Abstract
Prenatal maternal depression and a multilocus genetic profile of two susceptibility genes implicated in the stress response were examined in an interaction model predicting negative emotionality (NE) in the first 3 years. In 179 mother-infant dyads from the Maternal Adversity, Vulnerability and Neurodevelopment cohort, prenatal depression (CES-D) was assessed at 24 to 36 weeks. The multilocus genetic profile score consisted of the number of susceptibility alleles from 5-HTTLPR (No LA (S/S, S/LG or LG/LG) vs. any LA) and Dopamine Receptor D4 (6–8R vs. 2–5R). NE was extracted from the IBQ-R at 3 and 6 months and the ECBQ at 18 and 36 months. Mixed and confirmatory regression analyses indicated that prenatal depression and the multilocus genetic profile interacted to predict NE from 3 to 36 months. Results were characterised by a differential susceptibility model at 3 and 6 months and by a diathesis stress model at 36 months.
Keywords: temperament, childhood, prenatal maternal depression, gene-environment interactions, differential susceptibility, diathesis stress
Negative emotionality (NE) is derived from the temperamental dimensions of sadness, distress towards limitations, fear and excessive reactions to minor changes, and reflects a generally stable tendency to show increased emotional reactivity towards negative situations (Gartstein & Rothbart, 2003; Lemery, Goldsmith, Klinnert, & Mrazek, 1999). NE is associated with the development of later problematic behaviour and psychopathology (Eisenberg et al., 2009; Fox, Henderson, Rubin, Calkins, & Schmidt, 2001; Hyde, Mezulis, & Abramson, 2008). For example, fearful temperament is associated with childhood anxiety disorders (Degnan, Alma, & Fox, 2010; Goldsmith & Lemery, 2000), while NE is associated with depression (Phillips, Lonigan, & Driscoll, 2002) and maladjustment (Eisenberg et al., 2009). Understanding early influences of NE on socio-emotional development (Davis, Glynn, Schetter, Hobel, Chicz-demet, & Sandman, 2007; Davis, Snidman, Glynn, Dunkel Schetter, & Sandman, 2004; Hayden et al., 2010; Hayden et al., 2007) could inform efforts at prevention and early intervention. Recent contradictory findings about the role of genetic and prenatal adversity (Braithwaite et al., 2013; Pluess et al., 2011), suggest the need for replication (Duncan, 2013) and modeling of genetic risk with multiple genes (Plomin, 2013). Accordingly, we present the findings from a study of the development of NE from 3 to 36 months of age from the interaction of prenatal maternal depression and a multilocus genetic profile.
The Role of Prenatal Maternal Stress
Prenatal maternal stress, measured in diverse ways is associated with NE (Glover, 2011; O’Connor, Heron, & Glover, 2002a). For example, higher prenatal maternal cortisol is associated with fussier behaviour, more negative facial expressions, crying, as well as higher NE at 7 weeks of age (de Weerth, Hees, & Buitelaar, 2003). Symptoms of prenatal maternal anxiety and depression, loosely associated with the stress response predict behavioural reactivity at 4 months of age (Davis et al., 2004) and behavioural/emotional problems at 4 years of age (O’Connor, et al., 2002a). While most studies of prenatal maternal symptoms have examined prenatal anxiety (Glover, 2011; Pluess et al., 2011), there is evidence for the specific effect of prenatal depression with outcomes reported as early as 2 months of age (Davis et al., 2007; Field, 2011; McGrath, Records, & Rice, 2008). Specifically, Davis and colleagues (2007) reported higher negative reactivity at 2 months and McGrath and colleagues (2008) reported more difficult temperament at 2 and 6 months. The association between prenatal maternal stress and NE is inconsistent though (Susman, Ponirakis, & Griepy, 2001), suggesting other factors may serve as moderators.
The Role of Genotype
Twin and genetic linkage studies supporting genomic influence for temperament (Bouchard, 1994; Saudino, 2009) have recently been followed by studies of candidate genes. Genes in the serotonin cell signalling pathways have been of particular interest in the prediction of NE, given their role in regulating emotional responses (Sen, Burmeister, & Ghosh, 2004) and their activity during the third trimester of pregnancy (Geva & Feldman, 2008). There has been considerable emphasis on a functional variation in the promoter region of the serotonin transporter gene because of its association with anxiety, depression and affective regulation (Canli & Lesch, 2007; Hariri & Holmes, 2006). The SLC6A4 gene, which encodes for the serotonin transporter, contains a 43 bp variable-number tandem repeat polymorphism in the promoter region (5-HTTLPR) that is coupled to transcriptional efficiency. The ‘long’ (L) compared to the ‘short’ (S) variant shows an increased basal transcription of 5-HTT mRNA (Canli & Lesch, 2007). Within the L genotype (Uher, 2008), there is a functional polymorphism (A→G, rs25531) (Hu et al., 2006). The LA variant has greater transcriptional efficiency and greater 5-HTT binding potential in humans (Praschak-Rieder et al., 2007; Hu et al., 2006), while the LG variant has functionally similar effect on 5-HTT mRNA expression as the SS genotype. The frequency of the LG in Caucasians is not insignificant at 14% (Odgerell et al, 2013).
Carriers of the low expressing alleles (any S or LG (S/LG)) show enhanced processing of negative emotions (Pezawas et al., 2005), positive stimuli and general emotional processing (Canli, et al., 2005) that associates with structural differences in limbic brain regions (Hariri et al., 2002). Likewise carriers of the low expressing alleles (any S or LG (S/LG)) have more depressive and anxious symptoms (Caspi et al., 2003), and more depressive and anxious symptoms relative to carriers of the LALA allele (Canli & Lesch, 2007; Gonda et al., 2009). There is also some evidence indicating that carriers of the low expressing S allele rate higher in NE than LL carriers (Auerbach et al., 1999; Hayden et al., 2010; Hayden et al., 2007). For example, infants with the SS genotype are reported to rate higher in NE at 2 months (Auerbach, et al., 1999), higher in fearful temperament during childhood (Hayden et al., 2007), and higher in NE in the presence of low positive emotionality during childhood (Hayden et al., 2010).
Functional variants in the dopamine D4 receptor (DRD4) are also associated with NE (Auerbach et al., 1999; Auerbach, Benjamin, Faroy, Geller & Ebstein, 2001a; Auerbach, Faroy, Ebstein, Kahana, & Levine, 2001b). There is a 48-base pair variable number tandem repeats in exon 3 of the DRD4 gene ranging from 2 to 11 copies. The long (6- to 10-repeat) alleles and specifically the 7- repeat allele (7R) are associated with lower dopamine receptor signalling and are identified as the susceptibility alleles. For example, the 7R allele is associated with approach behaviours (e.g., Zohsel et al., 2014) and in a meta-analysis the 7R is associated with more externalizing behaviour in negative contexts and with less externalizing behaviour in positive contexts (Bakermans-Kranenburg &Van IJzendoorn, 2011). Reports of the main effect of 7R on NE are contradictory, with evidence that the 7R allele is associated both with lower scores (Auerbach et al., 1999; Auerbach et al., 2001b; De Luca et al., 2003) as well as higher scores (Auerbach et al., 2001a, Lakatos et al., 2003, Holmboe, Nemoda, Fearon, Sasvari-Szekely, & Johnson, 2011) of NE and associated features. Auerbach and colleagues (1999) report lower ratings of NE, distress towards limitations and distress to novel stimuli on the Infant Behavior Questionnaire (IBQ) at 2 months in carriers of the 6–8R allele than in carriers of the 2–5R allele. At 12 months, infants with the 6–8R allele show less active resistance, and struggle less than infants with the 2–5R (Auerbach et al., 2001b). However, the reverse has also been reported, with evidence that infants with the 7R allele are higher on NE as measured by the IBQ at 4 and 9 months of age (Holmboe et al., 2011), show less novelty preference at 12 months (Auerbach et al., 2001a), and show increased latency to accept a toy from strangers at 12 months (Lakatos et al., 2003).
Consistent with evidence that multiple genes act in an additive fashion for the expression of a particular phenotype (Masarik et al., 2014; Plomin, 2013), there is evidence for the joint effect of 5-HTTLPR and DRD4 in the prediction of emotionally reactive behaviours (Auerbach et al., 1999; Ebstein et al., 1998; Holmboe, et al., 2011; Lakatoes et al., 2003). These findings are consistent with the overlapping role of the serotonin and dopamine systems in regulating behaviours related to NE such as approach and escape. DRD4 is highly expressed in the amygdala and the prefrontal circuits (Oak, Oldenhof, & Van Tol, 2000) while 5-HTTLPR significantly influences the amygdala and amygdala-prefrontal coupling (Hariri et al., 2002). Reports of the interaction effect of 5-HTTLPR by DRD4 also point to contradictory findings about which alleles increase the likelihood for NE. Ebstein et al. (1998) report that the 5-HTTLPR SS genotype and the DRD4 2–5R allele are associated with lower orientation scores and reduced interactive behaviour in two weeks old neonates. Similarly, Auerbach and colleagues (1999) find that SS and 2–5R are associated with higher NE and distress to limitations in 2 month olds. Lakatos et al. (2003) however find that SS and 7R are associated with increased stranger anxiety duration and latency to smile at 12 months while Holmboe et al. (2011) report reversed findings such that LALA and 7R are associated with higher NE at 4 and 9 months. A number of possible explanations for these divergences are suggested (Holmboe et al., 2011) including unmeasured and inconsistently present moderator such as adversity (e.g., Pluess et al., 2011; Smith et al., 2012) as well as heterogeneous developmental age. The overall pattern of findings suggests that the DRD4 2–5R allele is associated with higher NE in the first months of infancy (Auerbach et al., 1999; Ebstein et al., 1998) and the 7R allele is associated with higher NE later in the first year of life (Holmboe et al., 2011; Lakatos et al., 2003). Save for one study (Holmboe et 2011) the 5-HTTLPR S and LG alleles seem consistently associated with higher NE.
Gene by Environment Interactions (GxE)
The interaction of the environment with genomic variants is consistent with findings in molecular biology that the activation of gene expression is contingent upon transcriptional signals that derive from the internal and the external environment (Meaney, 2009). GxE models used to investigate NE examine the continuum of environmental exposures from maternal pre-conception to postnatal periods. For example, infant carriers of the S allele of the 5-HTTLPR whose mothers experienced adversity during their childhood are reported to have higher NE at 18 and 36 months of age (Bouvette-Turcot et al., 2015). Similar results are found in studies investigating G x postnatal E models involving 5-HTTLPR. Carriers of the S allele with insecure attachment are reported to show elevated NE (Pauli-Pott, Friedl, Hinney, & Hebebrand, 2009) and decreased emotion regulation at age two to four (Kochanska, Philibert, & Barry, 2009), while S carriers with low levels of social support show increased behaviour inhibition at seven years of age (Fox et al., 2005). Similarly, Hayden et al. (2010) found that variants in the Brain-Derived Neurotrophic Factor (BDNF) gene and parental depression and marital discord interact to predict NE at 3 years of age.
