Abstract
MDMB-FUBINACA (aka MDMB(N)-Bz-F), chemical name Methyl (S)-2-(1-(4-fluorobenzyl)-1H-indazole-3-carboxamido)-3,3-dimethylbutanoate, a designer drug or a new psychoactive substance (NPS), was identified in three commercially available e-liquids formulated for electronic cigarette use. The e-liquids were evaluated using direct analysis in real time ion source attached to a time of flight mass spectrometer (DART-MS) and gas chromatograph mass spectrometer (GC-MS) to identify active ingredients/drugs, flavorants, and other possible constituents. The e-liquids were also evaluated for alcohol content by headspace gas chromatography with flame ionization detector (HS-GC-FID). The aerosol produced from the e-liquids by use of an e-cigarette was analyzed by solid phase micro-extraction gas chromatography mass spectrometry (SPME-GC-MS) to ensure delivery of the active ingredient/drug. Propylene glycol, vegetable glycerin, MDMB-FUBINACA, alcohol content and a flavor profile were determined for each of the e-liquids. MDMB-FUBINACA was determined to be the major active ingredient in all three e-liquids and was successfully detected by SPME-GC-MS in the aerosol generated by a KangerTech Aerotank clearomizer/electronic cigarette.
Keywords: Electronic Cigarette, MDMB-FUBINACA, E-liquids, Designer Drugs, Cannabimimetics
Graphical abstract

Introduction
MDMB-FUBINACA (aka MDMB(N)-Bz-F), chemical name Methyl (S)-2-(1-(4-fluorobenzyl)-1H-indazole-3-carboxamido)-3,3-dimethylbutanoate, is a fluoridated indazole-3-carboxamide synthetic compound (Figure 1) which is considered a designer drug or a new psychoactive substance (NPS). Regarding the pharmacological effect of this compound, it might be referred as a synthetic cannabinoid (SCB) or cannabimimetic drug. Even though MDMB-FUBINACA is available worldwide via the Internet and the abuse of the drug seems to be increasing [1] there is little to no scientific/medical data concerning the pharmacology, pharmacokinetics, biotransformation or toxicology of the drug. Animal studies of intraperitoneal (i.p.) injection of MDMB-FUBINACA in rats resulted in bradycardia and a dramatic hypothermia which were blocked by the CB1 receptor antagonist rimonabant, [2] an indication a cannabinoid CB1 activity. Hypothermia and bradycardia are typically observed following administration of cannabinoids like tetrahydrocannabinol (THC), as well as, with structurally distinct cannabimimetic indole and indazole derivatives, in rats [3] and mice [4, 5]. Additionally, in vitro binding studies have demonstrated that MDMB-FUBINACA is a potent agonist for the CB1 and CB2 receptors [1, 2]. MDMB-FUBINACA is presently unscheduled by US Drug Enforcement Agency (DEA).
Figure 1.
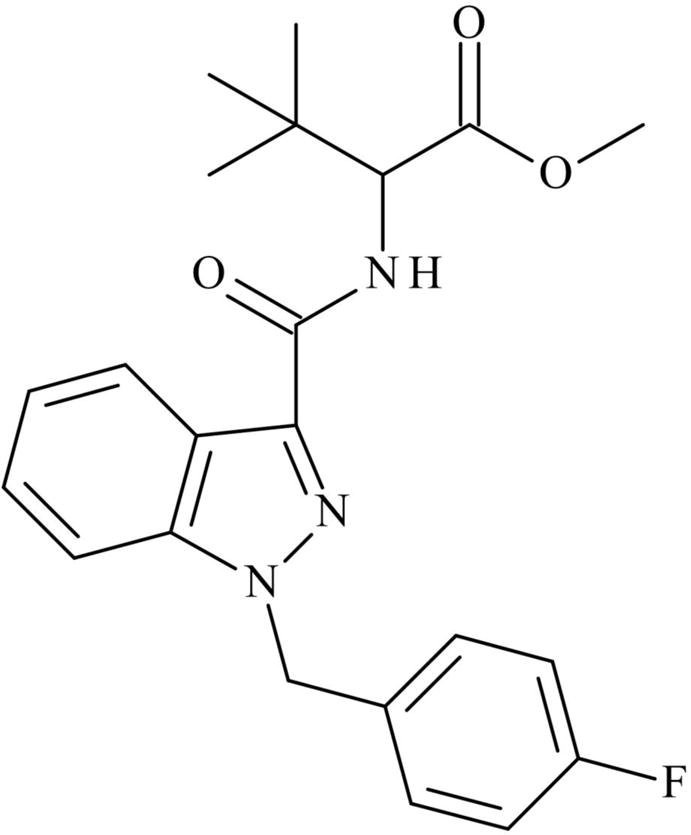
Chemical Structure of MDMB-FUBINACA
Electronic cigarettes (e-cigarettes) were developed as an alternative method for nicotine delivery. Their utility and popularity have transformed them into general drug-delivery devices that are inexpensive and easy to use. They work by either drawing negative pressure through the mouthpiece or depressing a button to activate a battery that heats a coil, containing a wick saturated with a formulation known as an e-liquid. E-liquids are made of propylene glycol and/or vegetable glycerin containing active ingredient(s) such as a pharmaceutical agent and/or herbal remedy. They often also contain flavoring agent(s). When the e-cigarette is activated, the e-liquid is vaporized, followed by rapid condensation into an aerosol that the user inhales [6, 7].
