Summary
Of the thousands of long noncoding RNAs expressed in embryonic stem cells (ESCs), few have known roles and fewer have been functionally implicated in the regulation of self-renewal and pluripotency, or the reprogramming of somatic cells to the pluripotent state. In ESCs, Cyrano is a stably expressed long intergenic noncoding RNA with no previously assigned role. We demonstrate that Cyrano contributes to ESC maintenance, as its depletion results in the loss of hallmarks of self-renewal. Delineation of Cyrano's network through transcriptomics revealed widespread effects on signaling pathways and gene expression networks that contribute to ESC maintenance. Cyrano shares unique sequence complementarity with the differentiation-associated microRNA, mir-7, and mir-7 overexpression reduces expression of a key self-renewal factor to a similar extent as Cyrano knockdown. This suggests that Cyrano functions to restrain the action of mir-7. Altogether, we provide a view into the multifaceted function of Cyrano in ESC maintenance.
Keywords: Cyrano, lncRNA, embryonic stem cells, self-renewal, cell survival, mir-7, nanog
Graphical Abstract
Highlights
-
•
Cyrano supports ESC self-renewal
-
•
Study reveals complexity of Cyrano's function including in cell survival and adhesion
-
•
Cyrano antagonizes mir-7 activity in ESCs
In this article, Magnuson and colleagues demonstrate a supportive role for the long noncoding RNA Cyrano in the maintenance of self-renewing embryonic stem cell colonies, including through antagonism of mir-7 activity. Mechanistically, Cyrano's role is complex, and its depletion results in an inability to self-renew due to aberrant gene expression, defects in cell adhesion and increased cell death.
Introduction
Pluripotent stem cells hold significant therapeutic potential in the context of degenerative disease. To use pluripotent cells in transplantation therapies, a thorough understanding of the molecular mechanisms that regulate immortality through self-renewal becomes a key requirement. The specialized cells that arise from pluripotent cells during development do so through temporal restrictions in their cellular plasticity. The blueprint behind this cell-fate determination is the transcriptome, whose status is based upon regulatory networks consisting of epigenetic machinery, transcription factors, and noncoding RNAs (ncRNAs).
Although well studied, protein-coding sequences account for only approximately 2% of the genome and 28%–40% of the transcriptome in humans (Alexander et al., 2010, Harrow et al., 2012). This suggests that non-protein-coding RNAs may have heretofore unidentified functions. Indeed, it has been demonstrated that ncRNAs are abundant regulatory components of vertebrate transcriptomes. This is particularly evident for long noncoding RNAs (lncRNAs; >200 nt long) and the noncoding class of small RNAs termed microRNAs (miRNAs), which influence numerous biological processes including proliferation, apoptosis, and differentiation.
Mechanistically, lncRNAs have emerged as multifaceted regulators of various cellular processes, with roles that include influencing epigenetic landscapes, transcriptional circuitry, and post-transcriptional regulatory processes (Rinn and Chang, 2012, Wang and Chang, 2011). While lncRNAs generally have no significant open reading frame, many share characteristics of mRNAs such as 5′ capping, splicing, and polyadenylation (Cabili et al., 2011, Guttman et al., 2010). Typically, tissue-specific expression of lncRNAs is more demarcated than that of mRNAs, and it follows that several lncRNAs have been implicated in organ development and cell-fate specification (Fatica and Bozzoni, 2014). These data point to the need for the elucidation of lncRNA function in both specialized and unspecialized cell types.
To date, thousands of lncRNAs have been identified through transcriptomics, particularly RNA sequencing (RNA-seq), in embryonic stem cells (ESCs) (Cabili et al., 2011, Guttman et al., 2010), yet well-defined biological functions are known for very few. However, loss-of-function approaches can provide insight into roles for lncRNAs in the maintenance of self-renewal and pluripotency, reprogramming, and differentiation (Guttman et al., 2011, Kelley and Rinn, 2012, Kim et al., 2015, Lin et al., 2014, Loewer et al., 2010). Such functional characterization would address precise roles for individual lncRNAs in pluripotent stem cell maintenance.
Cyrano (linc-oip5, 1700020I14Rik) is a long intergenic ncRNA (lincRNA) transcribed in mouse ESCs (Chew et al., 2013, Guttman et al., 2010, Ulitsky et al., 2011) that was first characterized in zebrafish (Ulitsky et al., 2011). In zebrafish, it is a key regulator that functions in brain, eye, and nasal development (Ulitsky et al., 2011). This is at least in part mediated by a short region of high sequence conservation among vertebrate genomes that is critical for function. Rescue experiments in zebrafish utilizing higher-order orthologs provided the first insight that Cyrano may have a functional role in mice (Ulitsky et al., 2011).
miRNAs, which are much shorter ncRNAs (approximately 22 nt), have also been assigned regulatory roles in numerous biological processes. Historically, miRNAs have been thought to function through pairing with complementary sequences in the 3′ UTR of target mRNAs to repress gene expression at the post-transcriptional level (Bartel, 2009). More recently, broader miRNA functionality has been recognized. This includes noncanonical binding to non-3′ UTR regions including the coding sequence of target genes, as well as cross-regulatory interactions that exist between miRNAs and lncRNAs to affect either miRNA or lncRNA stability and/or function, and the regulation of downstream targets (Jeggari et al., 2012, Paraskevopoulou et al., 2013).
One such lncRNA/miRNA interaction has been postulated between Cyrano and mir-7 (Ulitsky et al., 2011). At the cellular level, mir-7 is associated with differentiation (Cui et al., 2013, Kong et al., 2012, Nguyen et al., 2010), with its levels increasing during neural specification from neural stem cells (Cui et al., 2013). In various cellular contexts, it acts by inhibiting receptor-mediated signaling pathways, including EGFR and STAT3 signaling, to promote differentiation and modulate cellular adhesion (Kefas et al., 2008, Nguyen et al., 2010, Tazawa et al., 2012, Zhang et al., 2014). Antagonism of mir-7 function, mediated by sequestration and inactivation via molecular sponges or decoy RNAs, is a well-known strategy for moderating its activity on target transcripts. One of the best-studied examples is the circular RNA CD1Ras/CiRS-7, which possesses multiple seed matches to miR-7 (Hansen et al., 2013, Memczak et al., 2013). Sponge-based regulation of miRNA activity is also employed in the ESC regulatory landscape to prevent post-transcriptional degradation of key pluripotency factors including Oct4, Sox2, and Nanog (Wang et al., 2013).
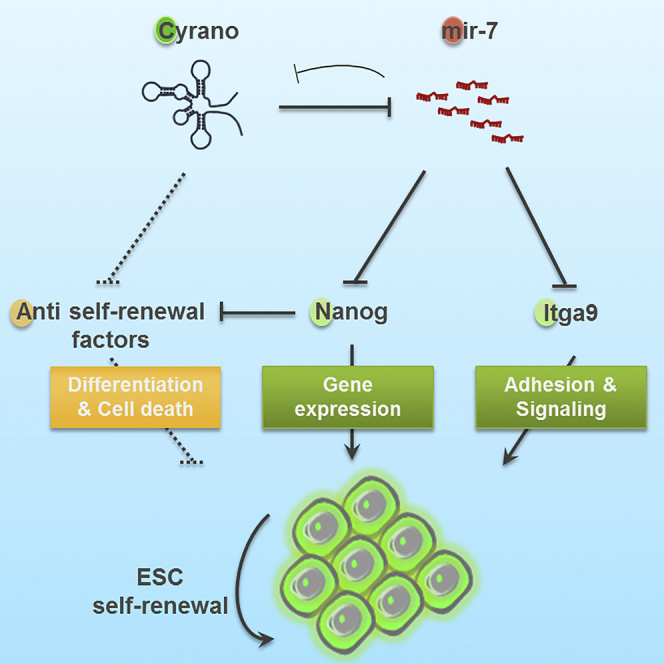
Here, we demonstrate that Cyrano is essential for maintenance of self-renewing ESCs. Our studies revealed that interplay between Cyrano and mir-7 affects key properties including cell adhesion in colony maintenance to support ESC immortality. Importantly, Cyrano depletion disrupts self-renewal signaling and gene expression regulatory networks, particularly the expression of Nanog. Aberrations in these properties including the loss of Nanog expression, cell adhesion, and colony survival to maintain self-renewal capacity are recapitulated in mir-7 gain-of-function experiments. This supports the existence of a competing relationship between mir-7 and Cyrano in ESCs.
Results
lncRNA Cyrano Exhibits Stability and Is Broadly Localized in ESCs
While lncRNAs exhibit a range of localization patterns (Cabili et al., 2015), their basic localization provides preliminary insight into their cellular functions. For instance, nuclear-domain localized lncRNAs, including Xist and Kcnq1ot1, function to silence vast chromatin domains, while the cytoplasmic lncRNA H19 is a primary miRNA precursor (Cai and Cullen, 2007, Keniry et al., 2012).
