Abstract
Objectives
The objective was to review the use of Impella devices (Abiomed Inc, Danvers, MA) for temporary circulatory support in pediatric and adolescent patients (age ≤21 yrs).
Background
Options for minimally invasive circulatory support in children are limited, and published data are confined to case reports and small case series.
Methods
This was a retrospective, multicenter review of Impella implants in pediatric and adolescent patients from 2009–15, using standardized data collection and INTERMACS definitions.
Results
A total of 39 implants were performed in 38 patients from 16 centers. Median age and weight were 16 yrs (4–21 yrs) and 62 kg (15–134 kg). The primary indication for implant was cardiogenic shock in 28 patients (72%). Cardiac allograft rejection, myocarditis, or cardiomyopathy were the underlying diagnosis in 23 patients (59%); 11 patients had congenital heart disease. The median duration of support was 45 hrs (1–1224 hrs). Indications for explant included ventricular recovery in 16 patients, transition to another device in 12, death in 5, and transplant in 1. Survival was 85% at 7 days and 68% at 30 days. Major adverse events occurred in 8 patients: hemolysis in 3, bleeding in 2, stroke in 1 (unclear if related to Impella), sepsis in 1, and critical leg ischemia in 1. An increase in aortic regurgitation was noted in 3 patients, with no evidence of valve injury.
Conclusion
Temporary circulatory support with Impella devices is feasible in pediatric and adolescent patients, with acceptable risk profiles. More experience and follow up is needed to improve technical performance and patient selection.
Index terms: mechanical support, congenital heart disease, pediatrics
Introduction
Indications for mechanical circulatory support in children and young adults include heart failure related to congenital heart disease, cardiomyopathy, myocarditis and cardiac allograft failure. Options for temporary pediatric circulatory support are extremely limited. At present, the only devices approved by the US Food and Drug Administration (FDA) for temporary support are extracorporeal membrane oxygenation (ECMO) and intra-aortic balloon pump (IABP), both of which commonly require surgical placement for pediatric use. The invasive nature of ECMO and the limited hemodynamic support provided by IABP have led to rapid growth of percutaneous mechanical circulatory support devices in the adult population(1–3). While these devices were initially developed to support high-risk patients suffering from acute left ventricular (LV) dysfunction related to acute myocardial infarction (AMI), utilization has increased to longer-term management of cardiogenic shock (CGS). The Impella 2.5, Impella CP, Impella 5.0 and Impella LD (Abiomed, Danvers, MA) assist devices are now approved for short-term support (4–6 days depending on the device) for treatment of refractory or ongoing CGS after AMI or following open-heart surgery(4–6).
As experience with these devices has demonstrated safety and efficacy in adults, off-label use in the pediatric population has grown, in part because the Impella provides both hemodynamic support and cardiac unloading, which may be a more appealing approach to short-term circulatory support than ECMO, which can fail to reduce LV wall stress or myocardial oxygen demand or IABP, which is not an active flow or LV unloading device and may be limited in its hemodynamic effect by end-diastolic flow reversal demonstrated in animal models(3,7–15). Thus far, the pediatric experience has been limited to isolated case reports and small case series(9–11). More data are needed regarding safety, efficacy, and indications for use of the Impella devices in pediatric and adolescent patients. Therefore, we performed a multicenter retrospective study in order to describe a larger pediatric experience with the Impella family of catheters, with particular focus on outcomes and device-related complications.
