Abstract
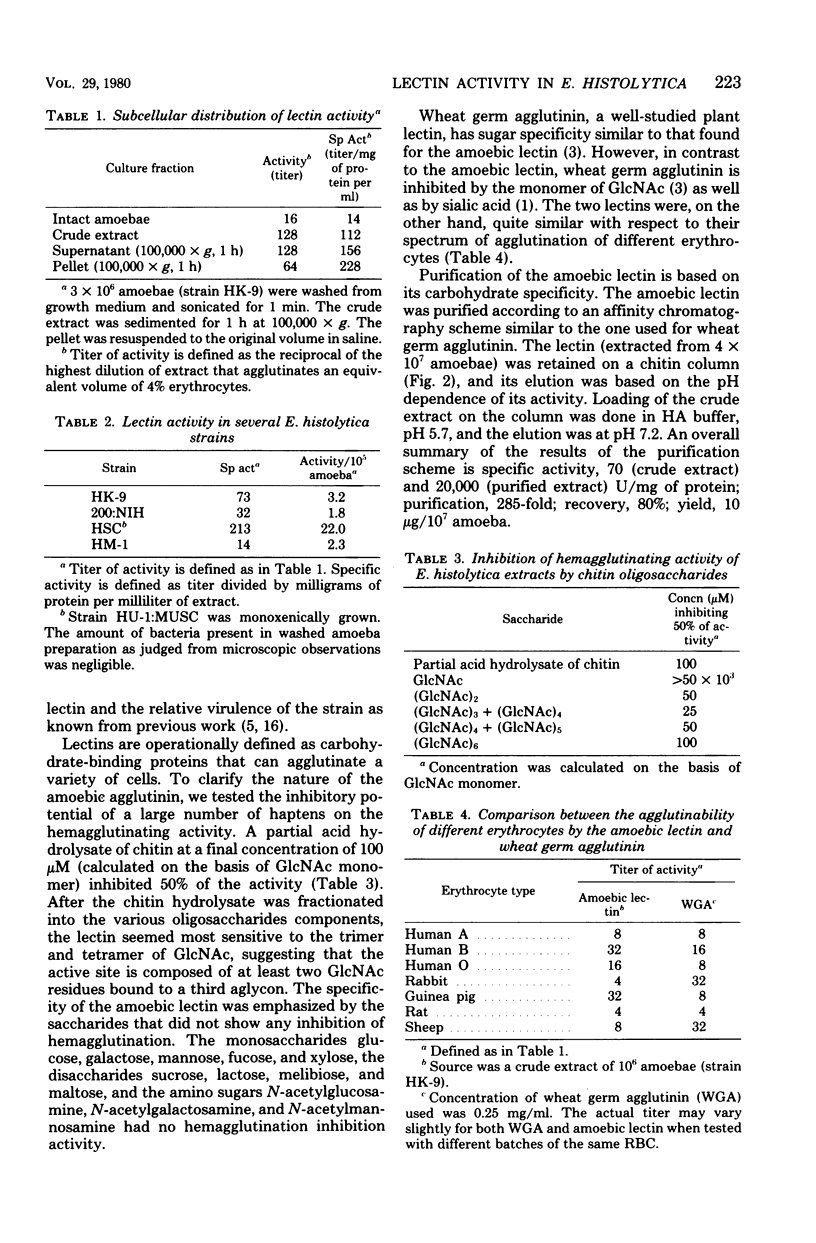
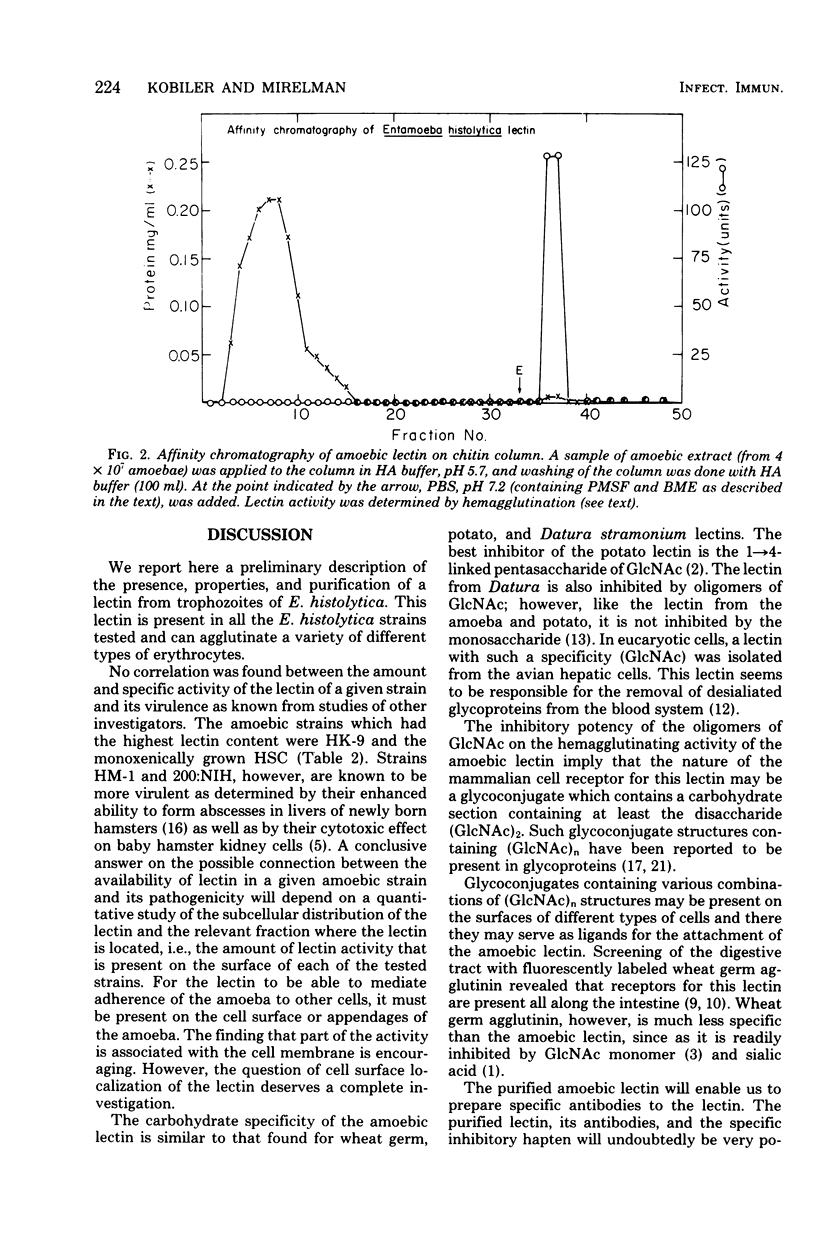
A lectin (carbohydrate-binding protein) has been found in extracts of a number of axenically grown trophozoites of Entamoeba histolytica strains. The strains grown in TYI-S-33 medium (Diamond et al., Trans. R. Soc. Trop. Med. Hyg. 72: 431-432, 1978) were HK-9, 200:NIH, and HM-1:IMSS. Strain HU-1:MUSC (HSC) was grown monoxenically in the same medium. The amoebic lectin agglutinated glutaraldehyde-fixed erythrocytes. This activity was pH dependent and heat and oxidation sensitive, and was destroyed by proteolysis upon autoincubation. The relative agglutinating potency of the different strains of amoebae was investigated. Strain HSC had the highest specific activity (210 U/mg of protein), and strain HM-1 had the lowest (14 U/mg). One unit of hemagglutinating activity is defined as the amount of lectin present in 1 ml of extract which will agglutinate 1 ml of 4% erythrocytes. Upon subcellular fractionation of the lectin present in extracts of strain HK-9, two-thirds of the activity was detected in the soluble, nonsedimentable (100,000 × g, 60 min) fraction. Partial hydrolysate of chitin was found to inhibit the hemagglutinating activity. Among the oligosaccharides of N-acetylglucosamine, the trimer and tetramer were the most potent inhibitors. The lectin was purified approximately 300-fold by a one-step affinity chromatography on a chitin column. The loading and elution from the column were based on the pH dependence of the lectin activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A. K., Neuberger A., Sharon N. The purification, composition