On May 5, 2016, the Food and Drug Administration (FDA) announced the extension of their authority to regulate all tobacco products, including e-cigarettes [8]. A significant reason to do so was to address the quality assurance of e-cigarette products, including the devices and the nicotine-based e-liquids they contain. The e-liquid formulations have been found to vary significantly from the advertised content [9–13].
Electronic cigarettes have become a popular means for using pharmaceuticals other than nicotine. This study characterized commercially available e-liquids with no advertised content. Chemical composition of the e-liquids was analyzed by direct analysis in real time ion source attached to a time of flight mass spectrometer (DART-MS) and a gas chromatograph mass spectrometer (GC-MS), concerning active ingredients, flavoring agents and other possible constituents, whereas the presence of alcohol was performed by headspace gas chromatography with flame ionization detector (HS-GC-FID). Lastly, the aerosol produced by an e-cigarette filled with e-liquid was analyzed by solid phase micro-extraction gas chromatography mass spectrometry (SPME-GC-MS).
Material and methods
Three different e-liquid samples, labeled with the designations “Blueberry B Juice”, “Regular B Juice” and “Vampire B Juice”, were purchased from the online store www.buzz-juice.com. No information was provided as to the contents of these e-liquids either on the website or on the vials containing the e-liquid. The 5.0 mL plastic e-liquid vials containing the e-liquids were labeled but did not list a lot number or date of production (Figure 2). Undissolved white crystalline powder could be seen at the bottom of each e-liquid. MDMB-FUBINACA primary reference standard (purity: ≥ 98%) was purchased from Cayman Chemicals (Ann Arbor, MI, USA) HPLC-grade methanol used for all dilutions, stock and working solution preparations was purchased from Pharmco-Aaper (Brookfield, CT, USA). Polyethylene glycol (PEG) with an average molecular mass of 600 Da used for DART-MS calibration was obtained from ULTRA Inc. (North Kingstown, RI, USA). Certified ACS grade Ammonium acetate, 1,4-butanediol, formic acid, HPLC grade methanol and de-ionized (DI) water, optima grade acetone, ethanol, methanol, n-propanol and isopropanol were purchased from Fisher Scientific (Hanover Park, IL, USA). The Ethanol 80 and Volatile Controls were purchased from UTACK® Laboratories, Inc. (Valencia, CA, USA). Nitrogen and helium gases were acquired from Praxair and Airgas (Richmond, VA, USA). Propylene glycol and glycerin were purchased from Wizard Labs (Altamonte Springs, FL, USA). The electronic cigarette used was a KangerTech Aerotank clearomizer (v2) attached to an eGo-V v2 variable voltage battery, purchased from 101vape.com (Carlsbad, CA, USA). A preassembled single coil purchased from Discount Vapes was wrapped in non-contact configuration with 34 gauge Nichrome wire to 1.8 Ω and a 2 mm diameter silica string was used as a wick (Orlando, FL). The flow meter used for the trap system was purchased from Cole Palmer (Vernon Hills, IL, USA). Solid-phase micro-extraction (SPME) fibers, 7 μm polydimethylsiloxane (PDMS) were purchased from Supelco (Bellefont, PA, USA). All tubing, glassware, and the fritted gas dispersion tubes were purchased from Colonial Scientific (Richmond, VA, USA).