Few studies though have examined the role of prenatal exposure, and to date, its effect on the development of NE is unclear. Pluess and colleagues (2011) found that carriers of the 5-HTTLPR S allele who were exposed to higher levels of maternal prenatal anxiety have increased NE at 6 months. However, Braithwaithe and colleagues (2013) did not replicate this finding. Considering the emerging importance of models that include multilocus genetic profiles (Plomin, 2013), the inconsistent GxE findings may be in part explained by the use of models that include only a single genomic variant. A composite genetic factor, akin to the well-established factor for cumulative exposure to trauma (Sameroff, Gutman, & Peck, 2003) has been used to test a (cumulative) GxE model (Belsky & Beaver, 2011; Sonuga-Barke et al., 2009). Belsky and Beaver (2011) found that the association between supportive parenting and regulation is stronger as the number of plasticity (or susceptibility) genes increases. Plasticity genes have been defined as variants in genes implicated in cellular responses to environmental signals (e.g., synaptic plasticity) and associated with increased biological sensitivity to environmental conditions (Boyce & Ellis, 2005). There is now considerable evidence for the idea that variants of the 5-HTTLPR and DRD4 genotype serve as such plasticity/susceptibility genes (Belsky & Beaver, 2011). A GxE model that includes a multilocus genetic profile with DRD4 and 5-HTTLPR would reflect evidence of joint influences of these two genotypes on NE and might determine if such an influence is stronger than that of only one gene. Further, as there has been variability in findings in the susceptibility alleles for both DRD4 and 5-HTTLPR at different times during development (Auerbach et al., 1999; Holmboe et al., 2011; Lakatos et al., 2003), investigating DRD4 and 5-HTTLPR in a model with a multilocus genetic profile at multiple times during development would reflect whether the same alleles are susceptible across an age span.
Characterising the model of GxE
The diathesis-stress and the differential susceptibility models potentially characterize how genes associated with functional outcomes could, under conditions of prenatal depression, produce variation in NE. In the diathesis stress model, carriers of the genotype that associate with an increased risk for disease (for example, the S/LG for 5-HTTLPR), when exposed to prenatal depression, would have a greater likelihood of developing higher NE. Non-carriers would be insensitive to any environment with respect to NE, while in the absence of adversity, individuals with or without the ‘risk’ variant would show comparable developmental outcome.
Unlike the diathesis stress model, the differential susceptibility model allows for the possibility of positive outcomes as a function of the quality of the relevant environmental condition (Ellis, Boyce, Belsky, Bakermans-Kranenburg, & Van IJzendoorn, 2011; Belsky & Pluess, 2009). The differential susceptibility model reframes risk as susceptibility in light of reanalyses of studies demonstrating that the same genotypes that confer a greater vulnerability under adverse conditions, promote the development of phenotypes associated with resistance to mental disorders under more favorable conditions (Belskey & Pluess, 2009; Pluess, Belsky, & Neuman, 2009). The differential susceptibility model suggests that ‘risk’ genotypes are better considered ‘plasticity’ or ‘susceptibility’ genotypes, and that carriers are more susceptible to both adverse and enriched environments. To date, the prediction of NE from prenatal exposure and genetic risk is only explained by a diathesis stress model (Pluess et al., 2011).
Purpose of the Study
In this study we aimed to investigate whether prenatal maternal depression, child 5-HTTLPR and DRD4 genotype exert a joint influence on the development of early age NE and whether this association is stable over 3, 6, 18 and 36 months of life. Specifically, we examined: (i) The two way interaction of prenatal depression and the 5-HTTLPR genotype predicting NE, (ii) The two way interaction of prenatal depression and DRD4 predicting NE; (iii) The two way interaction of a multilocus genetic profile consisting of DRD4 and 5-HTTLPR and prenatal depression in predicting NE, and whether the joint influence of 5-HTTLPR and DRD4 is more predictive than the individual effects of 5-HTTLPR or DRD4 alone in a GxE model; and, (iv) If the GxE model with the multilocus genetic profile is best explained by the diathesis stress or the differential susceptibility model. We used Confirmatory Analysis of Interaction Models (Widaman et al., 2012) a novel statistical method favoured over exploratory and conservative models, such as regression and simple slopes methods. Confirmatory models directly test four competing predictions (i.e., weak and strong versions of diathesis-stress and differential susceptibility models) to identify which GxE model best explains the significant interaction findings (Appendix A) (Belsky, Pluess & Widaman, 2013).
METHOD
Participants
The participants were a community based sample of mother-infant dyads from Montreal, Quebec and Hamilton, Ontario, who are part of the Maternal Adversity, Vulnerability and Neurodevelopment (MAVAN) Project. The MAVAN is an established community cohort that enrolled 578 mother-infant dyads between 2003 and 2009. Mothers were recruited from the general population at 13–20 weeks gestation during their routine ultrasound and were included in the study if they were at least 18 years old, and fluent in either French or English. Participants were excluded if they experienced serious obstetric complications during pregnancy or during the delivery of their child; if their child had any congenital diseases ascertained using the Bayley Scales of Infant Development-Second Edition (Bayley, 1993); or if they delivered prematurely (before 37 weeks gestation). Dyads involved in the MAVAN have been assessed up to 72 months. Subjects recruited in Hamilton were oversampled for maternal prenatal distress.
Retention rates for the MAVAN subjects were 97.4% at 6 months, 84.04% at 18 months, and 80.5% at 36 months, reducing the total sample size to 464 dyads at 36 months. Compared to mothers who remained in the MAVAN study at all time points, mothers who left the study before reaching the 72 months time point did not differ significantly on measures of age at birth, depression, or education. Children whose mothers left the MAVAN study before reaching the 72 months time point did not differ significantly on outcomes of NE between 3 and 36 months; however they had significant lower birth weights than children of mothers who stayed in the study at all time points. Greater details are available elsewhere (O’Donnell et al., 2014). The present study included a sub sample of 179 mother-child dyads, with all measures complete including genotype (refer to Table 1 for sample characteristics). The reduction in sample size from 464 to 179 participants is explained as follows: 60 children were lost due to missing prenatal data (involved in the study prior to implementation of all study measures); 199 were missing genomic data (due to partial funding for genotyping); 21 were missing data on the Infant Behaviour Questionnaire or from the maternal depression rating scale; and, 5 were outliers. A comparison of the study sample and the cohort sample indicated that this study sample had higher income and lower NE.
Table 1.
Demographic characteristics of subjects from MAVAN included in 36 months analyses
| Variables | Montreal (N = 104) N (%) |
Hamilton (N = 75) N (%) |
|---|---|---|
| Mothers | ||
| Age* | M = 29.1, SD = 4.6 | M = 31.2, SD = 4.2 |
| Education | ||
| High school or less and partial College | 19 (18.2%) | 14 (18.6%) |
| Completed college or some university | 30 (28.9%) | 26 (34.7%) |
| University graduate or more | 55 (52.9%) | 35 (46.7%) |
| Income | ||
| <15,000 | 5 (4.8%) | 1 (1.3%) |
| 15,00–<30,000 | 45 (13.4%) | 5 (6.7%) |
| 30,000–<50,000 | 24 (23.1%) | 18 (24%) |
| 50,000–<80,000 | 27 (26%) | 22 (29.3%) |
| >80,000 | 34 (32.7%) | 29 (38.7%) |
| Alcohol Consumption* | ||
| Never | 50 (48.1%) | 53 (73.6%) |
| 1–2 times/month or more | 54 (51.9%) | 19 (26.4%) |
| Postnnatal Depression 6–36 months | M = 10.5, SD = 6.46 | M = 12.7, SD = 9.37 |
| Prenatal CES-D* | M = 10.6, SD = 7.91 | M = 14.93, SD = 11.9 |
| Children | ||
| Gender | ||
| Male | 52 (50%) | 34 (45.3%) |
| Female | 52 (50%) | 41 (54.7%) |
| Birth Weight* (percentile) | M = 34.9, SD = 24.2 | M = 58.29, SD = 29.5 |
| Genotype 5-HTTLPR | ||
| SS | 17 (16.4%) | 19 (25.3%) |
| LS | 49 (47.1%) | 35 (46.7%) |
| LL | 38 (36.5%) | 21 (28%) |
| LG or S | 24 (23.1%) | 26 (34.7%) |
| One La | 48 (46.1%) | 30 (40%) |
| Two La | 32 (30.8%) | 19 (25.3%) |
| Genotype DRD4 | ||
| 2–5R | 67 (64.4%) | 43 (57.3%) |
| 6–8R | 37 (35.6%) | 32 (42.7%) |
| Negative Emotionality | ||
| 3 Months* | M = −0.33, SD = 0.50 | M = −0.01, SD = 0.59 |
| 6 Months | M = −0.12, SD = 0.59 | M = −0.03, SD = 0.49 |
| 18 Months | M = −0.10, SD = 0.55 | M = −0.15, SD = 0.55 |
| 36 Months | M = −0.05, SD = 0.54 | M = −0.02, SD = 0.62 |
Note.
Significant difference between Montreal and Hamilton.
Procedure
The mothers were interviewed between 24 and 36 weeks of pregnancy and the dyads were assessed at 3, 6, 12, and 18 months and yearly from 24 months onwards. Maternal health and well-being were assessed each year using validated measures of maternal mental health, social and family functioning and socio-economic status (Kramer et al., 2009). The children were assessed with age-appropriate measures of temperament, socio-emotional development and psychopathology. Informed consent was obtained at the time of recruitment and at each time point of data acquisition. Ethics Review Board approval was obtained from the institution of each study site.
Measures
Negative emotionality
NE at 3 and 6 months were obtained from the Infant Behaviour Questionnaire – Revised (IBQ-R) (IBQ-R; Gartstein & Rothbart, 2003), a reliable and valid parent-completed measure of 15 scales of temperament (Parade & Leekes, 2008). As per the recommendations of the authors, NE was extracted from our sample, given the younger age of our sample than that in the published sample (M. Gartstein, personal communication, May 13, 2012). Using promax oblique rotation, subscale loadings were determined using a 1% level of significance (subscales with loadings greater than 0.29) (Stevens, 1986). At 3 and 6 months, 7 of the 15 sub-scales (activity level, distress to limitations, falling reactivity (negative loading), fear, sadness, cuddliness (negative loading), and soothability (negative loading)) substantially loaded on the NE factor (see Appendix B for factor loadings). All subscales with significant loadings were transformed into z scores, aggregated and averaged to create a final NE factor as per the authors of the IBQ-R (Garstein & Rothbart, 2003). There was good internal consistency with Cronbach alphas (0.74 at 3 months and 0.71 at 6 months).
NE at 18 and 36 months was obtained from the Early Child Behavior Questionnaire (ECBQ) (Putnam, Gartstein, & Rothbart, 2006), a reliable and valid parent-completed measure of temperament (Putnam et al., 2006). The ECBQ is comprised of 18 different scales measuring different temperamental dimensions and is considered an upward extension of the IBQ-R. It contains 11 scales that are similar in form to the IBQ-R (Garstein & Rothbart, 2003; Putnam et al., 2006), and both instruments have yielded a similar three factor temperament structure that include NE with many consistent factor loadings across both measures (Putnam, Ellis, & Rothbart, 2001). Convergent validity and structural continuity between the IBQ-R and the ECBQ has been demonstrated (Putnam, Rothbart, & Gartstein, 2008). NE was extracted as per the method for the IBQ-R and consisted of the same subscales (fear, frustration, motor activation, perceptual sensitivity, sadness, discomfort, shyness and soothability (negative loading)) as per the original report (Putnam, Rothbart, & Gartstein, 2008). Impulsivity (negative loading) only loaded at 18 months. Given the almost perfect correlation with and without impulsivity (r = 0.98447), it was omitted for the purposes of consistency (18 and 36 months) and in accord with the NE factor derived by Putnam et al. (2006) (see Appendix C for factor loadings). Internal consistency was 0.76 and 0.75 at 18 and 36 months, respectively.
