Abstract
Codling moth (Cydia pomonella L.) is an internal feeding pest of apples and can cause substantial economic losses to fruit growers due to larval feeding which in turn degrades fruit quality and can result in complete crop loss if left uncontrolled. Although this pest originally developed in central Asia, it was not known to occur in China until 1953. For the first three decades the spread of codling moth within China was slow. Within the last three decades, addition of new commercial apple orchards and improved transportation, this pest has spread to over 131 counties in seven provinces in China. We developed regional (China) and global ecological niche models using MaxEnt to identify areas at highest potential risk of codling moth establishment and spread. Our objectives were to 1) predict the potential distribution of codling moth in China, 2) identify the important environmental factors associated with codling moth distribution in China, and 3) identify the different stages of invasion of codling moth in China. Human footprint, annual temperature range, precipitation of wettest quarter, and degree days ≥10 °C were the most important predictors associated with codling moth distribution. Our analysis identified areas where codling moth has the potential to establish, and mapped the different stages of invasion (i.e., potential for population stabilization, colonization, adaptation, and sink) of codling moth in China. Our results can be used in effective monitoring and management to stem the spread of codling moth in China.
Keywords: habitat modeling, species distribution modeling, invasive pest, MaxEnt, niche modeling
Codling moth, Cydia pomonella (Lepidoptera: Tortricidae) is an invasive insect pest of apple in China. Although this pest originally developed in central Asia Minor, most likely Kazakhstan, it was not reported in China until 1953 (Zhang 1957). This first report was located in Korla city in Xinjiang province (Zhang et al. 2012). Codling moth distribution remained in Xinjiang Province, but spread from Korla city to 18 additional counties from 1953 to 1989 (Zhang 1957, Xu et al. 2015). From 1989 to 2000 codling moth was reported in five new counties and one new province, Gansu province Xu & Gaun 2008. From 2003 to 2009 codling moth was detected in three new provinces, Ningxia, Inner Mongolia and Heilongjiang, for a total of five provinces. From 2009 to 2015 codling moth had spread to two more provinces, Jilin and Liaoning, and a total of 131 counties (Zhang et al. 2012, Xu et al. 2015). During this time, China was becoming the global leader in apple production with 48.4% of the world production and 42.5% of the world cultivated areas of apples (Xu et al. 2015). The economic damage assessment of codling moth in China was estimated to cause as much as $605 million (Zhu 2010). Its rapid spread could most likely be attributed to increased domestic and international trade, and transportation of trees, fruits, packing materials, and farm equipment (Evangelista and Kumar 2011).
Codling moth has a facultative diapause which is dependent on photoperiod and temperature (Neven 2012, 2013). In most temperate climates, the overwintering generation emerges synchronously in the spring followed by one to two slightly overlapping emergence peaks later on in the season. The life cycle can be affected by the temperature and day length, resulting in different emergence patterns (Neven 2012, 2013). In China, codling moth usually has two and half to three generations a year (Zhang et al. 1957, Lin et al. 2006, Liu et al. 2012, Xu et al. 2012).
Codling moth coevolved with wild apples (Lin et al. 1996) in central Asia. Along with the increasing cultivation of apples worldwide, codling moth distribution expanded as a result of human activities and climatic conditions (Shel’Deshova 1967). In 1989, the European Plant Protection Organization reported that codling moth had a limited distribution in western China, and that reports of this pest in the eastern part of the country were “most certainly mistakes” (Zhu 2010, EPPO 2015). Zhu (2010) reported that codling moth had a distribution in 53 counties in five provinces in China. Codling moth have now been reported in 131 counties within seven provinces in China (Zhang et al. 2012, Kumar et al. 2015a), and is the origin of the occurrence data (Supp Table 1 [online only]). The infestation pattern of codling moth in China appears to follow a disjunctive distribution, both in Northwestern and Northeastern of China (Zhao et al. 2015).
A study to predict the potential spread of codling moth in China, Lin et al. (1996) concluded that most of the regions in Northwest and Northeast China were at high risk of invasion by this pest. However, their CLIMEX and GIS prediction models did not include the apple growing areas of Shandong and Neimenggu provinces. Jin et al. (1996, 1997) used the Biological Climatic Geographic Information System (BCGIS) to complete the preliminary analysis of potential habitation, refuting claims of Lin et al. (1996). Liang et al. (2010) also used CLIMEX to analyze the potential distribution of codling moth using ArcGIS, which showed that production areas like Shandong and Liaoning have comparatively lower probabilities of infestation than other apple production regions. Lei (2010) applied the climate similarity distance to perform the distribution prediction of three insects including codling moth, indicating that the increased precipitation during summer time could be one of the limiting factors for this pest’s invasion into the Bohai Bay apple production area. However, it did not include actual occurrence data, so the results were not as reliable.
In a previous publication, Kumar et al. (2015a) found that the most important variables impacting the global distribution of codling moth were latitude (a surrogate for day length) and mean annual temperature. Many of the abiotic factors influencing codling moth global distribution were described by Neven (2012, 2013) in which the duration of low temperature exposure (≤ 10 °C) of diapausing 5th instars directly impacted post-diapause emergence, as well as day length. These findings supported Shel’Deshova (1967) and Willett et al. (2009) in that codling moth cannot sustain a population where the day length is <15 h, and the duration of winter temperatures ≤10 °C are insufficient to meet the chilling requirement for diapause completion in this species.
Ecological niche models (ENM) have become popular tools for mapping potential distribution of invasive pests and investigating effects of climate change. These models combine species occurrence data with spatial environmental variables and predict relative environmental suitability for a species (Peterson et al. 2011). ENM have been effectively used to assess risk of establishment of insect pests (Kumar et al. 2014a,b; 2015a,b), invasive aquatic species (Kumar et al. 2009, Poulos et al. 2012, Montecino et al. 2014), invasive plants (Stohlgren et al. 2010, West et al. 2015), human diseases (Du et al. 2014), vertebrates (Bogosian et al. 2012, Boria et al. 2014), and pathogens (Murray et al. 2011, Flory et al. 2012). Potential distribution modeling of codling moth in China has been attempted earlier by Yang (2008) and Zhao et al. (2015), however, none of these studies identified different stages of codling moth invasion. Our study integrates regional (China) distributions and recently published global potential distribution map for codling moth (Kumar et al. 2015a) to identify the leading edges of invasion.