Figure 2.

The three different e-liquid samples obtained form buzz-juice.com
Analysis of E-liquids by DART-MS
A JEOL JMS T100LC Accu-TOF™ DART-MS controlled by Mass Center software version 1.3.4 m (JEOL Inc. Tokyo, Japan) was used to screen the e-liquids for the presence of active ingredient(s) or drug(s), propylene glycol, glycerin, flavoring agents, and other possible components. Previously published DART-MS methods were adopted for this application [13, 14]. Each e-liquid was analyzed by three different sampling methods. The e-liquids were mixed by hand for 20 seconds before aliquoting. In the first sampling method, a capillary tube was dipped into an aliquot of the e-liquid and then the capillary tube was introduced into the helium stream of a DART-MS for the analysis. In the second sampling method, an aliquot of the e-liquid was held below the helium gas stream which allowed the analysis of only the volatile compounds in the e-liquids. In the third sampling method, a sample of the undissolved white crystalline powder from the bottom of the e-liquid vial was removed from the e-liquid, dried and then dissolved in methanol. This sample was then analyzed using a capillary tube as described in the first sampling method. Each sample was analyzed in positive-ion mode with a helium stream temperature of 350 °C. The flow rate was 2.0 L/min with a discharge electrode needle voltage at 150 V, and the grid electrode at 250 V. The ion guide peak voltage was 400 V, reflectron voltage was 900 V, orifice 2 was set to 5 V, and the ring lens was set to 3 V with orifice 1 set operating in function switching mode at 20, 60, or 90 V with a single data file created for all three voltages. The resolving power of the mass spectrometer was 6000 FWHM. The range of masses measured was from 40 to 1100 Da. Each e-liquid was analyzed five separate times to ensure reproducibility of the results. The data was analyzed by the creation of averaged, background subtracted, centroided mass spectra that was calibrated using the PEG 600. The active ingredients, propylene glycol and glycerin, were identified when the exact mass was detected within 5 mDa of its calculated monoisotopic mass (M+H)+ and the fragmentation pattern in function switching mode matched that of the primary references materials. All other compounds detected in the e-liquid were identified using a NIST 11.0 library.
Analysis of the E-liquid and the Aerosol Generated by E-liquids
The e-liquids were mixed by hand for 20 seconds before aliquoting. For the direct analysis of the e-liquids, 100 μL aliquots of each e-liquid along with primary reference materials consisting of MDMB-FUBINACA, at a concentration of 10 μg/mL in methanol, 100 % propylene glycol, and 100 % glycerin) were diluted 1:10 with methanol. These samples with methanol blanks in-between were analyzed by GC-MS. For the analysis of the aerosol, a KangerTech electronic cigarette containing approximately 0.5 mL aliquot of e-liquid was used to generate an aerosol. The electronic cigarette was activated for 4 seconds. aerosolizing approximately 10 μL of the e-liquid. The aerosol was introduced into a trap consisting of two Erlenmeyer flasks in tandem attached to a vacuum pump with a flow rate of 2.3 L/min. DI-water was placed in the flasks with a gas dispersion tube through which the aerosol is bubbled into the water. Glass wool was placed in between the 2 traps to contain the aerosol in the first trap. A 7 μm PDMS SPME fiber was inserted through a stopper in the top of the first trap. The fiber was introduced into the trap when the electronic cigarette was activated and the aerosol filled the trap. After five minutes, the fiber was retracted and removed. The fiber was then inserted into the injection port of the GC-MS and allowed to thermally desorb for 15 mins.
An Agilent 6890N Gas Chromatograph coupled to a 5973 Mass Selective Detector (Santa Clara, CA, USA) was used for both the e-liquid and aerosol analyses. The chromatographic separation was performed on a HP-5MS column 30 m × 0.25 mm id × 0.25 μm (Agilent, Santa Clara, CA, USA). The GC-MS was operated in splitless mode. The carrier gas was helium at a linear velocity of 35 cm/s. Oven temperature was programming from 120 °C to 300 °C at a rate of 10 °C/min and held for 12 min. The total run time was 30 min. The scan range for the mass spectrometer was 40–500 m/z. SPME fibers were thermally cleaned between runs following manufacturer specifications in order to ensure no carryover occurred between samples. The comparison of the retention time and mass spectrum of the samples verses those of the primary reference materials was used to identify MDMB-FUBINACA, propylene glycol, glycerin in both the direct analysis of the e-liquids and the aerosolized e-liquids. Flavoring agents were identified using a NIST 11.0 library (Figure 4).