Methods
Patients
This was a multicenter, retrospective study of Impella support in pediatric and adolescent patients with either acquired or congenital heart disease. Interventional cardiologists at centers who had implanted Impella devices in patients ≤21 years of age were contacted and invited to submit data for this study, based on a list of implanting centers provided by the device manufacturer (Abiomed, Inc). Individual centers were not required to submit data for the study. The age threshold for defining the pediatric patient population (≤21 years of age) was based on the 2014 Food and Drug Administration guidance(16). Baseline demographic data were collected for each patient, as were procedural variables, patient survival and duration of mechanical support. Major adverse events were recorded as defined by INTERMACS/PediMACS(17). Patients were also classified according to the pre-implant INTERMACS level of illness profile(18). The INTERMACS profile of advanced heart failure is a 7 point scale that provides a general clinical description of patients undergoing mechanical circulatory support. INTERMACS 1 is the most severe category, and describes a patient with critical cardiogenic shock, escalating inotropic support requirements and organ hypoperfusion while INTERMACS 7 describes a patient with New York Heart Association (NYHA) class 3 symptoms.
Impella Support
This study included cases in which an Impella device was used to support the systemic circulation due to acute ventricular failure/dysfunction or during high-risk catheter-based procedures. All versions of the Impella designed for the left heart were included (2.5, 5.0, CP and LD). Utilization of the Impella device, including device selection, access vessel, method of implant, and device management were at the discretion of the managing physicians. There were no procedural or patient care protocols in place to guide management decisions for this retrospective study. The use of additional circulatory support devices (ECMO, IABP, other left ventricular assist device [LVAD]) before, during, or after Impella support was recorded along with the indications for additional support.
Data Collection and Analysis
Participating centers completed and returned case report forms developed specifically for this study. The primary outcomes were 7-day and 30-day survival, patient and device disposition, and adverse events. Adverse events of particular interest included stroke, hemolysis, access vessel complications, device malfunction, bleeding, infection, and increase in aortic regurgitation. Adverse events were classified as major or minor according to INTERMACS/PediMACS definitions(17). Data were tabulated and presented largely in descriptive form. Limited exploratory analysis was conducted in an effort to identify differences in survival, adverse events according to patient, procedure, and device related variables. Nominal or categorical variables were expressed as number and percent of total. Continuous variables were expressed as median (minimium-maximum).
Results
Patients
Over 160 pediatric and adolescent patients have been supported with Impella to date. Data were available for 39 implants in 38 patients from 2009 to 2015. One patient underwent 2 separate device implants around a brief period of ECMO support, but all others were in unique patients. Baseline patient demographics are presented in Table 1. The median age at implant was 16 years (4–21 years), with 6 patients ≤10 years of age. The median weight was 62 kg (15–134 kg) and 6 patients were ≤30 kg. The mean body surface area was 1.62 ± 0.36. Patients with congenital heart disease (28%), acute rejection following heart transplant (26%), and deteriorating function with dilated cardiomyopathy (DCM; 23%) were the most commonly supported in this cohort. The indications for implant were ventricular dysfunction with acute CGS in 28 patients, planned support during high-risk arrhythmia-ablation procedures in 4 patients, support of chronic heart failure refractory to other management in 6 patients, and arrhythmia in 1 patient. The INTERMACS profile was 1 in 28 patients and 2 in 7 patients, while 4 patients had an Impella for high-risk procedural support.
Table 1.