5-HTTLPR and DRD4 genotype
Child genotype was obtained with the use of buccal swabs at 36 months. The method for genotyping the SLC6A4LPR (Bouvette-Turcot et al., 2015) and DRD4 (Lichter et al., 1993; Silveira et al. 2014, 2016) variants and for establishing reliability have been reported previously. For each marker tested, 10% of samples were re-typed as a form of quality control (QC). If there was conflict between the original genotype and the QC genotype, a new working dilution of the sample was made from stock, and the test was run again using both the old and new dilution to resolve the conflict.
We examined two categorizations of 5-HTTLPR. For the biallelic categorization, 5-HTTLPR was coded as (i) L/L, the highest expressing genotype and (ii) S/S or S/L, the lowest expressing genotype. For the triallelic categorization it was coded as (i) LALA, the highest expressing genotype, (ii) any LA (LA/S, LALG), and (iii) no LA (S/S, S/LG, LGLG) (Hu et al., 2006). The analyses with the biallelic categorization did not yield any significant findings. As such, only analyses using the triallelic categorization are reported here. Analyses with the triallelic categorization revealed comparable predictions in subjects with the presence of any LA allele, (i.e . LA/LA, LA/S or LALG). As such, the 5-HTTLPR genotype variable was recoded as a dichotomous variable: (i) any LA vs (ii) no LA. This facilitated the construction of a multilocus genetic profile score.
We also examined two categorization of DRD4. Specifically, DRD4 was coded as 6–8 Repeat (6–8R) or 2–5 Repeat (2–5R), as per Auerbach et al. (1999). DRD4 was also coded as 7R vs. other genotypes, as per Holmboe et al. (2011). Both categorizations yielded the same results. Findings are presented using the Auerbach (1999) classification (6–8R vs. 2-5R). For both the Montreal and Hamilton samples the distribution of DRD4 conformed to the Hardy Weinberg equilibrium (p = .58; p = .95, respectively). Similarly, the genotype distribution for 5-HTTLPR conformed to the Hardy Weinberg equilibrium for the Montreal (p = .60) and Hamilton (p = .06) samples. There were no gender differences for 5-HTTLPR or DRD4 (χ2(1) = .01, p = .92; χ2(1) = .4, p = .52, respectively) (Table 1).
Other plasticity genes that were examined in secondary analyses included the dopamine receptor D2 (DRD2 - A/G or A/A vs. G/G), dopamine transporter (DAT - 10/10 vs. 9/9 or 9/10), catechol-O-methyltransferase gene (COMT - G/G vs. A/G or A/A), monoamine oxidase A (MAOA - 3/4, 4/4 or 4/5), and STin2 VNTR polymorphism (STin2 – 10/12 or 10/10 vs. 12/12).
Multilocus genetic profile score
A genetic factor was obtained by summing the number of risk/susceptibility genotype: no LA (S/S, S/LG or LG/LG (S/LG)) for 5-HTTLPR and any 6–8R for DRD4 (Auerbach et al., 1999). The child’s value ranged from 0 (no risk/susceptibility genotype) to 2 (both risk/susceptibility genotypes).
Prenatal depression
Maternal depressive symptoms were obtained with the Center for Epidemiologic Studies Depression Scale (CES-D; Radloff, 1977) at 24 to 36 weeks of pregnancy. Items on the CES-D are designed to measure symptoms of depression in community-based populations, and include 20 questions about mood, appetite, and sleep, rated on a Likert-scale ranging from 0–3. Scores on the CES-D can range from 0–60 with a score of 16 or higher indicating the presence of a depressive illness. The CES-D has been validated in a sample of pregnant women (e.g., Field et al., 2004). In the present study, scores were centered to facilitate interpretation of regression coefficients, with higher scores indicating more severe depressive symptoms. Internal consistency was 0.92.
Covariates
Child birth-weight was obtained from the chart of the birthing unit. Other covariates were obtained from the Health and Well Being of Mothers and their Newborns questionnaire (Kramer et al., 2009) administered prenatally and at 6, 12 and 36 months postnatally. Postnatal depression was assessed with the CES-D at 6, 12 and 36 months. Maternal education, assessed prenatally, was dichotomized as ‘University graduate or higher’ or ‘others’. The original categories (Table 1) were collapsed into two groups in light of small sized categories.
Covariates were identified by preliminary analyses driven by theoretical conception. Variables were retained as covariates for the final analyses when they were associated with a predictor or the outcome. This included maternal postnatal depression, mother’s age at birth, site, child SES (represented by maternal education), child gender and maternal 5-HTTLPR genotype in the models that also contained child 5-HTTLPR genotype. Variables considered as covariates but not retained for the final analyses were maternal DRD4 genotype, maternal alcohol consumption during pregnancy, and child birth-weight. Postnatal depression was assessed with the CES-D at 6, 12 and 36 months and included in all analyses, except at 3 months when a measure of concurrent maternal depressive symptoms is unavailable.
Statistical analysis
Intraclass correlation (ICC), which depicts the proportion of variance in NE accounted for by site of recruiting, was 0.07 at 3 months and 0.00 at 6 months, 18 and 36 months (p’s > 0.05). Given the negligible proportion of the total variance of NE explained by site level, site was not added as a fixed effect. However, with significant differences between both sites in prenatal CES-D score (t(197) = −2.22, p = 0.03) and on NE at 3 months (t(127) = −2.55, p = 0.01), site was entered as a covariate.
Outliers were assessed by examining values of studentized residuals with magnitudes greater than 2.8 (probability of 0.005) or with values greater than 2.0 (probability of 0.05) with a combined leverage larger then 2p/n (Hoaglin, & Welsch, 1978). Five cases were removed. We corrected for heteroscedasticity with the use of consistent standard errors and p-values applied to the graphical representations of each significant mixed model. All main predictor variables except for genotype were centered.
The data was analysed in two separate models to test the robustness of our findings: (i) a repeated measure model (mixed model) predicting NE across all four time points simultaneously, and a (ii) Confirmatory Analysis of Interaction Models to identify which type of GxE model best explains the interaction finding at each of the four time points.
Secondary analyses included exploration of other known plasticity genes, although lesser related to NE. Results revealed no significant main or cumulative effect. As such, findings are presented only for 5-HTTLPR and DRD4.
Mixed Model for Longitudinal Data
We implemented mixed models for repeated measures with an unstructured covariance matrix to test the prediction of NE from prenatal depression and genotype. Child gender, site, maternal age, maternal 5-HTTLPR genotype and education were entered as covariates. In a more conservative model, a measure of postnatal maternal depression (average score of maternal depression scores from 6 to 36 months) was entered. This is a more conservative model since some of the exposure to postnatal depression post-dates the outcome under consideration, NE from 3 to 36 months.
Missing values were imputed for the mixed model analysis with the multilocus genetic profile score using the MICE (multivariate imputation by chained equations) algorithm (van Buuren & Groothuis-Oudshoorn, 2011). The imputation model was used to explain the pattern of missing data and to obtain imputed values for these missing data.
As recommended by van Buuren (1999), we included all variables used in the mixed model analysis in addition to variables that explained a considerable amount of variance. No variable had to be removed because of excessive missing data. Imputed values were based on regression estimates. As recommended, five iterations of the algorithm were run. When the amount of missing values is high, it is recommended run between 20 and 100 imputed data sets so, as a precaution, we created 50 imputed data sets. R software (version 3.1.0; The R Foundation for Statistical Computing, Vienna, Austria) was used to perform imputation.
Confirmatory regression
To specifically test the whether carriers of the 5-HTTLPR S/LG and DRD4 6–8R alleles were at risk (diathesis-stress) or susceptible (differential susceptibility) when exposed to prenatal depression, the regression was re-parameterized using the following equation (Widaman et al., 2012):
The parameters in this equation were the intercept (β0), the slope for carriers of the non-risk/susceptibility allele (β1), the slope for carriers of the risk/susceptibility allele (β2), and the cross-over point between the two slopes (C). Both the diathesis-stress and differential susceptibility models assume that carriers with no susceptibility alleles would not be influenced by the environment (prenatal depression), i.e. that β1 = 0. However, since there remains the possibility that the environment exerts a slight effect even on non-carriers, the diathesis-stress and differential susceptibility models are further separated into two groups: a weak model (β1 ≠ 0 and β1 < β2) and a strong model (β1 = 0).
The Akaike information criteria (AIC) with significance testing at a 95% confidence interval was used to determine which of the four models (i.e., weak vs. strong diathesis-stress, and weak vs. strong differential susceptibility) best fit the data at each time-point. Only the strong diathesis-stress and strong differential susceptibility model testing are reported; however, all four models were tested. The models presented are calculated for average maternal age, non-university maternal education, the non-susceptible/risk genotype and no prenatal or postnatal maternal depression (details available in Appendix A).
RESULTS
Descriptives
There was an almost equal distribution of males and females (Table 1). The mean age for mothers was 30, with half of the mothers highly educated. Specifically, 90 had a university degree or higher. Unstandardized prenatal depression scores ranged from 0 to 49 (M = 12.41, SD = 9.96, α = 0.92). Consistent with oversampling strategies, 26.26% of the women met the threshold for depression at 24 to 36 weeks of pregnancy. The mean prenatal CES-D score for mothers in Montreal (M = 10.6, SD = 7.91) was significantly lower than the mean in Hamilton (M = 14.93, SD = 11.9) (t(177) = −2.94, p = 0.004). For the biallelic categorization of 5-HTTLPR, there were 143 infants with at least one Lallele and 36 infants with only the S alleles. For the triallelic categorization of 5-HTTLPR there were 129 infants with at least one LA allele and 50 infants with only the LG or S alleles. For DRD4, there were 69 infants at least one 6–8R allele and 110 infants with two 2–5R alleles. Similar distributions have been found in other North American Caucasian samples for DRD4 (Chang, Kidd, Livak, Pakstis, & Kidd, 1996) and 5-HTTLPR (Hu et al., 2006). There was no significant association between maternal DRD4 genotype, prenatal depression or NE. Maternal 5-HTTLPR genotype was associated with prenatal maternal depression and NE at 3 and 6 months. There was also no significant association between child 5-HTTLPR, DRD4 genotype, or the multilocus genetic profile score and prenatal depression or NE (refer to Appendix D for correlation matrix). The demographic and socioeconomic distribution of women in this study was similar to that of women from the Generation R Study and the Avon Longitudinal Study of Parents and their Children, two comparable prenatal cohort studies (van Batenburg-Eddes et al., 2013). Given the sample size and the complexity of the interaction model, we examined the number of subjects in all key cells (presence and absence of the susceptibility genes and prenatal depression (dichotomized according to the established cut-off), and found an adequate number of participants in every risk group.