Figure 4.
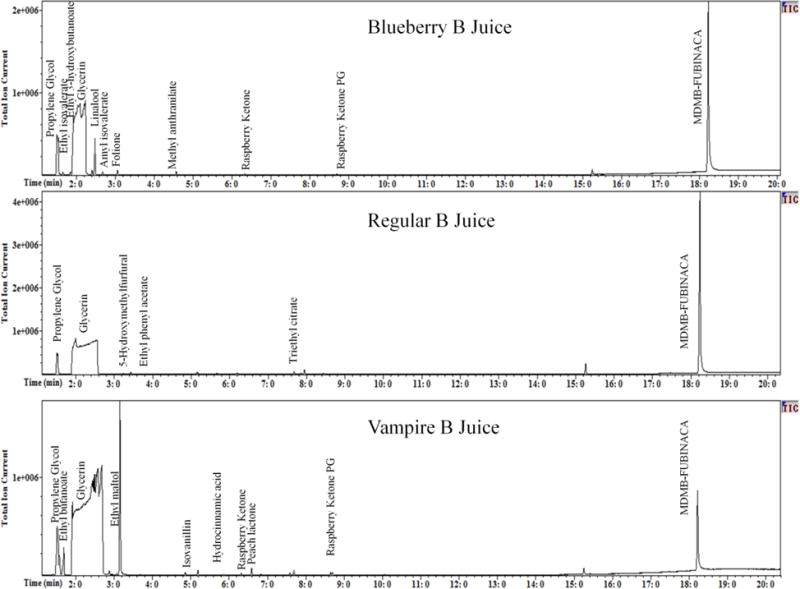
GC-MS total Ion chromatographs of Blueberry B Juice, Regular B Juice and Vampire B Juice e-liquids
Determination of Glycol Composition of E-liquids
The glycol composition of the e-liquids was determined using a modified method for glycol analysis in toothpaste used by the U.S. Food and Drug Administration (FDA) [15]. E-liquid samples were diluted 100 μL into 900 μL of de-ionized water containing and calibrators were prepared at 0.52, 1.04, 2.56, 5.18 and 10.36 mg/L propylene glycol and 0.63, 1.26, 3.15, 6.31 and 12.61 mg/L glycerin in water. An aliquot of 10 μL of internal standard, 71.4 mg/L of 1,4-butanediol, was added to each preparation. Samples were injected onto a Hewlett Packard 6890 Gas Chromatograph coupled to a 5973 Mass Spectrometer Detector (Hewlett Packard, Santa Clara, CA, USA) with a 30m Stabilwax column, 30m × 0.25mm ID × 0.25μm (Restek®, Bellefonte, PA, USA). The GC-MS was operated in split injection mode at a ratio of 20:1, with 1 μL injection volume. The carrier gas was helium at a flow of 35 cm/s. The inlet temperature was set to 250°C. Oven temperature programmed from 100°C held for 1 min then ramped to 250°C at a rate of 10 °C/min and held for 4 mins. The mass spectrometer was operated with a source temperature of 230°C in scan mode with a range of 29–400 m/z operated at 70 eV with a mass spectrometer source temperature of. Quantification was performed using the following mass ions (m/z): propylene glycol, 45; glycerin, 61; and 1,4-butanediol, 71. The total run time was 20 mins. The scan range for the mass spectrometer was 29–400 m/z. Propylene glycol and glycerin concentrations were determined by linear regression. The calibration curves were constructed using the peak area ratio counts of the quantifying ions for propylene glycol or glycerin and the internal standard versus the calibrator concentrations.
