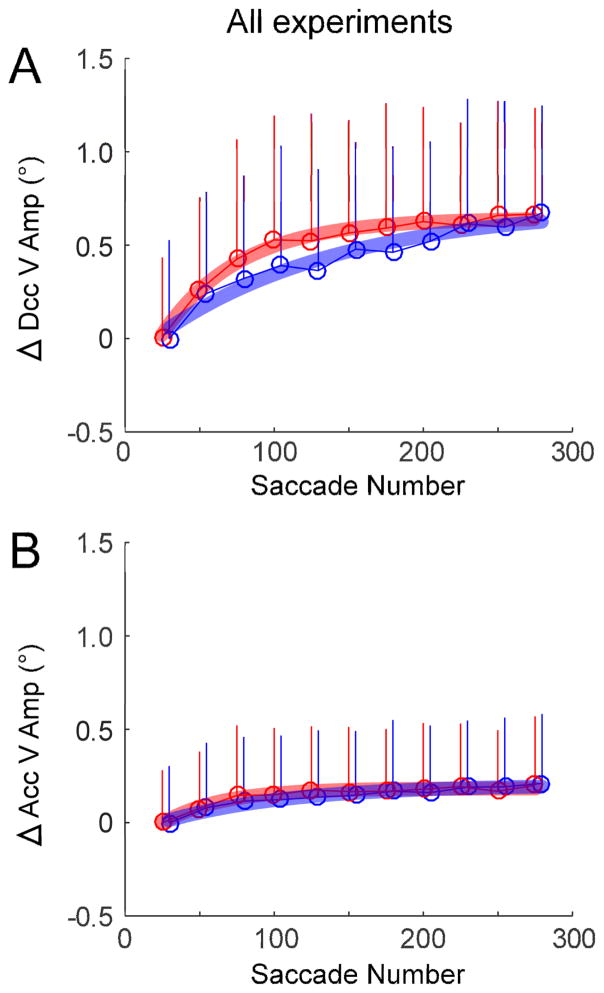
The courses of adaptation of saccade vertical amplitude in the rewarded and non-rewarded directions were significantly different in 16/20 (80%) experiments (Table 1). Four experiments did not show a significant difference (“ns”). Fig. 5 summarizes the data averaged across all 20 experiments. Each experiment contained at least 375 saccade trials for the rewarded direction and 275 for the non-rewarded direction. We compared data from only the first to the 275th saccade number from each experiment for both directions. The reaction time in the rewarded direction was significantly shorter than in the non-rewarded direction (Fig. 5A, p <0.05; Table 1 “Population”). Adaptation of the change in saccade vertical amplitude was significantly faster for saccades in the rewarded (rate constant: 47 saccades) than non-rewarded (rate constant: 112 saccades) directions (Fig. 5B, p <0.05; Table 1 “Population”).
As a control, we tested the effect of delivering the same reward for left- and rightward saccades during adaptation. For a representative control experiment (Fig. 6A), there was no significant difference (p >0.05) in reaction times for left- and rightward saccades. Moreover, the change of saccade vertical amplitude was not significantly different for left- and rightward saccades (p >0.05; Fig. 6B). For all 3 control experiments (2 on monkey D, one on monkey A), there never was any significant right-left difference in either the average reaction time (p >0.05) or saccade vertical amplitude (p >0.05) when each direction was rewarded equally (Fig. 6C, D).
The reward schedule caused more invalid trials in the non-rewarded than rewarded direction so equal saccade numbers in the two directions occurred at different times during adaptation. Therefore, the plots of the change in saccade vertical amplitude as a function of saccade number considered thus far occurred over different time periods in the rewarded and non-rewarded directions. For example, in the experiment of Fig. 1C, the 332nd saccade occurred after 1942 sec in the rewarded direction but after 3026 sec in the non-rewarded direction. To determine whether this time difference could account for our results, we also plotted the average change in saccade vertical amplitude with time in 250 sec bins for our entire population of experiments (Fig. 7). The minimum amount of adaptation time across all 20 experiments was 2500 sec so we took data only from the first to the 2500th sec in each experiment. As expected, there are fewer saccades, on average, in the non-rewarded direction (top inset of Fig. 7A). The average reaction time of primary saccades in the rewarded direction was significantly shorter than that in the non-rewarded direction (Fig. 7A, p <0.05). As with our previous analysis of adaptation using saccade number, adaptation in the rewarded direction still was significantly faster than in the non-rewarded direction (Fig. 7B, p <0.05). Thus, our results cannot be explained by the use of unequal adaptation times.
Fig. 7.