Prediction of NE from prenatal maternal depression and 5-HTTLPR from 3 to 36 months
Mixed model analyses indicated prenatal maternal depression and 5-HTTLPR interacted to predict NE from 3 to 36 months (b = 0.016, SE = 0.01, p = 0.04 (Table 2). Specifically, the association between prenatal depression and NE depended on the presence of child risk/susceptibility alleles (no LA). There was a main effect of child genotype and mothers genotype such that it predicted child’s NE (b = 0.139, SE = 0.07, p = 0.04; b = −.137, SE = 0.06, p = 0.03, respectively). Further, the absence of any university education in the mother was associated with an elevated NE score in the child (b = 0.197, SE = 0.08, p = 0.02) and the average score for postnatal maternal depression covariate (measured at 6, 18 and 36 months) was associated with elevated NE scores (b = 0.016, SE = 0.00, p < 0.001).
Table 2.
The interaction of prenatal maternal depression and child 5-HTTLPR, DRD4 and a multilocus genetic profile score in the prediction of Negative Emotionality from 3 to 36 months (mixed models)
| Predictors | 5-HTTLPR | DRD4 | Multilocus Genetic Profile Score |
|---|---|---|---|
| Prenatal depression | 0.001 | −0.000 | −0.003 |
| Genotype | 0.139* | −0.004 | 0.049 |
| Prenatal depression X genotype(s)(interaction) | 0.016* | 0.015* | 0.013** |
| Covariates | |||
| Postnatal depression | 0.016*** | 0.018*** | 0.018*** |
| Maternal education – College | 0.197* | 0.179* | 0.186* |
| Maternal education – University | 0.150t | 0.165* | 0.157* |
| Gender | 0.009 | 0.016 | 0.022 |
| Mother age of birth | −0.015 | −0.015* | −0.017* |
| Site | 0.038 | 0.057 | 0.051 |
| Maternal 5-HTTLPR | −0.137* | - | −0.102 |
Note. Multilocus genetic profile score − 0, 1 or 2 risk/susceptibility genotype (no LA (S/S, S/LG or LG/LG)(5-HTTLPR); 6–8R (DRD4)). In the model with 5-HTTLPR McFadden’s pseudo R2 = .065, χ2(9) = 112.16*** (AIC: 920.8) -, in the model with DRD4 McFadden’s pseudo R2 = .059, χ2(9) = 112.49***, in the model with the multilocus genetic profile score McFadden’s pseudo R2 = .066, χ2(9) = 112.04*** (AIC – 922).
p < .10
p < .05
p < .01
p < .001
Prediction of NE from prenatal maternal depression and DRD4 from 3 to 36 months
Mixed model analyses indicated that prenatal maternal depression and DRD4 interacted to predict NE from 3 to 36 months (b = 0.015, SE = 0.01, p = 0.02) (Table 2). The absence of any university education in the mother was associated with an elevated NE score in the child (b = 0.18, SE = 0.08, p = 0.03). Postnatal maternal depression (b = 0.018, SE = 0.00, p < 0.001) and mothers age at birth (b = −0.015, SE = 0.01, p = 0.03) were also significant predictors of NE.
Prediction of NE from prenatal maternal depression and the multilocus genetic profile from 3 to 36 months
Mixed model analyses indicated that prenatal maternal depression and the multilocus genetic profile interacted to predict NE from 3 to 36 months (b = 0.013, SE = 0.00, p = 0.004) (Table 2). Maternal education was a significant predictor of NE (b = 0.19, SE = 0.08, p = 0.02), as was postnatal maternal depression (b = 0.018, SE = 0.00, p < 0.001) and mothers age at birth (b = −0.017, SE = 0.01, p = 0.02). The parameter estimates for the interaction in the model with 5-HTTLPR and DRD4 were 0.016 and 0.015, respectively, while that for multilocus genetic profile score ranged from 0.013, for one susceptibility genotype, to 0.026, for two susceptibility genotypes. Similar results were found when the imputed values for missing data were used (for the interaction, b = 0.008, SE = 0.00, p = 0.04).
Further investigation of the change in McFadden’s pseudo R2 after entering the covariates, main effects and interaction in 3 steps revealed that the fit of the model increased from 0.021 to 0.051 after the inclusion of postnatal depression and then increased to 0.066 after the inclusion of main effect and interaction effects of prenatal depression and the multilocus genetic profile. These results are unique to prenatal depression as a model constructed to predict NE at 18 and 36 months from the interaction of postnatal depression (at 6 and 12 months) and the multilocus genetic profile score was not significant.
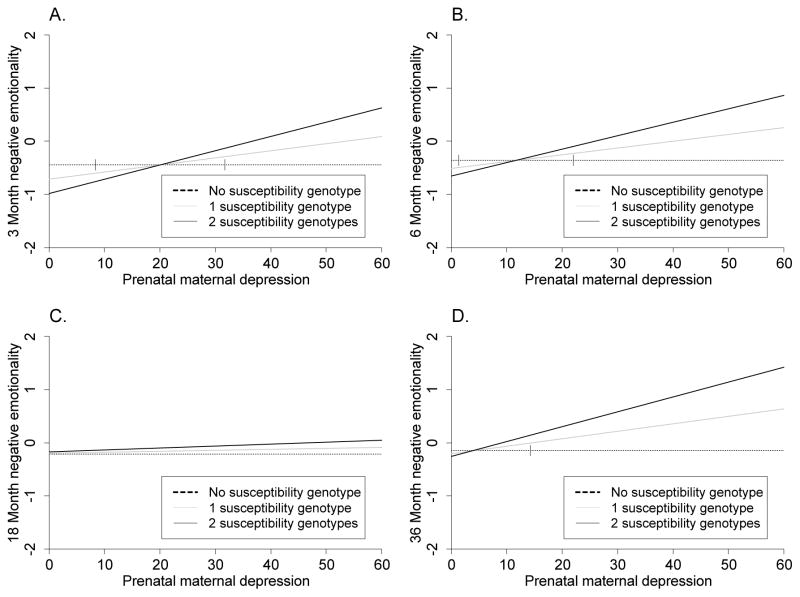
Confirmatory analyses found the best model to be strong differential susceptibility model at 3 and 6 months, and diathesis stress at 36 months. At 3 months (Table 3, Figure 1), the interaction was significant, and the 95% interval for the cross-over point fell within the range of the CES-D (between 8.35 and 31.74) and was significantly different from zero (b = 20.04, p < 0.001). We point out that the non-zero value of the cross-over point indicates that carriers of at least one susceptibility allele (No LA or 6–8R) can have a better outcome with respect to NE than carriers of both the LA and 2–5R allele when exposed to low levels of prenatal depression. At 6 months, the interaction was significant and the 95% interval for the cross-over point fell within the range of the CES-D (1.36 and 22) and was significantly different from zero (b = 11.68, p = 0.03). The estimates of the crossover points at 3 and 6 months (20.04 and 11.68, respectively) were close to the cut off point for the presence of a depressive illness indicating that children with one or two susceptibility alleles tended to be rated lower in NE than those with no susceptibility alleles if the mother was below the clinical cut-off score of 16. Further, these children had higher NE than those without the LA or 6–8R genotype if the mother was above the clinical cut-off score of 16.
Table 3.
The interaction of prenatal maternal depression and child multilocus genetic profile score (5-HTTLPR and DRD4) in the prediction of Negative Emotionality at 3, 6, 18 and 36 months (confirmatory regression)
| Predictors | 3 months β |
6 months B |
18 months B |
36 months B |
|---|---|---|---|---|
| Intercept | −0.445** | −0.362** | −0.215t | −0.145 |
| Cross-over pointa | 20.043*** | 11.68* | −11.362 | 4.108 |
| Interaction | 0.013** | 0.013* | 0.002 | 0.014** |
| Covariates | ||||
| Postnatal depression | - | 0.005 | 0.02*** | 0.018*** |
| Maternal education – College | 0.238t | 0.302* | 0.218t | 0.07 |
| Maternal education – University | 0.333* | 0.205t | 0.133 | 0.047 |
| Gender | 0.099 | 0.136t | −0.088 | −0.054 |
| Mother age of birth | −0.012 | −0.026** | −0.025** | −0.015 |
| Site | 0.318*** | 0.112 | −0.012 | −0.02 |
| Maternal 5-HTTLPR | −0.263* | −0.153t | −0.032 | −0.005 |
Note. Multilocus genetic profile score - 0, 1 or 2 risk/susceptibility genotype (no LA (S/S, S/LG or LG/LG)(5-HTTLPR); 6–8R (DRD4)). At 3 months R2 = .24, F(8, 123) = 4.86***, at 6 months R2 = .18, F(9, 157) = 3.91***, at 18 months R2 = .20, F(9, 152) = 4.33***, and at 36 months R2 = .20, F(9, 169) = 4.61***.
Strong differential susceptibility models are indicated by statistically significant cross over points at 3 and 6 months.
p < .10
p < .05
p < .01
p < .001
Figure 1.
The interaction of prenatal maternal depression and child multilocus genetic profile score (5-HTTLPR and DRD4) in the prediction of Negative Emotionality at 3, 6, 18 and 36 months (Confirmatory analyses). Multilocus Genetic Profile Score − 0, 1 or 2 risk/susceptibility genotype (no LA (S/S, S/ LG or LG/LG) (5-HTTLPR); 6–8R (DRD4)). Graphs depict models of strong differential susceptibility at 3 and 6 months and diathesis stress at 36 months.
The interaction at 18 months was not significantly different from zero. At 36 months, the interaction was significant. However, the 95% interval for the cross-over point was not significant from zero indicating a model of diathesis stress.
Figure 1 depicts the GxE models for the prediction of NE (standardized) at 3, 6, 18 and 36 months. Carriers of the LA and 2–5R alleles were insensitive to prenatal depression exposure, with stable scores of NE throughout the period of study. Carriers of one susceptibility allele (No LA or 6–8R), however, had higher levels of NE as a function of exposure to greater levels of prenatal depression, while carriers of both alleles had even higher levels of NE. With lower prenatal depression, carriers of the susceptibility alleles had lower levels of NE than non-carriers at 3 and 6 months. This effect is not significant at 36 months. From these results it appears, as the child gets older, the advantaged conferred from having the susceptible genes and low prenatal exposure diminishes.
In both analyses, the model that included the multilocus genetic profile was the strongest predictor of NE, as confirmed from the parameter estimates, the AIC model fit statistic and McFadden pseudo R-squared.
DISCUSSION
The findings of our study suggest that prenatal maternal depression and a child multilocus genetic profile (No LA (only S or LG) for 5-HTTLPR and 6–8R for DRD4) interact to predict early age NE from 3 to 36 months of age. The interaction identified in the mixed-model analysis was replicated at 3, 6, and 36 months of age in confirmatory analyses. Specifically, exposure to prenatal maternal depression was associated with higher NE across the first 3 years of life when carriers had the S/LG allele of the 5-HTTLPR and the 6–8R allele of the DRD4 genotypes. These unique findings are strengthened by the design of the study (i.e., prenatal longitudinal data with multiple time-points, refined functional genotyping of the L allele, and complimentary (mixed-model regression) and novel analyses (confirmatory analysis).