Baseline demographics
| n = 39 | |
|---|---|
| Age at implant (yrs) | 16 (4–21) |
| Weight (kg) | 62 (15–134) |
| Body surface area (m2) | 1.62 ± 0.36 |
| Male (n, %) | 27 (69%) |
| Impella device type | |
| 2.5 | 15 (38%) |
| CP | 19 (49%) |
| 5.0 | 5 (13%) |
| Diagnosis | |
| Congenital heart disease | 11 (28%) |
| Post heart transplant | 10 (26%) |
| Dilated cardiomyopathy | 9 (23%) |
| Myocarditis | 4 (10%) |
| Post-partum cardiomyopathy | 2 (5%) |
| Arrhythmia | 2 (5%) |
| Drug overdose | 1 (3%) |
| Indication for support | |
| Cardiogenic shock | 28 (72%) |
| Planned procedural support | 4 (10%) |
| Refractory heart failure | 6 (15%) |
| Arrhythmia | 1 (3%) |
| Ejection Fraction (%) | 15 (5–51) |
Data are presented as mean±SD, median (minimum-maximum) or number (%)
Procedural and Outcome Data
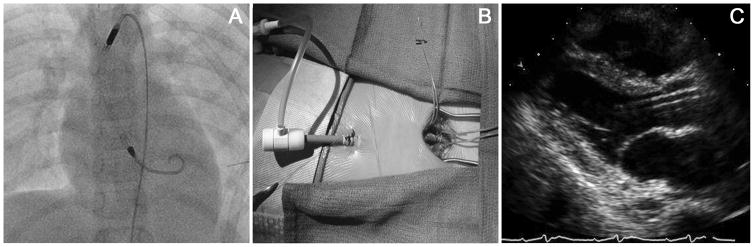
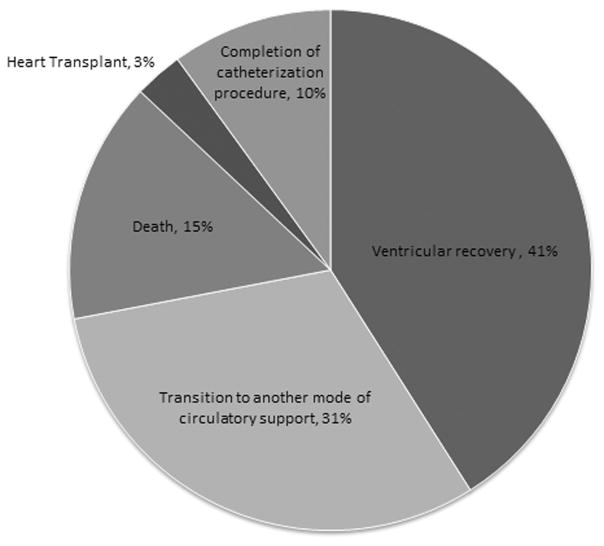
Most patients were supported with Impella 2.5 and CP devices, typically via femoral arterial access, regardless of age or size (Table 2). As previously described, a surgically placed chimney graft was employed in 31% of cases, 7 through a femoral artery and 5 through an axillary artery (Figure 1)(19). Device repositioning after the initiation of support and any immediate adjustments was required in 38% (15 of 38) of cases with no significant difference in the need for repositioning by access type. The median duration of support was 45 hours, 6 patients were supported for longer than 10 days (240 hours), and 1 was supported for 51 days. In the majority of patients the device was explanted due to ventricular recovery (41%) or transition to a different mode of mechanical support (31%)(Figure 2).
Table 2.
Procedural data
| n=39 | |
|---|---|
| Implant site | |
| Femoral artery | 33 (85%) |
| Axillary artery | 6 (15%) |
| Chimney graft (yes, %) | 12 (31%) |
| Repositioning required (yes, %) | 15 (38%) |
| Additional mechanical support | 19 (49%) |
| Prior to Impella placement | 1 |
| Impella used to decompress LV on ECMO | 3 |
| ECMO added after Impella support | 3 |
| Transition to ECMO | 6 |
| Transition to LVAD | 6 |
| Duration of support (hrs) | 45 (1–1224) |
| Indication for explant | |
| Ventricular recovery | 16 (41%) |
| Transition to different mode of support | 12 (31%) |
| Completion of high-risk catheterization procedure | 4 (10%) |
| Death | 6 (15%) |
| Heart transplant | 1 (3%) |
Data are presented as median (minimum-maximum) or number (%)
Figure 1.
Placement of an Impella 2.5 in an 11 year old weighing 30.9 kg. (A) This chest radiograph demonstrates appropriate placement of the Impella via femoral artery cutdown and chimney graft. (B) This is a femoral artery cutdown with a Gore-Tex chimney graft tunneled subcutaneously with a modified 14-French sheath secured within the graft for Impella placement. (C) Parasternal long axis echocardiographic image demonstrating appropriate positioning of the Impella within the left ventricular outflow tract away from the mitral valve.
Figure 2.