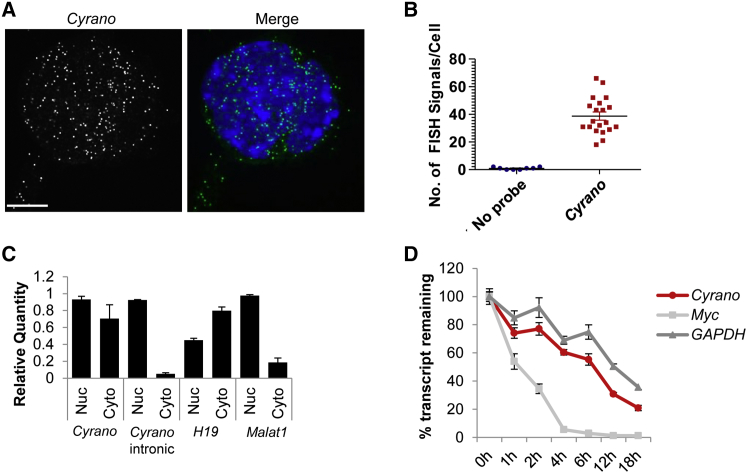
To characterize the function of Cyrano in ESCs, we first used single-molecule fluorescence in situ hybridization (smFISH) and fractionation methods to examine Cyrano's subcellular localization. Through z-stack imaging of multiple ESC lines, smFISH revealed distinct signals in the nucleus and cytoplasm throughout ESC colonies (Figures 1A and S1A–S1D), averaging ∼40 molecules per cell (Figure 1B). This distribution was confirmed by subcellular fractionation comparing the localization of spliced Cyrano to the unspliced nuclear form, cytoplasmic H19 (Keniry et al., 2012) and nuclear speckle-localized Malat1 (Miyagawa et al., 2012) (Figure 1C). Furthermore, ENCODE data from human ESCs (ENCODE Project Consortium, 2012, Yue et al., 2014) showed similar subcellular localization of the unspliced and spliced human ortholog, OIP5-AS1 (Figure S1E). Consistent with the lack of enrichment in either cellular compartment (Clark et al., 2012, Tani et al., 2012), we found that Cyrano displayed moderate stability of t1/2 ∼6 hr in ESCs (Figure 1D). Cyrano's distribution in the cell could indicate that nuclear and cytoplasmic Cyrano pools have distinct functions or that it interacts with proteins that shuttle from the nucleus to the cytoplasm.
Figure 1.
Cyrano Displays Dispersed Subcellular Localization and Exhibits Stability in ESCs
(A) smFISH analysis of a representative ESC colony shows Cyrano localization in the nucleus and cytoplasm of ESCs. Nuclei, blue (DAPI). Scale bar, 10 μm.
(B) Quantitation of Cyrano molecules/ESC.
(C) Subcellular fractionation and qRT-PCR confirms Cyrano's presence in the nucleus and cytoplasm.
(D) Assessment of the stability of Cyrano.
Data are from three independent experiments. Error bars represent SEM. See also Figure S1.
Cyrano Is Required for Maintenance of ESC Self-Renewal
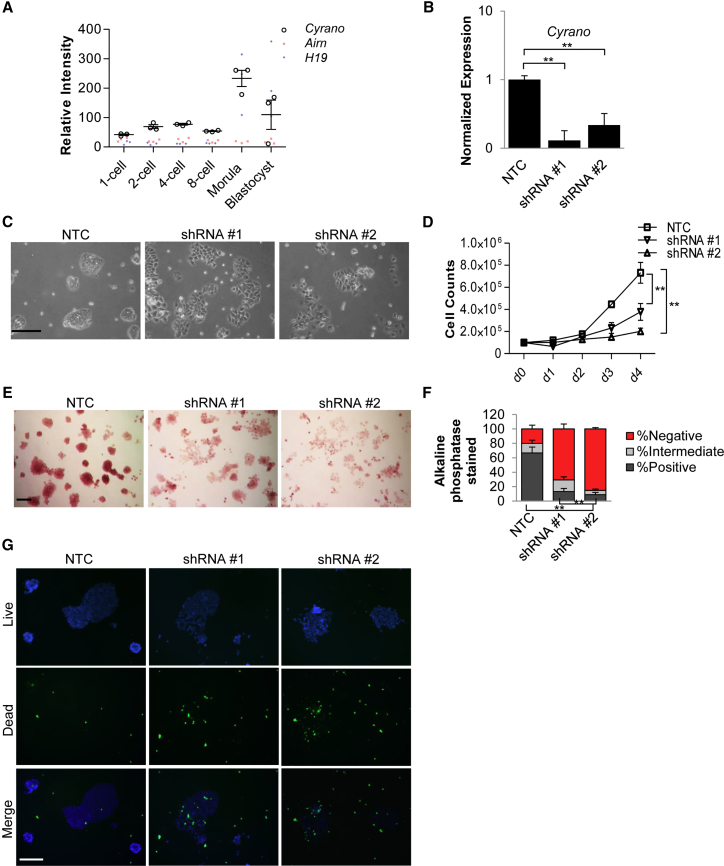
In addition to consistent localization and expression among ESC lines, microarray analysis of early embryonic developmental stages (Xie et al., 2010) revealed an increase in Cyrano expression in morulae and blastocysts relative to two well-studied lncRNAs, H19 and Airn (Figure 2A). Similar results were obtained upon examination of single-cell RNA-seq data (Deng et al., 2014) from ESCs (Figure S2A). ESCs are derived from blastocysts and are an excellent model system for early developmental processes.
Figure 2.
Cyrano Deficiency Impairs ESC Self-Renewal
(A) Expression analysis (GEO: GSE18290) of Cyrano, Airn, and H19 lncRNAs in early development.
(B) qRT-PCR shows significant reduction in Cyrano expression upon KD using independent shRNAs, compared with a nontargeting control. Experiments were performed in triplicate, normalized to GAPDH, with error bars representing 95% CI.
(C) Cyrano KD results in loss of the ESC characteristic colony morphology. Scale bar, 100 μm.
(D) KD of Cyrano results in a decrease in cell numbers as determined by cell counts beginning with plating on day 1 post transfection.
(E and F) Significant reduction in alkaline phosphatase staining of ESC colonies after Cyrano KD. n > 200. Scale bar, 100 μm.
(G) Increases in cell death observed upon Cyrano KD 2 days post transfection. Nuclei (blue, live; green, dead). Scale bar, 200 μm.
Counts were performed in triplicate with error bars representing SD. Data are from three independent experiments. ∗∗p < 0.01. See also Figure S2.
We used short hairpin RNA (shRNA) knockdown (KD) to examine the effect of loss of Cyrano expression in ESCs. Independent shRNAs reproducibly achieved greater than 60%–85% reduction of Cyrano levels (Figures 2B, S2B, and S2C) compared with a nontargeting control (NTC). Within 2–3 days of expression of shRNAs, we found that Cyrano-depleted cells were unable to robustly maintain a typical ESC phenotype of tightly packed cells assembled in a dome-shaped colony in leukemia inhibitory factor (LIF)-containing ESC medium (Figure 2C). Because of possible shortcomings in KD efficiency due to multiple lncRNA splice variants, differential subcellular localization, and possible nontargeting of shRNAs, the loss-of-self-renewal phenotype was confirmed using additional shRNAs (Figures S2C and S2D) and in an independent ESC line (Figure S2E). Specifically, we observed increased numbers of cells floating in the medium and prominent partitioning of cell-cell contacts. This is unlike the standard pluripotent ESC state in which cell-cell boundaries within a colony are difficult to define. Along with this breakdown in the colony maintenance of self-renewing ESCs, we observed a sharp reduction in cell numbers in Cyrano-depleted cells (Figure 2D). Loss of self-renewal was further underscored by a qualitative and quantitative loss of alkaline phosphatase activity (Figures 2E, 2F, S2D, and S2E) and increases in cell death (Figure 2G). Altogether, these data indicate that Cyrano is required for maintenance of ESC self-renewal.
