Abstract
Background
Acute pancreatitis (AP) is a sudden inflammation of the pancreas. It results in multiple, severe complications, and 15–20% of patients develop severe acute pancreatitis (SAP) with mortality as high as 30%. Consequently, it is imperative to develop an effective therapy for SAP.
Material/Methods
We used 30 adult male Sprague Dawley (SD) rats. Rats were randomly divided into 3 groups – sham, SAP, and fentanyl+SAP – with 10 rats in each group. An automatic biochemical analyzer was used to analyze the concentration of creatine kinase isoenzyme (CK-MB) and lactate dehydrogenase (LDH). Terminal-deoxynucleotidyl transferase-mediated nick-end labeling (TUNEL) assay was applied to assess the cell apoptosis rate. Pathological changes in pancreas/heart were detected with hematoxylin and eosin (HE) staining. Western immunoblot assay was used to analyze protein levels of interleukin (IL)-1β, IL-6, and IκB.
Results
Fentanyl pre-treatment inhibits SAP-induced elevation of CK-MB/LDH concentrations in serum. Compared with the sham group, SAP generates a higher brown/yellow staining rate, which is abated by fentanyl. In the pancreas, SAP generated more serious interstitial edema/hemorrhage and fat necrosis than in the sham group, which are attenuated by fentanyl. Likewise, compared to the sham group, SAP generates swelled/disordered myocardial fibers and congested blood vessels in myocardium, which are ameliorated by fentanyl. In the sham group, there was little IL-1β/IL-6, and fentanyl significantly inhibited SAP-induced up-regulation of IL-1β/IL-6 levels. Compared with the sham group, SAP significantly reduced IκB level, which was rescued by fentanyl.
Conclusions
Fentanyl effectively alleviates SAP-induced pancreas and heart injuries through regulating the nuclear factor-κB (NF-κB) signaling pathway.
MeSH Keywords: Cardiomyopathies; Fentanyl; Pancreatitis, Acute Necrotizing
Background
AP is a sudden inflammation in the pancreas [1], with severe complications and high mortality despite treatment. Unfortunately, in 15–20% of patients, AP progresses into SAP, with high (30%) mortality [2,3]. Patients with mild SAP can be effectively treated by conservative therapies (fasting and intravenous fluid rehydration), while patients with severe SAP need intensive care. SAP is an abdominal disease frequently encountered by surgeons [4]. Despite mounting achievements in modern medicine and increasing clinical/experimental studies, the specific pathogenesis of SAP remains elusive [5,6]. Surprisingly, nuclear factor-κB (NF-κB), inflammatory cytokines, leukocytic infiltration, and oxidative stress have been shown to be pivotal factors during the process of SAP [7,8]. Inflammation is responsible for the morbidity and mortality of AP [9]. Regrettably, there remains no effective therapy for SAP [10].
SAP causes multiple-organ injuries (e.g., renal failure [11]), and the morbidity of SAP-induced myocardial injury is reported to be 60.5% [12].
Fentanyl (intravenous) has recently been used for pain relief in AP, especially in renal impairment [13], and is increasingly used due to its good safety profile. Consequently, the morbidity of SAP-induced complications has not been substantially reduced to date. It is urgent to seek an effective medicine to successfully treat SAP and SAP-induced myocardial injury. The present study investigated the possible protective role of fentanyl against SAP-induced myocardial injury in rats and to explore the possible molecular mechanism.
Material and Methods
Animals
We randomly separated 30 male Sprague Dawley (SD) rats weighing 200–250 g into 3 different groups – sham, SAP, and fentanyl treatment – with 10 rats in each group. Rats were housed under pathogen-free conditions with controlled temperature (22±2°C), a relative humidity of 30–70%, 12-h light-dark cycle, and provided with water/food ad libitum. Each animal experiment was conducted in accordance with the animal care guidelines of the Chinese National Institutes of Health and the study was approved by the Central Hospital of Wuhan Animal Care and Use Committee.
Model preparation
In brief, anesthetization of rats was performed with 10% chloral hydrate (3 ml/kg) prior to abdomen incision. Upon the location of biliopancreatic duct and opening of duodenal papilla, a syringe needle was placed. Thereafter, the cholangitic porta hepatis was clipped with a small artery clamp, 5% sodium taurocholate (1 ml/kg) was injected into the pancreatic duct at the speed of 0.1 ml/min. Five min later, the clamp was removed, followed by incision suture and abdomen closing.