The objectives of this study were to 1) effectively map the progression of codling moth expansion through China from the 1950s to 2015, 2) develop an ecological niche model to appropriately determine environmental factors related to codling moth establishment and expansion, 3) determine the leading edge of the expansion of codling moth and stages of invasion in China, and 4) develop a predictive model to identify those production areas within China at highest risk of codling moth population expansion.
Materials and Methods
Species Occurrence Data
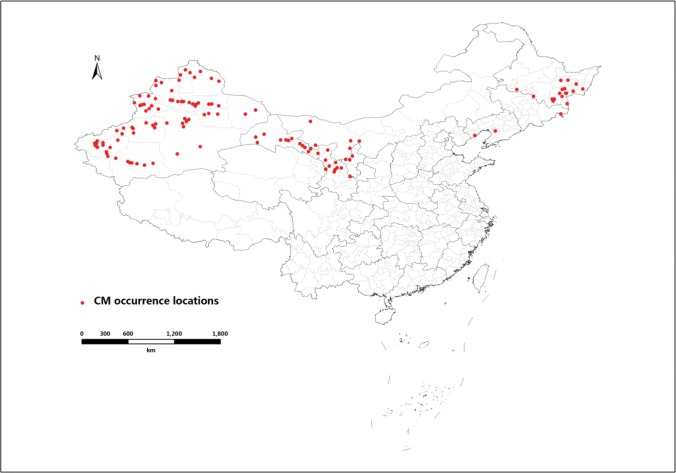
The codling moth occurrence data were collected from 131 counties within seven provinces in China where is it is currently known to occur (see Supplementary data A [online only]). These spatially unique C. pomonella occurrences were used in the niche model (Fig. 1). They were collected from three different sources: 1) early documentations including research articles and dissertations; 2) related supportive materials and documents of Chinese National Plant Protection agencies, previous reports and National Information Distribution of Quarantine Pests issued by the Chinese Department of Agricultural in 1996. Occurrence data from codling moth large-area monitoring and management workshops in which our research group (i.e., Institute of Zoology-Identification and Management of Invasive Alien Species; IOZ-IMIAS) was involved for many years was also included here (Du 2011). Some provincial Plant Protection and Quarantine Services provided occurrence data of infested locations during 1987 to 2005. 3) Other occurrence data collected during 2005–2013 were obtained from the reports released by Department of Agriculture (Xu et al. 2015). Occurrence data for developing global models were obtained from a variety of sources including books, articles and databases (see more details in Kumar et al. 2015a).
Fig. 1.
Current known occurrences of C. pomonella in China.
Environmental Data
A total of 26 environmental variables were considered in modeling the potential distribution of codling moth in China including climatic, topographic, and anthropogenic variables (see Supplementary data B [online only]). These variables were selected based on their potential effects on codling moth biology and its ecological requirements, and their use in previous insect pest niche modeling studies (Evangelista et al. 2011; Sambaraju et al. 2012; Zhu et al. 2012; Kumar et al. 2014a,b). Nineteen bioclimatic variables and elevation data were downloaded from the WorldClim dataset (Hijmans et al. 2005). Monthly average temperature data layers from the WorldClim were used to generate “degree days at ≥10 °C” variable (Shel’Deshova 1967). Other variables representing different aspects of climate included aridity index, annual potential evapotranspiration (Trabucco and Zomer 2009), and solar radiation. We also considered human footprint index (Sanderson et al. 2002) and human population density (year 2010) to represent human aided dispersal of codling moth and apple orchards/plantations distribution (see Supplementary data B [online only]). All variables were resampled to ∼5km spatial resolution to match the 19 bioclimatic variables. Multicollinearity among all environmental variables was assessed by calculating pairwise Pearson correlation coefficients and variables with |r| >0.75 were removed (see Supplementary data C [online only]). For example, elevation, solar radiation, annual potential evapotranspiration, maximum temperature of warmest month, mean temperature of warmest quarter, and degree days at ≥10 °C were highly correlated (|r| >0.75; P <0.0001), we included degree days at ≥10 °C and removed others (see Supplementary data C [online only]). Environmental variables with very low predictive power (i.e., training gain) in the MaxEnt model were also dropped.
Potential Distribution Modeling
MaxEnt correlative niche model (version 3.3.3k; Phillips et al. 2006) was used for integrating codling moth occurrences with environmental variables to model the risk of establishment. The MaxEnt model was chosen because of its higher performance (Elith et al. 2006, Kumar et al. 2009) and its use in numerous studies on insect pest risk establishment (Li et al. 2009; Evangelista et al. 2011; Lozier and Mills 2011; Zhu et al. 2012; Kumar et al. 2014a,b). The MaxEnt model is better suited for this study because it uses presence (i.e., locations where species was found present) and background (i.e., random pseudo-absence points) data. Ten thousand background points for MaxEnt were drawn using a Kernel Density Estimator (KDE) surface to account for variation in sampling intensity and potential sampling bias (see Supplementary data D [online only]); see more details in Elith et al. 2011, Kumar et al. 2014a). The MaxEnt was run with different settings using different combinations of “feature types” and regularization multiplier (Table 1). ENMTools (Warren et al. 2010) were used for model selection using Akaike’s Information Criterion corrected for small sample size (AICc) and information theoretic approach (Burnham and Anderson 2002). The “fade-by-clamping” in MaxEnt was used to avoid spurious model projections (Owens et al. 2013). Percent variable contribution and jackknife tests were used for assessing relative importance of different environmental variables. Response curves generated by MaxEnt were used for investigating C. pomonella responses to environmental variables.
Table 1.
