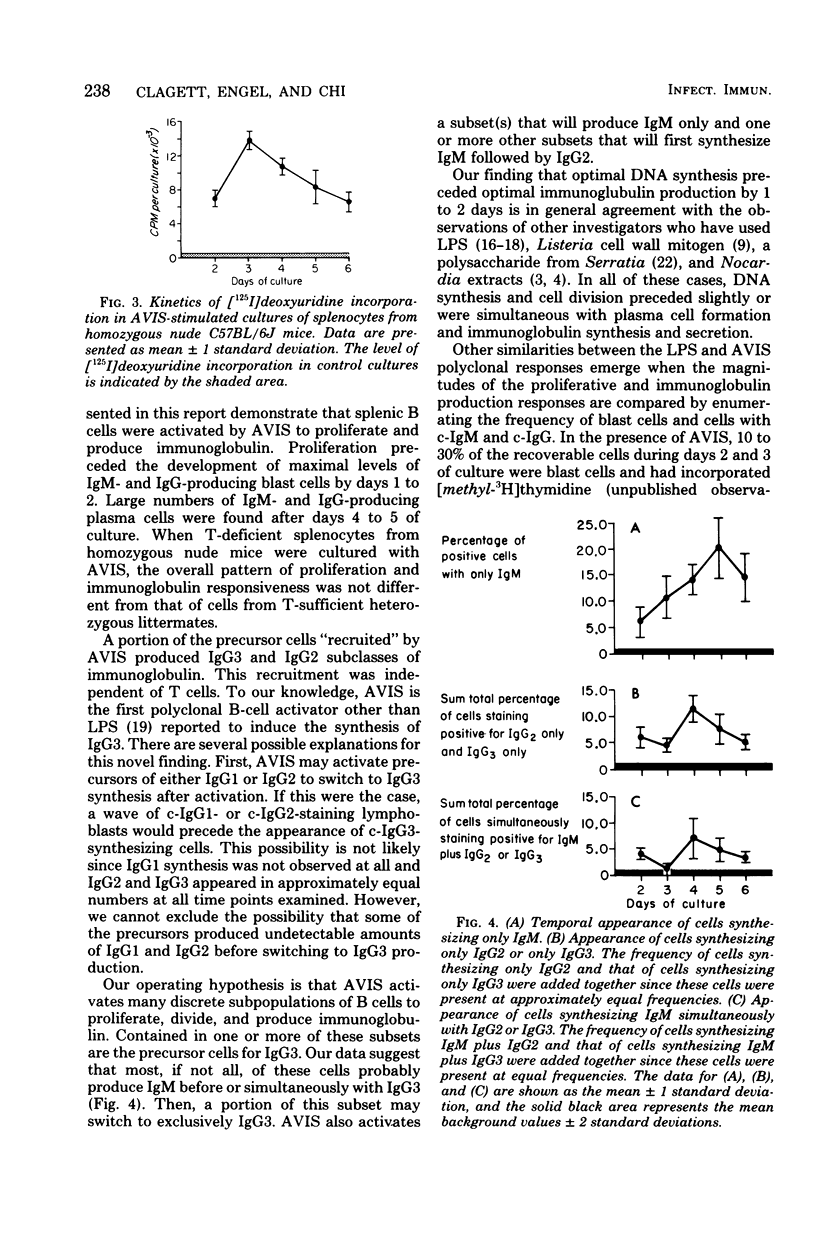
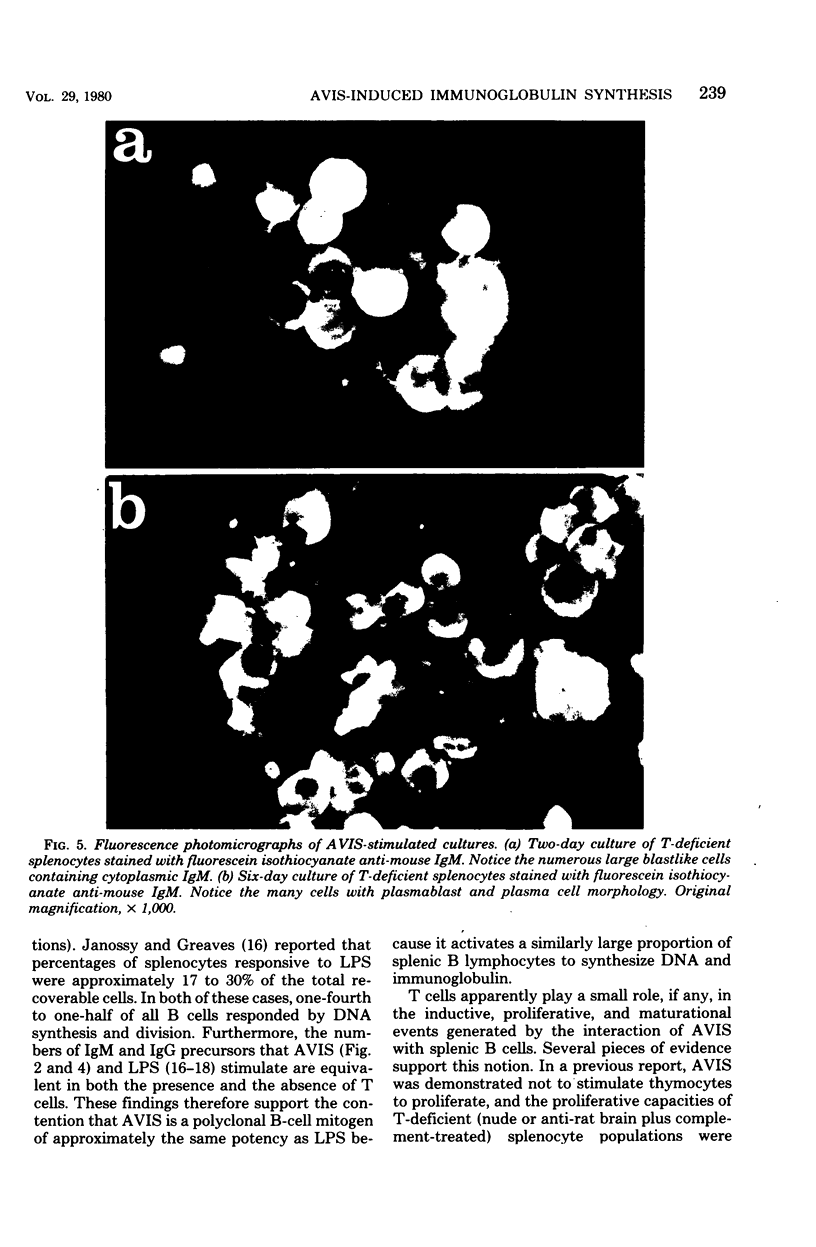
Abstract
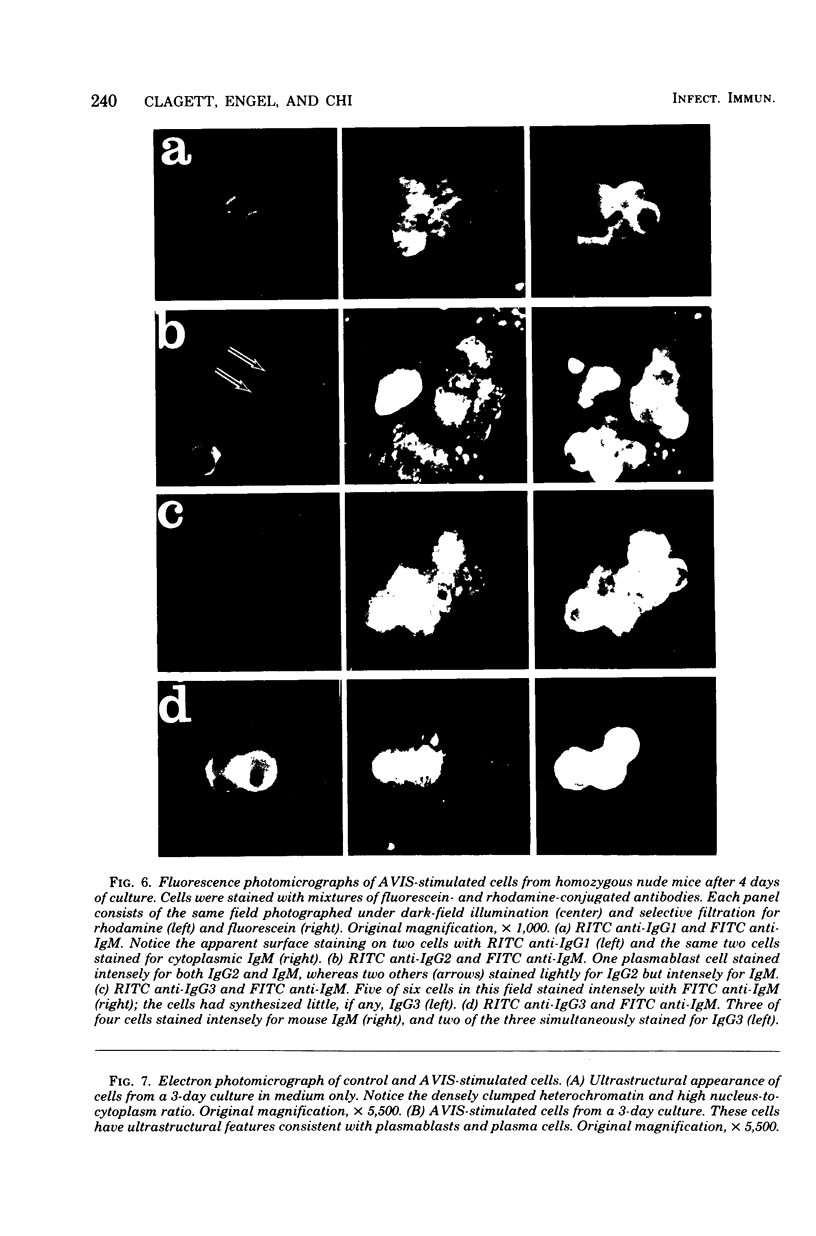
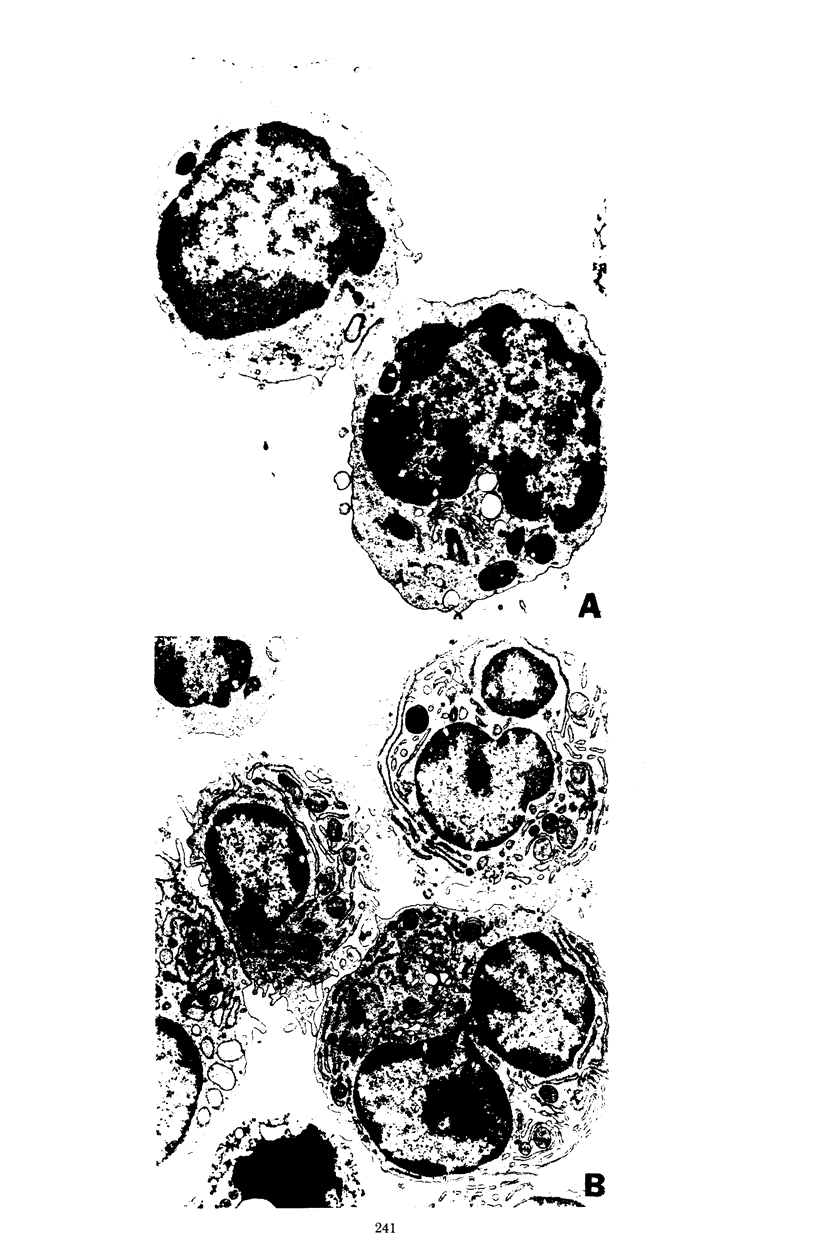
A cell wall extract from the gram-positive bacterium Actinomyces viscosus contains the mitogen AVIS, a potent polyclonal B-cell activator for murine B lymphocytes. Cultures of splenocytes from heterozygous nude mice in the presence of an optimal concentration of AVIS responded by a deoxyribonucleic acid synthesis response, and proliferaction reached maximal levels after 3 to 4 days. There was no requirement for T cells in the deoxyribonucleic acid synthesis, proliferactive, immunoglobulin M (IgM), or IgG responses. Significant numbers of IgM-producing cells were present as early as day 2 of culture, whereas later in the culture periods (days 3 to 6) IgG-producing plasmablasts and plasma cell were observed. In cultures of splenocytes from nude mice stimulated with AVIS for 4 to 5 days, 20 to 25% of the recoverable cells synthesized IgM, and 10% contained only IgG2 or IgG3; 5 to 8% of the cells stained for both IgM and IgG2 or both IgM and IgG3. Fine-structure analysis of AVIS-stimulated splenocytes from heterozygous nude mice after 3 days of culture demonstrated that 20 to 25% of the cells were activated to various degrees. Of most importance, all of the activated cells had the characteristic of B