Summary
Human embryonic stem cells (hESCs) are promising in regenerative medicine. Although several hESC-based clinical trials are under way, a widely accepted standard of clinical-grade cells remains obscure. To attain a completely xeno-free clinical-grade cell line, the system must be free of xenogenic components, the cells must have a comprehensive set of functions, and good manufacturing practice conditions must be used. In this study, following these criteria, we successfully derived two hESC lines, which were thereby considered “clinical-grade embryonic stem cells”. In addition to the primary capacity for pluripotency, these two cell lines were efficiently differentiated into various types of clinical-grade progeny. Importantly, the cells were recognized by the National Institutes for Food and Drug Control of China for further eligible accreditation. These data indicate that we have established completely xeno-free clinical-grade hESC lines and their derivatives, which will be valuable for the foundation of an international standard for clinical-grade cells for therapy.
Keywords: clinical-grade hESCs, xeno-free, functional differentiation, biosafety, chemically defined
Graphical Abstract
Highlights
-
•
Two clinical-grade hESC lines were generated
-
•
The hESCs were demonstrated to have sustained pluripotency
-
•
The hESCs could be differentiated into functional cells
-
•
The hESCs passed biosafety tests and were recognized by the NIFDC
In this article, Zhou, Hao, and colleagues generate two completely xeno-free clinical-grade human embryonic stem cell lines which have demonstrated pluripotency and biosafety under the investigation from an accredited organization.
Introduction
Human embryonic stem cells (hESCs), after directed differentiation, are valuable in regenerative medicine. However, clinical trials using hESC-derived cells remain scarce primarily because hESC lines available worldwide are mostly of nonclinical grade. To generate clinical-grade cells, good manufacturing practices (GMPs), which cover operating procedures and product quality control, must be employed (Ausubel et al., 2011). GMP is a quality assurance system that requires the traceability of materials and the validation of standard operating procedures (SOPs) (Unger et al., 2008). Stem cells are a type of human cellular and tissue-based product (HCT/P). In many countries, HCT/Ps are regulated under guidelines such as 21 CFR 1270 and 21 CFR 1271 issued by the US Food and Drug Administration (FDA) (FDA, 2012). Stem cell-based products must also meet the requirements of other therapeutic products including drugs, medical devices, xeno-transplants, and biological products (Fink, 2009). Therefore, a number of countries and professional associations (e.g., International Society for Stem Cell Research) have issued preliminary regulatory policies for the clinical application of stem cells, and GMP serves as the basic requirement for the generation of clinical-grade hESCs (Fink, 2009, Hyun et al., 2008, Wilkerson et al., 2013). The existing guidelines incorporate guidelines produced by the British Standards Institute for cell-based clinical application (Ratcliffe et al., 2013). Recently, in China, drafts of a stem cell-specific clinical therapy quality control standard and management of stem cell-based clinical experiments were implemented by the China Food and Drug Administration (CFDA) (http://www.sda.gov.cn) and National Health and Family Planning Commission of the People’s Republic of China (NHFPC) (http://www.nhfpc.gov.cn/). Each of these guidelines focuses on the efficacy, safety, and pharmaceutical quality, which are influenced by the cell sources, manufacturing systems, and specific therapeutic protocols (George, 2011, Huang and Fu, 2014). In addition to validating the biosafety of the hESCs, the CFDA and FDA both require rigorous testing of the donors' eligibility, thus differentiating these guidelines from the present NIH guidelines on human stem cell research (George, 2011, Huang and Fu, 2014, Jonlin, 2014).
The generation of hESCs involves numerous reagents, including growth factors, small molecules, and media. To avoid infection by animal-sourced components, mouse feeder cells can be substituted by human fibroblasts (Ellerstrom et al., 2006, Genbacev et al., 2005). Chemically defined hESC culture media such as mTeSR1, mTeSR2, and E8 have been developed to complement future hESC clinical applications (Chen et al., 2011, Ludwig et al., 2006a, Ludwig et al., 2006b). To facilitate and standardize the development of safe and effective clinical-grade hESCs, most clinical hESC studies have been reported as xeno-free or clinical-grade; a summary of current globally available xeno-free hESCs is listed in Table S1 (Crook et al., 2007, Ilic et al., 2012, Klimanskaya et al., 2006, Rajala et al., 2010, Schwartz et al., 2012, Tannenbaum et al., 2012). However, studies have only rarely demonstrated the biosafety investigated by an accredited organization. Here, we propose generation of clinical-grade hESCs with the following requirements: (1) donor consent, (2) donor eligibility requirements, (3) the use of completely xeno-free reagents, (4) biosafety tests from authorized organizations, and (5) stability, self-renewal characteristics, and differentiation capability.
Two clinical-grade hESC lines (Q-CTS-hESC-1 and Q-CTS-hESC-2) were successfully derived under GMP-controlled conditions in completely xeno-free culture media. Both cell lines were pluripotent and passed biosafety evaluations. For a further validation of their pluripotency and biosafety, both cell lines were reviewed and deemed eligible by the National Institutes for Food and Drug Control (NIFDC). Additionally the cell line Q-CTS-hESC-1 is parthenogenetic. Theoretically, parthenogenetic cells express high level of homologous human leukocyte antigen without recombination during meiosis I; thus, they have low histocompatibility and are applicable to more allograft recipients (Kim et al., 2007). Finally, the cell line Q-CTS-hESC-2 was selected for direct differentiation and was able to differentiate into clinical-grade cell types with representatives of three germ layers (ectoderm: retinal pigment epithelium [RPE] cells and neuronal progenitors; mesoderm: cardiomyocytes; endoderm: hepatocytes). Furthermore, clinical-grade neuronal progenitors differentiated from Q-CTS-hESC-2 cells survived and further differentiated into tyrosine hydroxylase (TH)-positive mature dopamine (DA) neurons after transplantation into Parkinson's disease (PD) rat models. These results demonstrate the value of these two clinical-grade hESC lines as sources for future hESC-based clinical trials or therapies.
Results
Procedures of Clinical-Grade hESC Derivation and Clinical-Grade Human Foreskin Fibroblast Isolation
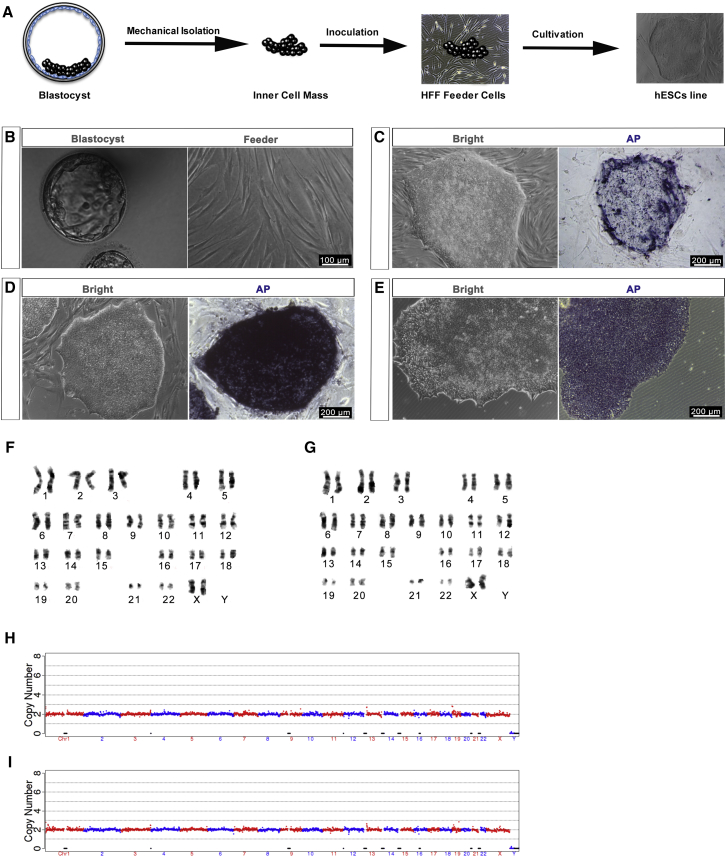
Figure 1 presents the flowchart for xeno-free clinical-grade hESC derivation. The requirements for each step are shown in the gray box. The study was approved by the Animal and Medical Ethics Committee of the Institute of Zoology, Chinese Academy of Sciences (Ethical No. IOZ15033 and IOZ15038) (Supplemental Experimental Procedures). To ensure safety, traceability, and reproducibility, hESC generation must comply with GMP throughout the process. One GMP-compliant laboratory has been established (Supplemental Information and Figure S1A) and tested by the Beijing Institute for Drug Control (Beijing Center for Health Food and Cosmetics Control) after a series of rigorous tests (Data S1, which is the original report document). To trace the cell line, we use a digital system to record the culture/passage and characterization history (Figures S1B and S1C). The GMP-compliant xeno-free reagents were chosen for hESC derivation and differentiation (Table S2). For the supply of safe cell sources for potential clinical applications, screening for eligible embryos and tissue donors is necessary. The embryos and foreskin donors signed informed consent and underwent strict medical tests.