Summary of all 20 experiments with adaptation considered as a function of the time during the adaptation instead of saccade number. A, Population average of log reaction time for successive 250 sec bins (open circles) during adaptation fit with linear regressions. Error bars indicate 1SD. Reaction times across the population were significantly shorter for saccades in the rewarded direction (F-test, p <0.05). Inset at top shows the average number of saccades in each 250 sec bin. B, population averages of change in saccade vertical amplitude in successive 250 sec bins (open circles) fit with exponentials. Saccades in the rewarded direction (red) showed significantly faster adaptation than those in the non-rewarded direction (F-test, p <0.05).
Change in saccade deceleration with selective reward
Earlier experiments on the adaptation of saccade direction in humans have shown that changes in saccade direction occur because of faster adjustments in the deceleration than in the acceleration phase of the saccade (Chen-Harris et al., 2008). Therefore, we examined here whether the faster adaptation of rewarded saccades also was manifest in faster changes in the deceleration phase.
Fig. 8 shows the velocity traces for the horizontal and vertical saccade components for the same experiment described in Fig. 3. For both the rewarded and non-rewarded directions, the average traces of the horizontal velocity of the first and last 50 saccades overlapped (Fig. 8A, B, top panel). The peak velocity was higher for rewarded than non-rewarded saccades (Fig. 8C, top panel). In contrast, the vertical component velocity was greater for the last than the first 50 saccades in both directions (Fig. 8A, B, bottom panel). However, the vertical component velocity of the last 50 saccades was larger in the rewarded than non-rewarded direction (Fig. 8C bottom panel). Also, the time to saccade peak vertical velocity in the rewarded direction occurred later than for the horizontal component (broken vertical line in Fig. 8A, B).
Fig. 8.
Average velocity traces of the vertical and horizontal components of saccades in the rewarded and non-rewarded directions during adaptation aligned on saccade onset. Broken lines indicate 1SD. A and B compare the first and last 50 saccades in the rewarded (yellow and red, respectively) and non-rewarded (green and blue, respectively) directions. Positive values indicate right and up. C, direct comparison of the average velocities of the first and last 50 saccades in the rewarded and non-rewarded directions of adaptation. Non-rewarded position traces are plotted with a reversed sign to superimpose data from the two reward conditions.
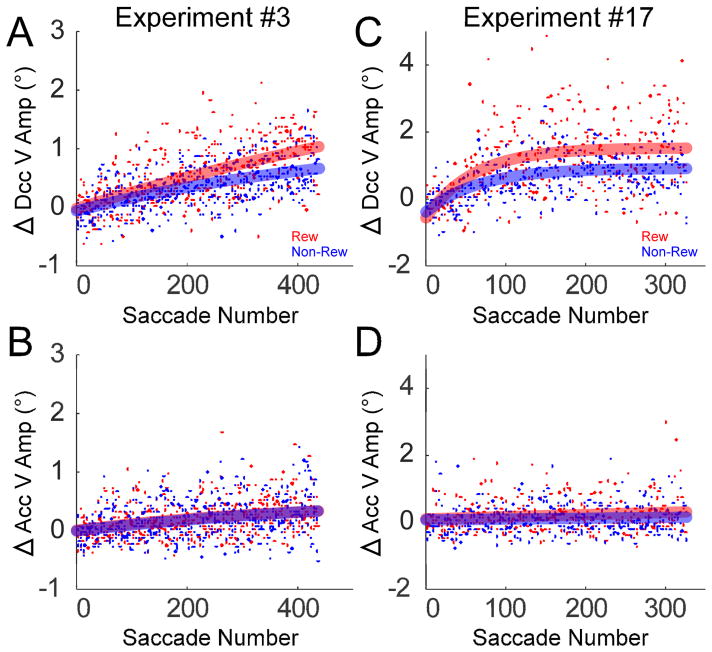
Fig. 9 shows the course of the change of saccade vertical amplitude during the deceleration (A, C) and acceleration (B, D) phases for the same two experiments described in Fig. 4. The change in vertical amplitude during deceleration was significantly faster in the rewarded than in the non-rewarded direction (Fig. 9A, C, p <0.05; Table 1, “*” in column “Dcc V amp Change”). In contrast, the change in vertical amplitude during acceleration in the rewarded and non-rewarded directions was not significantly different (Fig. 9B, D, p >0.05; Table 1, “*” in column “Acc V amp Change”).
Fig. 9.
Change in the deceleration and acceleration of saccade vertical component amplitude in the rewarded (red) and non-rewarded (blue) directions for experiments #3 and #17 in Table 1. Data from each saccade trial (dots) are fit by exponentials. The change in the deceleration of vertical component amplitude (A, C) was significantly faster in the rewarded direction (F-test, p <0.05), whereas the change in acceleration vertical amplitude (B, D) was not (F-test, p >0.05).