lymphoblasts, plasmablasts, or plasma cells. This is the first demonstration of a polyclonal B-cell activator other than lipopolysaccharide which induces IgG3 synthesis. We suggest that AVIS may be a useful probe for the exploration of the functional activities of subpopulations of B cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asantila T., Toivanen P. Potentation by fluorodeoxyuridine of 125I-deoxyuridine uptake by human and chicken lymphocytes in the quantitation of mitogenic response. J Immunol Methods. 1974 Dec;6(1-2):73–82. doi: 10.1016/0022-1759(74)90091-x. [DOI] [PubMed] [Google Scholar]
- Biberfeld G., Gronowicz E. Mycoplasma pneumoniae is a polyclonal B-cell activator. Nature. 1976 May 20;261(5557):238–239. doi: 10.1038/261238a0. [DOI] [PubMed] [Google Scholar]
- Bona C., Damais C., Chedid L. Blastic transformtion of mouse spleen lymphocytes by a water-soluble mitogen extracted from Nocardia. Proc Natl Acad Sci U S A. 1974 May;71(5):1602–1606. doi: 10.1073/pnas.71.5.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bona C., Yano A., Dimitriu A., Miller R. G. Mitogenic analysis of murine B-cell heterogeneity. J Exp Med. 1978 Jul 1;148(1):136–147. doi: 10.1084/jem.148.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burckhardt J. J., Guggenheim B., Hefti A. Are Actinomyces viscosus antigens B cell mitogens? J Immunol. 1977 Apr;118(4):1460–1465. [PubMed] [Google Scholar]