Codling moth (C. pomonella) model evaluation and validation for MaxEnt models for China
| Model | Variables/settings | AUCcv | AUCTest | AICc | ΔAICc | 0% Omission rate |
5% Omission rate |
||
|---|---|---|---|---|---|---|---|---|---|
| Sensitivity | pAUC ratio (±SD) | Sensitivity | pAUC ratio (±SD) | ||||||
| Model1 | Human footprint, deg. days10, bio7, bio11, bio15, bio16 (Default; RM = 1.0) | 0.915 | 0.932 | 2337.1 | 44.6 | 1.0 | 1.920 (±0.04) | 0.92 | 1.647 (±0.14) |
| Model2 (best model) | Human footprint, deg. days10, bio7, bio11, bio15, bio16 (Default; RM = 2.5) | 0.920 | 0.932 | 2292.5 | 0.0 | 1.0 | 1.918 (±0.04) | 0.92 | 1.660 (±0.13) |
| Model3 | Human footprint, deg. days10, bio7, bio11, bio15, bio16 (LQP; RM = 1.0) | 0.913 | 0.924 | 2289.0 | 3.5 | 1.0 | 1.908 (±0.05) | 0.92 | 1.613 (±0.17) |
| Model4 (climate only) | Deg. days10, aridity, bio3, bio7, bio11, bio15, bio19 (Default; RM = 2.5) | 0.838 | 0.860 | 2515.7 | 223.2 | 1.0 | 1.834 (±0.06) | 0.92 | 1.510 (±0.12) |
Default settings are with Linear (L), Quadratic (Q), Product (P), Threshold (T), and Hinge (H) features included; RM is regularization multiplier; bio1 to bio19 are Bioclim variables (see Supplementary data A [online only] for full names); AUCcv is AUC based on 10-fold cross-validation; AUCTest is AUC based on 20% withheld test data; AICc is Akaike’s Information Criterion for small sample size; Partial AUC (pAUC) ratio is from Peterson et al. (2008).
Model performance was assessed using MaxEnt generated area under the receiver operating characteristic (ROC) curve (AUC, Phillips et al. 2006), Partial AUC ratio (pAUC) statistic (Peterson et al. 2008), and sensitivity (i.e., fraction of correctly predicted presences). Eighty percent of the occurrence data (n = 105) were used for training the model and remaining 20% (n = 26) for assessing model performance. MaxEnt’s internal 10-fold cross-validation procedure was used for calculating training (AUCcv) and test AUC (AUCTest) values. The pAUC was calculated using a Visual Basic program (Barve, http://biodiversity-informatics-training.org). The sensitivity was calculated at 0% training omission rate, and 5% training omission rate (see details in Liu et al. 2013, Kumar et al. 2014a).
Mapping Leading Edge of Expansion of Codling Moth and Its Stages of Invasion
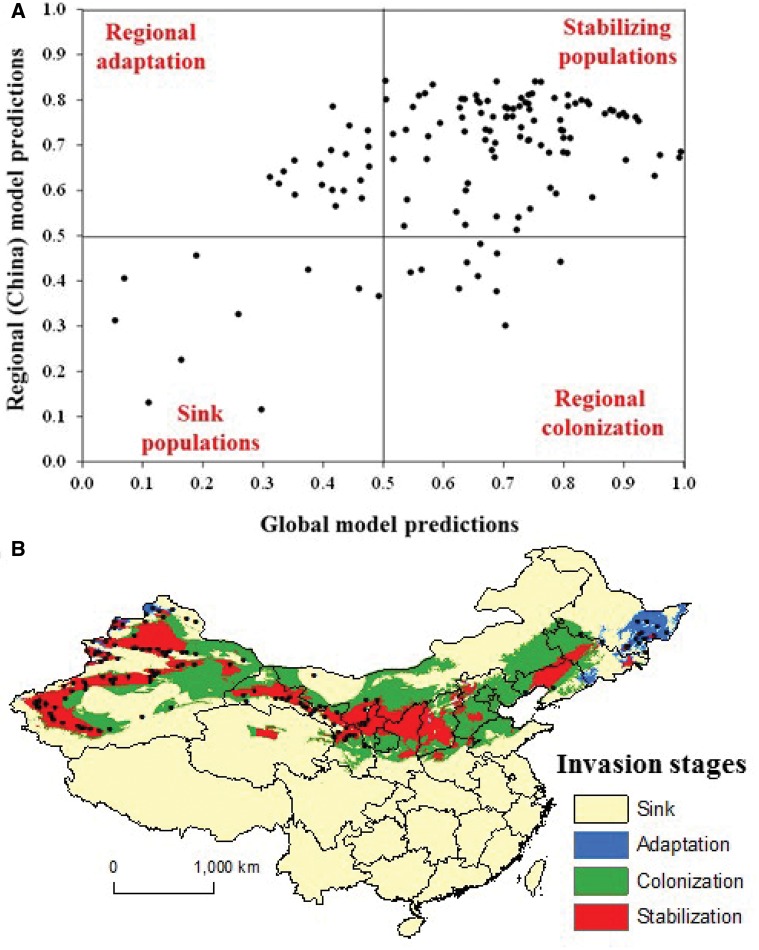
The theoretical framework of Gallien et al. (2012) was used for mapping leading edges of invasion by codling moth, and for making inferences about different stages of invasion for codling moth observed populations/occurrences in China by plotting predicted probabilities from the regional (China) model (this study) against the global model from Kumar et al. (2015a). This framework takes advantage of the differences between a species’ regional realized niche (i.e., species’ climatic niche in China in this case) and the global niche (i.e., species’ climatic niche for the entire world). According to this framework, a species would be at quasi-equilibrium if the regional and global models predict higher probabilities (e.g., >0.5) for species’ presence (i.e., stabilizing populations). In contrast, if both models predict lower probabilities for species presences, these locations may represent populations sinks (see more details in Gallien et al. 2012, Kumar et al. 2015b). If the species presences cover the global niche but not the regional realized niche, this suggests colonization from different sources including already invaded areas in the regional invaded range. In contrast, if species presences cover realized niche but not the global niche, this indicates that these populations may be adapting to new environmental conditions (local adaptation).