Volatile analysis of E-liquids
The ethanol content of the e-liquids was determined using a method routinely employed for the analysis of clinical and forensic samples for ethanol, acetone, isopropanol and methanol [16]. The e-liquid samples were diluted 100 μL into 900 μL of de-ionized water. Aliquots of 100 μL of the e-liquid dilutions, calibrator (100, 157, 393, 984, 1574, 3148 and 8000 mg/L ethanol), and ethanol free controls consisting of DI water, UTAK controls, (Ethanol 80 and Volatile controls), were added to 900 μL of 234 mg/L n-propanol serving as internal standard. Analysis was performed using a Tekmar HT3 Headspace sampler attached to a Shimadzu 2014 Gas Chromatograph (GC) with a flame ionization detector (FID). The chromatographic separation was performed on a RTX-BAC1, 30 m × 0.32 mm id × 1.80 μm column (Restek Corp, Bellefonte, PA, USA). The headspace oven and transfer line temperatures were set to 160 °C with a standby flow rate of 200 mL/min. The platen sample temperature was set to 80 °C with the mixer on. The sample equilibrium time was 3.5 min with a sample injection time of 0.5 min. The GC has an oven temperature of 50 °C and the injection temperature of 200 °C run in split mode with a 1:20 ratio. The column flow rate was 6.85 mL/min with purge flow of 0.5 mL/min. The detector temperature was 225 °C with both hydrogen and air set at 50 kPa. Ethanol concentrations were determined by linear regression. The calibration curve was constructed using peak ratio of the area counts of ethanol and the internal standard versus the calibrator concentrations.
Results
The contents of the e-liquid formulations were determined using a combination of primary reference material and a NIST 11.0 library when analyzing the DART-MS (Figure 3) and GC-MS (Figure 4) data. The DART-MS spectra produced while holding the e-liquids under the helium stream were used to indentify volatile compounds in the e-liquid formulations. This analysis resulted in the previously identified flavorants in each of the samples. The main identified active ingredient in each of the e-liquid formulations as well as the white crystalline powder found at the bottom of e-liquid vials was determined to be MDMB-FUBINACA. Identification of MDMB-FUBINACA, propylene glycol and glycerin was confirmed by comparing the retention times and the full-scan mass spectra to the primary references material to that of the samples (Figure 5). It was also confirmed by SPME-GC-MS analysis that the MDMB-FUBINACA was aerosolized using the KangerTech electronic cigarette.
Figure 3.
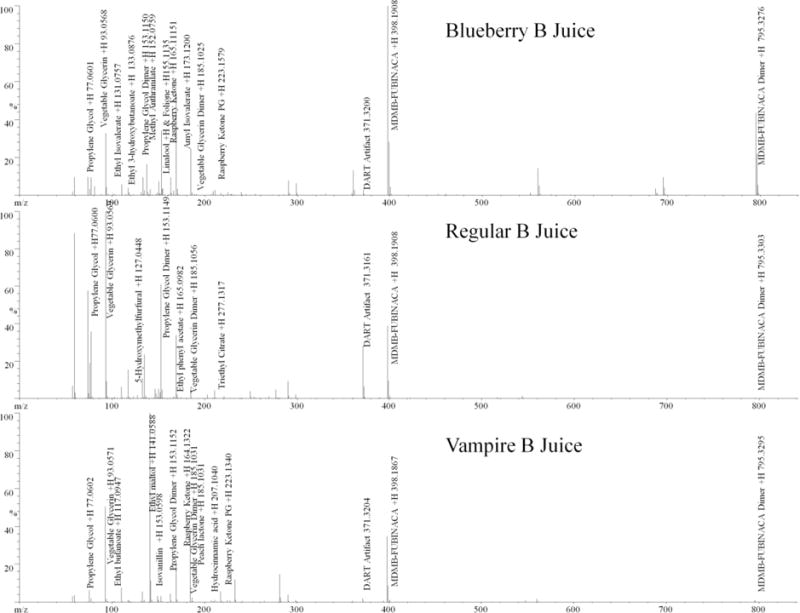
DART-MS spectra of Blueberry B Juice, Regular B Juice and Vampire B Juice e-liquids
Figure 5.