At 23–23.5 h after SAP preparation, rats in the fentanyl group received an intravenous administration of fentanyl (30 ug/kg diffused in 0.5 ml saline) and sustained for 30–60 min, while rats in sham and SAP groups were intravenously injected with 0.5 ml saline. For rats in the sham group, an incision was made in the abdomen and pancreatic tissue was marginally rotated several times, then the abdomen was closed. At 24 h after SAP preparation, rats were killed and prepared for further studies.
Specimen collection
Rats were anesthetized by intraperitoneal injection with 10% chloral hydrate, followed by abdomen opening, pancreas/heart removal, and abdominal aorta blood collection under sterile conditions. Heart tissues were used for the investigation of myocardial cell apoptosis and analysis of pathological changes, and pancreas tissues were utilized for determining pathological scores.
Assessment of myocardial injury
We let the obtained arterial blood coagulate naturally at room temperature for about 1 h. Afterwards, serum was collected via centrifuging the coagulated arterial blood at the speed of 1006.2×g at 4°C for 10 min. Concentrations of CK-MB and LDH were detected by use of a Vitros 250 automatic biochemical analyzer.
TUNEL staining
TUNEL staining was carried out using a cell death detection kit in accordance with manufacturer’s instructions to detect the apoptotic rates of myocardial cells in different groups. Nuclei were stained by 3, 3-diaminobenzidine (DAB). Nuclei of healthy cells were stained blue, while those in apoptotic cells presented brown/yellow staining. Ten areas were randomly selected from each section. Double-blind condition was adopted for the counting of TUNEL-positive cells.
HE staining
Pancreatic and heart tissues were fixed in 4% neutral paraformaldehyde (PFA) solution for approximately 24 h, dehydrated through graduated ethanol, embedded with paraffin, and finally sliced into 5-μm-thick sections. In brief, slices were dewaxed in xylene and rehydrated by upgraded ethanol. After rinsing 3 times with distilled water, slices were immersed in hematoxylin for approximately 5 min. Before slice differentiation with 1% HCl, a second rinsing was carried out. Thereafter, slices were further stained with eosin for about 4 min. At the end of the experiment, slices were dehydrated and differentiated in alcohol. Thereafter, morphological changes of tissues were studied under light microscopy.
Pathological score
According to changes in edema, infiltration of inflammatory cells, hemorrhage, and necrosis in pancreatic tissues, the severity of pathological changes and corresponding scores were recorded: 0, no; 1, mild; 2, moderate; 3, severe; and 4, very severe.
In accordance with pathological changes in infiltration of inflammatory cells and myocardial necrosis in heart tissues, severity of pathological changes and corresponding scores were recorded: 0, no; 1, mild; 2, moderate; 3, severe; 4, very severe; and 5, extremely severe.
Western immunoblot
Heart tissues from 3 different groups were homogenized in lysis buffer. In brief, samples were resolved via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electro-blotted to Immobilon-P nylon membranes. Filters were first treated with primary antibody at room temperature for about 3 h. Thereafter, filters were treated with the corresponding horseradish peroxidase (HRP)-labeled secondary antibody at room temperature for about 1 h. The immune complexes were visualized with enhanced chemiluminescence.
Statistical analysis
All of the experiments were performed in duplicate and repeated at least 3 times. Data were analyzed using two-way analysis of variance (ANOVA) and are expressed as mean ± standard deviation (SD). SPSS (Chicago, IL, USA) was used for statistical analysis. P<0.05 showed a significant difference.
Results
Fentanyl pre-treatment inhibits SAP-induced up-regulation of CK-MB and LDH in fentanyl+SAP group
At 24 hours after modeling, 2 rats in the SAP group and 1 rat in the fentanyl treatment group died, and there was no significant difference. Concentrations of CK-MB and LDH in the serum were detected. Results of automatic biochemical analyzer revealed that the concentration of CK-MB in the sham group was 505 u/ml, in the SAP group it was 1806 u/ml, and in the fentanyl+SAP group it was 1020 u/ml. The concentration of LDH in the sham group was 703 u/l, in SAP group it was 1516 u/ml, and in the fentanyl+SAP group it was 993 u/ml. In comparison with the sham group, SAP notably up-regulated concentrations of CK-MB and LDH, which were significantly repressed by fentanyl pre-treatment.