Results
Predicted Potential Distribution of Codling Moth in China
All models performed better than random with training and test AUC values >0.80, pAUC ratios >1.5, and had lower omission rates (OR) (Table 1). The best model (Model 2) included six predictor variables including human footprint, temperature annual range (bio7), precipitation of wettest quarter (bio16), degree days at 10 °C, precipitation seasonality (bio15), and mean temperature of coldest quarter (bio11), and had a test AUC value of 0.932, pAUC ratio of 1.918, and 1.0 and 0.92 sensitivity values at 0% OR and 5% OR, respectively (Table 1). The model with only climatic variables (i.e., Model 4) performed poorly (lower evaluation statistics and higher AIC value) than the model with climatic plus human footprint variables (Model 2) (Table 1).
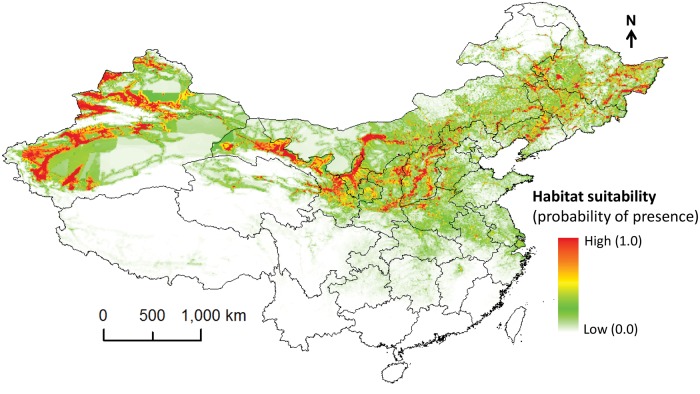
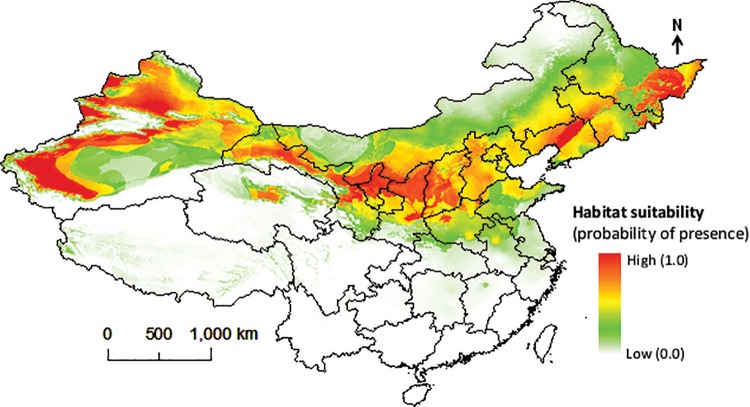
The best model predicted higher environmental suitability for codling moth in northern and northeastern parts of China including Xinjiang province in the west, and several provinces in east central China (Fig. 2). This model did not predict suitable areas for codling moth in southern provinces of China including Tibet, Yunnan, Guangxi, and Hainan; Taiwan was also predicted unsuitable (Fig. 2). The climate only model (i.e., without human footprint variable) predicted more suitable areas than the model with climatic plus human footprint variables (Fig. 3). The predicted environmental suitability covered all apple growing areas in China (Figs. 2 and 3).
Fig. 2.
Predicted potential distribution of C. pomonella in China using climate and human factors.
Fig. 3.
Predicted potential distribution of C. pomonella in China using only climatic factors.
Effects of Environmental Factors on Codling Moth Distribution in China
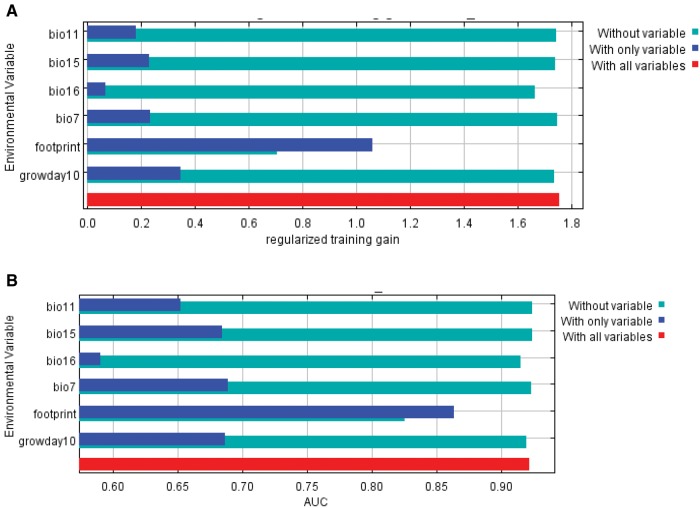
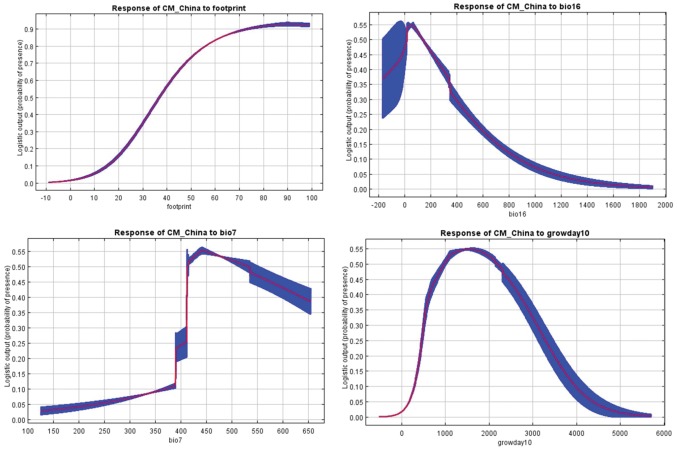
Human footprint was one of the most important predictors associated with codling moth distribution in China (Table 2). It contributed most (57.7% average contribution) to the best model and also had highest predictive power (i.e., highest training gain; Fig. 4a), and test AUC (Fig. 4b). Other important predictors included temperature annual range (bio7, precipitation of wettest quarter (bio16), and degree days at 10 °C with 15.9, 10.1, and 9.8% contributions, respectively (Table 2).
Table 2.
Percent contribution of different environmental variables to the best MaxEnt model (Model 2) for C. pomonella
| Environmental variable | Percent contribution | Permutation importance |
|---|---|---|
| Human footprint | 57.7 | 72.4 |
| Temperature annual range (Bio7; °C) | 15.9 | 4.0 |
| Precipitation of wettest quarter (Bio16; mm) | 10.1 | 14.8 |
| Degree days at ≥ 10 °C | 9.8 | 2.4 |
| Precipitation seasonality (CV) (Bio15) | 4.9 | 1.2 |
| Mean temperature of coldest quarter (Bio11; °C) | 1.5 | 5.3 |
Fig. 4.