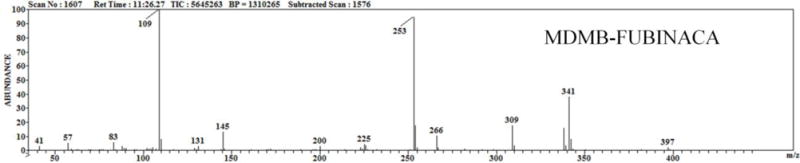
Ion spectra of MDMB-FUBINACA
All three e-liquids contained MDMB-FUBINACA as the main active ingredient. Blueberry B Juice contained the flavorants ethyl isovalerate (pineapple), ethyl 3-hydroxybutanoate (grape), linalool (citrus), amyl isobutyrate (apple), folione (fruit), raspberry ketone and raspberry ketone PG (raspberry) and 17.6% (w:v) ethanol with a propylene glycol to glycerin ratio of 74:26 (v:v). Regular B Juice contained the flavorants 5-hydroxymethyl furfural (butter), ethyl phenyl acetate (floral), and triethyl citrate (fruit) and 20.2% (w:v) ethanol with a propylene glycol to glycerin ratio of 52:48 (v:v). The Vampire B Juice e-liquid was found to contained the flavorants ethyl butanoate (pineapple), ethyl maltol (caramel), isovanillin (vanilla), hydrocinnamic acid (strawberry) peach lactone (peach) raspberry ketone and raspberry ketone PG (raspberry) and 24.6% (w:v) ethanol with a propylene glycol to glycerin ratio of 43:57 (v:v). A summary of the identified components of each e-liquid can be found in Table 1.
Table 1.
The identified components of each of the three Buzz brand e-liquids.
| Contents | Blueberry B Juice | Regular B Juice | Vampire B Juice |
|---|---|---|---|
| Ethanol | X | X | X |
| Glycerin | X | X | X |
| Propylene glycol | X | X | X |
|
| |||
| Flavorents | Blueberry B Juice | Regular B Juice | Vampire B Juice |
|
| |||
| Amyl isovalerate | X | ||
| Ethyl butanoate | X | ||
| Ethyl 3-hydroxybutanoate | X | ||
| Ethyl Isovalerate | X | ||
| Ethyl maltol | X | ||
| Ethyl phenyl acetate | X | ||
| Folione | X | ||
| Glycerin | X | ||
| Hydrocinnamic acid | X | ||
| 5-Hydroxymethylfurfural | X | ||
| Isovanillin | X | ||
| Linalool | X | ||
| Methyl anthranilate | X | ||
| Peach lactone | X | ||
| Raspberry Ketone | X | X | |
| Raspberry Ketone PG | X | X | |
| Triethyl citrate | X | ||
|
| |||
| Drug (NPS) | Blueberry B Juice | Regular B Juice | Vampire B Juice |
|
| |||
| MDMB-FUBINACA | X | X | X |
Discussion
Limited data concerning the effects of MDMB-FUBINACA are available at this time. A report from October 7, 2014 Russian Television attributed its use to 25 deaths, over 700 hospitalizations, and stated that “This synthetic cannabinoid is being dissolved into herbal tea, and then smoked”. The Federal Drug Control Service of the Russian Federation scheduled MDMB-FUBINACA as of March 17th, 2015 [17].
Commercially available e-liquids containing marijuana extracts and cannabidiol have been reported [7, 18]. In October 2015, Shanks KG presented an abstract at the annual Society of Forensic Toxicology meeting [19] that identified the presence of cannabimimetic drugs as the active ingredients in two e-liquids. These e-liquids, labeled as Buzz Juice Liquid Incense and Buzz Relax E-juice, were produced by the same company as the three e-liquids described. The major ingredient of the Buzz Juice Liquid Incense was identified as N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-1-(cyclohexylmethyl)-1H-indazole-3-carboxamide (MAB-CHMINACA) which was scheduled in the USA by the DEA as of September 16, 2015. The major active ingredient of the Buzz Relax E-juice was identified as (1-(5-fluoropentyl)-1H-indol-3-yl)(2,2,3,3-tetramethylcyclopropyl)methanone (XLR11), which was scheduled in the USA by the DEA as of May 16, 2013. MDMB-FUBINACA in the presented Buzz Juice e-liquids remains unscheduled in many countries, including the United States where these products were purchased.
Chemical composition of the e-liquids was screened for using DART-MS, which has been shown to be an effect technique for the identification active ingredients, flavoring agents and other possible constituents [13, 18]. The component of the e-liquids including the concentration of propylene glycol and glycerin were confirmed by using GC-MS methods. The presence and concentration of alcohol was performed by HS-GC-FID. MDMB-FUBINACA was determined to be the major active ingredient in all three e-liquids. SPME-CG-MS has historically been used to characterize the smoke of traditional cigarettes [20]. The present study sought to use a similar concept in order to analyze aerosol of e-liquids. The e-cigarette aerosolized the MDMB_FUBINACA as it was successfully extracted from the aerosol using SPME, then thermally desorbed by GC-MS.