Figure 1.
Process Flow and Requirements of Gold-Standard Clinical-Grade hESC Derivation, Characterization, and Biosafety Evaluation
The scheme indicates the steps for hESC derivation. All processes were standard operating procedures under GMP application and all reagents were xeno-free. After characterization and biosafety tests of each cell line, they were approved to authorized faculty NIFDC for accreditation. ICM, inner cell mass.
To establish a xeno-free human foreskin fibroblast (HFF) culture method, we used FibroGRO Xeno-Free Human Fibroblast Expansion Medium (SCFM) (Supplemental Information and Figure S2). Karyotype analysis indicated that the HFFs had normal chromosomes, and Ki-67 staining demonstrated that the feeder cells were unable to proliferate (Figures S2D and S2E) as a result of γ-irradiation, which induces G2/M cell-cycle arrest (O'Connell et al., 1998). After isolating and expanding a sufficient number of HFFs from the donated foreskin tissues, a series of biosafety-related experiments were performed according to the “Guidelines for Human Somatic Cell Therapies and Quality Control of Cell-based Products,” and the HFFs were verified as safe. These were obtained by the NIFDC in China (Table 1). At passages 8–10, the clinical-grade HFFs were inactivated by γ-irradiation and served as clinical-grade feeder cells. After verifying the absence of mycoplasma, endotoxin, and bacterial contamination, the clinical-grade feeder cells were cryopreserved using MesenCult ACF cryomedium for future clinical-grade hESC derivation and culture.
Table 1.
Biological Safety Analysis of the Clinical-Grade Cellsa
| Sterility and Pathogen | HFFs | Q-CTS-hESC-1 | Q-CTS-hESC-2 |
|---|---|---|---|
| Short tandem repeats (STRs) | each STR locus has 1–2 alleles | each STR locus has 1–2 alleles | each STR locus has 1–2 alleles |
| Isozyme analysis | B type of human origin | B type of human origin | B type of human origin |
| Species identification and cell cross-contamination between species | – | – | – |
| Bacteria and fungi | – | – | – |
| Mycoplasma | – | – | – |
| Human papilloma virus (HPV) | – | – | – |
| Human parvovirus B19 | – | – | – |
| HIV-I | – | – | – |
| HIV-II | – | – | – |
| John Cunningham virus (JCV) | – | – | – |
| Epstein-Barr virus (EBV) | – | – | – |
| Human hepatitis C virus (HCV) | – | – | – |
| Human hepatitis A virus (HAV) | – | – | – |
| Human cytomegalovirus (HCMV) | – | – | – |
| Human T-lymphotropic virus I (HTLV-I) | – | – | – |
| Human hepatitis B virus (HBV) | – | – | – |
| Human herpesvirus 6 (qPCR) | – | – | – |
| Human herpesvirus 7 (qPCR) | – | – | – |
| Human papillomavirus (molecular hybridization) | – | – | – |
| Reverse transcriptase activity | – | – | – |
| Bovine virus | – | – | – |
| Porcine virus | – | – | – |
| BSA residuals | <5 ng/mL | <5 ng/mL | <5 ng/mL |
| Endotoxin level | <0.5 EU/mL | <0.5 EU/mL | <0.5 EU/mL |
| Hemagglutination test of 9- to 11-day-old chick embryo allantoic fluid | negative | negative | negative |
| Survival rate of 5- to 6-day-old chick embryos | >90% | >90% | >90% |
| Intracerebral and intraperitoneal injections in suckling mice | survival rate >90% | survival rate >90% | survival rate >90% |
| Intracerebral and intraperitoneal injections in mice | survival rate >90% | survival rate >90% | survival rate >90% |
| Intraperitoneal injection in guinea pigs | survival rate >90% | survival rate >90% | survival rate >90% |
| Intracutaneous and subcutaneous injections in rabbits | survival rate >90% | survival rate >90% | survival rate >90% |
This table is translated from NIFDC report with numbers SH201601178 for Q-CTS-hESC-1, SH201402035 for Q-CTS-hESC-2, and SH201402033 for HFFs.
Derivation of Clinical-Grade hESCs
Figure 2A outlines the hESC derivation procedure. Blastocysts with a visible inner cell mass (ICM) were selected for the derivation of hESC lines (Figure 2B, left). To avoid animal-related reagents commonly used in immunosurgery, we separated the ICM from the blastocysts mechanically and then inoculated it onto the clinical-grade feeder cells (Figure 2B, right) in STEMedia. Two clinical-grade hESC lines were derived: one from a parthenogenetic embryo (Q-CTS-hESC-1) and the other from a fertilized embryo (Q-CTS-hESC-2). These two hESC lines were morphologically similar to human pluripotent stem cells and could be passaged for 40 generations on clinical-grade HFF feeder layers (Figures 2C and 2D) or in the absence of feeder cells (Figure 2E). The cells had high levels of alkaline phosphatase (Figures 2C–2E, right). Karyotype analysis confirmed the preservation of normal karyotypes (44 normal chromosomes and two X chromosomes) in the two cell lines for up to 40 passages (Figures 2F and 2G). Copy-number variation sequencing (CNV-seq) further indicated that there was no chromosome aneuploidy and no loss or repeat greater than 10-Mbp segments in either cell line (Figures 2H and 2I).
Figure 2.
Derivation of Clinical-Grade hESCs
(A) Schematic overview of hESC generation from the isolation of the ICM to stable passage on feeders.
(B) Left: human blastocyst used for ESC derivation. Right: the feeder cells for ICM attachment. Scale bars, 100 μm.
(C) The cell line Q-CTS-hESC-1 derivation with alkaline phosphatase (AP) activity. Scale bars, 200 μm.
(D) The cell line Q-CTS-hESC-2 derivation with AP activity. Scale bars, 200 μm.
(E) Clinical-grade hESCs on a feeder-free plate with AP activity. Shown are examples from the Q-CTS-hESC-2 line. Scale bars, 200 μm.
(F) Representative chromosome spreads of Q-CTS-hESC-1 cells with 44 euchromosomes and two X chromosomes.
(G) Representative chromosome spreads of Q-CTS-hESC-2 cells with 44 euchromosomes and two X chromosomes.
(H and I) CNV-seq for the cell line Q-CTS-hESC-1 (H) and CNV-seq for the cell line Q-CTS-hESC-2 (I).