Relative importance of different environmental predictors based on jack-knife tests for (a) training gain and (b) test AUC.
Probability of codling moth presence in China increased with increasing human footprint index (Fig. 5), and was highest at lower levels of precipitation of wettest quarter (bio16). The probability of codling moth presence was highest between 1,000 and 2,000 degree days at 10 °C (Fig. 5).
Fig. 5.
Response curves showing relationships between most important environmental factors and the probability of C. pomonella presence.
Stages of Codling Moth Invasion in China
The analysis of different stages of invasion for codling moth in China based on predictions from a regional model (i.e., China; this study) and a global model (Kumar et al. 2015a) showed that most of the stabilized populations of codling moth are present in inner parts of predicted suitable areas in northwestern Xinjiang, central Jiuquan, and northern Gansu and northern Ningxia provinces (Fig. 6). The analysis revealed that regional adaptation is occurring in Heilongjiang, Jilin and Liaoning provinces (Fig. 6) whereas colonization is occurring in outer parts of predicted suitable areas (Figs. 2 and 6). These results suggest that codling moth may be in quasi-equilibrium with the regional environment in China and it is still spreading to new environmentally suitable areas (i.e., blue and green areas in Fig. 6b). Most often invasive species are not in equilibrium with their environment as they may not have had enough time to disperse to all environmentally suitable areas after their introduction, which seems to be the case here (Rouget et al. 2004). Codling moth invasion in other parts of the country (i.e., yellow areas in Fig. 6b) may be limited by abiotic conditions, biotic interactions, invasion history and dispersal constraints (Wilson et al. 2007). For example, population sink areas predicted in northern Xinjiang, northeastern Inner Mongolia, and northwestern Heilongjiang provinces may be a result of extremely cold temperatures that limit codling moth potential distribution by exceeding its ability to overwinter (Fig. 6b). The predicted unsuitable areas in southern China may be due to a lack of host plant (i.e., apple) and warmer temperatures or dispersal constraints (Figs. 2 and 6b).
Fig. 6.
(a) Observed occurrences of Cydia pomonella at different stages of invasion based on global and regional (China) model predictions and (b) mapped areas in China showing potential (hypothesized) for population stabilization, adaptation, colonization, and sink.
Updates on the Current Distribution of Codling Moth in China
During the writing of this paper, there were some updates on the codling moth occurrences in China. At present, the occurrence sites are in 144 counties covering seven separate provinces, with total infestation area of 49,410 ha (Xu et al. 2015). As mentioned in the Methods and Materials section, we did not include those newer data into our modeling procedures. The data were updated just until the end of 2012 because the later occurrence were not that conspicuous and fast as the previous infestations, and also because we needed to define a time threshold to finish the prediction modeling. Moreover, these new occurrences corroborate our model results of codling moth potential suitable habitat.
Discussion
Our study is the first to identify and map different stages of codling moth invasion in China. These hypothesized stages of invasion can be used to guide codling moth control and management strategies in China. It may be easier to control and eradicate codling moth from areas where colonization and adaptation have been predicted by our models (Fig. 6). We also identified that human footprint, temperature annual range and precipitation of the wettest quarter are the most important factors associated with codling moth distribution in China.
Potential Risk to the Apple Producing Areas
The best model predicted highly suitable areas for codling moth in north-central, northwestern and northeastern parts of China suggesting higher risk of damage to apple production in these areas compared to other regions in southern parts of the country (Fig. 2). Our results showed that among seven highest apple producing provinces codling moth’s potential risk is highest in Gansu, Shaanxi, Shanxi, southern Hebei, central Liaoning, and northwestern Henan provinces. However, the best model predicted lower risk of codling moth infestation in Shandong province (Fig. 2). None or very low risk was predicted in southern provinces of the country. Medium to high risk was predicted in other apple producing provinces such as Ningxia, Heilongjiang and Jilin, whereas low or very low risk was predicted in Beijing, Tianjin, and Hubei provinces (Fig. 2).
Effects of Environmental Factors
Human footprint was one of the most important factors in predicting potential distribution of codling moth in China which may be because humans disperse codling moth propagules, and irrigation by humans make climatically unsuitable areas suitable for growing apple, a major host of codling moth. This finding matches recent studies by other investigators (Abulizi et al. 2015, Zhao et al. 2015, Cabra-Rivas et al. 2016) who also found human footprint as an important predictor of invasive species’ potential distribution. Precipitation of wettest quarter (bio16) was an important factor associated with codling moth distribution in China (Table 2) which could be because rainfall can affect the survival of first instar (Hagley 1976). Degree days at 10 °C was also an important predictor of codling moth distribution which is the lower development threshold for codling moth populations (Shel’Deshova 1967). Three important predictors in our best model, namely temperature annual range (bio7), precipitation of wettest quarter (bio16), and precipitation seasonality (bio15) were same as the global codling moth model of Kumar et al. (2015a). Not all predictors were same in our regional model and Kumar et al.’s (2015a) global model because different factors govern species distributions at different scales (Franklin 2009).
Comparison With Other Studies on Codling Moth
Two other studies by Kumar et al. (2015a) and Zhao et al. (2015) attempted to model potential distribution of codling moth at global and regional scales using niche models. However, our study was unique because it identified and mapped different stages of invasion of codling moth in China which other studies did not do. Predictions from our climate only model matched closely with that of Kumar et al. (2015a) predictions in China. However, predictions from our best model (human footprint + climate) matched with Zhao et al. (2015) in northern China but differed significantly in southern China. Our models, including that of Kumar et al. (2015a), are based on rigorous species-specific tuning and testing of multiple settings in MaxEnt, and model selection using AICc, which was not done by Zhao et al. (2015). Recent studies suggested that species-specific tuning is required for generating reliable and accurate species distribution models using MaxEnt; models with optimal complexity perform the best (Shcheglovitova and Anderson 2013; Syfert et al. 2013; Kumar et al. 2014a,b). Default settings in MaxEnt can produce highly complex models, biologically nonsensical species response curves (Fig. A3 in Kumar et al. 2014c), and thus produce highly erroneous model projections outside model training regions, which appears to be the case in Zhao et al. (2015) study.