The high concentration of ethanol in these e-liquids may have been used as a natural flavorant or as a solvent; however, the reason for the ethanol as an ingredient cannot be fully ascertained. The white crystalline powder in the bottom of the e-liquid vials identified as MDMB-FUBINACA was the result of saturation or insolubility of the drug. The undissolved crystalline powder in the e-liquid formulation may result in an inconsistent amount of drug being aerosolized by the electronic cigarette.
The FDA announced the extension of their authority to regulate all tobacco products, including e-cigarettes [8]. This extended authority may result in limiting the production of e-liquids by small manufactures in the USA, such as the products sold by www.buzz-juice.com. Other source not answerable to the FDA could continue to be problematic as many drugs or NPSs can easily be dissolved in an e-liquid and aerosolized.
Conclusion
Although e-liquids used in electronic cigarettes are commonly sold and labeled as containing nicotine many are also available that are labeled to contain other active ingredients such as cannabinoids [7, 18]. The evaluation of the three e-liquids, only labeled “Blueberry B Juice”, “Regular B Juice” and “Vampire B Juice” demonstrates that designer drugs such as MDMB-FUBINACA can be found in commercially available electronic cigarette products. Unlabeled e-liquids such as those discussed herein may lead to the unexpected consumption of potentially harmful drugs such as MDMB-FUBINACA. This drug was demonstrated to aerosolize and, therefore, has the potential for respiratory delivery.
Highlights.
Three commercially available e-liquids were analyzed by DART-MS, GC-MS, SPME-GC-MS and HS-GC-FID for their contents.
MDMB-FUBINACA, a cannabimimetic drug, was found to be the major active ingredient.
MDMB-FUBINACA was successfully aerosolized by an electronic cigarette.
Ethanol and numerous flavorants were also detected in the e-liquids.
Acknowledgments
This work was supported by the National Institutes of Health [grant numberP30DA033934] and National Institute of Justice, Office of Justice Programs, and the United States Department of Justice [award number 2014-R2-CX-K010]. The opinions, findings, and conclusions or recommendations expressed in this publication/program/exhibition are those of the author(s) and do not necessarily reflect those of the Department of Justice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shevyrin V, Melkozerov V, Nevero A, Eltsov O, Shafran Y, Morzherin Y, Lebedev AT. Identification and analytical characteristics of synthetic cannabinoids with an indazole-3-carboxamide structure bearing a N-1-methoxycarbonylalkyl group. Anal Bioanal Chem. 2015;407:6301–6315. doi: 10.1007/s00216-015-8612-7. [DOI] [PubMed] [Google Scholar]
- 2.Banister SD, Longworth M, Kevin R, Sachdev S, Santiago M, Stuart J, Mack JB, Glass M, McGregor IS, Connor M, Kassiou M. The pharmacology of valinate and tert-leucinate synthetic cannabinoids 5F-AMBICA, 5F-AMB, 5F-ADB, AMB-FUBINACA, MDMB-FUBINACA, MDMB-CHMICA, and their analogues. ACS Chem Neurosci. 2016;7:1241–1254. doi: 10.1021/acschemneuro.6b00137. [DOI] [PubMed] [Google Scholar]
- 3.Hine B, Torrelio M, Gershon S. Analgesic, Heart Rate, and Temperature Effects of O8-THC during Acute and Chronic Administration to Conscious Rats. Pharmacology. 1977;15:65–72. [PubMed] [Google Scholar]