In 80% (16/20) of all experiments, the change in the vertical amplitude during deceleration was significantly faster in the rewarded than non-rewarded direction (Table 1, column “Dcc V amp Change”). In contrast, the change in the vertical amplitude during acceleration was significantly faster in the rewarded than non-rewarded direction in only 20% (2/20) of the experiments (Table 1, column “Acc V amp Change”). Across all 20 experiments, the change in vertical amplitude during deceleration was significantly faster in the rewarded (rate constant: 52 saccades) than in the non-rewarded (rate constant: 122 saccades) direction (Fig. 10A, p <0.05; Table 1 “Population”). In contrast, the change in vertical amplitude during acceleration in the rewarded (rate constant: 40 saccades) and non-rewarded directions (rate constant: 84 saccades) was not significantly different across all 20 experiments (Fig. 10B, p >0.05; Table 1 “Population”).
Fig. 10.
Average change in the deceleration and acceleration of the vertical component of the saccade in the rewarded and non-rewarded direction across all 20 experiments. A, population average of the change in vertical component deceleration in successive 25-saccade bins (open circles). Error bars indicate 1SD. B, population average of the change in vertical component acceleration in successive 25-saccade bins (open circles). The change in saccade deceleration was significantly faster (F-test, p <0.05) for saccades in the rewarded direction (red circles) but the change in saccade acceleration was not (F-test, p >0.05). All fits are exponentials.
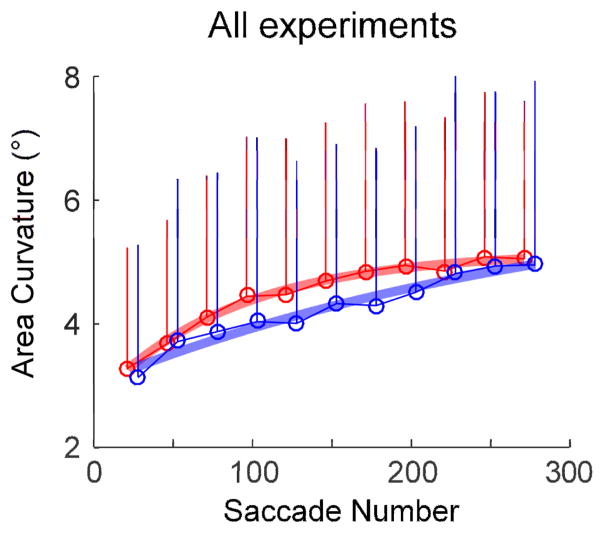
Fig. 11 shows the change in the curvature area during adaptation for the rewarded and non-rewarded directions. Across all 20 experiments, the change in curvature was significantly faster in the rewarded (rate constant: 88 saccades) than in the non-rewarded (rate constant: 311 saccades) direction (Fig. 11, p <0.05).
Fig. 11.
Average of the change in the area of curvature measure of saccade angle for all 20 experiments. Population averages for every 25-saccade bin (open circles) with exponential fits. Error bars indicate 1SD. Saccades adapted significantly faster in the rewarded than non-rewarded direction (F-test, p <0.05).
Effect of selective reward on the course of recovery
Fig. 12 summarizes the averaged course of recovery for all 20 experiments. Each experiment contained at least 275 recovery trials so we used data from the first to the 275th saccade number during recovery for each experiment. The reaction time for the rewarded and non-rewarded directions gradually returned to the same value (Fig. 12A). Because the vertical amplitude change at the end of the adaptation session in the rewarded direction was greater than in the non-rewarded direction (Fig. 5B, 7B), the recovery for the rewarded direction started from a greater vertical amplitude (Fig. 12B). The exponential fits for the rewarded (solid red line) and non-rewarded directions (solid blue line) were significantly different (p <0.05). However, once we normalized the offset, the exponential fits for recovery in the rewarded (dashed red line, time constant: 64 saccades) and non-rewarded directions (solid blue line, time constant: 63 saccades) were not significantly different (p >0.05).
Fig. 12.
Change in saccade vertical amplitude with saccade trial number during recovery of adaptation across all 20 experiments. A, population average of log reaction time for successive 25-saccade bins (open circles) fit with linear regressions. Error bars indicate 1SD. B, population average of the change in vertical component amplitude for successive 25-saccade bins (open circles) fit with exponentials. The fits in the rewarded (solid red line) and non-rewarded directions (solid blue line) were significantly different because the data were offset (F-test, p <0.05). After the rewarded data were shifted downward by 0.37 (dashed red line), the two fits were not significantly different (F-test, p >0.05).
Discussion
Selective reward speeds saccade adaptation
In all of our experiments, rewarding saccades in one direction caused a significant reduction of their reaction times relative to those in the non-rewarded direction (Fig. 3A, Table 1). We took this observation as an indication that the reward indeed had a selective effect on saccades in the rewarded direction. This selective reward was associated with a faster rate of adaptation of saccades in the rewarded direction than in the non-rewarded directions (Figs. 5).