Model Limitations and Caveats
Correlative niche models such as MaxEnt have inherent limitations and uncertainties associated with them (Jarnevich et al. 2015). For example, these models may be affected by multicollinearity, spatial autocorrelation, spatial resolution of predictor variables, species characteristics, and spatial errors in species’ occurrences, and sampling bias (Guisan et al. 2007a,b; Dormann et al. 2013; Syfert et al. 2013). The model predictions may also be affected by temporal mismatch in climatic data and species occurrences. Therefore results from these models should be interpreted cautiously. Our model included propagule pressure (represented by human footprint variable) which makes our predictions more reliable and accurate. Also, a number of correlative niche models are available for modeling potential distribution of a species and different modeling algorithms may result in slightly different predictions and vary in their performance (Qiao et al. 2015). In this study we only used MaxEnt model because it was best suited for our species since we only had presence data, no absence data were available. Some researchers have advocated the use of ensemble modeling to overcome this uncertainty (Araujo and New 2007) however others suggested using the best model (Mainali et al. 2015).
Conclusions and Management Implications
Our results may be useful in designing effective control and monitoring programs for management of codling moth in China. The results can also be used for designing science-based sampling scheme and setting up surveillance sites. Our study used a unique theoretical framework to identify and map different stages of invasion of codling moth in China. Information on leading edges of invasion, and about areas where adaptation and colonization is currently occurring can be especially useful in controlling codling moth spread to new areas, and monitoring its populations in currently infested areas. The theoretical and analytical framework presented in this study can be adopted for dealing with other harmful invasive species of concern in China and elsewhere in the world.
Supplementary Data
Supplementary data are available at Journal of Insect Science online.
Supplementary Material
Acknowledgments
Dr. S.K. and Dr. L.G.N. were supported by a grant from the Washington Tree Fruit Research Commission (WTFRC) from the Foreign Agricultural Service of the United States Department of Agriculture (USDA). We also thank the Natural Resource Ecology Laboratory at Colorado State University, USA, and USDA-ARS, Wapato, Washington, USA, for providing the logistical support.
References Cited
- Abulizi A., Feng Z., Yang J., Zayiti A., Xu Z. 2015. Invasion of the Himalayan hotspot by Acacia farnesiana: how the human footprint influences the potential distribution of alien species. Curr. Sci. 109: 183–189. [Google Scholar]
- Araujo M. B., New M. 2007. Ensemble forecasting of species distributions. Trends Ecol. Evol. 22: 42–47. [DOI] [PubMed] [Google Scholar]
- Bogosian V., Hellgren E. C., Sears M. W., Moody R. W. 2012. High-resolution niche models via a correlative approach: comparing and combining correlative and process-based information. Ecol. Model. 237–238. 63–73. [Google Scholar]
- Boria R. A., Olson L. E., Goodman S. M., Anderson R. P. 2014. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 275: 73–77. [Google Scholar]
- Burnham K. P., Anderson D. R. 2002. Model selection and multimodel inference: a practical information-theoretic approach, 2nd ed Springer, New York, NY. [Google Scholar]
- Cabra-Rivas I., Saldana A., Castro-Diez P., Gallien L. 2016. A multi-scale approach to identify invasion drivers and invaders’ future dynamics. Biol. Invasions 18: 411–426. [Google Scholar]
- Dormann C. F., Elith J., Bacher S., Buchmann C., Carl G., Carre G., Marquez J. R. G., Gruber B., Lafourcade B., Leitao P. J., et al. 2013. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36: 27–46. [Google Scholar]
- Du Z. H. 2011. Biological characters and Monitoring technique of the Codling Moth, Cydia pomonella (L.). Institute of Zoology, Chinese Academy of Sciences, (Ph.D. Dissertation).
- Du Z. H., Wang Z. Q., Liu Y. X., Wang H., Xue F. Z. 2014. Ecological niche modeling for predicting the potential risk areas of severe fever with thrombocytopenia syndrome. Int. J. Infect. Dis. 26: 1–8. [DOI] [PubMed] [Google Scholar]
- Elith J., Graham H., Anderson C. R.P., Ferrier M. D. S., Guisan A., Hijmans J. R., Huettmann F., Leathwick J.R., Lehmann A., Li J., et al. 2006. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 29: 129–151. [Google Scholar]
- Elith J., Phillips S. J., Hastie T., Dudik M., Chee Y. E., Yates C. J. 2011. A statistical explanation of MaxEnt for ecologists. Divers. Distributions 17: 43–57. [Google Scholar]
- EPPO. 2015. PQR – EPPO (European Plant Protection Organization) plant quarantine data retrieval system (version 5.3.5). http://www.eppo.int.