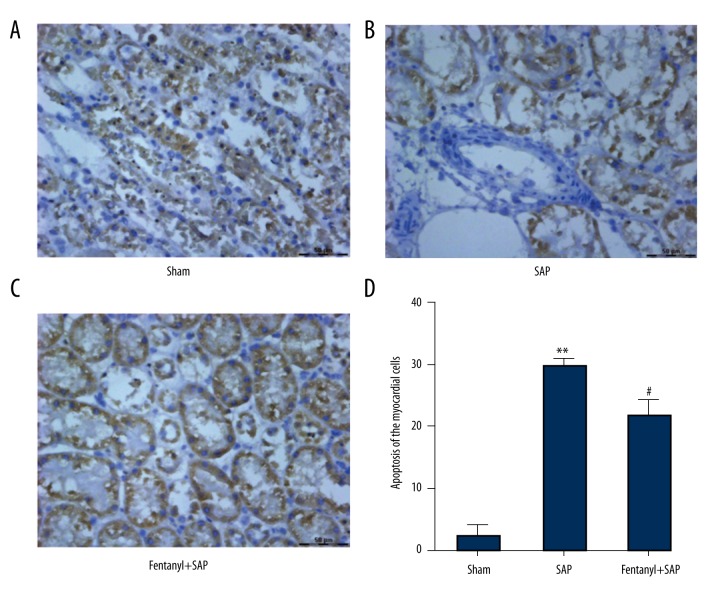
Fentanyl pre-treatment decreased SAP-induced elevation of myocardial cell apoptosis in the fentanyl+SAP group
TUNEL assay was carried out to identify whether fentanyl affected cell apoptosis of myocardial tissues induced by SAP. In the sham group, the majority of nuclei were stained blue (Figure 1A). Many nuclei were stained brown/yellow in the SAP group (Figure 1B), which was predominantly lowered by fentanyl, but was still higher than in the sham group (Figure 1C).
Figure 1.
Effect of fentanyl on SAP-induced apoptosis of myocardial cells. Cell apoptosis images in sham (A), SAP (B), and fentanyl + SAP (C) groups are presented. Statistical data are presented (D). ** P<0.01 vs. sham group; # P<0.05 vs. SAP group.
The apoptotic index was significantly higher in the SAP group than in the sham group (P<0.01), and was alleviated by fentanyl (P<0.05) (Figure 1D).
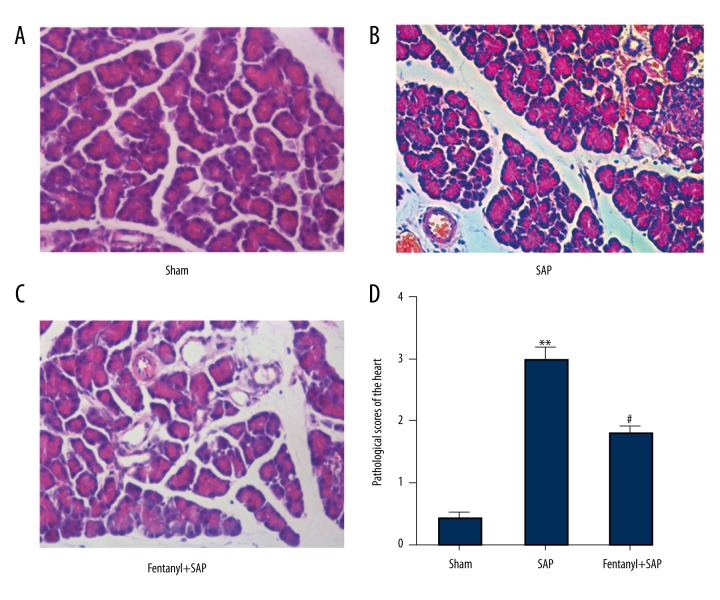
Fentanyl pre-treatment alleviated SAP-induced pathological features in pancreases in the fentanyl+SAP group
HE staining was conducted to detect the effects of fentanyl on pathological changes pancreases. Pancreases in the sham group exhibited normal morphology (Figure 2A). However, SAP resulted in serious morphological changes in interstitial edema, interstitial hemorrhage, and fat necrosis (Figure 2B). After treatment with fentanyl, pathological features were notably improved (Figure 2C).
Figure 2.