Three findings stand out. First, the interaction between child 5-HTTLPR and DRD4 genotype and prenatal maternal depression predicting NE is strengthened when the genetic factor includes both alleles in the same model. There were separate contributions for the 5-HTTLPR and DRD4 genotypes, such that both moderated the relationship between prenatal maternal depression and NE in separate models. The model that included the multilocus genetic profile was the strongest predictor of NE. These results not only indicate the importance of 5-HTTLPR and DRD4 in predicting early NE in the presence of prenatal maternal depression, but also show that multiple genes are likely involved in its development. Even exposure to low levels of depressive symptoms may influence differences in NE in children with the S/LG and 6–8R alleles since significant differences in NE were found between children with both susceptibility alleles and those with no susceptibly alleles even when their mother’s depressive symptoms did not meet the cutoff for a diagnosis of prenatal depression. The fact that the relationship between prenatal maternal depression and child NE was greatest in children with both susceptibility alleles also highlights the importance of considering genetic factors in main effect studies. As demonstrated by the small proportion of children in this study manifesting both alleles (10.06%), such findings may be influenced by a small number of highly susceptible children.
The association between prenatal maternal adversity and child development is consistent with the existing literature (e.g. Davis et al., 2007). For example, Pluess et al. (2011) have reported that prenatal maternal distress and the biallelic 5-HTTLPR predict 6 month NE. Seeing we were only able to reproduce this finding when the triallelic categorization was used, questions remain about how divergences in categorization of 5-HTTLPR can influence the detection of an association. If the biallelic categorization identifies some subjects as L, when functionally as LG, they better resemble S, one wonders about the role of measurement error in the underestimation and inconsistencies of GxE findings involving 5-HTTLPR. The role of power and precision in GxE analyses is nicely demonstrated in the sensitivity analyses of Wong et al (2003).
The joint effect of DRD4 and 5-HTTLPR is also consistent with the prior literature reviewed. Variants in the genotypes for both 5-HTTLPR (Hayden et al., 2010; Hariri & Holmes, 2006) and DRD4 (Belsky, Bakermans-Kranenburg & van Ijzendoorn, 2007) are associated with mood regulation and activation. Specifically, Serotonin and Dopamine have complementary and opposing action, with shared anatomy (e.g., direct projections from the 5-HT raphe nuclei to DA neurons in the substantia nigra (Dray, Gonye, Oakley & Tanner, 1976)) and neurophysiological activity (electrical stimulation of the raphe inhibits Dopamine neurons in the Substantia nigra, an effect mediated by Serotonin (Tsai, 1989)). The reward processing circuits and adversity processing circuits “compete to bias decision-making, motivation and well-being, by the opposite effect of dopamine and serotonin on the activation of each circuit (Vadovicova & Gasparotti, 2013, p. 7),” such that drive, hope, and impulsivity work in opposition with negative affect, discomfort, depression and worries. With several preclinical models revealing that dopamine and serotonin systems interact to determine corticolimbic responses to environmental adversity (e.g., Cools, Nakamura, & Daw, 2011), we extend these findings to demonstrate that the two genotypes also interact with prenatal depression to predict NE. The finding that the S/LG and 6–8R alleles operate as the susceptibility risk/alleles is consistent with recent studies and meta-analysis (Bakermans-Kranenburg & van IJzendoorn, 2011; Holmboe et al., 2011). Nonetheless, we suggest that the presence of opposite findings open the door for a more complex understanding of these genes and cell signalling systems. Finally, there are recent studies reporting that prenatal depression influences structural changes (Sandman et al., 2015) and connectivity (Qiu et al., 2015) of brain regions that influence emotion regulation. As our findings show that prenatal depression influences the development of NE only in the presence of the DRD4 and 5-HTTLPR susceptibility genotypes, they may be the mechanisms through which these structural changes take place.
Second, the characterization of the interaction changes with the development of the child. A model of differential susceptibility characterizes our findings up to 6 months of age. This finding suggests that exposure to the prenatal environment might be moderated in a bi-directional manner by genotype, and that early in development S/LG and 6–8R carriers have the greatest capacity to benefit from positive environments. A model of diathesis stress characterizes the findings at 36 months where only exposure to higher levels of prenatal depression influenced higher levels of NE reported in S/LG and 6–8R carriers. Questions remain about how to explain divergences in the model across the lifespan such as developmental windows for positive outcomes and postnatal factors unmeasured in our model.
A key observation from our analyses is that findings were strengthened by the use of a confirmatory model. Confirmatory models maximize statistical power by aligning analyses with hypotheses of interest when two viable alternative models are possible, in this case diathesis-stress and differential susceptibility. Our glove-like statistical analyses might explain the differences in our results from Braithwaite and colleagues (2013). Furthermore, since the model testing the multilocus genetic profile score was stronger than either model testing the separate contribution of 5-HTTLPR and DRD4, we wonder whether their discrepant findings might not also be related to the unmeasured role of DRD4.
Third, the effect of maternal depression on NE operates across the continuum of exposure, from prenatal to the early postnatal. Our findings indicate an increasing main effect of postnatal depression on NE from 6 to 36 months. This is consistent with all accounts of the importance of maternal mood in the development of the child (Goodman et al., 2011). However, postnatal maternal depression did not fully explain the association between prenatal depression and NE, even in a postnatal by genotype interaction model. That the prediction model for NE improved when both pre- and post-natal depression were included suggests the importance of depression across the continuum and its increasing influence when sustained from the prenatal to the postnatal period. We are currently further exploring these findings to gain a more complete understanding of its contribution on the development of NE (Gordon Green, et al., 2014).
Limitations
The design does not allow us to exclude that a gene by environment correlation (rGE) would better explain our GxE models. We found that mother’s 5-HTTLPR genotype was correlated with prenatal maternal depression. Further, in the model examining the interaction of prenatal depression and 5-HTTLPR, mother’s 5-HTTLPR genotype was a significant predictor of child NE. Since the interaction was still a significant predictor of NE above and beyond maternal genotype, it is likely that 5-HTTLPR and prenatal maternal depression contribute to the development of NE via both rGE and GxE pathways. The absence of a correlation between infant or mother DRD4 genotype and prenatal depression and of a confounding effect of maternal DRD4 genotype makes it unlikely that passive rGE factors are at play in the model examining the interaction of prenatal depression and DRD4. An evocative rGE remains possible although less likely with a consistent prediction starting at 3 months of age.
Our NE factors were obtained from parent-report measures. Although parent report questionnaires benefit from a longer observation period (Rothbart, 1981), parental mood may influence the ratings given to the child (Atella, DiPietro, Smith, & St James-Roberts, 2009). Given that the IBQ-R and ECBQ specifically inquire about the frequency of observable behaviours, parent-reporting bias is minimized. The concern though that these two types of assessment might measure different aspects of temperament is supported by the low convergence rates between them (Seifer, Sameroff, Barrett, & Krafchuk, 1994). We tried to limit the effect of present parental mood on ratings of infant NE by controlling all our models for current maternal depressive symptoms.
There may be unmeasured confounds. Specifically, mothers who experience prenatal maternal depression may also be vulnerable to adverse environmental factors that could provoke a different type of stress experience by the foetus such as prenatal maternal anxiety. However, previous smaller analyses run by the authors of this investigation on the interaction of genetic susceptibility and anxiety and depression found that prenatal maternal depression had a stronger, separate effect on the development of infant NE than prenatal maternal anxiety (Gordon Green et al., 2014), indicating that prenatal maternal depression makes a separate contribution. Interestingly, recent brain imaging studies with neonates reflect differential effects of prenatal maternal depression compared to anxiety on corticolimbic brain structures (Qiu et al., 2015; Rifkin-Graboi et al., 2013).
When compared to other genetic studies, the MAVAN has a relatively smaller number of subjects. In turn, our power is strengthened by the accuracy of the genotyping method, the increased precision of functional genotyping from the triallelic categorization of 5-HTTLPR, and by the use of confirmatory models (Wong, Day, Luan, Chan, & Wareham, 2003).
Finally, we do not include data on prenatal antidepressant medication exposure. Community estimates of antidepressant use suggest that about 6% of our sample might have been exposed during pregnancy (Cooper, Willy, Pont, & Ray, 2007). There is a slight possibility that the association between prenatal depression and NE might be in part explained by the associated antidepressant exposure in a few cases. Even then, questions remain as to whether antidepressant exposure predicts developmental outcomes via direct causal processes, or represents a marker of the severity for the associated prenatal depression (Weikum et al., 2013).
Summary and Implications
We report that the relation between prenatal depression and NE is better explained by the interaction of prenatal depression and genotype, such that infants with the susceptibility alleles of 5-HTTLPR and DRD4 will develop higher or lower NE depending on the severity of the exposure to prenatal maternal depression. The findings highlight the importance of considering multiple genes in a GxE model with a multilocus genetic profile including genes which in monogenic models seem to have a modest if any effect at all. The present study reports on two candidate genes consistent with pre-existing literature. Although secondary analyses did not identify any other genes as significant predictors in the model, this does not preclude that other susceptibility genes could also be operating in the development of NE. For example, Hill et al. (2013) has identified monoamine oxidase A (MAOA) as a moderator of adverse prenatal experiences and the development of NE at 5 weeks. It will be important for future studies to examine these and other candidate genes in greater detail.
Many studies have reported that the association between prenatal environmental exposure and child development is dependent on timing of gestation (e.g., Davis et al., 2007; Davis & Sandman, 2010; O’Connor, Heron, Golding, Beveridge, & Glover, 2002b), although there is some evidence that behavioural and emotional outcomes are associated only with exposure during later gestation (Davis et al., 2007; O’Connor et al., 2002b). Further examination of exposure to prenatal maternal depression earlier or later during pregnancy is needed to determine if similar associations may be found during different developmental periods.
A closer look at the moderating effect of the postnatal environment is also indicated. Boyce and Ellis (2005) postulate that susceptibly is heritable and influenced by stressful and protective environments. As such, NE is considered a susceptibility factor with positive and negative developmental outcomes documented for infants and children in the presence of certain adaptive or maladaptive environments (see Belsky & Pluess, 2009 for a review). Our results reveal that NE is shaped in-part by experiences during pregnancy. Consistent with the notion that exposure to stress during pregnancy functions as a primer to increase susceptibility (or vulnerability) to the later environment (Pluess, 2015; Grant et al., 2015), such “prenatal programming” of postnatal susceptibility suggests the importance of close attention to the role of postnatal environment in the outcome of these children.
Finally, our findings underline the importance of identifying and treating prenatal depression (O’Connor, Monk, & Fitelson, 2014). As noted above, the link between NE and later problematic behaviour and psychopathology has been well established (e.g., Eisenberg et al., 2009). The moderating role of NE in treatment outcome aimed at improving internalizing and externalizing behaviour (Blair, Mitchell, & Blair, 2005) suggests that targeting contributing factors of NE could be effective in preventing the development of childhood psychopathology. The results from this study replicate previous findings that prenatal maternal depression has an influence on temperamental vulnerability in the offspring and supports the importance of prevention and early intervention of maternal depressive symptoms (O’Connor, et al., 2014).