Pluripotent Characterization of the Clinical-Grade hESCs
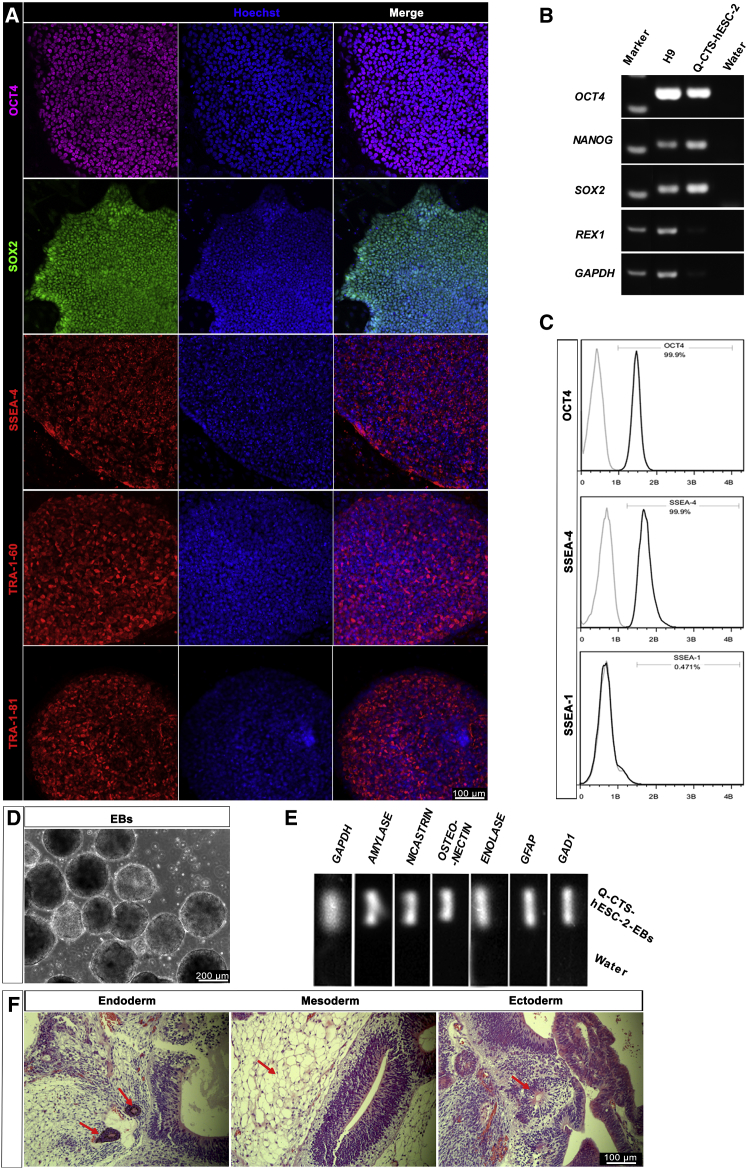
To evaluate the pluripotency of the Q-CTS-hESC-1 and Q-CTS-hESC-2 cells, we performed characterization at several levels. Positive immunofluorescence staining of the pluripotent markers OCT4 (POU5F1), SOX2, SSEA-4, TRA-1-60, and TRA-1-81 indicated the pluripotency of the two clinical-grade hESC lines (Figure 3A, Supplemental Information and Figure S3A). In parallel, RT-PCR analysis confirmed the expression of the pluripotent genes OCT4 (POU5F1), SOX2, NANOG, REX1, LIN28, and GDF3 in the two cell lines (Figures 3B and S3B). The chemically defined E8 medium has been reported to support feeder-free hESC growth and reduce the risk of hESC culture instability caused by variability between human serum album batches (Villa-Diaz et al., 2013). At passages 8–10, both cell lines were transferred to E8 medium and a feeder-free system, and both lines grew well without obvious signs of differentiation (Figure 2E). As further confirmation of the pluripotency of the feeder-free hESCs, flow-cytometric analysis demonstrated that more than 90% of the Q-CTS-hESC-2 and Q-CTS-hESC-1 cells expressed the pluripotent markers OCT4 and SSEA-4 (Figure 3C) and OCT4 and SSEA-3 (Supplemental Information and Figure S3C), respectively.
Figure 3.
Pluripotent Characterization of Q-CTS-hESC-2 Cells
(A) Immunofluorescence analysis of Q-CTS-hESC-2 cells. Positive nuclear transcription factors OCT4 (purple) and SOX2 (green) and clear expression of the ESC surface antigen SSEA4 (red), TRA-1-60 (red), and TRA-1-81 (red) were observed. Nuclei were stained with Hoechst 33342 (blue). Scale bars, 100 μm.
(B) RT-PCR analysis confirmed the expression of the ESC-specific genes (OCT4, NANOG, SOX2, and REX1). H9 cells were used as a positive control and water as the negative control. GAPDH was used as a housekeeping gene.
(C) Quantitative flow-cytometry analysis indicating robust expression of intracellular OCT4 and extracellular SSEA4 with almost no SSEA1 in feeder-free Q-CTS-hESC-2 cells.
(D) Q-CTS-hESC-2 cells can form EBs after suspension culture.
(E) RT-PCR of EBs showing transcripts for ectoderm (GAD1 and GFAP), mesoderm (ENOLASE and OSTEONECTIN), and endoderm (AMYLASE and NICASTRIN, also named NCSTN) markers.
(F) Teratoma formation. All three germ layer tissues were present on the teratoma dissection slices identified by staining with H&E. The red arrows indicate endoderm with glands (left), mesoderm with fat tissues (middle), and ectoderm with nervous tissues (right). Scale bars, 100 μm.
The cells were also capable of differentiation into embryoid bodies (EBs) in suspension cultures (Figures 3D and S3D). After 8 days the EBs were collected, and RT-PCR analysis confirmed the expression of all three germ layers: the ectoderm (GAD1 and GFAP), mesoderm (ENOLASE and OSTEONECTIN), and endoderm (NICASTRIN and AMYLASE) (Figures 3E and S3E). Finally, the two cell lines developed into teratomas 8 weeks after injection into the testes of SCID mice. Histological analysis revealed that all teratomas were composed of tissues of all three germ layers (Figures 3F and S3G). These results validated the pluripotency of the two clinical-grade hESC lines and their capacity to differentiate into all three germ layers in vitro and in vivo. Importantly, these results were reproduced by NIFDC when these procedures were replicated (data not shown).
Good Specialization of Clinical-Grade hESCs Reveals Different Lineages
To verify the applicability of these hESCs under clinical conditions, we differentiated the hESCs into specialized cell types. We tested the differentiation capability of Q-CTS-hESC-1 by inducing neural differentiation. When grown in neural stem cell medium, the EBs attached to the dish and differentiated into neuronal progenitor cells expressing the precursor marker PAX6 (Figure S3F, top). After further induction, the cells began to express TUJ1 (β-III tubulin antibody), a neuronal marker (Figure S3F, bottom).
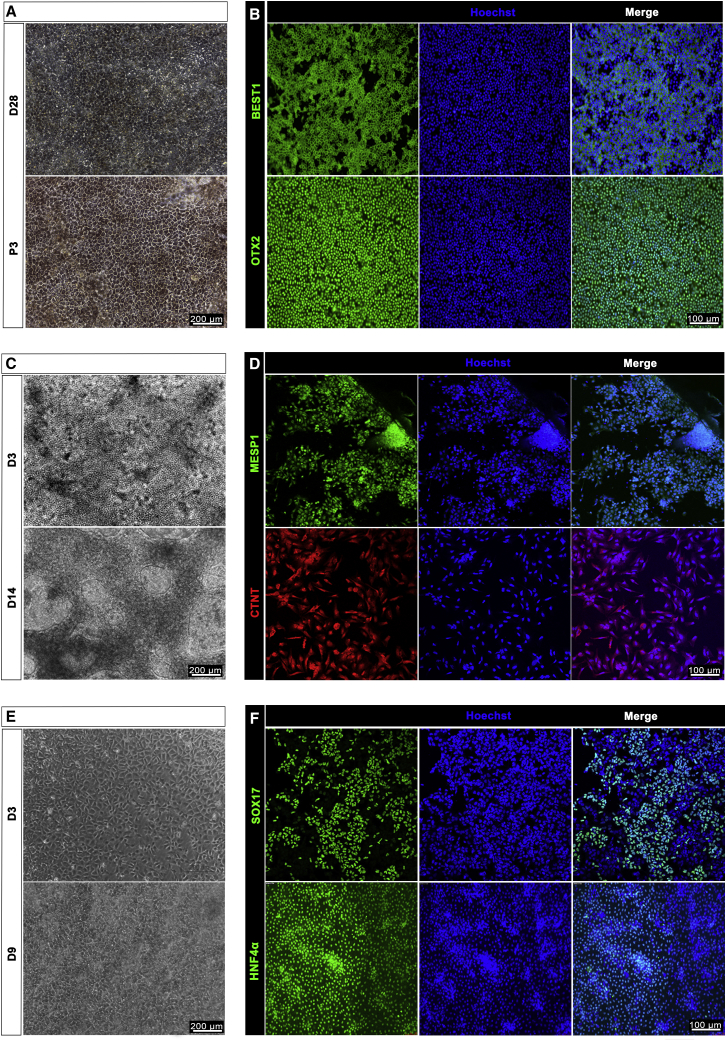
We used corresponding methodologies to derive RPE cells, myocardial precursors, and hepatocyte precursors from Q-CTS-hESC-2 cells. Xeno-free clinical-grade hESC basic medium was used throughout the RPE differentiation procedures (Table S2). Monolayer cuboidal-appearing RPE cells were observed on day 28 of differentiation (Figure 4A, top). The monolayers were sufficiently confluent for passage on days 40–60 of differentiation. The RPE markers OTX2 and BEST1 were highly expressed, and the cells were tightly connected as observed by immunofluorescence staining analysis (Figures 4A [bottom] and 4B) at passage three. To induce cardiomyocyte differentiation, we used a temporal WNT signal activation and inhibition method according to a previous report (Lian et al., 2013). We developed a chemically defined medium for cardiomyocyte differentiation (Tan et al., 2016). Cardiac mesoderm was formed with the expression of MESP1 (Figures 4C [top] and 4D [top]), which patterned the mesoderm into cardiac progenitors (Chan et al., 2013) on day 3 of differentiation. Cardiomyocytes were derived, and most of the cells expressed CTNT on day 14 (Figures 4C [bottom] and 4D [bottom]). Hepatocyte differentiation involved three stages: endoderm induction, hepatic initiation, and maturation (Cai et al., 2007). The endoderm cell layer was formed with expression of the endoderm marker SOX17 (Figures 4E [top] and 4F [top]) on day 3, and mature hepatocytes expressed liver marker HNF4α on day 9 (Figures 4E [bottom] and 4F [bottom]). The differentiation of RPE and hepatocytes was further validated by flow-cytometry analysis of MITF1 and HNF4α, respectively (Supplemental Information and Figure S3H).