Possible concerns about our conclusions
Does our selective reward speed adaptation or do the shorter latency saccades induced by selective reward exhibit faster adaptation? We feel that the later cannot explain our findings because adaptation of express and targeting saccades is not significantly different in human (Hopp and Fuchs, 2002) and non-human primates (Kojima et al., 2015). Moreover, in our study, although the reaction time of rewarded saccades was shorter than that of non-rewarded saccades, it was not as short as that of express saccades.
Does our selective reward induce faster or greater adaptation? The exemplar experiments showed that adaptation in the rewarded direction was greater and faster (Fig. 4B, D). Also, in the population average, the plot of saccade vertical amplitude as a function of time indicated that adaptation in the rewarded direction was greater and faster (Fig. 7). However, the analysis that averaged adaptations for the same number of saccades showed that adaptation was faster in the rewarded direction but did not appear to be greater (Fig. 5B, Fig. 11).
However, it is problematic to reach the conclusion that selective reward caused a faster but not greater adaptation because the adaptation in the rewarded direction in Fig. 5B had not gone to completion. Because there are more saccades in the rewarded than non-rewarded direction, we cut off the rewarded saccades at 275 for the comparison, i.e., the rewarded saccades after ~2000 sec in Fig. 7 are not included in Fig. 5. In Fig. 5, after the rewarded saccades from number 276 to ~375 (4 additional data points) were added, it is clear that the change in saccade vertical amplitude was in fact continuing to increase. Thus, we cannot conclude from our data that rewarded saccades adapt more or some combination of more and faster.
Mechanisms for changing adaptation speed
We show here that the faster change in saccade vertical amplitude can largely be accounted for by faster changes in the deceleration phases of the adapted saccades. This is consistent with a previous human studies, which showed that cross-axis and amplitude adaptation changes primarily the deceleration phase of the adapting saccade (Straube and Deubel, 1995; Chen-Harris et al., 2008; Collins et al., 2008). Also, for ordinary human targeting saccades, an increase in amplitude usually occurs through a prolonged duration of deceleration (Hyde, 1959; Baloh et al., 1975). Therefore, the effect we demonstrated on the deceleration phase of saccade kinematics during directional adaptation is not too surprising because the angle change is basically a gradual increase in the amplitude of the saccade’s vertical component.
Recent electrophysiological experiments allow us to speculate that the faster adaptation of saccade direction by reward we demonstrated here could be produced by the cerebellum. First, several studies suggest that saccade amplitude adaptation is probably the result of plastic changes in the oculomotor vermis (OMV) (Catz et al., 2005; Soetedjo and Fuchs, 2006; Catz et al., 2008; Soetedjo et al., 2008b, a; Kojima et al., 2010b). To the best of our knowledge, there are no studies on the neuronal basis of saccade directional adaptation. However, by analogy with the many experiments on amplitude adaptation, we suggest that the oculomotor cerebellum could also subserve the adaptation of saccade direction. Second, inactivation of the OMV (Kojima et al., 2010a) or the caudal fastigial nucleus (cFN) to which it projects (Goffart et al., 2004; Buzunov et al., 2013), affects the deceleration phase of saccades. Moreover, inactivation of the cFN affects the falling phase of the burst of abducens motoneurons (Kojima et al., 2014), which directly determines saccade deceleration. Third, the changes in saccade curvature produced by cross-axis adaptation occur primarily on the deceleration phase of the adapting saccade (Chen-Harris et al., 2008). Therefore, our results together with those of existing studies are consistent with our suggestion that a selective reward influences the plastic changes associated with saccade adaptation in the OMV.
How might reward influence the circuitry in the OMV? Reward modulates activity in the superior colliculus (SC) associated with saccades (Ikeda and Hikosaka, 2003; Isoda and Hikosaka, 2008). The SC, in turn, can modulate both types of action potential present on Purkinje cells in the OMV. First, simple spike activity, which reflects a saccade command signal, could be modulated by the SC activity that reaches the OMV via pathways through the nucleus reticularis tegmenti pontis (for review (Scudder et al., 2002)). Second, complex spike activity associated with the saccade error that drives adaptation could be modulated by the SC activity that reaches the OMV via the inferior olive (Harting, 1977; Huerta and Harting, 1984; Yamada and Noda, 1987; Kralj-Hans et al., 2007; Kaku et al., 2009; Soetedjo et al., 2009). Therefore, we speculate that reward influences the OMV through the SC. Finally, saccade-related activity in the basal ganglia, a major input to the SC, also is influenced by reward (Hikosaka, 2007). Indeed, saccade adaptation is slowed in patients with Parkinson’s disease, a disorder that affects the basal ganglia (MacAskill et al., 2002; Abouaf et al., 2012) (see next section for details). Taken together, these studies suggest a chain of neurons through which the effect of reward on saccade adaptation could be made manifest. Of course, the mechanism whereby this influence does occur is still unknown and must be elucidated by future experiments.