- Evangelista P. H., Kumar S., Stohlgren T. J., Young N. E. 2011. Assessing forest vulnerability and the potential distribution of pine beetles under current and future climate scenarios in the Interior West of the US. Forest Ecol. Manag. 262: 307–316. [Google Scholar]
- Evangelista P., Kumar S. 2011. Trade and transportation is changing the game. Curr. Zool. 57: I–I. [Google Scholar]
- Flory A. R., Kumar S., Stohlgren T. J., Cryan P. M. 2012. Environmental conditions associated with bat White-Nose Syndrome mortality in the north-eastern United States. J. Appl. Ecol. 49: 680–689. [Google Scholar]
- Franklin J. 2009. Mapping species distributions: spatial inference and prediction. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Gallien L., Douzet R., Pratte S., Zimmermann N. E., Thuiller W. 2012. Invasive species distribution models – how violating the equilibrium assumption can create new insights. Global Ecol. Biogeogr. 21: 1126–1136. [Google Scholar]
- Guisan A., Graham C. H., Elith J., Huettmann F. and NCEAS Species Distribution Modeling Group. 2007a. Sensitivity of predictive species distribution models to change in grain size. Divers. Distributions 13: 332–340. [Google Scholar]
- Guisan A., Zimmermann N. E., Elith J., Graham C. H., Phillips S., Peterson A. T. 2007b. What matters for predicting the occurrences of trees: techniques, data, or species’ characteristics? Ecol. Monogr. 77: 615–630. [Google Scholar]
- Hagley E. A. C. 1976. Effect of rainfall and temperature on codling moth oviposition. Environ. Entomol. 5: 967–969. [Google Scholar]
- Hijmans R. J., Cameron S. E., Parra J. L., Jones P. G., Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25: 1965–1978. [Google Scholar]
- Jarnevich C. S., Stohlgren T. J., Kumar S., Morisette J. T., Holcombe T. R. 2015. Caveats for correlative species distribution modeling. Ecol. Inform. 29: 6–15. [Google Scholar]
- Jin R., Zhang J., Bai Z. 1996. A preliminary study on the relationship between the rain and distribution of codling moth, Laspeyresia pomonella L. Plant Quarantine (China) 10: 129–141. [Google Scholar]
- Jin R., Zhang J., Bai Z., Lui L. 1996. An analysis risk of the codling moth [Cydia pomonella] in China. Acta Phytophylac. Sin. 23: 191–192. [Google Scholar]
- Kumar S., Neven L. G., Yee W. L. 2014a. Assessing the potential for establishment of western cherry fruit fly using ecological niche modeling. J. Econ. Entomol. 107: 1032–1044. [DOI] [PubMed] [Google Scholar]
- Kumar S., Graham J., West A. M., Evangelista P. H. 2014b. Using district-level occurrences in MaxEnt for predicting the invasion potential of an exotic insect pest in India. Comput. Electron. Agric. 103: 55–62. [Google Scholar]
- Kumar S., Neven L. G., Yee W. L. 2014c. Evaluating correlative and mechanistic niche models for assessing the risk of pest establishment. Ecosphere 5: 1–23. [Google Scholar]
- Kumar S., Neven L. G., Zhu H., Zhang R. 2015a. Assessing the global risk of establishment of Cydia pomonella (Lepidoptera: Tortricidae) using CLIMEX and MaxEnt niche models. J. Econ. Entomol. 108: 1708–1719. [DOI] [PubMed] [Google Scholar]
- Kumar S., LeBrun E. G., Stohlgren T. J., Stabach J. A., McDonald D. L., Oi D. H., LaPolla J. S. 2015b. Evidence of niche shift and global invasion potential of Tawny Crazy ant Nylanderia fulva. Ecol. Evol. 5: 4628–4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Spaulding S. A., Stohlgren T. J., Hermann K. A., Schmidt T. S., Bahls L. L. 2009. Potential habitat distribution for the freshwater diatom Didymosphenia geminata in the continental US. Front. Ecol. Environ. 7: 415–420. [Google Scholar]
- Lei. 2010. Prediction of invasive alien species potential distribution and risk management research. Nanjing University of Information Science & Technology, Nanjing (Master Thesis).
- Li B. N., Ma J., Hu X. N., Liu H. J., Zhang R. J. 2009. Potential geographical distributions of the fruit flies Ceratitis capitata, Ceratitis cosyra, and Ceratitis rosa in China. J. Econ. Entomol. 102: 1781–1790. [DOI] [PubMed] [Google Scholar]
- Liang L., Hui Y., Xingyue L., Junhua Z., Zaizhing C., Ding Y. 2010. Analysis of suitability of the codling moth, Cydia pomonella in China. Plant Prot. 36: 101–105. [Google Scholar]
- Lin W., Lin C. J., Chong J. 1996. Effect of ecological factors on the geographic distribution of codling moth. Plant Quarantine 10: 1–6. [Google Scholar]
- Lin W., Yu J., Xue G., Wang Y. 2006. Study on population fluctuations of Cydia pomonella (L.) and Grapholitha molesta Busck in Aksy, Xinjiang. Xinjiang Agric. Sci. 43: 100–102. [Google Scholar]
- Liu Y., Luo J., Zhou Z., Wei Y. 2012. Life tables of the experimental population of codling moth, Cydia pomonella (L.) at different temperatures. Acta Phytophyl. Sin. 39: 205–210. [Google Scholar]
- Liu C., White M., Newell G. 2013. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 40: 778–789. [Google Scholar]
- Lozier J. D., Mills N. J. 2011. Predicting the potential invasive range of light brown apple moth (Epiphyas postvittana) using biologically informed and correlative species distribution models. Biol. Invasions 13: 2409–2421. [Google Scholar]
- Mainali K. P., Warren D. L., Dhileepan K., McConnachie A., Strathie L., Hassan G., Karki D., Shrestha B. B., Parmesan C. 2015. Projecting future expansion of invasive species: comparing and improving methodologies for species distribution modeling. Global Change Biol. 21: 4464–4480. [DOI] [PubMed] [Google Scholar]
- Montecino V., Molina X., Kumar S., Castillo M. L. C., Bustamante R. O. 2014. Niche dynamics and potential geographic distribution of Didymosphenia geminata (Lyngbye) M. Schmidt, an invasive freshwater diatom in Southern Chile. Aquat. Invasions 9: 507–519. [Google Scholar]
- Murray K. A., Retallick R. W. R., Puschendorf R., Skerratt L. F., Rosauer D., McCallum H. I., Berger L., Speare R., VanDerWal J. 2011. Issues with modelling the current and future distribution of invasive pathogens. J. Appl. Ecol. 48: 177–180. [Google Scholar]