and specificity of wheat-germ agglutinin. Biochem J. 1973 Jan;131(1):155–162. doi: 10.1042/bj1310155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A. K., Neuberger A. The purification and properties of the lectin from potato tubers, a hydroxyproline-containing glycoprotein. Biochem J. 1973 Oct;135(2):307–314. doi: 10.1042/bj1350307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos H. J. Entamoeba histolytica: cytopathogenicity of intact amebae and cell-free extracts; isolation and characterization of an intracellular toxin. Exp Parasitol. 1979 Jun;47(3):369–377. doi: 10.1016/0014-4894(79)90089-4. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Diamond L. S., Harlow D. R., Cunnick C. C. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg. 1978;72(4):431–432. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- Essner E., Schreiber J., Griewski R. A. Localization of carbohydrate components in rat colon with fluoresceinated lectins. J Histochem Cytochem. 1978 Jun;26(6):452–458. doi: 10.1177/26.6.670681. [DOI] [PubMed] [Google Scholar]
- Etzler M. E., Branstrator M. L. Differential localization of cell surface and secretory components in rat intestinal epithelium by use of lectins. J Cell Biol. 1974 Aug;62(2):329–343. doi: 10.1083/jcb.62.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOARE C. A. The feed habits of Entamoeba histolytica in its commensal phase. Parasitology. 1952 Mar;42(1-2):43–47. doi: 10.1017/s0031182000084249. [DOI] [PubMed] [Google Scholar]
- Kawasaki T., Ashwell G. Isolation and characterization of an avian hepatic binding protein specific for N-acetylglucosamine-terminated glycoproteins. J Biol Chem. 1977 Sep 25;252(18):6536–6543. [PubMed] [Google Scholar]