Figure 4.
Immunophenotyping of RPE, Cardiomyocytes, and Hepatocyte Differentiated from Q-CTS-hESC-2 Cells
(A and B) RPE derivation. (A) Photomicrograph of adherent cultures showing pigmented RPE-like cells by day 28 of differentiation (top); the cells reacquire the morphology and pigmentation of RPE cells with high-density cultures after three passages (bottom). Scale bar, 200 μm. (B) Immunofluorescence staining showing that pigmented cells expressed the RPE markers BEST1 (green) and OTX2 (green). Nuclei were stained with Hoechst 33342 (blue). Scale bar, 100 μm.
(C and D) Cardiomyocyte derivation. (C) Phase-contrast image of hESCs differentiated into cardiomyocytes on day 3 (D3, top) and day 14 (D14, bottom). Scale bar, 200 μm. (D) Immunofluorescence staining showing cardiac mesoderm marker MESP1 (green) expression on day 3 (top) and cardiomyocyte marker CTNT (red) expression on day 14 (bottom). Nuclei were stained with Hoechst 33342 (blue). Scale bar, 100 μm.
(E and F) Hepatocyte derivation. (E) Phase-contrast images showing sequential morphological changes from definitive endoderm (top) on day 3 (D3) to hepatoblast (bottom) on day 9 (D9) of differentiation. Scale bars, 200 μm. (F) Immunofluorescence staining showing the expression of SOX17 (green) as a definitive endoderm marker on day 3 and HNF4α (green) for hepatoblasts on day 9. Nuclei were stained with Hoechst 33342 (blue). Scale bar, 100 μm.
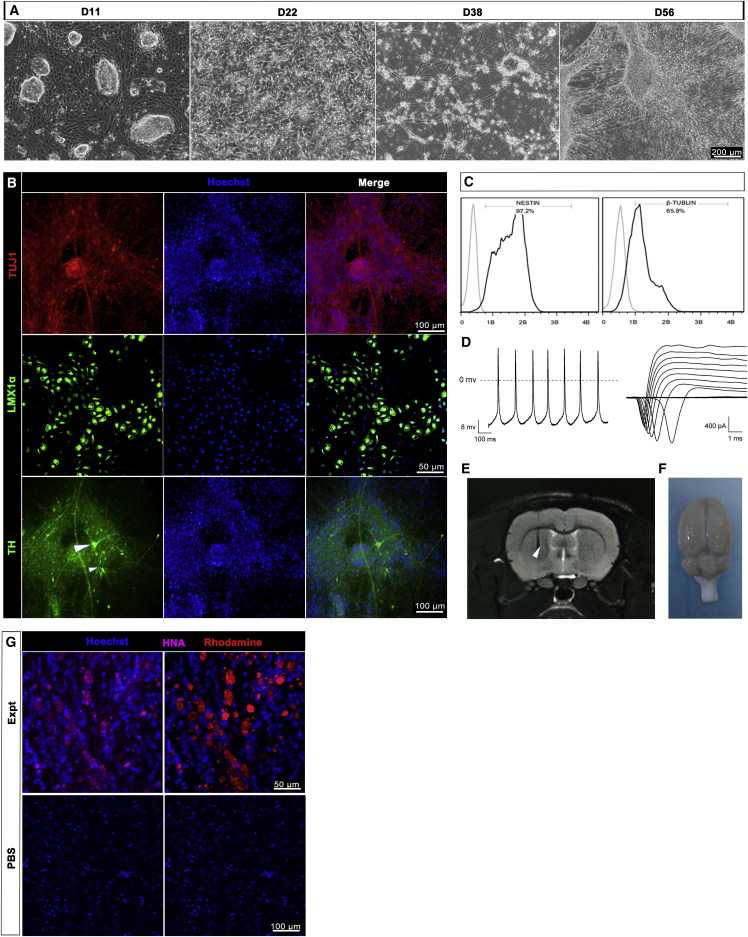
Dopamine Neuronal Progenitor Cells Differentiated from Q-CTS-hESC-2 Can Survive and Mature In Vivo
The survival and maturity of transplanted cells are prerequisites for their functions in vivo in cell replacement therapies. To determine whether DA neuronal progenitors specialized from Q-CTS-hESC-2 cells could survive and mature in vivo, we transplanted them into the brains of PD rat models according to a published method (Kriks et al., 2011) (Supplemental Experimental Procedures). However, the media used throughout the procedure were xeno-free. Neural ectoderm cells emerged on day 11 of differentiation, and short synapses were observed on day 22; these synapses were further elongated on day 38, 53 and 56 (Figure 5A). TUJ1-positive neurons (Figure 5B) were observed on day 38, and the cells expressed high levels of the DA lineage factor LMX1α (Figure 5B) on day 53. TH-positive DA neurons were also observed (Figure 5B) on day 56. Flow-cytometry analysis demonstrated greater than 90% expression of the neural precursor marker NESTIN and approximately 65% expression of the neuronal marker TUJ1 on day 38 of differentiation (Figure 5C), which indicated a high differentiation efficiency. Importantly, the DA neurons fired spontaneous action potentials (Figure 5D, left) and produced inward fast inactivating currents that were sensitive to the Na+ and K+ channel blocker tetrodotoxin (TTX; Figure 5D, right). To assess the long-term survival and functions of the cells in vivo, we transplanted the day 38-derived DA neuronal progenitors into PD rat models that had lesions caused by 6-hydroxydopamine (6-OHDA) (Deumens et al., 2002). One week later, cells were observed at the transplanted site by nuclear magnetic resonance (Figure 5E). Three months after transplantation, the rats were euthanized and the brains were dissected (Figure 5F). Immunofluorescence staining of the brain slices showed positive staining for human nuclear antigen (HNA) and rhodamine that was specific to Molday ION-containing cells (Figure 5G, top). However, HNA and rhodamine could not be detected at the PBS-grafted site (Figure 5G, bottom). These results confirmed that the clinical-grade DA neuronal progenitors survived and matured in the PD rat models.
Figure 5.
Functional DA Neurons Derived from Q-CTS-hESC-2 Cells Engrafted in Rats with PD
(A) Bright-field images of the typical morphology of hESC differentiation at different stages. Scale bars, 200 μm.
(B) Immunofluorescence staining for the expression of TUJ1 (red) on day 38 and LMX1α (green) on day 53 (two top panels) and TH (green, white arrowheads) on day 56 (bottom panel). Nuclei were stained with Hoechst 33342 (blue). Scale bars as indicated.
(C) Quantitative flow cytometry indicating the expression of NESTIN and TUJ1 on day 38.
(D) Electrophysiological analysis. Left: representative traces of evoked action potentials generated by neurons on day 56 of differentiation. Right: cells were hyperpolarized before applying depolarizing pulses to elicit Na+ and K+ currents by TTX treatment.
(E) Magnetic resonance image of the transplantation site (white arrowhead).