Acknowledgments
This research was made possible by grants from the Canadian Institutes of Health Research, the March of Dimes Foundation and the Fonds de Research du Quebec. The MAVAN project has been supported by funding from the McGill Faculty of Medicine, the Blema & Arnold Steinberg Family Foundation, and the Canadian Institutes for Health Research
We would like to thank all members and participants of the Maternal Adversity, Vulnerability, and Neurodevelopment (MAVAN) project for their time and commitment to this research. We would also like to thank David Brownlee, Vincent Jolivet, Amber Rider, Patricia Szymkow, Keith Widaman, and Michael Pluess for their contributions.
Appendix A
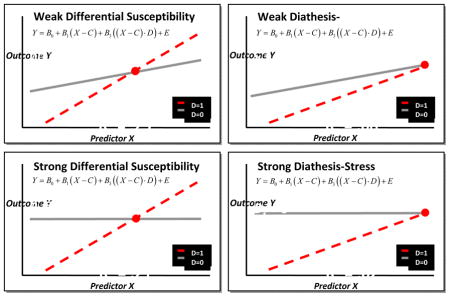
Example representation of confirmatory analysis, by Michael Pluess.
Appendix B
IBQ-R factor loadings at 3 months(N=328) and 6 months(N=418)
| Scale | Negative Emotionality | Positive Emotionality | ||
|---|---|---|---|---|
|
| ||||
| 3M | 6M | 3M | 6M | |
| Activity Level | .47 | .42 | .34 | .32 |
| Distress to Limitations | .78 | .78 | ||
| Fear | .33 | .39 | ||
| Duration of orienting | .69 | .56 | ||
| Smile and Laugh | .68 | .67 | ||
| High Pleasure | .67 | .64 | ||
| Low Pleasure | .64 | .63 | ||
| Soothability | −.39 | −.40 | .30 | .26 |
| Falling Reactivity | −.62 | −.60 | ||
| Cuddliness | −.41 | −.48 | .31 | |
| Perceptual Sensitivity | .60 | .60 | ||
| Sadness | .64 | .67 | ||
| Approach | .67 | .57 | ||
| Vocal Reactivity | .78 | .72 | ||
Appendix C
ECBQ-R factor loadings at 18 months(N=405) and 36 months(N=370)
| Scale | Negative Emotionality | Surgency-Extraversion | Regulation | |||
|---|---|---|---|---|---|---|
|
| ||||||
| 18M | 36M | 18M | 36M | 18M | 36M | |
| Activity Level | .76 | .60 | ||||
| Attention Focusing | .39 | .40 | ||||
| Attention Shifting | .58 | .70 | ||||
| Cuddliness | .52 | .54 | ||||
| Discomfort | .74 | .71 | ||||
| Fear | .78 | .64 | ||||
| Frustration | .52 | .55 | ||||
| High-Intensity Pleasure | .58 | .62 | ||||
| Impulsivity | −.33 | .58 | ||||
| Inhibitory Control | −.49 | −.32 | .39 | .47 | ||
| Low-Intensity Pleasure | .69 | .75 | ||||
| Motor Activation | .59 | .52 | ||||
| Perceptual Sensitivity | .39 | .53 | .52 | .41 | ||
| Sadness | .60 | .56 | ||||
| Shyness | .47 | .39 | −.44 | |||
| Sociability | .50 | .35 | .35 | |||
| Soothability | −.51 | −.31 | .32 | .51 | ||
Appendix D
Spearman correlation matrix
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Site | 1 | .08 | .14*** | .19*** | −.09* | .03 | −.05 | −.07 | .04 | .04 | .08 | −.14** | −.04 | .20*** | .10 | .12* | −.09 | −.02 |
| 2. Gender | 1 | .03 | .01 | .01 | .03 | .02 | .04 | .07 | .06 | .09 | −.06 | .04 | .03 | .09 | .10* | −.05 | .00 | |
| 3. MaternalAge | 1 | .34*** | −.02 | −.13** | −.17*** | −.15** | .05 | .08 | .09 | −.11* | .00 | .10* | .03 | −.10* | −.21*** | −.13* | ||
| 4. SES (Maternal Education) | 1 | .10* | −.32*** | −.33*** | −.30*** | .06 | .07 | .11 | −.05 | −.00 | −.02 | −.08 | −.12* | −.31*** | −.23*** | |||
| 5. Alcohol During pregnancy | 1 | −.01 | −.03 | −.06 | −.04 | .06 | .02 | −.01 | .00 | −.07 | −.03 | −.02 | .01 | −.01 | ||||
| 6. Depression Prenatal | 1 | .62*** | .50*** | −.02 | −.09 | −.08 | −.14** | .06 | .05 | .15** | .21*** | .24*** | .28*** | |||||
| 7. Depression 6 months | 1 | .65*** | −.08 | −.02 | −.06 | −.08 | .11* | .03 | .16** | .24*** | .32*** | .33*** | ||||||
| 8. Depression 12 months | 1 | −.05 | −.08 | −.09 | −.05 | −.05 | .01 | .16** | .25*** | .34*** | .31*** | |||||||
| 9.5-HTTLPR | 1 | .00 | .67*** | .37*** | .03* | .05 | −.04 | .04 | .05 | −.00 | ||||||||
| 10. DRD4 | 1 | .73*** | −.11* | .47*** | .04 | −.06 | −.05 | .01 | .01 | |||||||||
| 11. Multilocus genetic score | 1 | .19** | .35*** | .11 | −.14 | −.01 | .02 | −.02 | ||||||||||
| 12. Mother’s 5-HTTLPR | 1 | −.07 | −.08 | −.18** | −.15** | −.01 | −.07 | |||||||||||
| 13. Mother’s DRD4 | 1 | .03 | −.04 | −.01 | −.01 | −.00 | ||||||||||||
| 14. ChildBirth Weight | 1 | .02 | −.05 | −.02 | .01 | |||||||||||||
| 15. Child NE at 3 months | 1 | .67*** | .24*** | .30*** | ||||||||||||||
| 16. Child NE at 6months | 1 | .37*** | .36*** | |||||||||||||||
| 17. Child NE at 18 months | 1 | .62*** | ||||||||||||||||
| 18. Child NE at 36 months | 1 |
p< 0.05
p< 0.01
p< 0.001
References
- Atella LD, DiPietro JA, Smith BA, St James-Roberts I. More than meets the eye: Parental and infant contributors to maternal and paternal reports of early infant difficultness. Parenting: Science and Practice. 2003;3:265–284. doi: 10.1207/s15327922par0304_1. [DOI] [Google Scholar]
- Auerbach J, Geller V, Lezer S, Shinwell E, Belmaker RH, Levine J, Ebstein RP. Dopamine D4 receptor (DRD4) and serotonin transporter promoter (5-HTTLPR) polymorphisms in the determination of temperament in 2-month-old infants. Molecular Psychiatry. 1999;4:369–373. doi: 10.1038/sj.mp.4000531. [DOI] [PubMed] [Google Scholar]
- Auerbach JG, Faroy M, Ebstein R, Kahana M, Levine J. The association of the dopamine D4 receptor gene (DRD4) and the serotonin transporter promoter gene (5-HTTLPR) with temperament and 12-month-old infants. Journal of Child Psychology and Psychiatry. 2001b;42:777–783. doi: 10.1111/1469-7610.00774. [DOI] [PubMed] [Google Scholar]
- Auerbach JG, Benjamin J, Faroy M, Geller V, Ebstein R. DRD4 related to infant attention and information processing: a developmental link to ADHD? Psychiatric genetics. 2001a;11:31–35. doi: 10.1097/00041444-200103000-00006. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van Ijzendoorn MH. Differential susceptibility to rearing environment depending on dopamine-related genes: New evidence and a meta-analysis. Development and Psychopathology. 2011;23:39–52. doi: 10.1017/S0954579410000635. [DOI] [PubMed] [Google Scholar]
- Barker DJP. The developmental origins of adult disease. Journal of the American College of Nutrition. 2004;23:588S–595S. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- Bayley N. Bayley scales of infant development. 2. San Antonio, Texas: Psychological Corp; 1993. [Google Scholar]
- Belsky J, Beaver KM. Cumulative-genetic plasticity, parenting and adolescent self-regulation. The Journal of Child Psychology and Psychiatry. 2011;52:619–626. doi: 10.1111/j.1469-7610.2010.02327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16:300–304. doi: 10.1111/j.1467-8721.2007.00525.x. [DOI] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M, Widaman KF. Confirmatory and competitive evaluation of alternative gene-environment interaction hypotheses. Journal of Child Psychology and Psychiatry. 2013;54:1135–1143. doi: 10.1111/jcpp.12075. [DOI] [PubMed] [Google Scholar]
- Blair J, Mitchell D, Blair K. The Psychopath: Emotion and the Brain. Oxford: Blackwell Publishing; 2005. [Google Scholar]
- Bouchard TJ. Genes, environment, and personality. Science-New York Then Washington. 1994:1700–1700. doi: 10.1126/science.8209250. [DOI] [PubMed] [Google Scholar]
- Bouvette-Turcot AA, Fleming A, Wazana A, Sokolowski M, Gaudreau H, Gonzalez A, … Meaney MJ. Maternal childhood adversity and child temperament: An association moderated by child SLC6A4 genotype. Genes Brain and Behavior. 2015;14:229–237. doi: 10.1111/gbb.12205. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary–developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;2:271–301x. doi: 10.1017/s0954579405050145. x.doi.org/10.1017/S0954579405050145. [DOI] [PubMed] [Google Scholar]
- Braithwaite EC, Ramchandani PG, O’Connor TG, van IJzendoorn MH, Bakermans-Kranenburg MJ, Glover V, … Murphy SE. No moderating effect of 5-HTTLPR on associations between antenatal anxiety and infant behavior. Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52(5):519–526. doi: 10.1016/j.jaac.2013.02.010. http://dx.doi.org/10.1016/j.jaac.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nature neuroscience. 2007;10(9):1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Canli T, Omura K, Haas BW, Fallgatter A, Constable RT, Lesch KP. Beyond affect: a role for genetic variation of the serotonin transporter in neural activation during a cognitive attention task. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(34):12224–12229. doi: 10.1073/pnas.0503880102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffit T, Taylor A, Craig I, Harrington H, … Poulton R. Influences of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chang FM, Kidd JR, Livak KJ, Pakstis AJ, Kidd KK. The world-wide distribution of allele frequencies at the human dopamine D4 receptor locus. Human genetics. 1996;98(1):91–101. doi: 10.1007/s004390050166. [DOI] [PubMed] [Google Scholar]
- Cools R, Nakamura K, Daw ND. Serotonin and dopamine: unifying affective, activational, and decision functions. Neuropsychopharmacology. 2011;36(1):98–113. doi: 10.1038/npp.2010.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper WO, Willy ME, Pont SJ, Ray WA. Increasing use of antidepressants in pregnancy. American journal of obstetrics and gynecology. 2007;196(6):544–e1. doi: 10.1016/j.ajog.2007.01.033. http://dx.doi.org/10.1016/j.ajog.2007.01.033. [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, Sandman CA. Prenatal exposure to maternal depression and cortisol influences infant temperament. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46(6):737–746. doi: 10.1097/chi.0b013e318047b775. http://dx.doi.org/10.1097/chi.0b013e318047b775. [DOI] [PubMed] [Google Scholar]
- Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Development. 2010;81:131–148. doi: 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Snidman N, Wadhwa PD, Glynn LM, Dunkel Schetter C, Sandman CA. Prenatal maternal anxiety and depression predict negative behavioral reactivity in infancy. Infancy. 2004;6:319–331. doi: 10.1207/s15327078in0603_1. [DOI] [Google Scholar]
- Degnan KA, Almas AN, Fox NA. Temperament and the environment in the etiology of childhood anxiety. Journal of Child Psychology and Psychiatry. 2010;51(4):497–517. doi: 10.1111/j.1469-7610.2010.02228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca A, Rizzardi M, Buccino A, Alessandroni R, Salvioli GP, Filograsso N, … Dallapiccola B. Association of dopamine D4 receptor (DRD4) exon III repeat polymorphism with temperament in 3-year-old infants. Neurogenetics. 2003;4:207–212. doi: 10.1007/s10048-003-0146-z. [DOI] [PubMed] [Google Scholar]