Effect of fentanyl on SAP-induced pathological changes of the pancreas. Pathological images in sham (A), SAP (B), and fentanyl + SAP (C) groups are presented. Statistical data are revealed (D). ** P<0.01 vs. sham group; # P<0.05 vs. SAP group.
Pathological scores of pancreases are displayed in Figure 2D. Compared with the sham group (1.6), pathological scores in the SAP group were significantly higher (10.3). Moreover, following treatment with fentanyl, pathological scores (5.7) were significantly lower than in the SAP group.
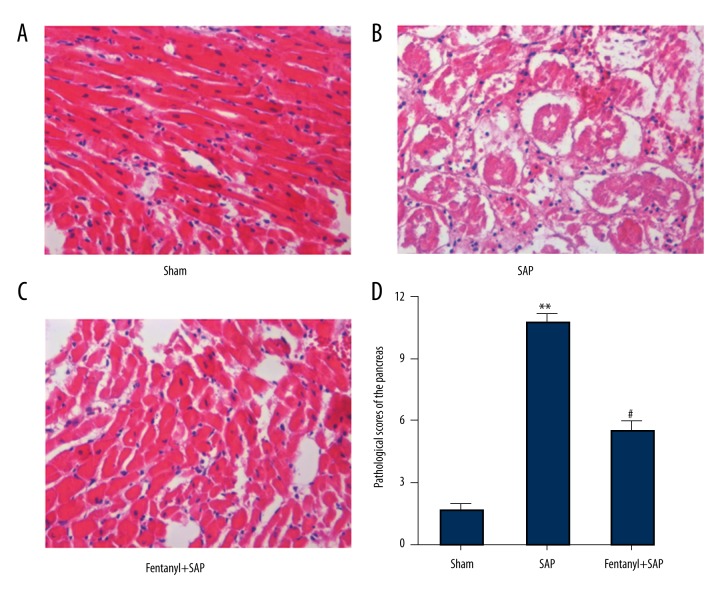
Fentanyl pre-treatment alleviated SAP-induced pathological features in hearts in the fentanyl+SAP group
Similarly, HE staining of myocardial tissue in the sham group exhibited normal morphology (Figure 3A), but in the SAP group myocardial fibers were seriously degenerated, swelled, and disordered and the blood vessels were congested (Figure 3B). After fentanyl treatment, pathological features of hearts were improved (Figure 3C).
Figure 3.
Effect of fentanyl on SAP-induced pathological changes of the heart. Pathological images in sham (A), SAP (B), and fentanyl + SAP (C) groups are exhibited. Statistical data are displayed (D). ** P<0.01 vs. sham group; # P<0.05 vs. SAP group.
Pathological scores of hearts are shown in Figure 3D. Pathological scores in the SAP group (2.9) were significantly higher than in the sham group (0.3), and were significantly (1.8) lower in the fentanyl group.
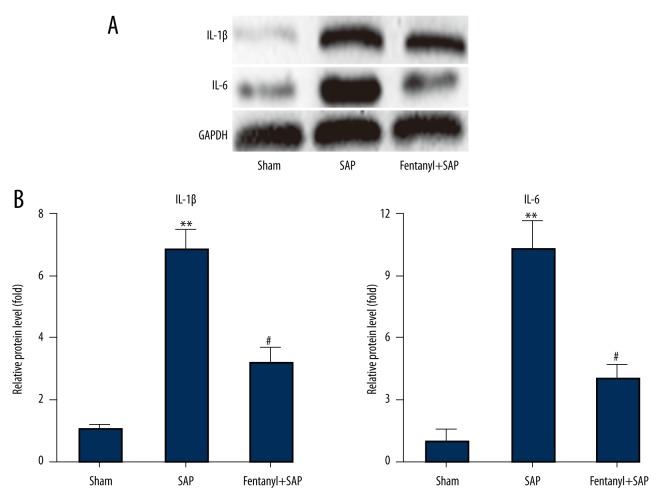
Fentanyl pre-treatment inhibited SAP-induced IL-1β/IL-6 up-regulation in hearts in the fentanyl+SAP group
Western blot results showed that in the sham group there was only a low level of IL-1β/IL-6, and fentanyl treatment significantly inhibited SAP-induced up-regulation of IL-1β/IL-6 level (Figure 4A).
Figure 4.
Effect of fentanyl on SAP-induced expression changes of IL-1β and IL-6 in heart (A). Data of band densities were presented (B). ** P<0.01 vs. sham group; # P<0.05 vs. SAP group.