The ESC Gene Expression Signature Is Disrupted by Cyrano Deficiency
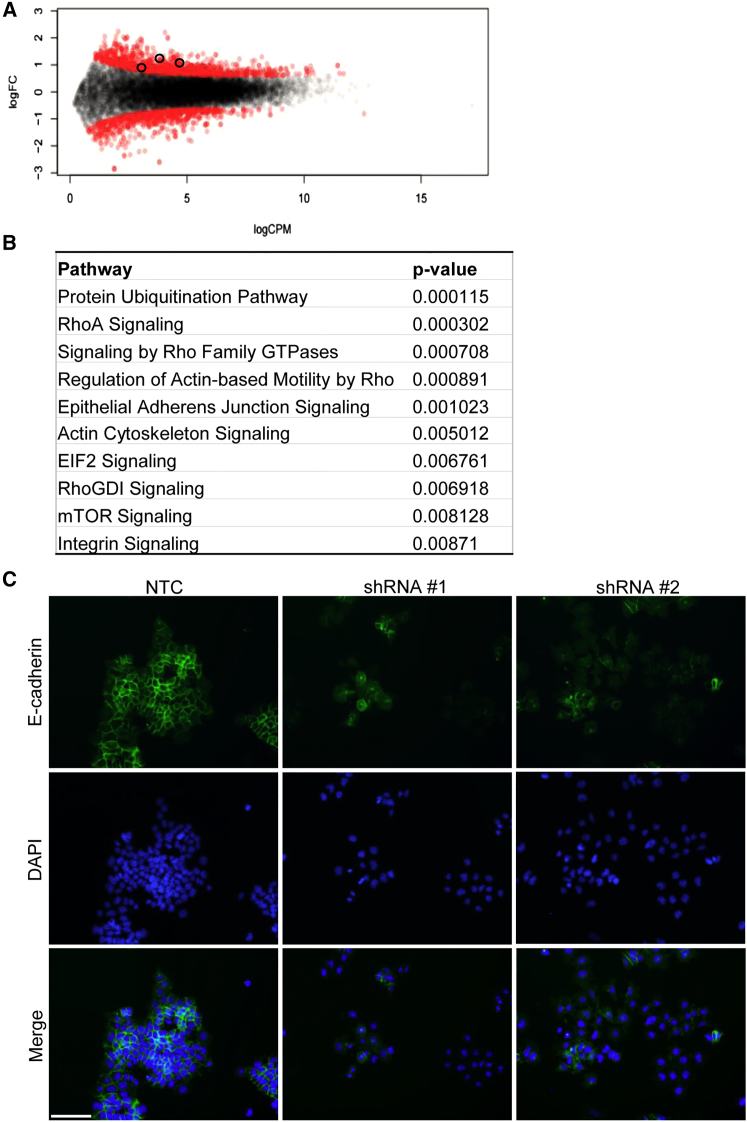
Pluripotent ESCs are characterized by a well-documented gene expression profile that supports their ability to self-renew while maintaining the capacity for differentiation into embryonic germ layer derivatives (Boyer et al., 2005). We next examined modifications in gene expression profiles upon Cyrano KD to determine whether cells assumed a particular cellular identity upon Cyrano loss. Total read number in RNA-seq experiments for samples ranged from 23 to 29 million reads, with mapped reads ranging from 88% to 90% of total read number. Concomitant with morphological anomalies, RNA-seq uncovered significant differential gene expression due to shRNA-mediated KD (Figure 3A). Sample comparisons revealed 489 and 380 up- and downregulated genes, respectively, between control and KD samples (Figure 3A). Validation of differentially expressed non-pluripotency-related genes was carried out by qRT-PCR (Figure S3A). Ingenuity Pathway Analysis (IPA) classification revealed that the top dysregulated pathways post KD were primarily related to cell adhesion, signaling, and motility (Figure 3B), observations consistent with the loss-of-function phenotype. Based on these results and phenotypic observations (Figure 2C), we further assessed anomalies in cell adhesion by examining localization of E-cadherin (Figure 3C) and F-actin (Figure S3B) in immunofluorescence assays. E-cadherin is a key pluripotency cell-signaling modulator (Redmer et al., 2011) that typically mediates cell-cell adhesion and is normally found at cell-cell boundaries in ESC colonies. We observed aberrant localization of cell membranous E-cadherin on Cyrano depletion (Figure 3C). Furthermore, phalloidin staining (Figure S3B) revealed a loss of F-actin localization as is typically seen at the cell cortex in ESCs (Schratt et al., 2002). In addition to anomalous cell and colony morphology, this uncharacteristic localization of qualitative markers, whose localization is normally indicative of self-renewing ESC colonies, further indicates atypical cell adhesion in colony maintenance.
Figure 3.
Cyrano Depletion Results in Aberrant Gene Expression in ESCs
(A) Smear plot comparing gene expression in NTC and KD samples reveals significant gene expression changes 3 days post KD. Differentially expressed genes are indicated in red, compared with insignificant genes in black (FDR < 0.05).
(B) IPA analysis of differentially expressed genes shows an enrichment of pathways after Cyrano KD.
(C) Immunofluorescence examination of E-cadherin in control and KD cells, showing aberrant localization. Nuclei, blue (DAPI); Scale bar, 100 μm.
See also Figure S3.
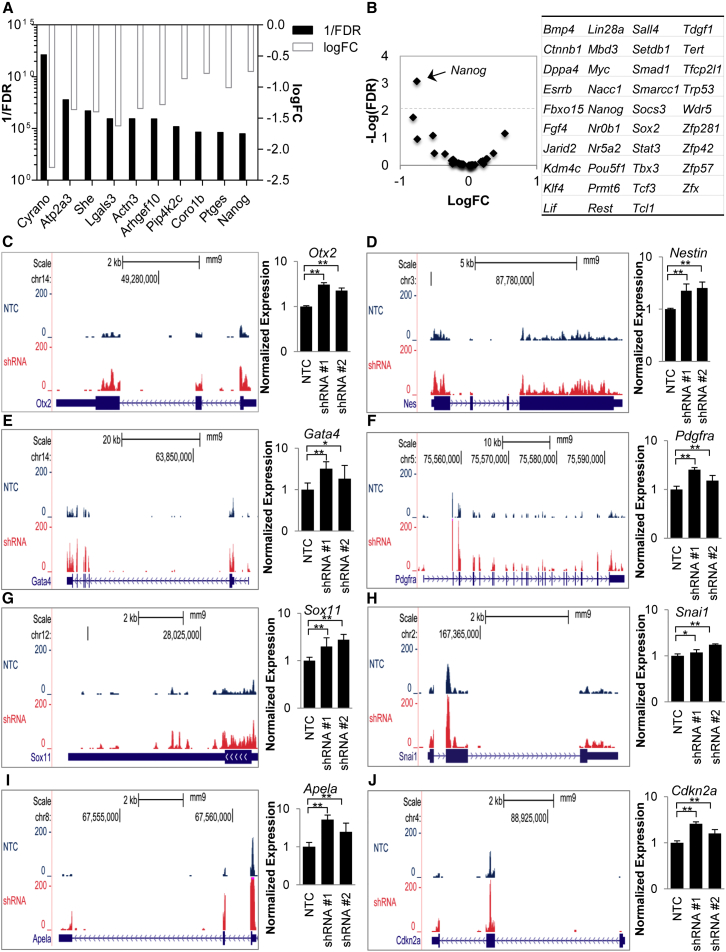
Cyrano Depletion Disrupts Nanog Expression
We next rank-ordered genes that showed a decrease in expression (false discovery rate [FDR] < 0.05). As expected, the reduction in Cyrano expression was most significant using this threshold (Figure 4A). Interestingly, the master pluripotency regulator Nanog was one of the more significantly downregulated genes (Figure 4A). Further examination of expression of other key factors in self-renewal maintenance (Xu et al., 2014) including Oct4, revealed that they remained mostly unchanged while there was a decrease in Nanog levels (Figures 4B, S4A–S4D). The specific downregulation of Nanog and not other master regulators, including Oct4, suggests that Cyrano affects a specific subset of self-renewal master genes to result in the observed phenotype, as opposed to being an indirect result of spontaneous differentiation.
Figure 4.
Depletion of Cyrano Results in Altered Expression of Nanog and Lineage-Related Genes
(A) Ranking of the top-10 decreased genes in RNA-seq based on FDR (black bars) shows significant decreases in Nanog levels with Cyrano KD. Fold change is also indicated (white bars).
(B) Assessment of pluripotency regulators on Cyrano KD reveals that Nanog displays the most significant differential regulation.
(C–J) UCSC genome browser plots shows RNA-seq reads mapped to mm9 and normalized to remove sequencing depth biases, and independent qRT-PCR examination upon Cyrano KD indicates increased expression of factors that antagonize self-renewal in mouse ESCs. Data are from three independent experiments with error bars representing 95% CI. ∗p < 0.05, ∗∗p < 0.01.
See also Figure S4.
Aberrant Expression of Anti-Self-Renewal Factors Is Associated with Cyrano Depletion
The phenotype upon Cyrano loss led us to more closely examine genes that showed significant increases in expression. This revealed key anti-pluripotency genes, including developmental regulators not associated with the ESC state. These include lineage specification markers (Otx2, Nestin, Gata4, Pdgfra, and Sox11; Figures 4C–4G) and the epithelial-mesenchymal transition factor, Snai1 (Figure 4H). Furthermore, we observed increases in Apela expression (Figure 4I), a recently described regulatory RNA with the ability to encode a small peptide hormone (Chng et al., 2013, Li et al., 2015b, Pauli et al., 2014). The upregulation of Apela is consistent with it being a genomic target of Nanog, whose expression increases upon RNAi-mediated depletion of Nanog (Loh et al., 2006), and its role in mesendoderm specification in zebrafish (Chng et al., 2013). Apela expression abruptly increases upon LIF withdrawal in mouse ESCs (Figure S4E), consistent with the reduced capacity to differentiate into mesoderm and endoderm in embryoid bodies with Apela depletion (Li et al., 2015b). As Apela has been recently shown to be important for self-renewal maintenance in human ESCs (Ho et al., 2015), the rapid upregulation may represent a transition from naive to primed pluripotency prior to spontaneous differentiation. Consistent with this, subsequent downregulation of Apela is seen later in mouse ESC differentiation (Figure S4E), similar to the decrease observed in human ESC differentiation (Chng et al., 2013). These results suggest that deviations from ideal Apela levels antagonize maintenance of the ESC state.