We did not find a significant difference in the change of the acceleration phase of rewarded and non-rewarded saccades. It may possible that the mechanisms for changing acceleration and deceleration for saccade adaptation are different and possibly are the product of independent neuronal mechanisms; however, our experiment does not address this possibility.
Other evidence that motivation influences motor learning in primates
To the best of our knowledge, this is the first demonstration that reward influences motor adaptation in the monkey. Although reward did not affect the learning speed of simian smooth pursuit eye movements, it apparently did influence the expression of the learned pursuit eye movements (Joshua and Lisberger, 2012). Also, learning to use a robotic arm can be influenced by reward (Nikooyan and Ahmed, 2015) or punishment (Galea et al., 2015) in healthy human subjects.
The depression that commonly follows a lesion of the central nervous system, such as stroke, brain or spinal cord injury (Chemerinski et al., 2001; Osteraker and Levi, 2005; Saxena et al., 2007; Graves and Bombardier, 2008), delays the recovery of motor function in humans (Saxena et al., 2007). These studies indicate that recovery of motor function, i.e., a type of motor adaptation, can be influenced by motivation. Also, saccade adaptation is slower in patients with Parkinson’s disease, where apathy is a major non-motor symptom, than in age-matched healthy control subjects. The robustness of the slowing depended on how far the disease had advanced. Adaptation was slightly, but not significantly, slower in Parkinsonian patients followed for 4.2 years (MacAskill et al., 2002), but significantly slower in those followed for an average of 10 years (Abouaf et al., 2012).
The effect of selective reward on the retention of adaptation
A previous study of robotic arm learning in humans showed that rewarded learning was better retained, i.e., the recovery was slower, by a rewarded subject group than by two other groups that were either punished or rewarded randomly (Galea et al., 2015). However, in our study, we found no difference in the recovery speed for the rewarded and non-rewarded saccade directions (Fig. 8). In so far as robotic arm learning is similar to saccade adaptation, why did our study not show better retention of the rewarded saccades? Perhaps the better retention of the adaptation of rewarded saccades was reduced by another factor. In humans, when saccade adaptation is faster, its retention is poorer (Chen-Harris et al., 2008). Perhaps this factor eliminated the better retention by reward, so the recovery speed was not different. Alternatively or concurrently, perhaps we did not see a difference in recovery speed because saccades in both directions were rewarded and the difference in the reaction time for both directions was smaller during the recovery period (Fig. 12A) than during the adaptation period (Fig. 7A).
Consideration of other adaptation phenomena
In this study, we induced saccade adaptation by creating a constant visual error and tested the effect of a selective reward for saccade direction on the on-going adaptation. We found that selective reward facilitated saccade adaptation by increasing adaptation speed in one direction and not the other. Thus, although reward motivation is not necessary to induce saccade adaptation per se (Frens and van Opstal, 1994; Hopp and Fuchs, 2004), it apparently can speed that adaptation by increasing the sensitivity to the error in driving adaptation, i.e., how much the brain “learns” from a given error (Marko et al., 2012; Hanajima et al., 2015).
Saccade gain also can be altered by non-visual error signals (Madelain et al., 2011). If a saccade’s visual target is extinguished and replaced with an auditory “reinforcement” signal when, for example, saccade gain is larger than 1.0, subjects make larger saccade more frequently. A similar “reinforcement” paradigm can alter the size of human arm movements (Izawa and Shadmehr, 2011). In our experiment, we tested reward motivation on saccade adaptation, but the adaptation itself was induced by a visual error. Therefore, our study should not be viewed as a reinforcement-induced adaptation.
Is it useful to view the effect of reward in this study as a context adaptation? For example, a subject can assume different gains for identical saccades depending on the proprioceptive or visual contexts in which they are made (for review: (Pelisson et al., 2010; Herman et al., 2013)). As an example of eye position as a context, it is possible to adapt rightward 10° saccades that start at 10° up to a gain of 1.2 and simultaneously adapt rightward 10° saccades that start at 10° down to a gain of 0.8 (Alahyane and Pelisson, 2004; Zimmermann and Lappe, 2011). In our experiment, rightward and leftward saccades do exhibit different changes in saccade vertical amplitude during adaptation, but the saccades are not the same because they differ in direction. Therefore, we think that the facilitated adaptation by reward that we demonstrate in this study should not be viewed as a context specific adaptation.
Conclusion
We tested whether a selective reward could influence saccade adaptation. We induced adaptation of both right- and leftward saccades simultaneously, but rewarded the monkeys only when they made a saccade in one direction and not the other. Our results showed that saccades in the rewarded direction usually adapted faster than did those in the non-rewarded direction.
Highlights.
We tested whether a selective reward could affect saccade motor learning.
When saccades were rewarded in only one direction, adaptation of saccades in that direction was faster.
Faster adapting saccades changed predominantly in their deceleration phase, a property controlled by the cerebellum.