- Dray A, Gonye TJ, Oakley NR, Tanner Ti. Evidence for the existence of a raphe projection to the substantia nigra in rat. Brain Res. 1976;113:45–57. doi: 10.1016/0006-8993(76)90005-6. [DOI] [PubMed] [Google Scholar]
- Duncan LE. Paying attention to all results, positive and negative. Journal of the American Academy of Child and Adolecent Psychiatry. 2013;52:462–465. doi: 10.1016/j.jaac.2013.02.007. http://dx.doi.org/10.1016/j.jaac.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Levine J, Geller V, Auerbach J, Gritsenko I, Belmaker RH. dopamine D4 receptor and serotonin transporter promoter in the determination of neonatal temperament. Molecular Psychiatry. 1998;3(3) doi: 10.1038/sj.mp.4000363. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Valiente C, Spinrad TL, Cumberland A, Liew J, Reiser M, … Losoya SH. Longitudinal relations of children’s effortful control, impulsivity, and negative emotionality to their externalizing, internalizing, and co-occurring behavior problems. Developmental psychology. 2009;45(4):988. doi: 10.1037/a0016213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, Van IJzendoorn MH. Differential susceptibility to the environment: An evolutionary–neurodevelopmental theory. Development and Psychopathology. 2011;23:7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Field T. Prenatal depression effects on early development: A review. Infant Behaviour and Development. 2011;34:1–14. doi: 10.1016/j.infbeh.2010.09.008. http://dx.doi.org/10.1016/j.infbeh.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Field T, Diego M, Dieter J, Hernandez-Reif M, Schanberg S, Kuhn C, … Bendell D. Prenatal depression effects on the fetus and the newborn. Infant Behavior & Development. 2004;27:216–229. http://dx.doi.org/10.1016/j.infbeh.2003.09.010. [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral Influences across the first four years of life. Child development. 2001;72(1):1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Fox NA, Nichols KE, Henderson HA, Rubin K, Schmidt L, Hamer D, … Pine DS. Evidence for a gene-environment interaction in predicting behavioral inhibition in middle childhood. Psychological Science. 2005;16(12):921–926. doi: 10.1111/j.1467-9280.2005.01637.x. [DOI] [PubMed] [Google Scholar]
- Gartstein MA, Rothbart MK. Studying infant temperament via the Revised Infant Behavior Questionnaire. Infant Behavior & Development. 2003;26(1):64–86. http://dx.doi.org/10.1016/S0163-6383(02)00169-8. [Google Scholar]
- Geva R, Feldman R. A neurobiological model for the effects of early brainstem functioning on the development of behavior and emotion regulation in infants: Implications for prenatal and perinatal risk. Journal of Child Psychology and Psychiatry. 2008;49:1031–1041. doi: 10.1111/j.1469-7610.2008.01918.x. [DOI] [PubMed] [Google Scholar]
- Glover V. Annual research review: Prenatal stress and the origins of psychopathology: An evolutionary perspective. Journal of Child Psychology and Psychiatry. 2011;52:356–367. doi: 10.1111/j.1469-7610.2011.02371.x. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Lemery KS. Linking temperamental fearfulness and anxiety symptoms: A behavior–genetic perspective. Biological Psychiatry. 2000;48(12):1199–1209. doi: 10.1016/s0006-3223(00)01003-9. http://dx.doi.org/10.1016/S0006-3223(00)01003-9. [DOI] [PubMed] [Google Scholar]
- Gonda X, Fountoulakis KN, Juhasz G, Rihmer Z, Lazary J, Laszik A, Akiskal HS, Bagdy G. Association of the s allele of the 5-HTTLPR with neuroticism-related traits and temperaments in a psychiatrically healthy population. Eur Arch Psychiarty Clin Neurosci. 2009;259:106–113. doi: 10.1007/s00406-008-0842-7. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Rouse MH, Connell AM, Broth MR, Hall CM, Heyward D. Maternal depression and child psychopathology: a meta-analytic review. Clin Child Fam Psychol Rev. 2011;11:1–27. doi: 10.1007/s10567-010-0080-1. [DOI] [PubMed] [Google Scholar]
- Green Gordon, et al. Prenatal depression and polygenic risk: Early contributors of childhood negative emotionality. Poster session presented at the World Congress on Brain, Behavior and Emotions; Montreal, Canada. 2014. Apr, [Google Scholar]
- Grant KA, Sandman CA, Wing DA, Dmitrieva J, Davis EP. Prenatal programming of postnatal susceptibility to memory impairments a developmental double jeopardy. Psychological Science. 2015;26:1054–1062. doi: 10.1177/0956797615580299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends in cognitive sciences. 2006;10(4):182–191. doi: 10.1016/j.tics.2006.02.011. http://dx.doi.org/10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, … Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297(5580):400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hayden EP, Dougherty LR, Maloney B, Durbin CE, Olino TM, Nurnberger JI, Jr, … Klein DN. Temperamental fearfulness in childhood and the serotonin transporter promoter region polymorphism: a multimethod association study. Psychiatric genetics. 2007;17(3):135–142. doi: 10.1097/YPG.0b013e3280147847. [DOI] [PubMed] [Google Scholar]
- Hayden EP, Klein DN, Sheikh HI, Olino TM, Dougherty LR, Dyson MW, … Singh SM. The serotonin transporter promoter polymorphism and childhood positive and negative emotionality. Emotion. 2010;10(5):696. doi: 10.1037/a0019527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J, Breen G, Quinn J, Tibu F, Sharp H, Pickles A. Evidence for interplay between genes and maternal stress in utero: monoamine oxidase A polymorphism moderates effects of life events during pregnancy on infant negative emotionality at 5 weeks. Genes, Brain and Behavior. 2013;12:388–396. doi: 10.1111/gbb.12033. [DOI] [PubMed] [Google Scholar]
- Hoaglin DC, Welsch RE. The Hat Matrix in Regression and ANOVA. The American Statistician. 1978;32:17–22. doi: 10.2307/2683469. [DOI] [Google Scholar]
- Holmboe K, Nemoda Z, Fearon RMP, Sasvari-Szekely M, Johnson MH. Dopamine D4 receptor and serotonin transporter gene effects on the longitudinal development of infant temperament. Genes, Brain and Behavior. 2011;10(5):513–522. doi: 10.1111/j.1601-183X.2010.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, … Goldman D. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive-disorder. The American Journal of Human Genetics. 2006;78:815–826. doi: 10.1086/503850. http://dx.doi.org/10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JS, Mezulis AH, Abramson LY. The ABCs of depression: integrating affective, biological, and cognitive models to explain the emergence of the gender difference in depression. Psychological review. 2008;115(2):291. doi: 10.1037/0033-295X.115.2.291. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Philibert RA, Berry RA. Interplay of genes and early mother-child relationship in the development of self-regulation from toddler to preschool age. Journal of Child Psychology and Psychiatry. 2009;50:1331–1338. doi: 10.1111/j.1469-7610.2008.02050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MS, Wilkins R, Gouler L, Seguin L, Lydon J, Kahn SR, … Platt Investigating socio-economic disparities in preterm birth: Evidence for selective study participants and selection bias. Paediatric and Perinatal Epidemiology. 2009;23:301–309. doi: 10.1111/j.1365-3016.2009.01042.x. [DOI] [PubMed] [Google Scholar]
- Lakatos K, Nemoda Z, Birkas E, Ronai Z, Kovacs E, Ney K, … Gervai J. Association of D4 dopamine receptor gene and serotonin transporter promoter polymorphisms with infants’ response to novelty. Molecular Psychiatry. 2003;8:90–97. doi: 10.1038/sj.mp.4001212. [DOI] [PubMed] [Google Scholar]
- Lemery KS, Goldsmith HH, Klinnert MD, Mrazek DA. Developmental models of infant and childhood temperament. Developmental Psychology. 1999;35:189–204. doi: 10.1037/0012-1649.35.1.189. [DOI] [PubMed] [Google Scholar]
- Lichter JB, Barr CL, Kennedy JL, Van Tol HH, Kidd KK, Livak KJ. A hypervariable segment in the human dopamine receptor D4 (DRD4) gene. Human Molecular Genetics. 1993;2:767–773. doi: 10.1093/hmg/2.6.767. [DOI] [PubMed] [Google Scholar]
- Masarik AS, Conger RD, Donnellan MB, Stallings MC, Martin MJ, Schofield TJ, … Widaman KF. For better and for worse: Genes and parenting interact to predict future behavior in romantic relationships. Journal of Family Psychology. 2014;3:357–367. doi: 10.1037/a0036818. [DOI] [PubMed] [Google Scholar]
- McGrath JM, Records K, Rice M. Maternal depression and infant temperament characteristics. Infant Behavior and Development. 2008;31(1):71–80. doi: 10.1016/j.infbeh.2007.07.001. http://dx.doi.org/10.1016/j.infbeh.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Epigenetics and the biological definition of gene x environment interactions. Child Development. 2009;81:41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Oak JN, Oldenhof J, Van Tol HH. The dopamine D(4) receptor: one decade of research. European journal of pharmacology. 2000;405(1):303–327. doi: 10.1016/s0014-2999(00)00562-8. http://dx.doi.org/10.1016/S0014-2999(00)00562-8. [DOI] [PubMed] [Google Scholar]
- Oak JN, Oldenhof J, Van Tol HH. The dopamine D< sub> 4</sub> receptor: one decade of research. European Journal of Pharmacology. 2000;405:303–327. doi: 10.1016/s0014-2999(00)00562-8. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Monk C, Fitelson EM. Practitioner Review: Maternal mood in pregnancy and child development–implications for child psychology and psychiatry. Journal of Child Psychology and Psychiatry. 2014;55(2):99–111. doi: 10.1111/jcpp.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor T, Heron J, Glover V. Antenatal anxiety predicts child behavioural/ emotional problems independently of postnatal depression. J AM Acaad Child Adolesc Psychiatry. 2002a;41:1470–1477. doi: 10.1097/00004583-200212000-00019. http://dx.doi.org/10.1097/00004583-200212000-00019. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children’s behavioural/emotional problems at 4 years Report from the Avon Longitudinal Study of Parents and Children. The British Journal of Psychiatry. 2002b;180:502–508. doi: 10.1192/bjp.180.6.502. [DOI] [PubMed] [Google Scholar]