- Kilpatrick D. C., Yeoman M. M. Purification of the lectin from Datura stramonium. Biochem J. 1978 Dec 1;175(3):1151–1153. doi: 10.1042/bj1751151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobiler D., Barondes S. H. Lectin activity from embryonic chick brain, heart, and liver: changes with development. Dev Biol. 1977 Oct 1;60(1):326–330. doi: 10.1016/0012-1606(77)90130-0. [DOI] [PubMed] [Google Scholar]
- Mattern C. F., Keister D. B. Experimental amebiasis. II. Hepatic amebiasis in the newborn hamster. Am J Trop Med Hyg. 1977 May;26(3):402–411. [PubMed] [Google Scholar]
- Mizuochi T., Yonemasu K., Yamashita K., Kobata A. The asparagine-linked sugar chains of subcomponent C1q of the first component of human complement. J Biol Chem. 1978 Oct 25;253(20):7404–7409. [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- RUPLEY J. A. THE HYDROLYSIS OF CHITIN BY CONCENTRATED HYDROCHLORIC ACID, AND THE PREPARATION OF LOW-MOLECULAR-WEIGHT SUBSTRATES FOR LYSOZYME. Biochim Biophys Acta. 1964 Nov 1;83:245–255. doi: 10.1016/0926-6526(64)90001-1. [DOI] [PubMed] [Google Scholar]
- Trissl D., Martínez-Palomo A., de la Torre M., de la Hoz R., Pérez de Suárez E. Surface properties of Entamoeba: increased rates of human erythrocyte phagocytosis in pathogenic strains. J Exp Med. 1978 Nov 1;148(5):1137–1143. doi: 10.1084/jem.148.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita K., Tachibana Y., Kobata A. The structures of the galactose-containing sugar chains of ovalbumin. J Biol Chem. 1978 Jun 10;253(11):3862–3869. [PubMed] [Google Scholar]