Therefore, we suggest that the cerebellum could implement the effect of selective reward on saccade motor learning.
Acknowledgments
This study was supported by National Institute of Health (NIH) grants EY023277 (R01 for YK), EY019258 (R01 for RS), and OD010425 (P51 for WaNPRC). The authors declare no competing financial interests. Albert Fuchs helped with the editing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abouaf L, Panouilleres M, Thobois S, Majerova V, Vighetto A, Pelisson D, Tilikete C. Saccadic system plasticity mechanisms in Parkinson disease patients. Journal francais d'ophtalmologie. 2012;35:242–250. doi: 10.1016/j.jfo.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Alahyane N, Pelisson D. Eye position specificity of saccadic adaptation. Investigative ophthalmology & visual science. 2004;45:123–130. doi: 10.1167/iovs.03-0570. [DOI] [PubMed] [Google Scholar]
- Baloh RW, Sills AW, Kumley WE, Honrubia V. Quantitative measurement of saccade amplitude, duration, and velocity. Neurology. 1975;25:1065–1070. doi: 10.1212/wnl.25.11.1065. [DOI] [PubMed] [Google Scholar]
- Buzunov E, Mueller A, Straube A, Robinson FR. When during horizontal saccades in monkey does cerebellar output affect movement? Brain research. 2013;1503:33–42. doi: 10.1016/j.brainres.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catz N, Dicke PW, Thier P. Cerebellar complex spike firing is suitable to induce as well as to stabilize motor learning. Current biology : CB. 2005;15:2179–2189. doi: 10.1016/j.cub.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Catz N, Dicke PW, Thier P. Cerebellar-dependent motor learning is based on pruning a Purkinje cell population response. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7309–7314. doi: 10.1073/pnas.0706032105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemerinski E, Robinson RG, Kosier JT. Improved recovery in activities of daily living associated with remission of poststroke depression. Stroke; a journal of cerebral circulation. 2001;32:113–117. doi: 10.1161/01.str.32.1.113. [DOI] [PubMed] [Google Scholar]
- Chen-Harris H, Joiner WM, Ethier V, Zee DS, Shadmehr R. Adaptive control of saccades via internal feedback. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:2804–2813. doi: 10.1523/JNEUROSCI.5300-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T. Probability of seeing increases saccadic readiness. PloS one. 2012;7:e49454. doi: 10.1371/journal.pone.0049454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T, Semroud A, Orriols E, Dore-Mazars K. Saccade dynamics before, during, and after saccadic adaptation in humans. Investigative ophthalmology & visual science. 2008;49:604–612. doi: 10.1167/iovs.07-0753. [DOI] [PubMed] [Google Scholar]
- Deubel H. Adaptivity of gain and direction in oblique saccades. In: O’Regan JK, Lévy-Schoen A, editors. Eye Movements: From Physiology to Cognition. Elsevier/North Holland; Amsterdam: 1987. pp. 181–190. [Google Scholar]
- Frens MA, van Opstal AJ. Transfer of short-term adaptation in human saccadic eye movements. Experimental brain research. 1994;100:293–306. doi: 10.1007/BF00227199. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. Journal of applied physiology. 1966;21:1068–1070. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- Galea JM, Mallia E, Rothwell J, Diedrichsen J. The dissociable effects of punishment and reward on motor learning. Nat Neurosci. 2015;18:597–602. doi: 10.1038/nn.3956. [DOI] [PubMed] [Google Scholar]
- Goffart L, Chen LL, Sparks DL. Deficits in saccades and fixation during muscimol inactivation of the caudal fastigial nucleus in the rhesus monkey. Journal of neurophysiology. 2004;92:3351–3367. doi: 10.1152/jn.01199.2003. [DOI] [PubMed] [Google Scholar]
- Graves DE, Bombardier CH. Improving the efficiency of screening for major depression in people with spinal cord injury. The journal of spinal cord medicine. 2008;31:177–184. doi: 10.1080/10790268.2008.11760709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Shadmehr R, Ohminami S, Tsutsumi R, Shirota Y, Shimizu T, Tanaka N, Terao Y, Tsuji S, Ugawa Y, Uchimura M, Inoue M, Kitazawa S. Modulation of error-sensitivity during a prism adaptation task in people with cerebellar degeneration. Journal of neurophysiology. 2015;114:2460–2471. doi: 10.1152/jn.00145.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harting JK. Descending pathways from the superior collicullus: an autoradiographic analysis in the rhesus monkey (Macaca mulatta) The Journal of comparative neurology. 1977;173:583–612. doi: 10.1002/cne.901730311. [DOI] [PubMed] [Google Scholar]