- Clagett J. A., Engel D. Polyclonal activation: a form of primitive immunity and its possible role in pathogenesis of inflammatory diseases. Dev Comp Immunol. 1978 Apr;2(2):235–241. doi: 10.1016/s0145-305x(78)80066-4. [DOI] [PubMed] [Google Scholar]
- Clagett J. A., Weigle W. O. Roles of T and B lymphocytes in the termination of unresponsiveness to autologous thyroglobulin in mice. J Exp Med. 1974 Mar 1;139(3):643–660. doi: 10.1084/jem.139.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clagett J. A., Wilson C. B., Weigle W. O. Interstitial immune complex thyroiditis in mice: the role of autoantibody to thyroglobulin. J Exp Med. 1974 Dec 1;140(6):1439–1456. doi: 10.1084/jem.140.6.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J., Rodriguez G. E., Kind P. D., Campbell P. A. Listeria cell wall fraction: a B cell mitogen. J Immunol. 1975 Mar;114(3):1132–1134. [PubMed] [Google Scholar]
- Engel D., Clagett J., Page R., Williams B. Mitogenic activity of Actinomyces viscosus. I. Effects on murine B and T lymphocytes, and partial characterization. J Immunol. 1977 Apr;118(4):1466–1471. [PubMed] [Google Scholar]
- FRASCA J. M., PARKS V. R. A ROUTINE TECHNIQUE FOR DOUBLE-STAINING ULTRATHIN SECTIONS USING URANYL AND LEAD SALTS. J Cell Biol. 1965 Apr;25:157–161. doi: 10.1083/jcb.25.