(F) The removed brain with cell engraftment for tissue processing.
(G) Immunohistochemistry for the human-specific marker human nuclear antigen (HNA, purple) and rhodamine (red). The top panels are the experimental group and the bottom panels the blank controls with PBS transplantation. Scale bars as indicated.
Clinical-Grade hESCs Are Sterile and Safe
The clinical-grade hESCs were subjected to a series of biosafety evaluations according to the “Guidelines for Human Somatic Cell Therapies and Quality Control of Cell-Based Products” (Food and Drug Administration, 1998), the regulations and management approach on stem cell clinical trials (CFDA and NHFPC) (Rosemann and Sleeboom-Faulkner, 2016), and FDA-equivalent guidelines from other countries (George, 2011). The NIFDC is the only legal institution for drug and food safety evaluations and is especially assigned by the CFDA. The NIFDC biosafety test items include cell identification, fungal and bacterial testing, mycoplasma testing, virus testing, and endotoxin and BSA tests as summarized in Table 1. The results of short tandem repeats analysis indicated that neither cell line was contaminated by other cells, and isozyme analysis showed that the two cell lines were B type of human origin. Furthermore, a series of tests demonstrated that the two cell lines were negative for bacteria, fungi, mycoplasma, and serious pathogenic microorganisms such as HIV and human papilloma virus (Table 1). To detect unknown potential pathogenic microorganisms, we injected the two cell lines into a suckling mouse intracerebrally and intraperitoneally, into a guinea pig intraperitoneally, into a rabbit intracutaneously and subcutaneously, and into the chorioallantoic membrane and yolk sac of chicks according to the methods described in “Pharmacopoeia of the People's Republic of China, Edition 2010, Volume III.” After an appropriate observation time, more than 90% of the animals remained alive (Table 1). Endotoxin testing results showed that the endotoxin level of the hESC culture medium met the requirements defined in the “Pharmacopoeia of the People's Republic of China, Edition 2010, Volume III” (Table 1). Moreover, BSA was not detected in the culture medium. All test results were verified by NIFDC. One example of the original report document for Q-CTS-hESC-2 is attached as Data S2 and is the basis of the data presented in Table 1. Teratoma formation analysis of the differentiated cells, DA neuronal progenitors, cardiomyocytes, and hepatocytes further supported the safety of these cells for future cell therapy (Supplemental Information and Figure S3I). These results confirmed that the two clinical-grade hESC lines are biologically safe.
Discussion
Clinical-grade hESCs have gained significant attention in recent years due to their potential applications in cell therapy. This study aimed to create an absolute clinical-grade hESC standard and to generate hESCs by adhering to this protocol. Xeno-free requirements can completely avoid heterogenetic immunogenicity risks while producing high-quality stem cells. In our study, we chose a number of commercial CTS reagents, which are high-quality products in the Clinical Therapy Systems from Life Technologies with Drug Master Files (DMFs)(https://www.thermofisher.com/cn/zh/home/life-science/bioproduction/bioproduction-resources/regulatory-support/cell-culture-drug-master-files.html), to minimize the burden of transition from research to clinical application. The generation of GMP-grade hESCs within xeno-free systems has been reported previously as shown in Table S1, but this report describes completely xeno-free hESC production complete with biosafety validation. NIFDC is a subordinate agency of CFDA, and its test results validate the pluripotency and biosafety of the hESCs; these results can be used to support clinical applications to the CFDA. Previously, three companies have used specialized hESCs for clinical trials, but not all components used in the initial stages of hESC generation were xeno-free (Klimanskaya et al., 2006). In the present study all reagents were xeno-free, the procedures complied with SOPs, the donors passed safety tests, and the cells passed biosafety tests with authorized certification. Human juvenile foreskin cells were chosen as the feeders to support ICM outgrowth and hESC passage. The STEMedia and E8 media were both xeno-free. Although the two cell lines can be cultured in a feeder-free environment such as on vitronectin-coated plates (Figure 2E), the cells were maintained on feeder cells to improve colony attachment and homogeneity. Two different passages of hESCs for NIFDC were chosen for testing each passage, consisting of 250 million cells. The hESCs remained stable with restricted capabilities for self-renewal and differentiation. During differentiation, all reagents used in a recently published protocol were replaced with xeno-free reagents. Long-term engraftment in a PD rat brain demonstrated the potential of the clinical-grade hESC-derived DA neuronal progenitors to mediate substantial improvement of PD symptoms in vivo. These results provide reasonable assurance of the efficacy of these cells for administration in clinical trials.
Although our report mainly focuses on the generation of clinical-grade hESCs, transplantation safety is also a concern. For example, a 9-year-old boy was diagnosed with a glioneuronal neoplasm after being transplanted with neural stem cells derived from two donors (Amariglio et al., 2009). Although we did not observe any teratoma formation in the animal brain, more data are required to define the safety of these cells for the treatment of PD. Banking of clinical-grade hESCs is necessary for stem cell-based therapies (Lin et al., 2009). Once a clinical-grade cell line has been proved eligible based on its characteristics and biosafety according to existing international and national regulations, it should be stored in at least three sublevels of cell banks (master cell bank, seed cell bank, and working cell bank). The implementation of the International Stem Cell Banking Initiative (ISCBI) attempts to harmonize banking, and a study has been released to develop best practices for ensuring the quality of clinical-grade pluripotent stem cells (Andrews et al., 2015). Storing a sufficient number of clinical-grade hESC lines in a stem cell bank is necessary to meet the demands of hESC-based cell therapies in the future.
In conclusion, biosafety is the priority. We have developed a biosafe system to generate clinical-grade hESCs and specialized cells differentiating from these hESCs. Our report proposes strict procedures for the generation of clinical-grade hESCs and provides appropriate analytical evaluation methods. We have obtained two absolute xeno-free hESC lines, and our results will promote the development of hESC-based products.
Experimental Procedures
All reagents used for clinical hESC derivation and differentiation are shown in Table S2.
Parthenogenetic Activation of Oocytes and Embryo Culture
The parthenogenetic activation of oocytes and embryo culture (both parthenogenetic and fertilized embryos) were conducted as previously described (Mai et al., 2007), except for the culture environment, which was 37°C, 5% CO2, and saturated humidity.
Derivation of HFFs and Feeder Cells
Juvenile foreskin tissues were collected in 50-mL centrifuge tubes containing CTS-DPBS with 2,000 U/mL xeno-free penicillin-streptomycin (P/S) and then transported to the laboratory on ice. After washing three times with CTS-DPBS, adipose and connective tissues were carefully removed from the foreskins, and the remaining epithelial tissue was cut into small pieces of approximately about 1 mm3. These pieces were then immersed in FibroGRO Xeno-Free Human Fibroblast Expansion Medium (SCFM) and transferred onto T25 tissue culture flasks pre-coated with MesenCult-XF Supplement (MesenCult) attachment substrates. After 24 hr of inverted culture, 1.5 mL of SCFM was added to each flask. Fibroblasts outgrew around the tissues after approximately 7 days of culture and were subsequently passaged routinely. HFFs at passages 8–10 were inactivated by γ-irradiation at a dosage of 5.5 Gy/min and a total dose of 55 Gy at the School of Physics, Peking University, to serve as “clinical-grade feeder cells”.
Isolation and Culture of Clinical-Grade hESCs
Human blastocysts that reached hatching stage were used for clinical-grade hESC derivation. The ICM of each blastocyst was isolated mechanically as previously described (Ström et al., 2007). The ICMs were then inoculated on clinical-grade feeder cells in NutriStem XF/FF Culture Medium (STEMedia). After 5–9 days of culture at 37°C, 5% CO2, and saturated humidity, outgrowths were passaged onto new feeder cells mechanically. At earlier passages, a mechanical method was used to passage the clinical-grade hESCs. Once the proliferation of clinical-grade hESCs appeared to have been stabilized, the clinical-grade hESCs were passaged using collagenase NB6. At passages 8–10, the clinical-grade hESCs were transferred to feeder-free culture conditions in Essential 8 Medium (E8) on vitronectin-coated dishes as previously described (Ludwig et al., 2006b).