The constitutive cell cycle of ESCs, which lacks the periodicity of differentiated cell types, is associated with a lack of cyclin D regulation (White and Dalton, 2005). Consistent with the phenotypic change and decreased proliferative capacity, we observed an increase in levels of the cyclin D-Cdk inhibitor, Cdkn2a, in Cyrano-deficient cells relative to controls (Figure 4J).
Taken together, these gene expression alterations support the failure to retain self-renewal capacity upon Cyrano depletion. The function of Cyrano does not appear to primarily be via proximal cis mechanisms, as no significant gene expression changes were observed in RNA-seq (FDR < 0.05) for neighboring genes within a 100-kb window (Figure S4F), and consistent changes were not observed for both shRNAs in qRT-PCR analyses (Figure S4G).
Cyrano Counteracts mir-7 Action in ESCs to Support Self-Renewal Maintenance
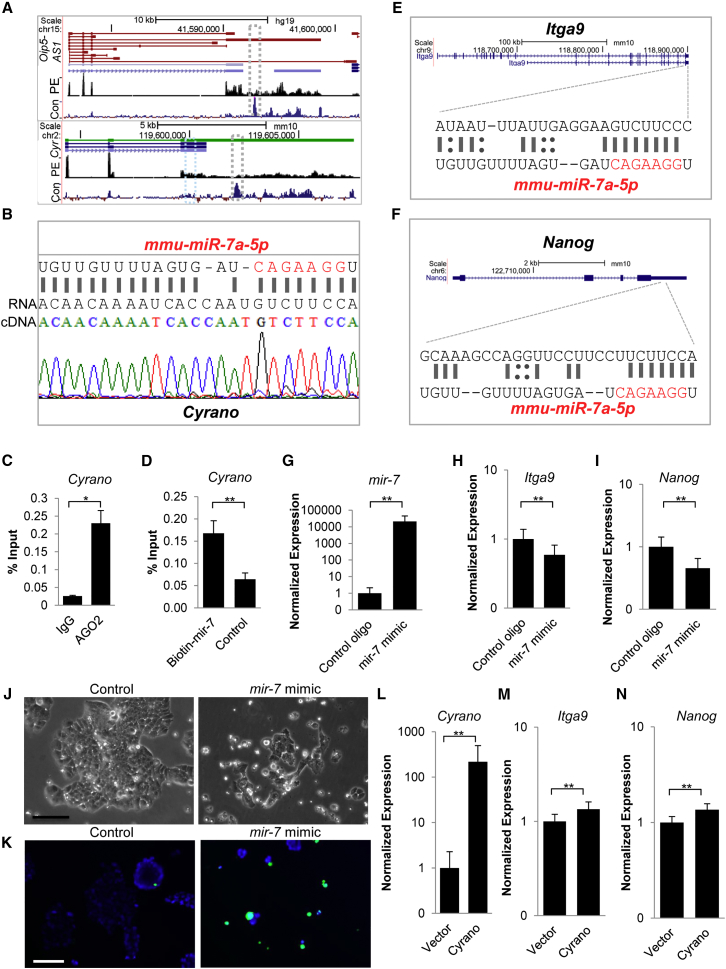
We sought to determine how Cyrano mediates these survival, adhesion, and anti-differentiation roles in ESCs, and hypothesized that Cyrano functions were at least partially determined through its unique relationship with mir-7 (Ulitsky et al., 2011). Several lines of evidence support this hypothesis. (1) In addition to a conventional seed match (Figure S5A and Table S1), the prominently expressed long splice variant (Figure 5A) of Oip5-AS1/Cyrano (Sigova et al., 2013) possesses an almost complete binding site to mir-7 in mouse ESCs (Figure 5B) and this binding site is conserved across vertebrates (Figure 5A and Ulitsky et al., 2011). (2) Similar to previous observations in mouse brain (Zhang and Darnell, 2011) and HEK293 (Kishore et al., 2011), Cyrano or its human ortholog is bound by Argonaute in ESCs (Figure 5C), similar to the known mir-7 target gene Igf1R (Figure S5B) (Jiang et al., 2010). (3) mir-7 is associated with differentiation in various cell types (Cui et al., 2013, Kong et al., 2012, Nguyen et al., 2010). (4) mir-7 is a documented Stat3 pathway antagonist (Zhang et al., 2014) and the LIF-Stat3-Nanog axis is required for mouse ESC self-renewal. (5) Similar to previous observations from Bartel and colleagues, we found that mir-7 and Cyrano physically interact (Figure 5D), as observed for the mir-7 target Igf1R (Jiang et al., 2010) (Figure S5C), suggesting that this molecular interaction could be a mechanism for Cyrano regulation of mir-7 function. Indeed, small RNA-seq data from ENCODE indicated that mir-7 is expressed and localized similarly to Cyrano in ESCs (Figure S5D). Furthermore, nuclear enrichment of mir-7 has been found in additional contexts including HEK293 and human carcinoma cell lines (Liao et al., 2010), similar to that observed for Cyrano (Figures 1A, 1C, and S1A–S1E).
Figure 5.
Cyrano Restrains mir-7 Activity to Support ESC Self-Renewal
(A) Genome browser blots showing Oip5-AS1/Cyrano splice variants. Despite the presence of multiple splice variants of Oip5-AS1, the long variant containing the conserved mir-7 binding site (gray box; segment of this region with the mir-7 interaction site is expanded in B) is prominently expressed in human ESCs (top panel, GEO: GSE41009) and mouse ESCs (bottom panel, GEO: GSE36799). Blue box marks additional conventional mir-7 seed sequence. Con denotes conservation: Vertebrate Multiz Alignment & Conservation; PE, paired-end RNA-seq; Cyr, Cyrano.
(B) Near-complete mir-7 sequence complementarity is observed in Cyrano sequenced from ESCs.
(C) RNA immunoprecipitation indicates that Cyrano is bound by Ago2 in ESCs. Experiments were performed in triplicate with error bars representing SEM.
(D) miRNA pull-down and qPCR indicates a physical interaction between Cyrano and mir-7. Experiments were performed in triplicate with error bars representing SEM.
(E and F) The 3′ UTRs of Itga9 and Nanog contain mir-7 target sites (see Figure S5; Tables S1 and S2 for further information on target sites).
(G–N) Overexpression of mir-7 (G) results in a decrease in Itga9 (H) and Nanog levels (I), loss of ESC self-renewal maintenance (J), and increased cell death (K) in ESCs 2 days post transfection. Nuclei (blue, live; green, dead); Scale bar, 100 μm. qRT-PCR monitoring of Cyrano (L), Itga9 (M), and Nanog (N) levels with Cyrano overexpression. Error bars for qRT-PCR expression analysis represent 95% CI.
Data are from three independent experiments. ∗p < 0.05, ∗∗p < 0.01. See also Figure S5; Tables S1 and S2.
We then determined whether mir-7 could affect ESC maintenance by downregulating key adhesion and self-renewal regulators. First, we used mirWalk (Dweep and Gretz, 2015) (Figure S5E and Table S2), TargetScan (Agarwal et al., 2015, Lewis et al., 2003, Lewis et al., 2005), and RNA22 (Miranda et al., 2006), and found that the 3′ UTR of the ESC-enriched integrin Itga9 (Nagano et al., 2008, Rugg-Gunn et al., 2012) (Figure 5E) contains a strong 8mer mir-7 seed sequence. Interestingly, examination of Nanog's 3′ UTR also revealed a 7mer-A1 mir-7 seed match (Figure 5F). Second, we transfected ESCs with a mir-7 mimic (Figures 5G, S5F, and S5G) and observed a decrease in Itga9 (Figure 5H) and Nanog levels (Figure 5I). Third, as the mir-7-Nanog interaction was based on a weaker prediction, we introduced the mir-7 mimic along with a luciferase reporter plasmid constituting of the Nanog 3′ UTR together with a transfection control and observed inhibition of luciferase expression (Figure S5H). Also, inhibition of mir-7 resulted in increased Nanog levels (Figure S5I). We also found that Cyrano levels decreased upon introduction of the mir-7 mimic, suggesting that mir-7 also regulates Cyrano in ESCs (Figure S5J). Importantly, when we transfected ESCs with the mir-7 mimic and monitored the ESC phenotype in LIF-containing medium, we observed a loss of ESC colony maintenance within 2 days of mir-7 introduction (Figure 5J). This was accompanied by anomalies in cell and colony adhesion with numerous detached cells (Figure 5J) and increases in cell death (Figure 5K), similar to observations made upon Cyrano depletion. Finally, as Itga9 and Nanog levels decreased upon mir-7 overexpression as well as upon Cyrano depletion (Figures S5K and S4B–S4D), we hypothesized that Itga9 and Nanog levels would increase with modulation of Cyrano levels and found that increased expression of Cyrano (Figure 5L) augmented their expression (Figures 5M and 5N). This suggests that Cyrano and mir-7 act in opposition to one another in ESCs.