We also did band density analysis, and data were in line with that in Western blot experiments (Figure 4B).
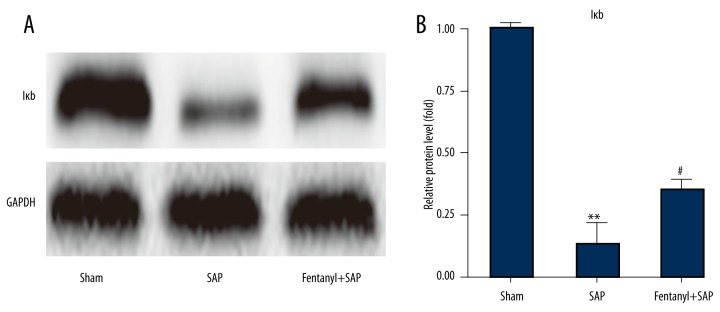
Fentanyl pre-treatment rescued SAP-induced reduction of IκB level in rat hearts in the fentanyl+SAP group
Results demonstrated that, compared with the sham group, SAP significantly reduced IκB level, and fentanyl rescued SAP-induced IκB reduction (Figure 5A).
Figure 5.
Effect of fentanyl on SAP-induced expression change of IκB in heart (A). Data of band densities were exhibited (B). ** P<0.01 vs. sham group; # P<0.05 vs. SAP group.
The corresponding statistical data are presented in Figure 5B.
Discussion
AP is a fatal disease whose pathogenesis remains unclear despite remarkable advances achieved over the past 25 years [14]. Fentanyl was reported to be used for pain relief in AP [13] and is being increasingly used by virtue of its safety profile, especially in renal impairment. The present study aimed to investigate whether fentanyl has a protective role in SAP-induced myocardial injury in rats and to provide a possible molecular mechanism.
CK-MB and LDH in the serum are markers of myocardial injury [15,16]. We first detected changes in CK-MB and LDH concentrations in the present study. Results demonstrated that up-regulation of CK-MB and LDH levels in SAP rats were notably inhibited by fentanyl, which provides direct evidence that fentanyl indeed exerted myocardial protective effects in rats with SAP.
The myocardial protective effects of fentanyl were further verified by TUNEL assay, which displayed that the apoptosis index in the fentanyl group was lower than in the SAP group.
Pathological features in pancreatic and myocardial tissues in the SAP group were remarkably attenuated after treatment with fentanyl, which is consistent with SAP-induced injuries. Consistently, the pathological scores of pancreases and hearts in the fentanyl treatment group were significantly lower than those in the SAP group, indicating that fentanyl can improve SAP-induced pathological changes.
Taken together, our present results verified that fentanyl effectively reduced serum levels of CK-MB and LDH, and inhibited myocardial cell apoptosis, ultimately reducing SAP-generated myocardial injuries. However, the corresponding precise molecular mechanism remains elusive. As the role of fentanyl in pain relief in AP [13] and co-regulators between pain and inflammation [17], we hypothesized the possible mechanism based on previous studies.
AP, together with sepsis, trauma, burns, and surgery, has the potential to generate systemic inflammatory response syndrome (SIRS) [18], in which cytokines act as initiators, enhancers, and damaging agents [19].
Cytokines can activate numerous signaling pathways; for instance, NF-κB can deteriorate inflammation [20,21]. NF-κB is activated at the initial stage of AP and plays a crucial role in inflammatory response during AP [8,22]. Moreover, Satoh et al. reported an obviously elevated activation of NF-κB following the presence of SAP [23]. Free NF-κB is translocated to the nucleus and is bound with the κB site, thus causing massive transcription of plentiful inflammation-correlated genes, including IL-1β and IL-6 [24,25].
Therefore, fentanyl might play a protective role in SAP-induced myocardial injury via inhibiting the activation of the NF-κB signaling pathway, ultimately attenuating the synthesis and release of inflammatory factors. We carried out further studies to investigate whether the aforementioned proposed hypothesis was right.
We first detected the protein levels of IL-1β and IL-6, showing that they were significantly elevated in SAP rats compared to the sham group rats, which were inhibited by fentanyl treatment.