Embryoid Body Formation
With the exception of the use of clinical-grade collagenase NB6 for cell dissociation and xeno-free hESC basic medium for EB culture, the entire EB formation procedure was conducted as previously described (Wang et al., 2013). The clinical-grade hESC basic medium was composed of CTS KnockOut DMEM (CTS-KO-DMEM), 20% CTS KnockOut SR XenoFree Medium (CTS-KOSR), 100 μM nonessential amino acids (NEAA), 2 mM CTS GlutaMAX-I Supplement (CTS-GlutaMAX), and 55 μM β-mercaptoethanol.
Neural Differentiation
The neural differentiation of Q-CTS-hESC-1 cells was performed as previously described (Hu and Zhang, 2010, Zhang and Zhang, 2010). In brief, hESCs were cultured in suspension in clinical-grade hESC basic medium for 4 days to form EBs and then cultured in NIM (CTS-KO-DMEM/F12 with 1% CTS-N2) for an additional 3 days for neural induction. The round EBs attached to plates pre-coated with 20 μg/mL laminin (Sigma, L6274). Rosette-like neuroepithelial cells appeared after 2 weeks of differentiation.
The differentiation of Q-CTS-hESC-2 cells to DA progenitors was performed according to the mononuclear differentiation method as previously described (Kriks et al., 2011), with the exception that the reagents were completely xeno-free (Table S2). In brief, clinical-grade hESCs were dissociated into single cells using CTS-TrypLE and plated on CTS CELLstart Substrate (CTS-CELLstart)-coated dishes at a density of 4 × 105 cells per cm2 on day 0. Initially, the differentiation medium was CTS-KO-DMEM supplemented with 15% CTS-KOSR, 100 μM NEAA, 2 mM CTS-GlutaMAX, and 55 μM β-mercaptoethanol. On day 5, the differentiation medium was gradually changed to CTS-KO-DMEM supplemented with 1% CTS-N2 as described previously (Kriks et al., 2011). On day 11, the medium was replaced by CTS Neurobasal Medium (CTS-Neurobasal) supplemented with 2% CTS B-27 Supplement (CTS-B27). The concentrations of and duration of treatment with growth factors and small molecules in the differentiation media were according to previously described methods (Kriks et al., 2011). The electrophysiology of differentiated neurons was studied as previously described (Sheng et al., 2012).
RPE Cell Differentiation
For RPE differentiation, hESCs were cultured in E8 medium on vitronectin-coated dishes. Upon reaching 90% confluence, the E8 medium was replaced by RPE differentiation medium, RPE-DM (CTS-KO-DMEM supplemented with 20% CTS-KOSR, 2 mM CTS-GlutaMAX, 100 μM NEAA, and 55 μM β-mercaptoethanol). After 40–60 days of culture, monolayers containing primary RPE cells appeared and were isolated for further passage and characterization.
Cardiomyocyte Differentiation
Cardiomyocyte differentiation was conducted based on a previous report (Lian et al., 2013) and with modifications to a chemically defined xeno-free grade in our laboratory (Tan et al., 2016). Confluent clinical-grade hESCs were digested into single cells by StemPro Accutase Cell Dissociation Reagent (Accutase) and seeded onto vitronectin-coated dishes at a density of 1.25 × 105 cells per cm2. 10 μM Y27632 was added to the culture medium for one day to improve the survival of single cells. After allowing the cells to grow in E8 medium for 2–3 days to reach 90% confluence, the medium was replaced with the chemically defined medium Car-CDM, which was prepared from RPMI-1640 supplemented with self-modified chemically defined B27 (1 mg/L L-carnitine, 2 mg/L ethanolamine, 12 mg/L putrescine, 0.016 mg/L selenium, 1 mg/L linolenic acid, 1 mg/L linoleic acid, 5 mg/L transferrin, 50 μM N-acetylcysteine, and 200 mg/L L-ascorbic acid-2-phosphate). During differentiation, 4 μM CHIR99021 was added on days 0–1. On day 2 of differentiation, the medium was changed to Car-CDM with the addition of 5 μM IWR-1 (an inhibitor of the canonical Wnt signaling response, 4-(1,3,3a,4,7,7a-hexahydro-1,3-dioxo-4,7-methano-2H-isoindol-2-yl)-N-8-quinolinyl-benzamide). The medium was then replaced with Car-CDM on day 5 of differentiation. On day 7 of differentiation, the medium was changed to Car-CDM plus 5 μg of insulin. The medium was changed every 3 days thereafter.
Hepatic Cell Differentiation
Hepatic cell differentiation was also modified from previously published protocols (Si-Tayeb et al., 2010, Song et al., 2009). Three types of medium were used in this experiment: endoderm differentiation medium (EDM; RPMI-1640 supplemented with 100 ng/mL activin A [R&D, 338-AC-050/CF] and 50 ng/mL Wnt3a [R&D, 5036-WN-010]); hepatic initiation medium (HIM; CTS-KO-DMEM/F12 supplemented with 20% CTS-KOSR, 2 mM CTS-GlutaMAX, 100 μM NEAA, 0.1 mM β-mercaptoethanol, and 1% DMSO), and maturation medium (MM; Iscove’s modified Dulbecco’s medium supplemented with 30 ng/mL oncostatin M, 50 ng/mL HGF, and 10 μM dexamethasone). On day 1 of differentiation, clinical-grade hESCs were digested into single cells and plated onto 6-well plates pre-coated with vitronectin in E8 medium. On day 3, when the cells reached 60%–80% confluence, E8 medium was replaced with EDM. On day 6 and day 10, the medium was changed to HIM and MM, respectively.
Cryopreservation for Banking
Fibroblasts were banked in batches of 25–30 cryovials at passages 3 and 5 with 1.5 × 107 cells per vial. At passage 8, the fibroblasts were mitotically inactivated by 55 Gy of γ-irradiation. The feeders were then banked in batches of 50–60 cryovials at two different densities of 5 × 106 cells per vial and 1.5 × 107 cells per vial. The cryomedium used to freeze the fibroblasts and feeders, MesenCult ACF freezing medium, is a defined, serum-free, and animal component-free medium. hESC lines were harvested in batches of five vials at early passages (5–8) with 2 × 106 cells per vial. Cultures were then banked every five passages in batches of 10–15 vials with 1 × 107 cells per vial in serum-free and animal component-free cryomedia, STEM-CELLBANKER GMP (CELLBANKER). The cell number was counted using a Cell Counter (Millipore, Scepter 2.0), and the vials were sealed with a label printer (BMP21-LAB, Brady). After freezing in Nalge Nunc Cryo containers overnight in a freezer at −80°C, the vials were transported in the gas phase of a liquid nitrogen tank.
Karyotyping
When the clinical-grade hESCs reached 60%–70% confluence, karyotype analysis and G-binding were conducted at the Chinese Academy of Medical Science & Peking Union Medical College.
RT-PCR Analysis
Total RNA of clinical-grade hESCs and EBs was extracted using TRIzol reagent (Life Technologies, 10296010), and RQ1 RNase-free DNase (Promega, M6101) was used to remove residual genomic DNA. The RNA was then reverse transcribed to cDNA using M-MLV reverse transcriptase and random primers (Promega, M1701) according to the manufacturer's instructions. RT-PCR was performed as previously described (Wang et al., 2013). The sequences of the primers used in this study are shown in Table S3.
Alkaline Phosphatase Staining
Alkaline phosphatase staining was performed using the Alkaline Phosphatase Assay Kit (Beyotime, P0321) as described by the manufacturer.
Immunofluorescence Staining
Immunofluorescence staining was performed as previously described (Gu et al., 2014). Cell samples were fixed with 4% (w/v) paraformaldehyde (PFA; P6148, Sigma) for 10 min and then permeabilized with 0.5% Triton X-100 (Sigma) and blocked in 2% BSA (Sigma) at 37°C for 10 min after rinsing. Samples were subsequently incubated with primary antibodies against OCT4 (Santa Cruz Biotechnology, sc-8628, 1:200), SOX2 (Santa Cruz, sc-17320, 1:200), TRA-1-60 (Millipore, MAB4360, 1:200), TRA-1-81 (Millipore, MAB4381A4, 1:200), SSEA4 (Millipore, MC-813-70, 1:200), BEST1 (Abcam, ab14929, 1:150), TH (Santa Cruz, sc-14007, 1:200), PAX6 (Abcam, ab5790, 1:200), OTX2 (Millipore, AB9566, 1:200), MESP1 (Aviva System Biology, OAAB09387, 1:50), CTNT (Abcam, ab8295, 1 μg/mL), TUJ1 (Cell signaling, 2125), LMX1α (Abcam, ab31006, 1:50), SOX17 (R&D Systems, AF1924, 1:200), or HNF4α (Epitomics, 2803-1, 1:200) at 4°C overnight. On day 2, the samples were rinsed and then incubated with the appropriate secondary antibodies donkey-anti-mouse-cy5 (Jackson ImmunoResearch, 103856, 1:200), donkey-anti-goat-Cy5 (Millipore, AP1805A6, 1:200), donkey-anti-rabbit-FITC (Jackson ImmunoResearch, 99320, 1:200), or donkey-mouse-cy3 (Jackson ImmunoResearch, 715-165-150, 1:200) for 1 hr at 37°C. Nuclei were visualized by staining with Hoechst 33342 (10 μg/mL) or propidium iodide (5 μg/mL) for 10 min at room temperature.