- 4.Wiley JL, Marusich JA, Martin BR, Huffman JW. 1-Pentyl-3-phenylacetylindoles and JWH-018 share in vivo cannabinoid profiles in mice. Drug Alcohol Depen. 2012;123:148–153. doi: 10.1016/j.drugalcdep.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiley JL, Marusich JA, Lefever TW, Grabenauer M, Moore KN, Thomas BF. Cannabinoids in disguise: Delta9-tetrahydrocannabinol-like effects of tetramethylcyclopropyl ketone indoles. Neuropharmacology. 2013;75:145–154. doi: 10.1016/j.neuropharm.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breland A, Soule E, Lopez A, Ramôa C, El-Hellani A, Eissenberg T. Electronic cigarettes: what are they, and what do they do? Ann N Y Acad Sci. 2016 doi: 10.1111/nyas.12977. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peace MR, Stone JW, Poklis JL, Turner JBM, Poklis A. Analysis of a commercial marijuana e-cigarette formulation. J Anal Toxicol. 2016;40:374–378. doi: 10.1093/jat/bkw021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.U.S. Food and Drug Administration [FDA] FDA Takes Significant Steps to Protect Americans from Dangers of Tobacco through New Regulation. 2016 http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm499234.htm [accessed May 12, 2016]
- 9.Etter JF, Zather E, Svensson S. Analysis of refill liquids for electronic cigarettes. Addiction. 2013;108:1671–1679. doi: 10.1111/add.12235. [DOI] [PubMed] [Google Scholar]
- 10.Goniewicz ML, Kuma T, Gawron M, Knysak J, Kosmider L. Nicotine levels in electronic cigarettes. Nicotine Tob Res. 2013;15:158–166. doi: 10.1093/ntr/nts103. [DOI] [PubMed] [Google Scholar]
- 11.Kavvalakis MP, Stivaktakis PD, Tzatzarakis MN, Kouretas D, Liesivuori J, Alegakis AK, et al. Multicomponent analysis of replacement liquids of electronic cigarettes using chromatographic techniques. J Anal Toxicol. 2015;39:262–269. doi: 10.1093/jat/bkv002. [DOI] [PubMed] [Google Scholar]
- 12.Pagano T, DiFrancesco AG, Smith SB, George J, Wink G, Rahman I, et al. Determination of nicotine content and delivery in disposable electronic cigarettes available in the united states by gas chromatography-mass spectrometry. Nicotine Tob Res. 2015;18:700–707. doi: 10.1093/ntr/ntv120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peace MR, Baird TR, Smith N, Wolf CE, Poklis JL, Poklis A. Concentration of nicotine and glycols in 27 electronic cigarette formulations. J Anal Toxicol. 2016;40:403–407. doi: 10.1093/jat/bkw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poklis JL, Raso SA, Alford KN, Poklis A, Peace MR. Analysis of 25I-NBOMe, 25B-NBOMe, 25C-NBOMe and Other Dimethoxyphenyl-N-[(2-Methoxyphenyl) Methyl]Ethanamine derivatives on blotter paper. J Anal Toxicol. 2015;39:617–623. doi: 10.1093/jat/bkv073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Litzau J, Mulligan K. Gas Chromatography – Mass Spectrometry (GC-MS) Screening Procedure for the Presence of Diethylene Glycol and Ethylene Glycol in Toothpaste. U.S Food and Drug Administration; 2007. [Google Scholar]
- 16.O’Neal CL, Wolf CE, Levine B, Kunsman G, Poklis A. Gas chromatographic procedures for determination of ethanol in postmortem blood using t-butanol and methyl ethyl ketone as internal standards. Forensic Sci Int. 1996;83:31–38. doi: 10.1016/0379-0738(96)02007-5. [DOI] [PubMed] [Google Scholar]
- 17.25 killed, over 700 hospitalized: Cheap “Spice” designer drug causes severe poisoning across Russia. RT Question More. Published October 7, 2014. https://www.rt.com/news/193700-russia-spice-deadly-drug/ Accessed October 23, 2016.
- 18.Peace MR, Butler KE, Wolf CE, Poklis JL, Poklis A. Evaluation of Two Commercially Available Cannabidiol Formulations for Use in Electronic Cigarettes. Front Pharmacol. 2016;7:279. doi: 10.3389/fphar.2016.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shanks KG. P12 Detection of Synthetic Cannabinoids in Two E-Cigarette Liquids. http://www.soft-tox.org/files/meeting_abstracts/SOFT_2015_meeting_abstracts.pdf (accessed 12.08.16)
- 20.Watson CH, Trommel JS, Ashley DL. Solid-phase microextraction-based approach to determine free-base nicotine in trapped mainstream cigarette smoke total particulate matter. J Agric Food Chem. 2004;52:7240–7245. doi: 10.1021/jf049455o. [DOI] [PubMed] [Google Scholar]