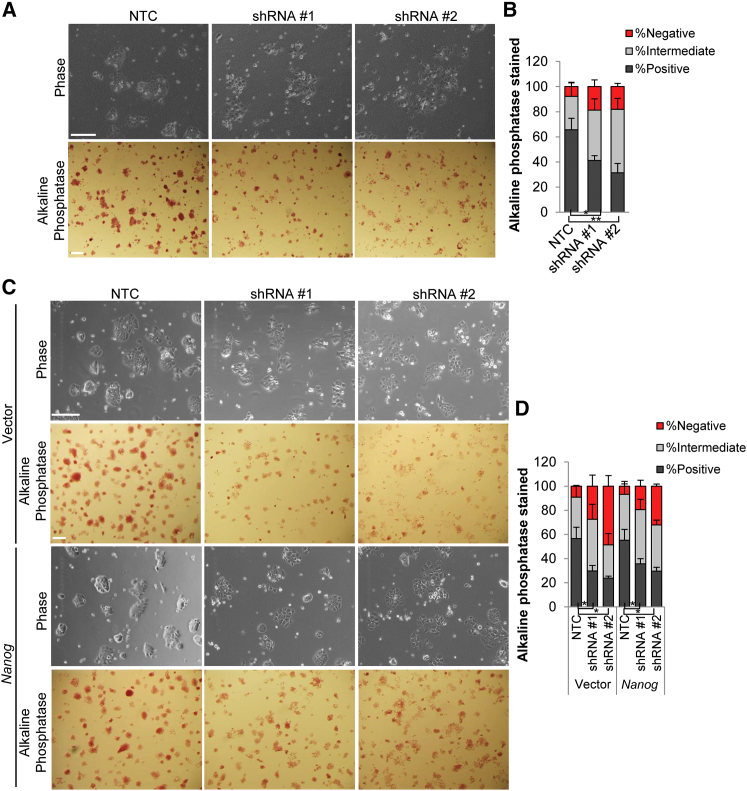
It is now well established that compared with culture in serum + LIF, mouse ESCs can be cultured more homogenously in the presence of GSK3 and MEK inhibitors (2i, Ying et al., 2008). Due to the heterogeneity observed in serum + LIF, we also tested the effect of Cyrano depletion under conditions of more homogeneity. Under 2i conditions, Nanog expression is somewhat higher than that of growth conditions in serum-containing medium (Abranches et al., 2014), whereas Cyrano's levels remain unchanged (Figure S6). To further delineate whether Cyrano functions upstream of Nanog, we examined the effect of Cyrano KD in ESCs under conditions of higher Nanog expression: (1) cells cultured in 2i and (2) Nanog overexpression. Similar to observations in serum-containing LIF medium, KD of Cyrano in 2i resulted primarily in a loss of self-renewal maintenance, and increased Nanog expression resulted in partial recovery of self-renewal capacity (Figures 6A–6D). This suggests that additional mechanisms of Cyrano action are functional in ESC maintenance. Delineation of Cyrano function can be further enhanced through single-cell analyses of ESC lines harboring endogenously encoded reporters and mutant alleles of Cyrano and putative direct and indirect targets.
Figure 6.
Ability of Increased Nanog Expression to Rescue the Cyrano KD Phenotype
(A and B) A reduction in alkaline phosphatase staining is observed in ESC colonies after Cyrano KD in 2i medium.
(C and D) Nanog overexpression results in qualitative/partial rescue of Cyrano KD phenotype. n > 120.
Scale bar, 100 μm. Data are from three independent experiments and error bars represent SD. ∗p < 0.05, ∗∗p < 0.01. See also Figure S6.
Altogether, we provide evidence for Cyrano's role in cell survival and colony maintenance in self-renewing ESCs. Furthermore, our results provide evidence for one direct mechanism by which Cyrano functions, namely the existence of a negative-feedback loop between Cyrano and mir-7 (see Graphical Abstract), to support the maintenance of ESC self-renewal through factors including Nanog and Itga9 that sustain key properties of pluripotent cells.
Discussion
Of the nearly 9,000 known lncRNAs (Derrien et al., 2012, Harrow et al., 2006, Harrow et al., 2012), the mechanism of action is understood for only a small fraction. We have described a previously undefined role for Cyrano in the maintenance of the self-renewing state of mammalian pluripotent cells, a model cell type for establishing mechanisms of action relevant to early development and regenerative medicine.
In our characterization of Cyrano function, we show that depletion of Cyrano results in disarray in the pluripotent gene expression signature and defects in self-renewal maintenance. Driving this is aberrations in colony survival and preservation, which requires maintenance of cellular adhesion and signaling, as well as the decrease in Nanog expression, which itself is required for cell growth and apoptosis avoidance in ESCs (Chen et al., 2012). Furthermore, it is well established that Nanog function has far-reaching implications in the repression of negative regulators of the ESC state, such as Dkk1 and Gata6 (Loh et al., 2006, Singh et al., 2007). Mining gene expression data produced in a recent shRNA screen for lncRNAs that regulate pluripotency further supports Cyrano's key role (Lin et al., 2014).
Misregulation and aberrant expression of lncRNAs are increasingly associated with disease states (Wapinski and Chang, 2011, Batista and Chang, 2013, Fatica and Bozzoni, 2014). Similar signaling pathways (e.g., Jak-Stat, PI3K/AKT) and transcription factors (e.g., Myc, Stat3) activities support stem cell growth and survival as well as tumorigenesis (Kim et al., 2010). As Cyrano displays particularly high expression in ENCODE cancer cell lines, a priority will be to determine the requirement of Cyrano in tumor cell survival and cellular reprogramming to a malignant state. Indeed, similar to our findings in ESCs, Cyrano supports glioma cell proliferation in addition to cell migration and tumorigenesis (Hu et al., 2017).
Complex relationships exist between miRNAs and lncRNAs in transcriptional, post-transcriptional, and translational regulatory processes. Previous studies have shown that lncRNAs can function as miRNA precursors and miRNA targets, or compete as decoys/sponges/competing endogenous RNAs to prevent inhibition of mRNA targets (Jeggari et al., 2012, Paraskevopoulou et al., 2013). In the context of skeletal muscle differentiation, H19 antagonizes let-7, releasing the repression of let-7 targets such as HMGA2 (Kallen et al., 2013). Similarly, if Cyrano has a negative influence on mir-7 function, it would be expected that depletion of Cyrano would free mir-7 to repress its targets, while overexpression of Cyrano would boost mir-7 target-transcript levels, consistent with our observations.
Moreover, the combination of multiple sites for binding to Cyrano, including the unique ultra-conserved binding site, along with the intermediate expression of mir-7 in ESCs (Tang et al., 2006), suggests that it is feasible for Cyrano to attenuate mir-7 activity to sustain self-renewal. This is particularly relevant for supporting the maintenance of expression of targets such as Nanog, which display some heterogeneity in expression in ESCs (Abranches et al., 2014, Singh et al., 2007). While Nanog transcripts range from 0 to 500 mRNA molecules per ESC in normal culture conditions, a fraction of pluripotent cells exhibit low Nanog expression in the range of <25 molecules/cell (Abranches et al., 2014). One hypothesis is that Cyrano is one factor that presents a barrier to lineage commitment in conditions of suboptimal fluctuations in Nanog levels. Additionally or alternatively, Cyrano may also directly repress lineage specification factors. Further studies may reveal mechanisms behind the molecular interplay between Cyrano and its interacting RNAs at the single-molecule and single-cell levels.
Based on our and previously available data, we postulate that regulating RNAs and proteins through interactions is a prominent role of the 8.2-kb Cyrano molecule. Indeed, the ortholog OIP5-AS1 has been found to bind 37 RNA binding proteins (RBPs) (Li et al., 2015a), and a recent report provided further evidence for Cyrano's interaction with other RNAs and highlighted the capacity for Cyrano to function as a sponge of RBPs from mRNAs (Kim et al., 2016). Overall, our observations along with other emerging data on Cyrano, which in various systems include anti-proliferative functions and roles in organogenesis/embryonic development, are strongly indicative of complex biological function, which clearly warrants further study.
Experimental Procedures
Cell Culture and RNAi
Mouse R1 (XY, Nagy et al., 1993) ESCs were maintained in complete medium supplemented with LIF on gelatin-coated dishes. Mouse ES2-1 (XX, Royce-Tolland et al., 2010) were maintained in complete medium with LIF on feeders or gelatin-coated dishes. Embryoid body differentiation was carried out on low adherent dishes under LIF withdrawal conditions.