Teratoma Formation
Upon reaching 80%–90% confluence, clinical-grade hESCs were harvested using collagenase NB6 digestion and suspended in CTS-DPBS at a density of 5 × 107 cells/mL. Then 20 μL of cell suspension was carefully injected into each testis of 6- to 8-week-old SCID mice using a sterile glass needle under a sterile stereo microscope. Two months later, the mice were euthanized and the teratomas were examined following the guidelines of the Institutional Animal Care and Use Committee. The teratomas were then fixed, sliced, and stained with H&E for histology analysis.
Flow Cytometry
Cells were dissociated into single cells and then fixed with 4% PFA for 15 min at room temperature. After permeabilization in 0.1% Triton X-100 for 30 min at room temperature, the cells were stained with primary antibodies, followed by secondary antibodies diluted in PBS plus 2% BSA. Data were collected on the flow cytometer and analyzed using FlowJo software. For the antibodies used, please refer to the Immunofluorescence Staining section. hESCs treated only with secondary antibodies were used as a gating control in the experiments of pluripotency characterization. Undifferentiated hESCs treated with the same antibodies were used as negative control for gating in differentiation experiments.
Generation of Rat Models of Parkinson's Disease
Female Sprague-Dawley rats purchased from Beijing Vital River Company with a body weight of 200–250 g were chosen to generate the PD models. After anesthetizing the rat with chloral hydrate, the head was fixed with the brain locator. The midline head skin was then sliced open with a scalpel to expose the bregma. With respect to bregma, the medial forebrain bundle's position is anteroposterior −4.3 mm, mediolateral +1.5 mm. A dose of 16 μg of 6-OHDA base dissolved in 5 μL of saline solution was injected into the medial forebrain bundle to produce unilateral lesions of the left nigrostriatal pathway. The injection depth was dorsoventral −7.5 mm with respect to the dura, and the injection rate was 1 μL/min. After injection, the needle remained in situ for a further 3 min to prevent diffusion. Three to four weeks later, apomorphine (0.5 mg/kg, Sigma)-induced rotation was tested. Five minutes after apomorphine treatment, rats that showed a mean of >5 contralateral turns per minute over a 25-min period were regarded as successful models.
Biosafety Evaluation
The tested items are listed in Table 1. The “Pharmacopoeia of the People's Republic of China, Edition 2010, Volume III” was used as a reference for the testing methods.
Author Contributions
Q.Z. and J.H. conceived the project and supervised the experiments. Q.G., J.W., and L.W. wrote the manuscript with help from all of the authors. Q.G., L.W., J.W., Z.-X.L., W.-W.Z., Y.-Q.T, W.-F.H, C.-J.F., J.-H.F., L.L., L.W., X.-Y.Z., B.-Y.H and W.L. participated in the experiments and data analysis.
Acknowledgments
The authors are grateful to Elise Stewart and Siti Abdul Rahim for their revision of the grammar of the manuscript. This work was supported by the China National Basic Research Program (2013CB967100, to L.L.) and the National Key Research and Development Program of China-Stem Cell and Translational Research 2016YFA0101502 (to L.W.).
Published: May 11, 2017
Footnotes
Supplemental Information includes three figures, three tables, and two data files and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2017.04.017.
Contributor Information
Jie Hao, Email: haojie@ioz.ac.cn.
Qi Zhou, Email: zhouqi@ioz.ac.cn.
Supplemental Information
References
- Amariglio N., Hirshberg A., Scheithauer B.W., Cohen Y., Loewenthal R., Trakhtenbrot L., Paz N., Koren-Michowitz M., Waldman D., Leider-Trejo L. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009;6:e1000029. doi: 10.1371/journal.pmed.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P.W., Baker D., Benvinisty N., Miranda B., Bruce K., Brustle O., Choi M., Choi Y.M., Crook J.M., de Sousa P.A. Points to consider in the development of seed stocks of pluripotent stem cells for clinical applications: International Stem Cell Banking Initiative (ISCBI) Regen. Med. 2015;10:1–44. doi: 10.2217/rme.14.93. [DOI] [PubMed] [Google Scholar]
- Ausubel L.J., Lopez P.M., Couture L.A. GMP scale-up and banking of pluripotent stem cells for cellular therapy applications. Methods Mol. Biol. 2011;767:147–159. doi: 10.1007/978-1-61779-201-4_11. [DOI] [PubMed] [Google Scholar]
- Cai J., Zhao Y., Liu Y., Ye F., Song Z., Qin H., Meng S., Chen Y., Zhou R., Song X. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45:1229–1239. doi: 10.1002/hep.21582. [DOI] [PubMed] [Google Scholar]
- Chan S.S.-K., Shi X., Toyama A., Arpke R.W., Dandapat A., Iacovino M., Kang J., Le G., Hagen H.R., Garry D.J. Mesp1 patterns mesoderm into cardiac, hematopoietic, or skeletal myogenic progenitors in a context-dependent manner. Cell Stem Cell. 2013;12:587–601. doi: 10.1016/j.stem.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Gulbranson D.R., Hou Z., Bolin J.M., Ruotti V., Probasco M.D., Smuga-Otto K., Howden S.E., Diol N.R., Propson N.E. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods. 2011;8:424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook J.M., Peura T.T., Kravets L., Bosman A.G., Buzzard J.J., Horne R., Hentze H., Dunn N.R., Zweigerdt R., Chua F. The generation of six clinical-grade human embryonic stem cell lines. Cell Stem Cell. 2007;1:490–494. doi: 10.1016/j.stem.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Deumens R., Blokland A., Prickaerts J. Modeling Parkinson's disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp. Neurol. 2002;175:303–317. doi: 10.1006/exnr.2002.7891. [DOI] [PubMed] [Google Scholar]
- Ellerstrom C., Strehl R., Moya K., Andersson K., Bergh C., Lundin K., Hyllner J., Semb H. Derivation of a xeno-free human embryonic stem cell line. Stem Cells. 2006;24:2170–2176. doi: 10.1634/stemcells.2006-0130. [DOI] [PubMed] [Google Scholar]
- FDA . US Food and Drug Administration; 2012. Guidance for Industry: Eligibility Determination for Donors of Human Cells, Tissues, and Cellular and Tissue-Based Products. [PubMed] [Google Scholar]
- Fink D.W., Jr. FDA regulation of stem cell-based products. Science. 2009;324:1662–1663. doi: 10.1126/science.1173712. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration . US Food and Drug Administration; 1998. Guidance for Industry: Guidance for Human Somatic Cell Therapy and Gene Therapy. [Google Scholar]
- Genbacev O., Krtolica A., Zdravkovic T., Brunette E., Powell S., Nath A., Caceres E., McMaster M., McDonagh S., Li Y. Serum-free derivation of human embryonic stem cell lines on human placental fibroblast feeders. Fertil. Steril. 2005;83:1517–1529. doi: 10.1016/j.fertnstert.2005.01.086. [DOI] [PubMed] [Google Scholar]
- George B. Regulations and guidelines governing stem cell based products: clinical considerations. Perspect. Clin. Res. 2011;2:94–99. doi: 10.4103/2229-3485.83228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q., Hao J., Hai T., Wang J., Jia Y., Kong Q., Wang J., Feng C., Xue B., Xie B. Efficient generation of mouse ESCs-like pig induced pluripotent stem cells. Protein Cell. 2014;5:338–342. doi: 10.1007/s13238-014-0043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B.Y., Zhang S.C. Directed differentiation of neural-stem cells and subtype-specific neurons from hESCs. Methods Mol. Biol. 2010;636:123–137. doi: 10.1007/978-1-60761-691-7_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Fu X. Stem cell therapies and regenerative medicine in China. Sci. China Life Sci. 2014;57:157–161. doi: 10.1007/s11427-014-4608-3. [DOI] [PubMed] [Google Scholar]