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronowicz E., Biberfeld P., Wahren B., Coutinho A. Characterization of dextran-sulfate-sensitive cells. Scand J Immunol. 1976;5(5):573–582. doi: 10.1111/j.1365-3083.1976.tb00312.x. [DOI] [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A. Heterogeneity of B cells: direct evidence of selective triggering of distinct subpopulations by polyclonal activators. Scand J Immunol. 1976;5(1-2):55–69. doi: 10.1111/j.1365-3083.1976.tb02992.x. [DOI] [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A. Responsiveness of lymphoid precursors to polyclonal B-cell activators. Scand J Immunol. 1975 Sep;4(5-6):429–437. doi: 10.1111/j.1365-3083.1975.tb02648.x. [DOI] [PubMed] [Google Scholar]
- Janossy G., Greaves M. Functional analysis of murine and human B lymphocyte subsets. Transplant Rev. 1975;24:177–236. doi: 10.1111/j.1600-065x.1975.tb00169.x. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Lawton A. R. B lymphocyte differentiation induced by lipopolysaccharide. I. Generation of cells synthesizing four major immunoglobulin classes. J Immunol. 1975 Sep;115(3):671–676. [PubMed] [Google Scholar]
- Kearney J. F., Lawton A. R. B lymphocyte differentiation induced by lipopolysaccharide. II. Response of fetal lymphocytes. J Immunol. 1975 Sep;115(3):677–681. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenard J. Rapid and effective removal of sodium dodecyl sulfate from proteins. Biochem Biophys Res Commun. 