- Herman JP, Blangero A, Madelain L, Khan A, Harwood MR. Saccade adaptation as a model of flexible and general motor learning. Experimental eye research. 2013;114:6–15. doi: 10.1016/j.exer.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O. Basal ganglia mechanisms of reward-oriented eye movement. Annals of the New York Academy of Sciences. 2007;1104:229–249. doi: 10.1196/annals.1390.012. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Nakahara H. Basal ganglia orient eyes to reward. Journal of neurophysiology. 2006;95:567–584. doi: 10.1152/jn.00458.2005. [DOI] [PubMed] [Google Scholar]
- Hopp JJ, Fuchs AF. Investigating the site of human saccadic adaptation with express and targeting saccades. Experimental brain research. 2002;144:538–548. doi: 10.1007/s00221-002-1077-x. [DOI] [PubMed] [Google Scholar]
- Hopp JJ, Fuchs AF. The characteristics and neuronal substrate of saccadic eye movement plasticity. Progress in neurobiology. 2004;72:27–53. doi: 10.1016/j.pneurobio.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Harting JK. The mammalian superior colliculus studies of its morphology and connections. In: Vanegas H, editor. Comparative neurology of the optic tectum. Plenum Press; New York: 1984. pp. 687–773. [Google Scholar]
- Hyde JE. Some characteristics of voluntary human ocular movements in the horizontal plane. American journal of ophthalmology. 1959;48:85–94. doi: 10.1016/0002-9394(59)90290-9. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Hikosaka O. Reward-dependent gain and bias of visual responses in primate superior colliculus. Neuron. 2003;39:693–700. doi: 10.1016/s0896-6273(03)00464-1. [DOI] [PubMed] [Google Scholar]
- Isoda M, Hikosaka O. A neural correlate of motivational conflict in the superior colliculus of the macaque. Journal of neurophysiology. 2008;100:1332–1342. doi: 10.1152/jn.90275.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa J, Shadmehr R. Learning from sensory and reward prediction errors during motor adaptation. PLoS computational biology. 2011;7:e1002012. doi: 10.1371/journal.pcbi.1002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshua M, Lisberger SG. Reward action in the initiation of smooth pursuit eye movements. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:2856–2867. doi: 10.1523/JNEUROSCI.4676-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision research. 1980;20:535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- Kaku Y, Yoshida K, Iwamoto Y. Learning signals from the superior colliculus for adaptation of saccadic eye movements in the monkey. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:5266–5275. doi: 10.1523/JNEUROSCI.0661-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y, Soetedjo R, Fuchs AF. Effects of GABA agonist and antagonist injections into the oculomotor vermis on horizontal saccades. Brain research. 2010a;1366:93–100. doi: 10.1016/j.brainres.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y, Soetedjo R, Fuchs AF. Changes in simple spike activity of some Purkinje cells in the oculomotor vermis during saccade adaptation are appropriate to participate in motor learning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010b;30:3715–3727. doi: 10.1523/JNEUROSCI.4953-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y, Robinson FR, Soetedjo R. Cerebellar fastigial nucleus influence on ipsilateral abducens activity during saccades. Journal of neurophysiology. 2014;111:1553–1563. doi: 10.1152/jn.00567.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y, Fuchs AF, Soetedjo R. Adaptation and adaptation transfer characteristics of five different saccade types in the monkey. Journal of neurophysiology. 2015;114:125–137. doi: 10.1152/jn.00212.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralj-Hans I, Baizer JS, Swales C, Glickstein M. Independent roles for the dorsal paraflocculus and vermal lobule VII of the cerebellum in visuomotor coordination. Experimental brain research. 2007;177:209–222. doi: 10.1007/s00221-006-0661-x. [DOI] [PubMed] [Google Scholar]
- Ludwig CJ, Gilchrist ID. Measuring saccade curvature: a curve-fitting approach. Behavior research methods, instruments, & computers : a journal of the Psychonomic Society, Inc. 2002;34:618–624. doi: 10.3758/bf03195490. [DOI] [PubMed] [Google Scholar]
- MacAskill MR, Anderson TJ, Jones RD. Adaptive modification of saccade amplitude in Parkinson's disease. Brain : a journal of neurology. 2002;125:1570–1582. doi: 10.1093/brain/awf168. [DOI] [PubMed] [Google Scholar]
- Madelain L, Paeye C, Wallman J. Modification of saccadic gain by reinforcement. Journal of neurophysiology. 2011;106:219–232. doi: 10.1152/jn.01094.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marko MK, Haith AM, Harran MD, Shadmehr R. Sensitivity to prediction error in reach adaptation. Journal of neurophysiology. 2012;108:1752–1763. doi: 10.1152/jn.00177.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S. Parametric adjustment in saccadic eye movements. Perception and Psychophysics. 1967;2:359–362. [Google Scholar]