RNA Extraction and qRT-PCR
Total RNA was isolated using the RNeasy Mini Kit (Qiagen) or the Quick-RNA MiniPrep Kit (Zymo), followed by DNase treatment (Ambion) and reverse transcription using the iScript reagent (Bio-Rad). qRT-PCR for lncRNA and mRNA transcripts was performed using primers listed in Table S3 with the SsoFast Evagreen Supermix (Bio-Rad) or Taqman Assays (Applied Biosystems).
miRNAs were extracted with the Quick-RNA MiniPrep Kit (Zymo) and reverse transcription carried out with the TaqMan MicroRNA Reverse Transcription Kit. miR-7 and the U6 control were amplified using the TaqMan Universal Master Mix II, no UNG and TaqMan microRNA assays for mmu-miR-7a-5p and U6, respectively.
Data were analyzed based on the 2−ΔΔCt, after log transformation, mean centering, and autoscaling (Willems et al., 2008). For these and other quantitative data, experiments were carried out in at least three independent replicates and unpaired, two-tailed Student's t-tests used to determine statistical significance (∗∗p < 0.01, ∗p < 0.05, †p < 0.1 in figures).
Alkaline Phosphatase Assay
Alkaline phosphatase activity was detected using a leukocyte alkaline phosphatase staining kit (Sigma), and the effect of Cyrano KD assessed on day 3 post-transfection.
smFISH
A pool of 36 FISH probes for Cyrano was used for hybridization (Biosearch Technologies) for cells cultured on coverslips for approximately 24–30 hr. After fixing with 4% paraformaldehyde (PFA) and permeabilization, cells were hybridized in 100 mg/mL dextran sulfate, 1% formamide, and 2× saline sodium citrate overnight at 37°C.
Transcriptomics
Libraries for RNA-seq were prepared from total RNA with a modified dUTP strand-specific method (Zhong et al., 2011). RNA-seq reads were aligned to the mm9 mouse genome using TopHat (Trapnell et al., 2009) and reads per gene were counted using HTSeq-count (Anders et al., 2015). Normalized bedgraphs were generated for the UCSC genome browser using bedtools (Quinlan and Hall, 2010). Differential expression between NTC and shRNA KD was performed using edgeR (Robinson et al., 2010) after removing genes with fewer than 20 reads, mapping to them across all replicates. Expression changes were visualized using ggplot2 (Wickham, 2009).
Publicly available microarray data (GEO: GSE18290) (Xie et al., 2010) of 1-cell, 2-cell, 4-cell, 8-cell, morula, and blastocyst embryos were used to examine the expression of Cyrano, Airn, and H19 lncRNAs. Publicly available data (GEO: GSE45719, Deng et al., 2014) were used for single-cell RNA-seq analysis of Cyrano (1700020I14Rik) expression in preimplantation embryos.
Publicly available paired-end RNA-seq data (GEO: GSE36799, GSE41009; Sigova et al., 2013) were used to examine expression of Cyrano splice variants in mouse and human ESCs.
RNA Immunoprecipitation and miRNA Target Isolation
Coimmunoprecipitation of Argonaute-bound RNAs was performed generally as previously described (Moran et al., 2012). Ago2 antibody (04-642, Millipore) or immunoglobulin G control was coupled to protein A/G magnetic beads (Thermo Fisher) and incubated with cellular lysate prepared from approximately 1.0 × 107 cells previously crosslinked with 0.3% formaldehyde. Immunoprecipitation was carried out overnight at 4°C with gentle rotation followed by extensive washes. RNA was eluted in the presence of proteinase K and incubated at 65°C for 2 hr to reverse crosslinks. RNA was purified using the RNA Clean & Concentrator Kit (Zymo) followed by reverse transcription (iScript, Bio-Rad) for use in qPCR.
Cells for miRNA pull-down were transfected with biotin-tagged mir-7 at a concentration of 50 nM and harvested approximately 30 hr post transfection. Cleared cellular lysate was incubated with streptavidin-magnetic beads preblocked with RNase-free BSA and yeast tRNA for 1 hr at 4°C. After washing, RNA was isolated with TRIzol, followed by reverse transcription (iScript) for use in qPCR.
Western Blot Analysis and Immunofluorescence
Antibodies were used for lamin A/C (E-1, sc-376248, Santa Cruz Biotechnology), Oct4 (C-10, sc-5279, Santa Cruz), Nanog (A300-397A, Bethyl Laboratories; #8822, Cell Signaling Technologies), E-cadherin (13-1900, Zymed), and β-actin (ab8226, Abcam) on cell extracts prepared using a modified RIPA buffer or fixed for immunofluorescence experiments with 4% PFA.
miRNA Binding Site Prediction and Luciferase Assays
miRNA binding sites for Itga9 and Nanog were predicted using miRWalk (Dweep and Gretz, 2015), TargetScan (Agarwal et al., 2015, Lewis et al., 2003, Lewis et al., 2005), and RNA22 (Miranda et al., 2006). Cells were cotransfected in 24-well plates using Lipofectamine 3000 (Thermo Fisher) for Dual-Luciferase Reporter Assays (Promega) according to the manufacturer's protocol. miRNA mimics (50 nM, Dharmacon) were introduced along with 50 ng of the firefly luciferase vector pGL3 containing the Nanog 3′ UTR (Luc-Nanog-3′ UTR was a gift from Lin He, Addgene plasmid #63893) (Choi et al., 2011) and 5 ng of the control Renilla luciferase vector, pRL-TK (Promega). Firefly luciferase and Renilla luciferase activities were consecutively measured 48 hr post transfection.
Author Contributions
Conceptualization, K.N.S. and T.M.; Methodology, K.N.S. and J.S.; Investigation, K.N.S. and S.C.M.; Formal Analysis, K.N.S, J.S. and T.M.; Data Curation, J.S.; Writing – Original Draft, K.N.S.; Writing – Review & Editing, K.N.S., J.S., S.C.M., P.S., and T.M.; Funding Acquisition, T.M.; Resources, P.S. and T.M.; Supervision, K.N.S and T.M.
Acknowledgments
We thank Dr. Barbara Panning for the ES2-1 line, Dr. Mauro Calabrese for helpful discussions and critical comments on the manuscript, and the members of the T.M. laboratory for comments. Grant support: NIH R01 GM101974 to T.M.
Published: June 1, 2017
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, six figures, and three tables and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2017.05.005.
Accession Numbers
Gene expression data are publicly available and can be retrieved from the GEO, NCBI under accession number GEO: GSE98297.