- Hyun I., Lindvall O., Ahrlund-Richter L., Cattaneo E., Cavazzana-Calvo M., Cossu G., De Luca M., Fox I.J., Gerstle C., Goldstein R.A. New ISSCR guidelines underscore major principles for responsible translational stem cell research. Cell Stem Cell. 2008;3:607–609. doi: 10.1016/j.stem.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Ilic D., Stephenson E., Wood V., Jacquet L., Stevenson D., Petrova A., Kadeva N., Codognotto S., Patel H., Semple M. Derivation and feeder-free propagation of human embryonic stem cells under xeno-free conditions. Cytotherapy. 2012;14:122–128. doi: 10.3109/14653249.2011.623692. [DOI] [PubMed] [Google Scholar]
- Jonlin E.C. Differing standards for the NIH Stem Cell Registry and FDA approval render most federally funded hESC lines unsuitable for clinical use. Cell Stem Cell. 2014;14:139–140. doi: 10.1016/j.stem.2013.12.014. [DOI] [PubMed] [Google Scholar]
- Kim K., Lerou P., Yabuuchi A., Lengerke C., Ng K., West J., Kirby A., Daly M.J., Daley G.Q. Histocompatible embryonic stem cells by parthenogenesis. Science. 2007;315:482–486. doi: 10.1126/science.1133542. [DOI] [PubMed] [Google Scholar]
- Klimanskaya I., Chung Y., Becker S., Lu S.J., Lanza R. Human embryonic stem cell lines derived from single blastomeres. Nature. 2006;444:481–485. doi: 10.1038/nature05142. [DOI] [PubMed] [Google Scholar]
- Kriks S., Shim J.W., Piao J., Ganat Y.M., Wakeman D.R., Xie Z., Carrillo-Reid L., Auyeung G., Antonacci C., Buch A. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian X., Zhang J., Azarin S.M., Zhu K., Hazeltine L.B., Bao X., Hsiao C., Kamp T.J., Palecek S.P. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions. Nat. Protoc. 2013;8:162–175. doi: 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G., Xie Y., Ouyang Q., Qian X., Xie P., Zhou X., Xiong B., Tan Y., Li W., Deng L. HLA-matching potential of an established human embryonic stem cell bank in China. Cell Stem Cell. 2009;5:461–465. doi: 10.1016/j.stem.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Ludwig T.E., Bergendahl V., Levenstein M.E., Yu J., Probasco M.D., Thomson J.A. Feeder-independent culture of human embryonic stem cells. Nat. Methods. 2006;3:637–646. doi: 10.1038/nmeth902. [DOI] [PubMed] [Google Scholar]
- Ludwig T.E., Levenstein M.E., Jones J.M., Berggren W.T., Mitchen E.R., Frane J.L., Crandall L.J., Daigh C.A., Conard K.R., Piekarczyk M.S. Derivation of human embryonic stem cells in defined conditions. Nat. Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- Mai Q., Yu Y., Li T., Wang L., Chen M.J., Huang S.Z., Zhou C., Zhou Q. Derivation of human embryonic stem cell lines from parthenogenetic blastocysts. Cell Res. 2007;17:1008–1019. doi: 10.1038/cr.2007.102. [DOI] [PubMed] [Google Scholar]
- O'Connell A.C., Lillibridge C.D., Zheng C., Baum B.J., O'Connell B.C., Ambudkar I.S. Gamma-irradiation-induced cell cycle arrest and cell death in a human submandibular gland cell line: effect of E2F1 expression. J. Cell Physiol. 1998;177:264–273. doi: 10.1002/(SICI)1097-4652(199811)177:2<264::AID-JCP8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Rajala K., Lindroos B., Hussein S.M., Lappalainen R.S., Pekkanen-Mattila M., Inzunza J., Rozell B., Miettinen S., Narkilahti S., Kerkela E. A defined and xeno-free culture method enabling the establishment of clinical-grade human embryonic, induced pluripotent and adipose stem cells. PLoS One. 2010;5:e10246. doi: 10.1371/journal.pone.0010246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe E., Glen K.E., Naing M.W., Williams D.J. Current status and perspectives on stem cell-based therapies undergoing clinical trials for regenerative medicine: case studies. Br. Med. Bull. 2013;108:73–94. doi: 10.1093/bmb/ldt034. [DOI] [PubMed] [Google Scholar]
- Rosemann A., Sleeboom-Faulkner M. New regulation for clinical stem cell research in China: expected impact and challenges for implementation. Regen. Med. 2016;11:5–9. doi: 10.2217/rme.15.80. [DOI] [PubMed] [Google Scholar]
- Schwartz S.D., Hubschman J.P., Heilwell G., Franco-Cardenas V., Pan C.K., Ostrick R.M., Mickunas E., Gay R., Klimanskaya I., Lanza R. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379:713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- Sheng C., Zheng Q., Wu J., Xu Z., Wang L., Li W., Zhang H., Zhao X.-Y., Liu L., Wang Z. Direct reprogramming of Sertoli cells into multipotent neural stem cells by defined factors. Cell Res. 2012;22:208–218. doi: 10.1038/cr.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si-Tayeb K., Noto F.K., Nagaoka M., Li J., Battle M.A., Duris C., North P.E., Dalton S., Duncan S.A. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010;51:297–305. doi: 10.1002/hep.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z., Cai J., Liu Y., Zhao D., Yong J., Duo S., Song X., Guo Y., Zhao Y., Qin H. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res. 2009;19:1233–1242. doi: 10.1038/cr.2009.107. [DOI] [PubMed] [Google Scholar]
- Ström S., Inzunza J., Grinnemo K.-H., Holmberg K., Matilainen E., Strömberg A.-M., Blennow E., Hovatta O. Mechanical isolation of the inner cell mass is effective in derivation of new human embryonic stem cell lines. Hum. Reprod. 2007;22:3051–3058. doi: 10.1093/humrep/dem335. [DOI] [PubMed] [Google Scholar]
- Tan Y., Han P., Gu Q., Chen G., Wang L., Ma R., Wu J., Feng C., Zhang Y., Wang L. Generation of clinical-grade functional cardiomyocytes from human embryonic stem cells in chemically defined conditions. J. Tissue Eng. Regen. Med. 2016 doi: 10.1002/term.2381. [DOI] [PubMed] [Google Scholar]
- Tannenbaum S.E., Turetsky T.T., Singer O., Aizenman E., Kirshberg S., Ilouz N., Gil Y., Berman-Zaken Y., Perlman T.S., Geva N. Derivation of xeno-free and GMP-grade human embryonic stem cells–platforms for future clinical applications. PLoS One. 2012;7:e35325. doi: 10.1371/journal.pone.0035325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger C., Skottman H., Blomberg P., Dilber M.S., Hovatta O. Good manufacturing practice and clinical-grade human embryonic stem cell lines. Hum. Mol. Genet. 2008;17:R48–R53. doi: 10.1093/hmg/ddn079. [DOI] [PubMed] [Google Scholar]
- Villa-Diaz L.G., Ross A.M., Lahann J., Krebsbach P.H. Concise review: the evolution of human pluripotent stem cell culture: from feeder cells to synthetic coatings. Stem Cells. 2013;31:1–7. doi: 10.1002/stem.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Gu Q., Hao J., Bai D., Liu L., Zhao X., Liu Z., Wang L., Zhou Q. Generation of induced pluripotent stem cells with high efficiency from human umbilical cord blood mononuclear cells. Genomics Proteomics Bioinformatics. 2013;11:304–311. doi: 10.1016/j.gpb.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson A., Wongsatittham K., Johnston J. The NIH Stem Cell Registry: an absence of gamete donor consent. Cell Stem Cell. 2013;12:147–148. doi: 10.1016/j.stem.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Zhang X.Q., Zhang S.C. Differentiation of neural precursors and dopaminergic neurons from human embryonic stem cells. Methods Mol. Biol. 2010;584:355–366. doi: 10.1007/978-1-60761-369-5_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.