NF-κB can generate transcription of IL-1β/IL-6 and is involved in the process of AP [22–25]. The NF-κB/Rel family contains NF-κB1 (p50), NF-κB2 (p52), Rel A (p65), Rel B, r-Rel, and c-Rel [26–30]. NF-κB is sequestered when bound to IκB (inhibitory element) and stimulated IκB releases NF-κB [29–31]. Furthermore, no detectable changes were found in expression levels of either p50 or p65; IκB levels were reduced in early pancreatitis and elevated with resolving pancreatitis [25]. Thus, we tested IκB levels by Western blot analysis. Results demonstrated that SAP-inhibited activation of IκB was rescued by fentanyl, which is consistent with a previous study [25].
Furthermore, Isik A et al. reported the protective effect of ozone and naringin on intestinal I/R injury [32], and astragaloside IV was recently reported to attenuate AP via reducing the activation of NF-κB in rats [33]. The PI3K/Akt-NF-κB pathway was recently discovered to be activated in AP rats [34]. Our findings are in line with previous studies showing the involvement of the NF-κB pathway in AP, thus providing a possible treatment for SAP via modulating the NF-κB pathway.
Conclusions
Fentanyl had a protective role in SAP-induced myocardial injury in rats via repressing the NF-κB signaling pathway, ultimately reducing the protein level of inflammatory factors.
Footnotes
Disclosure
There are no conflicts of interest to declare.
Source of support: This study was funded by the Natural Science Foundation of China (No. 81470482)
References
- 1.Sommermeyer L. Am J Nurs. Philadelphia, PA: Lippincott Williams & Wilkins; 1935. Acute pancreatitis; pp. 1157–61. [Google Scholar]
- 2.Long J, Song N, Liu XP, et al. Nuclear factor-kappaB activation on the reactive oxygen species in acute necrotizing pancreatitic rats. World J Gastroenterol. 2005;11:4277–80. doi: 10.3748/wjg.v11.i27.4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: Bench to the bedside. Gastroenterol. 2007;132:1127–51. doi: 10.1053/j.gastro.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 4.Harper SJ, Cheslyn-Curtis S. Acute pancreatitis. Ann Clin Biochem. 2011;48:23–37. doi: 10.1258/acb.2010.010196. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Zak Y, Hernandez-Boussard T, et al. The epidemiology of idiopathic acute pancreatitis, analysis of the nationwide inpatient sample from 1998 to 2007. Pancreas. 2013;42:1–5. doi: 10.1097/MPA.0b013e3182572d3a. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki M, Sai JK, Shimizu T. Acute pancreatitis in children and adolescents. World J Gastrointest Pathophysiol. 2014;5:416–26. doi: 10.4291/wjgp.v5.i4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sweiry JH, Mann GE. Role of oxidative stress in the pathogenesis of acute pancreatitis. Scand J Gastroenterol Suppl. 1996;219:10–15. doi: 10.3109/00365529609104992. [DOI] [PubMed] [Google Scholar]
- 8.Gukovskaya AS, Gukovsky I, Zaninovic V, et al. Pancreatic acinar cells produce, release, and respond to tumor necrosis factor-alpha. Role in regulating cell death and pancreatitis. J Clin Invest. 1997;100:1853–62. doi: 10.1172/JCI119714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatia M, Brady M, Shokuhi S, et al. Inflammatory mediators in acute pancreatitis. J Pathol. 2000;190:117–25. doi: 10.1002/(SICI)1096-9896(200002)190:2<117::AID-PATH494>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 10.Zerem E. Treatment of severe acute pancreatitis and its complications. World J Gastroenterol. 2014;20:13879–92. doi: 10.3748/wjg.v20.i38.13879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan YG, Ai ZL, Liu ZS, Xu G. [Protective effect of angelica injection on acute hemorrhagic necrotizing pancreatitis complicated with renal injury]. Chin J Gen Surg. 2000;9:228–30. [in Chinese] [Google Scholar]
- 12.Calleja GA, Barkin JS. Acute pancreatitis. Med Clin North Am. 1993;77:1037–56. doi: 10.1016/s0025-7125(16)30209-7. [DOI] [PubMed] [Google Scholar]
- 13.Stevens M, Esler R, Asher G. Transdermal fentanyl for the management of acute pancreatitis pain. Appl Nurs Res. 2002;15:102–10. doi: 10.1053/apnr.2002.29532. [DOI] [PubMed] [Google Scholar]