- Neven L. G. 2012. Fate of codling moth (Lepidoptera: Tortricidae) in harvested apples held under short photoperiod. J. Econ. Entomol. 105: 297–303. [DOI] [PubMed] [Google Scholar]
- Neven L. G. 2013. Effects of short photoperiod on codling moth diapause and survival. J. Econ. Entomol. 106: 520–523. [DOI] [PubMed] [Google Scholar]
- Owens H. L., Campbell L. P., Dornak L. L., Saupe E. E., Barve N., Soberon J., Ingenloff K., Lira-Noriega A., Hensz C. M., Myers C. E., et al. 2013. Constraints on interpretation of ecological niche models by limited environmental ranges on calibration areas. Ecol. Model. 263: 10–18. [Google Scholar]
- Peterson A. T., Soberon J., Pearson R. G., Anderson R. P., Martinez-Meyer E., Nakamura M., Araujo M. B. 2011. Ecological niches and geographic distributions. Princeton University Press, Princeton, NJ. [Google Scholar]
- Peterson A. T., Papes M., Soberon J. 2008. Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecol. Model. 213: 63–72. [Google Scholar]
- Phillips S. J., Anderson R. P., Schapire R. E. 2006. Maximum entropy modeling of species geographic distributions. Ecol. Model. 190: 231–259. [Google Scholar]
- Poulos H. M., Chernoff B., Fuller P. L., Butman D. 2012. Ensemble forecasting of potential habitat for three invasive fishes. Aquat. Invasions 7: 59–72. [Google Scholar]
- Qiao H., Soberón J., Peterson A. T. 2015. No silver bullets in correlative ecological niche modelling: insights from testing among many potential algorithms for niche estimation. Methods Ecol. Evol. 6: 1126–1136. [Google Scholar]
- Rouget M., Richardson D. M., Nel J. L., Le Maitre D. C., Egoh B., Mgidi T. 2004. Mapping the potential ranges of major plant invaders in South Africa, Lesotho and Swaziland using climatic suitability. Divers. Distributions 10: 475–484. [Google Scholar]
- Sambaraju K. R., Carroll A. L., Zhu J., Stahl K., Moore R. D., Aukema B. H. 2012. Climate change could alter the distribution of mountain pine beetle outbreaks in western Canada. Ecography 35: 211–223. [Google Scholar]
- Sanderson E. W., Jaiteh M., Levy M. A., Redford K. H., Wannebo A. V., Woolmer G. 2002. The human footprint and the last of the wild. Bioscience 52: 891–904. [Google Scholar]
- Shcheglovitova M., Anderson R. P. 2013. Estimating optimal complexity for ecological niche models: a jackknife approach for species with small sample sizes. Ecol. Model. 269: 9–17. [Google Scholar]
- Shel’Deshova G. G. 1967. Ecological factors determining distribution of the codling moth, Laspeyresia pomonella L. (Lepidoptera: Tortricidae), in northern and southern hemispheres. Entomol. Rev. 46: 349–361. [Google Scholar]
- Stohlgren T. J., Ma P., Kumar S., Rocca M., Morisette J. T., Jarnevich C. S., Benson N. 2010. Ensemble habitat mapping of invasive plant species. Risk Anal. 30: 224–235. [DOI] [PubMed] [Google Scholar]
- Syfert M. M., Smith M. J., Coomes D. A. 2013. The effects of sampling bias and model complexity on the predictive performance of MaxEnt species distribution models. PLoS One 8: e55158.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabucco A., Zomer R. J. 2009. Global Aridity Index (Global-Aridity) and Global Potential Evapo-Transpiration (Global-PET) Geospatial Database. CGIAR Consortium for Spatial Information. Published online, available from the CGIAR-CSI GeoPortal. (http://www.csi.cgiar.org) (accessed 27 June 2016).
- Warren D. L., Glor R. E., Turelli M. 2010. ENMTools: a toolbox for comparative studies of environmental niche models. Ecography 33: 607–611. [Google Scholar]
- West A. M., Kumar S., Wakie T., Brown C. S., Stohlgren T. J., Laituri M., Bromberg J. 2015. Using high-resolution future climate scenarios to forecast Bromus tectorum invasion in Rocky Mountain National Park. PLoS One 10: e0117893.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett M. J., Neven L., Miller C. E. 2009. The occurrence of codling moth in low latitude countries: validation of pest distribution reports. Horttechnology 19: 633–637. [Google Scholar]
- Wilson J. R. U., Richardson D. M., Rouget M., Proches S., Amis M. A., Henderson L., Thuiller W. 2007. Residence time and potential range: crucial considerations in modelling plant invasions. Divers/ Distributions 13: 11–22. [Google Scholar]
- Xu Z., Guan M. 2008. The occurrence of Laspeyresia pomonella in Zhangye area, Gansu province and its control. China Fruits 4: 70–71. [Google Scholar]
- Xu J., Jiang H.-X., Aliya G. J., Zhang R. 2012. Growth and patterns of population decline in Cydia pomonella adults in Gansu, Xinjiang and Inner Mongolia. Chin. J. Appl. Entomol. 49: 89–95. [Google Scholar]
- Xu J., Liu W., Liu H., Wu L., Zhang R. 2015. Spread and impact of the codling moth Cydia pomonella (L.) in China. J/ Biosafety 24: 327–336. [Google Scholar]
- Yang R. 2008. Study on habitat suitability of Cydia pomonella (L.) in China. MS, Northwest University of Science and Technology Yangling, Shaanxi, China. [Google Scholar]
- Zhang X. Z. 1957. Taxonomic notes on the codling moth, Carpocapsa pomonella L. in Sinkiang. Acta Entomol. Sin. 7: 467–472. [Google Scholar]
- Zhang R. Z., Wang F. X., Zhang Y. L., Chen H. J., Luo J. C., Wang Q. Y., Liu W. X., Ainiwaer M., Pu C. J., Yan Y. G., et al. 2012. Progress on monitoring and control of the codling moth, Cydia pomonella (L.). Chin. J. Appl. Entomol. 49: 37–42. [Google Scholar]
- Zhao L., Hou P., Zhu G., Li M., Xie T., Liu Q. 2015. Mapping the disjunct distribution of introduced codling moth Cydia pomonella in China. Agric. Forest Entomol. 17: 214–222. [Google Scholar]
- Zhu H. Y. 2010. The distribution and threats of invasive codling moth in China. Presentation at Potential Invasive Pests Workshop; Miami, FL: (http://conference.ifas.ufl.edu/TSTAR/presentations/Tuesday/am/11%2030am%20H.ZHU.pdf) (accessed 27 June 2016). [Google Scholar]
- Zhu G., Bu W., Gao Y., Liu G. 2012. Potential geographic distribution of brown marmorated stink bug invasion (Halyomorpha halys). PLoS One 7: e31246.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.