1971 Nov 5;45(3):662–668. doi: 10.1016/0006-291x(71)90467-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Shimonishi C., Ootsu K. Effects of an acidic polysaccharide procuced by Serratia piscatorum on immune responses in mice. I. mitogenicity and stimulation of plaque-forming cells (PFC) in vitro. J Immunol. 1975 May;114(5):1574–1580. [PubMed] [Google Scholar]
- Melchers F., Andersson J. Synthesis, surface deposition and secretion of immunoglobulin M in bone marrow-derived lymphocytes before and after mitogenic stimulation. Transplant Rev. 1973;14:76–130. doi: 10.1111/j.1600-065x.1973.tb00103.x. [DOI] [PubMed] [Google Scholar]
- Primi D., Hammarström L., Smith C. I., Möller G. Characterization of self-reactive B cells by polyclonal B-cell activators. J Exp Med. 1977 Jan 1;145(1):21–30. doi: 10.1084/jem.145.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid M. P., Hoffmann M. K., Komuro K., Hämmerling U., Abbott J., Boyse E. A., Cohen G. H., Hooper J. A., Schulof R. S., Goldstein A. L. Differentiation of T cells induced by preparations from thymus and by nonthymic agents. J Exp Med. 1973 Oct 1;138(4):1027–1032. doi: 10.1084/jem.138.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultzer B. M., Nilsson B. S. PPD tuberculin--a B-cell mitogen. Nat New Biol. 1972 Dec 13;240(102):198–200. doi: 10.1038/newbio240198a0. [DOI] [PubMed] [Google Scholar]