- Milstein DM, Dorris MC. The influence of expected value on saccadic preparation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:4810–4818. doi: 10.1523/JNEUROSCI.0577-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein DM, Dorris MC. The Relationship between Saccadic Choice and Reaction Times with Manipulations of Target Value. Frontiers in neuroscience. 2011;5:122. doi: 10.3389/fnins.2011.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motulsky H, Christopoulos A. Fitting Models to Biological Data Using Linear and Nonlinear Regression: A Practical Guide to Curve Fitting. 1. Oxford University Press; 2004. Using global fitting to test a treatment effect in one experiment; pp. 163–165. [Google Scholar]
- Nikooyan AA, Ahmed AA. Reward feedback accelerates motor learning. Journal of neurophysiology. 2015;113:633–646. doi: 10.1152/jn.00032.2014. [DOI] [PubMed] [Google Scholar]
- Noto CT, Watanabe S, Fuchs AF. Characteristics of simian adaptation fields produced by behavioral changes in saccade size and direction. Journal of neurophysiology. 1999;81:2798–2813. doi: 10.1152/jn.1999.81.6.2798. [DOI] [PubMed] [Google Scholar]
- Osteraker AL, Levi R. Indicators of psychological distress in postacute spinal cord injured individuals. Spinal cord. 2005;43:223–229. doi: 10.1038/sj.sc.3101703. [DOI] [PubMed] [Google Scholar]
- Pelisson D, Alahyane N, Panouilleres M, Tilikete C. Sensorimotor adaptation of saccadic eye movements. Neuroscience and biobehavioral reviews. 2010;34:1103–1120. doi: 10.1016/j.neubiorev.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Robinson DA. A METHOD OF MEASURING EYE MOVEMENT USING A SCLERAL SEARCH COIL IN A MAGNETIC FIELD. IEEE transactions on bio-medical engineering. 1963;10:137–145. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Noto CT, Bevans SE. Effect of visual error size on saccade adaptation in monkey. Journal of neurophysiology. 2003;90:1235–1244. doi: 10.1152/jn.00656.2002. [DOI] [PubMed] [Google Scholar]
- Saxena SK, Ng TP, Koh G, Yong D, Fong NP. Is improvement in impaired cognition and depressive symptoms in post-stroke patients associated with recovery in activities of daily living? Acta neurologica Scandinavica. 2007;115:339–346. doi: 10.1111/j.1600-0404.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- Scudder CA, Kaneko CS, Fuchs AF. The brainstem burst generator for saccadic eye movements: a modern synthesis. Experimental brain research. 2002;142:439–462. doi: 10.1007/s00221-001-0912-9. [DOI] [PubMed] [Google Scholar]
- Soetedjo R, Fuchs AF. Complex spike activity of purkinje cells in the oculomotor vermis during behavioral adaptation of monkey saccades. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:7741–7755. doi: 10.1523/JNEUROSCI.4658-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soetedjo R, Kojima Y, Fuchs A. Complex spike activity in the oculomotor vermis of the cerebellum: a vectorial error signal for saccade motor learning? Journal of neurophysiology. 2008a;100:1949–1966. doi: 10.1152/jn.90526.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soetedjo R, Kojima Y, Fuchs A. Complex spike activity signals the direction and size of dysmetric saccade errors. Progress in brain research. 2008b;171:153–159. doi: 10.1016/S0079-6123(08)00620-1. [DOI] [PubMed] [Google Scholar]
- Soetedjo R, Fuchs AF, Kojima Y. Subthreshold activation of the superior colliculus drives saccade motor learning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:15213–15222. doi: 10.1523/JNEUROSCI.4296-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube A, Deubel H. Rapid gain adaptation affects the dynamics of saccadic eye movements in humans. Vision research. 1995;35:3451–3458. doi: 10.1016/0042-6989(95)00076-q. [DOI] [PubMed] [Google Scholar]
- Takikawa Y, Kawagoe R, Hikosaka O. A possible role of midbrain dopamine neurons in short- and long-term adaptation of saccades to position-reward mapping. Journal of neurophysiology. 2004;92:2520–2529. doi: 10.1152/jn.00238.2004. [DOI] [PubMed] [Google Scholar]
- Van der Stigchel S, Meeter M, Theeuwes J. Eye movement trajectories and what they tell us. Neuroscience and biobehavioral reviews. 2006;30:666–679. doi: 10.1016/j.neubiorev.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Yamada J, Noda H. Afferent and efferent connections of the oculomotor cerebellar vermis in the macaque monkey. The Journal of comparative neurology. 1987;265:224–241. doi: 10.1002/cne.902650207. [DOI] [PubMed] [Google Scholar]
- Zimmermann E, Lappe M. Motor signals in visual localization. Journal of vision. 2010;10:2. doi: 10.1167/10.6.2. [DOI] [PubMed] [Google Scholar]
- Zimmermann E, Lappe M. Eye position effects in oculomotor plasticity and visual localization. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:7341–7348. doi: 10.1523/JNEUROSCI.6112-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]