Supplemental Information
References
- Abranches E., Guedes A.M., Moravec M., Maamar H., Svoboda P., Raj A., Henrique D. Stochastic NANOG fluctuations allow mouse embryonic stem cells to explore pluripotency. Development. 2014;141:2770–2779. doi: 10.1242/dev.108910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal V., Bell G.W., Nam J., Bartel D.P. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4:e05005. doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander R.P., Fang G., Rozowsky J., Snyder M., Gerstein M.B. Annotating non-coding regions of the genome. Nat. Rev. Genet. 2010;11:559–571. doi: 10.1038/nrg2814. [DOI] [PubMed] [Google Scholar]
- Anders S., Pyl P.T., Huber W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista P.J., Chang H.Y. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152:1298–1307. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer L.A., Lee T.I., Cole M.F., Johnstone S.E., Levine S.S., Zucker J.P., Guenther M.G., Kumar R.M., Murray H.L., Jenner R.G. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabili M.N., Trapnell C., Goff L., Koziol M., Tazon-Vega B., Regev A., Rinn J.L. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011;25:1915–1927. doi: 10.1101/gad.17446611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabili M.N., Dunagin M.C., McClanahan P.D., Biaesch A., Padovan-Merhar O., Regev A., Rinn J.L., Raj A. Localization and abundance analysis of human lncRNAs at single-cell and single-molecule resolution. Genome Biol. 2015;16:20. doi: 10.1186/s13059-015-0586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X., Cullen B.R. The imprinted H19 noncoding RNA is a primary microRNA precursor. RNA. 2007;13:313–316. doi: 10.1261/rna.351707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Du J., Lu G. Cell growth arrest and apoptosis induced by Oct4 or Nanog knockdown in mouse embryonic stem cells: a possible role of Trp53. Mol. Biol. Rep. 2012;39:1855–1861. doi: 10.1007/s11033-011-0928-6. [DOI] [PubMed] [Google Scholar]
- Chew G.L., Pauli A., Rinn J.L., Regev A., Schier A.F., Valen E. Ribosome profiling reveals resemblance between long non-coding RNAs and 5' leaders of coding RNAs. Development. 2013;140:2828–2834. doi: 10.1242/dev.098343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chng S.C., Ho L., Tian J., Reversade B. ELABELA: a hormone essential for heart development signals via the apelin receptor. Dev. Cell. 2013;27:672–680. doi: 10.1016/j.devcel.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Choi Y.J., Lin C., Ho J.J., He X., Okada N., Bu P., Zhong Y., Kim S.Y., Bennett M.J., Chen C. miR-34 miRNAs provide a barrier for somatic cell reprogramming. Nat. Cell Biol. 2011;13:1353–1360. doi: 10.1038/ncb2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M.B., Johnston R.L., Inostroza-Ponta M., Fox A.H., Fortini E., Moscato P., Dinger M.E., Mattick J.S. Genome-wide analysis of long noncoding RNA stability. Genome Res. 2012;22:885–898. doi: 10.1101/gr.131037.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Xiao Z., Chen T., Wei J., Chen L., Liu L., Chen B., Wang X., Li X., Dai J. The miR-7 identified from collagen biomaterial-based three-dimensional cultured cells regulates neural stem cell differentiation. Stem Cells Dev. 2013;23:393–405. doi: 10.1089/scd.2013.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q., Ramsköld D., Reinius B., Sandberg R. Single-cell RNA-seq reveals dynamic, random monoallelic gene expression in mammalian cells. Science. 2014;343:193–196. doi: 10.1126/science.1245316. [DOI] [PubMed] [Google Scholar]
- Derrien T., Johnson R., Bussotti G., Tanzer A., Djebali S., Tilgner H., Guernec G., Martin D., Merkel A., Knowles D.G. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22:1775–1789. doi: 10.1101/gr.132159.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dweep H., Gretz N. miRWalk2. 0: a comprehensive atlas of microRNA-target interactions. Nat. Methods. 2015;12:697. doi: 10.1038/nmeth.3485. [DOI] [PubMed] [Google Scholar]
- ENCODE Project Consortium An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatica A., Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat. Rev. Genet. 2014;15:7–21. doi: 10.1038/nrg3606. [DOI] [PubMed] [Google Scholar]
- Guttman M., Garber M., Levin J.Z., Donaghey J., Robinson J., Adiconis X., Fan L., Koziol M.J., Gnirke A., Nusbaum C. Ab initio reconstruction of cell type-specific transcriptomes in mouse reveals the conserved multi-exonic structure of lincRNAs. Nat. Biotechnol. 2010;28:503–510. doi: 10.1038/nbt.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman M., Donaghey J., Carey B.W., Garber M., Grenier J.K., Munson G., Young G., Lucas A.B., Ach R., Bruhn L. lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature. 2011;477:295–300. doi: 10.1038/nature10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen T.B., Jensen T.I., Clausen B.H., Bramsen J.B., Finsen B., Damgaard C.K., Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- Harrow J., Denoeud F., Frankish A., Reymond A., Chen C.K., Chrast J., Lagarde J., Gilbert J.G., Storey R., Swarbreck D. GENCODE: producing a reference annotation for ENCODE. Genome Biol. 2006;7(Suppl 1):S4.1–S4.9. doi: 10.1186/gb-2006-7-s1-s4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrow J., Frankish A., Gonzalez J.M., Tapanari E., Diekhans M., Kokocinski F., Aken B.L., Barrell D., Zadissa A., Searle S. GENCODE: the reference human genome annotation for the ENCODE Project. Genome Res. 2012;22:1760–1774. doi: 10.1101/gr.135350.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L., Tan S.Y., Wee S., Wu Y., Tan S.J., Ramakrishna N.B., Chng S.C., Nama S., Sczerbinska I., Chan Y. ELABELA is an endogenous growth factor that sustains hESC self-renewal via the PI3K/AKT pathway. Cell Stem Cell. 2015;17:435–447. doi: 10.1016/j.stem.2015.08.010. [DOI] [PubMed] [Google Scholar]
- Hu G., Wu L., Kuang W., Chen Y., Zhu X., Guo H., Lang H. Knockdown of linc-OIP5 inhibits proliferation and migration of glioma cells through down-regulation of YAP-NOTCH signaling pathway. Gene. 2017;610:24–31. doi: 10.1016/j.gene.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Jeggari A., Marks D.S., Larsson E. miRcode: a map of putative microRNA target sites in the long non-coding transcriptome. Bioinformatics. 2012;28:2062–2063. doi: 10.1093/bioinformatics/bts344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Liu X., Chen Z., Jin Y., Heidbreder C.E., Kolokythas A., Wang A., Dai Y., Zhou X. MicroRNA-7 targets IGF1R (insulin-like growth factor 1 receptor) in tongue squamous cell carcinoma cells. Biochem. J. 2010;432:199–205. doi: 10.1042/BJ20100859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen A.N., Zhou X., Xu J., Qiao C., Ma J., Yan L., Lu L., Liu C., Yi J., Zhang H. The imprinted H19 lncRNA antagonizes let-7 microRNAs. Mol. Cell. 2013;52:101–112. doi: 10.1016/j.molcel.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefas B., Godlewski J., Comeau L., Li Y., Abounader R., Hawkinson M., Lee J., Fine H., Chiocca E.A., Lawler S., Purow B. microRNA-7 inhibits the epidermal growth factor receptor and the Akt pathway and is down-regulated in glioblastoma. Cancer Res. 2008;68:3566–3572. doi: 10.1158/0008-5472.CAN-07-6639. [DOI] [PubMed] [Google Scholar]
- Kelley D., Rinn J. Transposable elements reveal a stem cell-specific class of long noncoding RNAs. Genome Biol. 2012;13:R107. doi: 10.1186/gb-2012-13-11-r107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keniry A., Oxley D., Monnier P., Kyba M., Dandolo L., Smits G., Reik W. The H19 lincRNA is a developmental reservoir of miR-675 that suppresses growth and Igf1r. Nat. Cell Biol. 2012;14:659–665. doi: 10.1038/ncb2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Woo A.J., Chu J., Snow J.W., Fujiwara Y., Kim C.G., Cantor A.B., Orkin S.H. A Myc network accounts for similarities between embryonic stem and cancer cell transcription programs. Cell. 2010;143:313–324. doi: 10.1016/j.cell.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.H., Marinov G.K., Pepke S., Singer Z.S., He P., Williams B., Schroth G.P., Elowitz M.B., Wold B.J. Single-cell transcriptome analysis reveals dynamic changes in lncRNA expression during reprogramming. Cell Stem Cell. 2015;16:88–101. doi: 10.1016/j.stem.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Abdelmohsen K., Yang X., De S., Grammatikakis I., Noh J.H., Gorospe M. LncRNA OIP5-AS1/cyrano sponges RNA-binding protein HuR. Nucleic Acids Res. 2016;44:2378–2392. doi: 10.1093/nar/gkw017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore S., Jaskiewicz L., Burger L., Hausser J., Khorshid M., Zavolan M. A quantitative analysis of CLIP methods for identifying binding sites of RNA-binding proteins. Nat. Methods. 2011;8:559–564. doi: 10.1038/nmeth.1608. [DOI] [PubMed] [Google Scholar]
- Kong D., Piao Y., Yamashita S., Oshima H., Oguma K., Fushida S., Fujimura T., Minamoto T., Seno H., Yamada Y. Inflammation-induced repression of tumor suppressor miR-7 in gastric tumor cells. Oncogene. 2012;31:3949–3960. doi: 10.1038/onc.2011.558. [DOI] [PubMed] [Google Scholar]