- 14.Sah RP, Saluja A. Molecular mechanisms of pancreatic injury. Curr Opin Gastroenterol. 2011;27:444–51. doi: 10.1097/MOG.0b013e328349e346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dahlin LG, Kågedal B, Nylander E, et al. Early identification of permanent myocardial damage after coronary surgery is aided by repeated measurements of CK MB. Scand Cardiovasc J. 2002;36:35–40. doi: 10.1080/140174302317282366. [DOI] [PubMed] [Google Scholar]
- 16.Dai JZ, Fan H. [Protective effects of Danhong injection against heart injury in rats with severe acute pancreatitis]. Shi Jie Hua Ren Xiao Hua Za Zhi. 2009;10:969–75. [in Chinese] [Google Scholar]
- 17.Erin DM, Linda RW. Pathological and protective roles of glia in chronic pain. Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nyström PO. The systemic inflammatory response syndrome: Definitions and aetiology. J Antimicrob Chemoth. 1998;41:1–7. doi: 10.1093/jac/41.suppl_1.1. [DOI] [PubMed] [Google Scholar]
- 19.Closa D, Folch-Puy E. Oxygen free radicals and the systemic inflammatory response. IUBMB Life. 2004;56:185–91. doi: 10.1080/15216540410001701642. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Martindale JL, Liu Y, et al. The cellular response to oxidative stress: influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem J. 1998;333:291–300. doi: 10.1042/bj3330291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahman I, MacNee W. Regulation of redox glutathione levels and gene transcription in lung inflammation: therapeutic approaches. Free Radic Biol Med. 2000;28:1405–20. doi: 10.1016/s0891-5849(00)00215-x. [DOI] [PubMed] [Google Scholar]
- 22.Grady T, Liang P, Ernst SA, Logsdon CD. Chemokine gene expression in rat pancreatic acinar cells is an early event associated with acute pancreatitis. Gastroenterol. 1997;113:1966–75. doi: 10.1016/s0016-5085(97)70017-9. [DOI] [PubMed] [Google Scholar]
- 23.Satoh A, Shimosegawa T, Fujita M. Inhibition of nuclear factor-κB activation improves the survival of rats with taurocholate pancreatitis. Gut. 1999;44:253–58. doi: 10.1136/gut.44.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Algül H, Tando Y, Schneider G, et al. Acute experimental pancreatitis and NF-kappaB/Rel activation. Pancreatol. 2002;2:503–9. doi: 10.1159/000066090. [DOI] [PubMed] [Google Scholar]
- 25.Ethridge RT, Hashimoto K, Chung DH, et al. Selective inhibition of NF-kappaB attenuates the severity of cerulein-induced acute pancreatitis. J Am Coll Surg. 2002;195:497–505. doi: 10.1016/s1072-7515(02)01222-x. [DOI] [PubMed] [Google Scholar]
- 26.Mercurio F, Manning AM. Multiple signals converging on NF-κB. Curr Opin Cell Biol. 1999;11:226–32. doi: 10.1016/s0955-0674(99)80030-1. [DOI] [PubMed] [Google Scholar]
- 27.Baeuerle PA, Baltimore D. NF-κB: Ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh S, May MJ, Kopp EB. NF-κB and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–60. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 29.Baldwin AS., Jr The NF-κB and IκB proteins: New discoveries and insights. Annu Rev Immunol. 1996;14:649–83. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 30.May MJ, Ghosh S. Signal transduction through NF-kappa B. Immunol Today. 1998;19:80–88. doi: 10.1016/s0167-5699(97)01197-3. [DOI] [PubMed] [Google Scholar]
- 31.Thanos D, Maniatis T. NF-κB: A lesson in family values. Cell. 1995;80:529–32. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 32.Isik A, Peker K, Gursul C, et al. The effect of ozone and naringin on intestinal ischemia/reperfusion injury in an experimental model. Int J Surg. 2015;21:38–44. doi: 10.1016/j.ijsu.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 33.Qiu L, Yin GJ, Cheng L, et al. Astragaloside IV ameliorates acute pancreatitis in rats by inhibiting the activation of nuclear factor-κB. Inter J Mol Med. 2015;35:625–36. doi: 10.3892/ijmm.2015.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang WY, Chen Y, Su X, et al. Resistin-like molecule-α causes lung injury in rats with acute pancreatitis by activating the PI3K/Akt-NF-κB pathway and promoting inflammatory cytokine release. Curr Mol Med. 2016;16:677–87. doi: 10.2174/1566524016666160802145700. [DOI] [PubMed] [Google Scholar]