- O’Donnell K, Gaudreau H, Colalillo S, Steiner M, Atkinson L, Moss E, … Meaney M. The Maternal Adversity Vulnerability and Neurodevelopment (MAVAN) Project: Theory and methodology. The Canadian Journal of Psychiatry. 2014;9:497. doi: 10.1177/070674371405900906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odgerel Z, Talati1 A, Hamilton SP, Levinson DF, Weissman MM. Genotyping serotonin transporter polymorphisms 5-HTTLPR and rs25531 in European- and African-American subjects from the National Institute of Mental Health’s Collaborative Center for Genomic Studies. Translational Psychiatry. 2013;3:e307. doi: 10.1038/tp.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parade SH, Leerkes EM. The reliability and validity of the Infant Behaviour Questionnaire-Revised. Infant Behavior and Development. 2008;31:637–646. doi: 10.1016/j.infbeh.2008.07.009. http://dx.doi.org/10.1016/j.infbeh.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli-Pott U, Friedl S, Hinney A, Hebebrand J. Serotonin transporter gene polymorphism (5-HTTLPR), environmental conditions, and developing negative emotionality and fear in early childhood. J Neural Transm. 2009;116:503–512. doi: 10.1007/s00702-008-0171-z. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, … Weinberger DR. 5-HTTLPR polymorphism impacts human cingulated-amygdala interactions: A genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Phillips BM, Lonigan CJ, Driscoll K, Hooe ES. Positive and negative affectivity in children: A multitrait-multimethod investigation. Journal of Clinical Child and Adolescent Psychology. 2002;31(4):465–479. doi: 10.1207/S15374424JCCP3104_6. [DOI] [PubMed] [Google Scholar]
- Plomin R. Child development and molecular genetics: 14 years later. Child development. 2013;84(1):104–120. doi: 10.1111/j.1467-8624.2012.01757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluess M. Individual differences in environmental sensitivity. Child Development Perspectives. 2015;9:138–143. doi: 10.1111/cdep.12120. [DOI] [Google Scholar]
- Pluess M, Belsky J. Prenatal programming of postnatal plasticity? Development and Psychopathology. 2011;23:29–38. doi: 10.1017/S0954579410000623. [DOI] [PubMed] [Google Scholar]
- Pluess M, Belsky J, Neuman RJ. Prenatal smoking and attention-deficit/hyperactivity disorder: DRD4–7R as a plasticity gene. Biological Psychiatry. 2009;66:5–6. doi: 10.1016/j.biopsych.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Pluess M, Velders PF, Belsky J, van IJzendoorn MH, Bakermns-Kranenburg MJ, Jaddoe VWV, … Tiemeier H. Serotonin transporter polymorphism moderates effects of prenatal maternal anxiety on infant negative emotionality. Biological Psychiatry. 2011;69:520–525. doi: 10.1016/j.biopsych.2010.10.006. http://dx.doi.org/10.1016/j.biopsych.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Praschak-Rieder N, Kennedy J, Wilson AA, Hussey D, Boovariwala A, Willeit M, … Meyer JH. Novel 5-HTTLPR allele associates with higher serotonin transporter binding in Putamen: A [11C] DASB positron emission tomography study. Biological Psychiatry. 2007;62:327–331. doi: 10.1016/j.biopsych.2006.09.022. http://dx.doi.org/10.1016/j.biopsych.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Putnam SP, Ellis LK, Rothbart MK. The structure of temperament from infancy through adolescence. Advances in Research on Temperament. 2001:165–182. [Google Scholar]
- Putnam SP, Gartstein MA, Rothbart MK. Measurement of Fine-Grained Aspects of Toddler Temperament: The Early Childhood Behavior Questionnaire. Infant Behavior & Development. 2006;29:386–401. doi: 10.1016/j.infbeh.2006.01.004. http://dx.doi.org/10.1016/j.infbeh.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam SP, Rothbart MK, Gartstein MA. Homotypic and heterotypic continuity of fine-grained temperament during infancy, toddlerhood, and early childhood. Infant and Child Development. 2008;17:387–405. doi: 10.1002/ICD.582. [DOI] [Google Scholar]
- Qiu A, Anh TT, Li Y, Chen H, Rifkin-Graboi A, Broekman BFP, … Meaney MJ. Prenatal maternal depression alters amygdala functional connectivity in 6-month-old infants. Translational Psychiatry. 2015;5:e508. doi: 10.1038/tp.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychological Measurement 1. 1977:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Rifkin-Graboi A, Bai J, Chen H, Hameed WBR, Sim LW, Tint MT, … Qiu A. Prenatal Maternal Depression Associates with Microstructure of Right Amygdala in Neonates at Birth. Biological psychiatry. 2013;74(11):837–844. doi: 10.1016/j.biopsych.2013.06.019. org/10.1016/j.biopsych.2013.06.019. [DOI] [PubMed] [Google Scholar]
- Rothbart M. Measurement of temperament in infancy. Child Development. 1981;53:569–578. [Google Scholar]
- Sameroff A, Gutman LM, Peck SC. Adaptation among youth facing multiple risks: Prospective research findings. In: Luthar S, editor. Resilience and Vulnerability: Adaptation in Context of Childhood Adversities. New York, NY: Cambridge University Press; 2003. pp. 364–391. [Google Scholar]
- Sandman CA, Buss C, Head K, Davis EP. Fetal exposure to maternal depressive symptoms is associated with cortical thickness in late childhood. Biological Psychiatry. 2015;77:324–334. doi: 10.1016/j.biopsych.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudino KJ. The development of temperament from a behavioural genetics perspective. Advances in Child Development and Behaviour. 2009;37:201–231. doi: 10.1016/S0065-2407(09)03705-7. [DOI] [PubMed] [Google Scholar]
- Seifer R, Sameroff AJ, Barrett LC, Krafchuk E. Infant temperament measured by multiple observations and mothers report. Child Development. 1994;65:1478–1490. doi: 10.1111/j.1467-8624.1994.tb00830.x. [DOI] [PubMed] [Google Scholar]
- Sen S, Burmeister M, Ghosh D. Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxiety-related personality traits. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2004;127(1):85–89. doi: 10.1002/ajmg.b.20158. [DOI] [PubMed] [Google Scholar]
- Silveira PP, Gaudreau H, Atkinson L, Fleming AS, Sokolowski MB, Steiner M, … Dubé L. Genetic Differential Susceptibility to Socioeconomic Status and Childhood Obesogenic Behavior: Why Targeted Prevention May Be the Best Societal Investment. JAMA pediatrics. 2016;170:359–364. doi: 10.1001/jamapediatrics.2015.4253. [DOI] [PubMed] [Google Scholar]
- Silveira PP, Portella AK, Kennedy JL, Gaudreau H, Davis C, Steiner M, … Loucks EB. Association between the seven-repeat allele of the dopamine-4 receptor gene (DRD4) and spontaneous food intake in pre-school children. Appetite. 2014;73:15–22. doi: 10.1016/j.appet.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HJ, Sheikh HI, Dyson MW, Olino TM, Laptook RS, Durbin CE, … Klein DN. Parenting and child DRD4 genotype interact to predict children’s early emerging effortful control. Child Development. 2012;83:1932–1944. doi: 10.1111/j.1467-8624.2012.01818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Oades RD, Psychogiou L, Chen W, Franke B, Buitelaar J, … Faraone SV. Dopamine and serotonin transporter genotypes moderate sensitivity to maternal expressed emotion: the case of conduct and emotional problems in attention deficit/hyperactivity disorder. Journal of Child Psychology and Psychiatry. 2009;50(9):1052–1063. doi: 10.1111/j.1469-7610.2009.02095.x. [DOI] [PubMed] [Google Scholar]
- Stevens J. Applied multivariate statistics for the social sciences. Hillsdale, NJ: Lawrence Eribaum; 1986. [Google Scholar]
- Susman EJ, Schmeelk KH, Ponirakis A, Gariepy JL. Maternal prenatal, postpartum, and concurrent stressorsand temperament in 3-year-olds: A person and variable analysis. Developmental Psychopathology. 2001;13:629–652. doi: 10.1017/s0954579401003121. http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- Tsai CT. Involvement of serotonin in mediation of inhibition of substantia nigra neurons by noxious stimuli. Brain Res Bull. 1989;23:121–127. doi: 10.1016/0361-9230(89)90170-6. [DOI] [PubMed] [Google Scholar]
- Uher R. The implications of gene-environment interactions in depression: Will cause inform cure? Molecular Psychiatry. 2008;13:1070–1078. doi: 10.1038/mp.2008.92. [DOI] [PubMed] [Google Scholar]
- Vadovicova K, Gasparotti R. Reward and adversity processing circuits: their competition and interaction with dopamine and serotonin signalling. Science Open Research. 2014 doi: 10.14293/S2199-1006.1.SOR-LIFE.AEKZPZ.v12. [DOI] [Google Scholar]
- van Batenburg-Eddes T, Brion MJ, Henrichs J, Jaddoe VWV, Hofman A, Verhulst FC, … Tiemeier H. Parental depressive and anxiety symptoms during pregnancy and attention problems in children: A cross-cohort consistency study. Journal of Child Psychology and Psychiatry. 2013;54:591–600. doi: 10.1111/jcpp.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Statistics in Medicine. 1999;18:681–694. doi: 10.1002/(sici)1097-0258(19990330)18:6<681::aid-sim71>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- van Buuren S, Groothuis-Oudshoorn K. MICE: Multivariate Imputation by Chained Equations in R. Journal of Statistical Software. 2011;45:1–67. [Google Scholar]
- de Weerth C, Hees Y, Buitelaar JK. Prenatal maternal cortisol levels and infant behaviour during the first 5 months. Early Human Development. 2003;74:139–151. doi: 10.1016/s0378-3782(03)00088-4. http://dx.doi.org/10.1016/S0378-3782(03)00088-4. [DOI] [PubMed] [Google Scholar]
- Widaman KF, Helm JL, Castro-Schilo L, Pluess M, Stallings MC, Belsky J. Distinguishing ordinal and disordinal interactions. Psychological Methods. 2012;17:615–622. doi: 10.1037/a0030003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weikum WM, Brain U, Chau CM, Grunau RE, Boyce WT, Diamond A, Oberlander TF. Prenatal serotonin reuptake inhibitor (SRI) antidepressant exposure and serotonin transporter promoter genotype (SLC6A4) influence executive functions at 6 years of age. Frontiers in cellular neuroscience. 2013:7. doi: 10.3389/fncel.2013.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MY, Day NE, Luan JA, Chan KP, Wareham NJ. The detection of gene-environment interaction for continuous traits: should we deal with measurement error by bigger studies or better measurement? Int J Epidemiol. 2003;2:51–57. doi: 10.1093/ije/dyg002. [DOI] [PubMed] [Google Scholar]
- Zohsel K, Buchmann AF, Blomeyer D, Hohm E, Schmidt MH, Esser G, … Laucht M. Mothers’ prenatal stress and their children’s antisocial outcomes–a moderating role for the Dopamine D4 Receptor (DRD4) gene. Journal of child psychology and psychiatry. 2014;55(1):69–76. doi: 10.1111/jcpp.12138. [DOI] [PubMed] [Google Scholar]