- Lewis B.P., Shih I., Jones-Rhoades M.W., Bartel D.P., Burge C.B. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- Lewis B.P., Burge C.B., Bartel D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Li J., Liu S., Zheng L., Wu J., Sun W., Wang Z., Zhou H., Qu L., Yang J. Discovery of protein-lncRNA interactions by integrating large-scale CLIP-seq and RNA-seq datasets. Front. Bioeng. Biotechnol. 2015;2:88. doi: 10.3389/fbioe.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Gou H., Tripathi B.K., Huang J., Jiang S., Dubois W., Waybright T., Lei M., Shi J., Zhou M. An apela RNA-containing negative feedback loop regulates p53-mediated apoptosis in embryonic stem cells. Cell Stem Cell. 2015;16:669–683. doi: 10.1016/j.stem.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J., Ma L., Guo Y., Zhang Y., Zhou H., Shao P., Chen Y., Qu L. Deep sequencing of human nuclear and cytoplasmic small RNAs reveals an unexpectedly complex subcellular distribution of miRNAs and tRNA 3′ trailers. PLoS One. 2010;5:e10563. doi: 10.1371/journal.pone.0010563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin N., Chang K., Li Z., Gates K., Rana Z.A., Dang J., Zhang D., Han T., Yang C., Cunningham T.J. An evolutionarily conserved long noncoding RNA TUNA controls pluripotency and neural lineage commitment. Mol. Cell. 2014;53:1005–1019. doi: 10.1016/j.molcel.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewer S., Cabili M.N., Guttman M., Loh Y., Thomas K., Park I.H., Garber M., Curran M., Onder T., Agarwal S. Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nat. Genet. 2010;42:1113–1117. doi: 10.1038/ng.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh Y., Wu Q., Chew J., Vega V.B., Zhang W., Chen X., Bourque G., George J., Leong B., Liu J. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat. Genet. 2006;38:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- Memczak S., Jens M., Elefsinioti A., Torti F., Krueger J., Rybak A., Maier L., Mackowiak S.D., Gregersen L.H., Munschauer M. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- Miranda K.C., Huynh T., Tay Y., Ang Y., Tam W., Thomson A.M., Lim B., Rigoutsos I. A pattern-based method for the identification of MicroRNA binding sites and their corresponding heteroduplexes. Cell. 2006;126:1203–1217. doi: 10.1016/j.cell.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Miyagawa R., Tano K., Mizuno R., Nakamura Y., Ijiri K., Rakwal R., Shibato J., Masuo Y., Mayeda A., Hirose T., Akimitsu N. Identification of cis- and trans-acting factors involved in the localization of MALAT-1 noncoding RNA to nuclear speckles. RNA. 2012;18:738–751. doi: 10.1261/rna.028639.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran V.A., Niland C.N., Khalil A.M. Co-immunoprecipitation of long noncoding RNAs. Methods Mol. Biol. 2012;925:219–228. doi: 10.1007/978-1-62703-011-3_15. [DOI] [PubMed] [Google Scholar]
- Nagano K., Yoshida Y., Isobe T. Cell surface biomarkers of embryonic stem cells. Proteomics. 2008;8:4025–4035. doi: 10.1002/pmic.200800073. [DOI] [PubMed] [Google Scholar]
- Nagy A., Rossant J., Nagy R., Abramow-Newerly W., Roder J.C. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc. Natl. Acad. Sci. USA. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H.T., Dalmasso G., Yan Y., Laroui H., Dahan S., Mayer L., Sitaraman S.V., Merlin D. MicroRNA-7 modulates CD98 expression during intestinal epithelial cell differentiation. J. Biol. Chem. 2010;285:1479–1489. doi: 10.1074/jbc.M109.057141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskevopoulou M.D., Georgakilas G., Kostoulas N., Reczko M., Maragkakis M., Dalamagas T.M., Hatzigeorgiou A.G. DIANA-LncBase: experimentally verified and computationally predicted microRNA targets on long non-coding RNAs. Nucleic Acids Res. 2013;41:D239–D245. doi: 10.1093/nar/gks1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli A., Norris M.L., Valen E., Chew G.L., Gagnon J.A., Zimmerman S., Mitchell A., Ma J., Dubrulle J., Reyon D. Toddler: an embryonic signal that promotes cell movement via Apelin receptors. Science. 2014;343:1248636. doi: 10.1126/science.1248636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan A.R., Hall I.M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmer T., Diecke S., Grigoryan T., Quiroga-Negreira A., Birchmeier W., Besser D. E-cadherin is crucial for embryonic stem cell pluripotency and can replace OCT4 during somatic cell reprogramming. EMBO Rep. 2011;12:720–726. doi: 10.1038/embor.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn J.L., Chang H.Y. Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M.D., McCarthy D.J., Smyth G.K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royce-Tolland M.E., Andersen A.A., Koyfman H.R., Talbot D.J., Wutz A., Tonks I.D., Kay G.F., Panning B. The A-repeat links ASF/SF2-dependent Xist RNA processing with random choice during X inactivation. Nat. Struct. Mol. Biol. 2010;17:948–954. doi: 10.1038/nsmb.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg-Gunn P.J., Cox B.J., Lanner F., Sharma P., Ignatchenko V., McDonald A.C., Garner J., Gramolini A.O., Rossant J., Kislinger T. Cell-surface proteomics identifies lineage-specific markers of embryo-derived stem cells. Dev. Cell. 2012;22:887–901. doi: 10.1016/j.devcel.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schratt G., Philippar U., Berger J., Schwarz H., Heidenreich O., Nordheim A. Serum response factor is crucial for actin cytoskeletal organization and focal adhesion assembly in embryonic stem cells. J. Cell Biol. 2002;156:737–750. doi: 10.1083/jcb.200106008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.M., Hamazaki T., Hankowski K.E., Terada N. A heterogeneous expression pattern for Nanog in embryonic stem cells. Stem Cells. 2007;25:2534–2542. doi: 10.1634/stemcells.2007-0126. [DOI] [PubMed] [Google Scholar]
- Sigova A.A., Mullen A.C., Molinie B., Gupta S., Orlando D.A., Guenther M.G., Almada A.E., Lin C., Sharp P.A., Giallourakis C.C. Divergent transcription of noncoding RNA/mRNA gene pairs in embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2013;110:2876–2881. doi: 10.1073/pnas.1221904110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F., Hajkova P., Barton S.C., Lao K., Surani M.A. MicroRNA expression profiling of single whole embryonic stem cells. Nucleic Acids Res. 2006;34:e9. doi: 10.1093/nar/gnj009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani H., Mizutani R., Salam K.A., Tano K., Ijiri K., Wakamatsu A., Isogai T., Suzuki Y., Akimitsu N. Genome-wide determination of RNA stability reveals hundreds of short-lived noncoding transcripts in mammals. Genome Res. 2012;22:947–956. doi: 10.1101/gr.130559.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazawa H., Yano S., Yoshida R., Yamasaki Y., Sasaki T., Hashimoto Y., Kuroda S., Ouchi M., Onishi T., Uno F. Genetically engineered oncolytic adenovirus induces autophagic cell death through an E2F1-microRNA-7-epidermal growth factor receptor axis. Int. J. Cancer. 2012;131:2939–2950. doi: 10.1002/ijc.27589. [DOI] [PubMed] [Google Scholar]
- Trapnell C., Pachter L., Salzberg S.L. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitsky I., Shkumatava A., Jan C.H., Sive H., Bartel D.P. Conserved function of lincRNAs in vertebrate embryonic development despite rapid sequence evolution. Cell. 2011;147:1537–1550. doi: 10.1016/j.cell.2011.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K.C., Chang H.Y. Molecular mechanisms of long noncoding RNAs. Mol. Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Xu Z., Jiang J., Xu C., Kang J., Xiao L., Wu M., Xiong J., Guo X., Liu H. Endogenous miRNA sponge lincRNA-RoR regulates Oct4, Nanog, and Sox2 in human embryonic stem cell self-renewal. Dev. Cell. 2013;25:69–80. doi: 10.1016/j.devcel.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Wapinski O., Chang H.Y. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21:354–361. doi: 10.1016/j.tcb.2011.04.001. [DOI] [PubMed] [Google Scholar]
- White J., Dalton S. Cell cycle control of embryonic stem cells. Stem Cell Rev. 2005;1:131–138. doi: 10.1385/SCR:1:2:131. [DOI] [PubMed] [Google Scholar]
- Wickham H. Springer Science & Business Media; 2009. ggplot2: Elegant Graphics for Data Analysis. [Google Scholar]
- Willems E., Leyns L., Vandesompele J. Standardization of real-time PCR gene expression data from independent biological replicates. Anal. Biochem. 2008;379:127–129. doi: 10.1016/j.ab.2008.04.036. [DOI] [PubMed] [Google Scholar]
- Xie D., Chen C.C., Ptaszek L.M., Xiao S., Cao X., Fang F., Ng H.H., Lewin H.A., Cowan C., Zhong S. Rewirable gene regulatory networks in the preimplantation embryonic development of three mammalian species. Genome Res. 2010;20:804–815. doi: 10.1101/gr.100594.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Ang Y., Sevilla A., Lemischka I.R., Ma'ayan A. Construction and validation of a regulatory network for pluripotency and self-renewal of mouse embryonic stem cells. PLoS Comput. Biol. 2014;10:e1003777. doi: 10.1371/journal.pcbi.1003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Q.L., Wray J., Nichols J., Batlle-Morera L., Doble B., Woodgett J., Cohen P., Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue F., Cheng Y., Breschi A., Vierstra J., Wu W., Ryba T., Sandstrom R., Ma Z., Davis C., Pope B.D. A comparative encyclopedia of DNA elements in the mouse genome. Nature. 2014;515:355–364. doi: 10.1038/nature13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Darnell R.B. Mapping in vivo protein-RNA interactions at single-nucleotide resolution from HITS-CLIP data. Nat. Biotechnol. 2011;29:607–614. doi: 10.1038/nbt.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Cai K., Wang J., Wang X., Cheng K., Shi F., Jiang L., Zhang Y., Dou J. MiR-7, inhibited indirectly by LincRNA HOTAIR, directly inhibits SETDB1 and reverses the EMT of breast cancer stem cells by downregulating the STAT3 pathway. Stem Cells. 2014;32:2858–2868. doi: 10.1002/stem.1795. [DOI] [PubMed] [Google Scholar]
- Zhong S., Joung J., Zheng Y., Chen Y., Liu B., Shao Y., Xiang J.Z., Fei Z., Giovannoni J.J. High-throughput Illumina strand-specific RNA sequencing library preparation. Cold Spring Harb. Protoc. 2011;2011:940–949. doi: 10.1101/pdb.prot5652. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.