Indications for Impella device explant. Of the 39 total device implants, 16 (41%) were explanted for ventricular recovery, 12 (31%) for transition to another mode of mechanical circulatory support, 4 (10%) following completion of a high risk catheterization procedure, 6 (15%) following patient death with the device in place, and 1 (3%) prior to heart transplant.
Additional mechanical support in the form of ECMO or VAD was used in 49% of patients before, during, or after the Impella. In 6 of those cases, the Impella was used to decompress the left heart on ECMO (n=3) or ECMO was added to augment the support provided by the Impella (n=3). In 12 patients, the Impella served as a bridge to ECMO or durable LVAD. The most common indication for transitioning from Impella to ECMO was the need for additional support, typically of the right ventricle. Heart transplant rejection patients maintained on another form of mechanical support were more likely to undergo Impella placement to augment that existing support modality than patients with other diagnoses (30% of transplant patients vs 3.6% of others, p=0.048), whereas patients with DCM were more likely to transition to another form of mechanical support from Impella relative to patients with other diagnoses (78% of DCM patients vs 30% of others, p=0.01).
Twelve of the 38 patients (32%) died within 30 days of implant, 6 within 7 days (15%). Six of the 12 deaths occurred after the Impella was explanted (median 12 days; 2–22 days), including 5 who had been transitioned to another device (ECMO in 3, VAD in 2), and 1 who died after bridging to heart transplant.
Major adverse advents were noted in 8 patients, as summarized in Table 3. Some patients experienced more than 1 adverse event. Vascular access-related events were reported in 2 patients, 1 of whom experienced critical limb ischemia necessitating device removal. This patient was initially supported with an Impella for myocarditis but deteriorated with intractable arrhythmias and was transitioned to ECMO. The Impella was utilized as a left-sided vent, resulting in bifemoral arterial access. Ischemia of the left lower extremity was noted on post-implant day 1, with no evidence of flow by Doppler, so the device was removed. Lower extremity perfusion remained compromised even after device removal and the patient ultimately developed severe rhabdomyolysis necessitating below-the-knee amputation. There was no further cardiac activity for 7 more days and ultimately the patient died of multisystem organ failure. Severe bleeding occurred in 2 patients. There were no reports of gastrointestinal bleeding. Significant hemolysis based on laboratory criteria occurred in 3 patients, leading to device removal and transition to ECMO in 2. One stroke was documented but it was unclear if this was directly related to the device itself. An increase in the severity of aortic regurgitation was reported in 3 patients, 2 who had baseline mild regurgitation and 1 who returned to baseline after device removal. None had documented evidence of valve injury following explant. Minor adverse events that did not meet INTERMACS definitions of major adverse events were also recorded and included minor hemolysis in 9 patients and access site bleeding in 6. Minor device related problems that did not result in device explant or patient complication occurred in 2 patients. There was no apparent association between age, size, underlying diagnosis, or indication for support and any of the outcomes reported.
Table 3.
Major Adverse events
| Patients experiencing adverse events | 8* |
| Device malfunction | 1 |
| Hemolysis | 3 |
| Neurologic dysfunction | 1 |
| Access related | 2 |
| Bleeding | 2 |
| Infection | 1 |
Some patients experienced more than 1 adverse event during the course of mechanical support.
Discussion
Options for temporary circulatory support remain limited for pediatric and adolescent patients. Extrapolation from the adult experience has led to limited off-label use in children and young adults, although there are few data on safety and efficacy in this patient population. This study reports the largest experience to date of mechanical support of pediatric and adolescent patients with the Impella family of devices. Immediate-term findings demonstrated an acceptable safety profile of the Impella family of catheters for temporary circulatory support in pediatric patients suffering from acute CGS. While 30-day mortality was high, it was similar to the largest adult study of Impella for CGS with similar or slightly lower complication rates(6,20).
Severe adverse events occurred in 8 patients. In smaller pediatric and adolescent patients concerns arise regarding vascular access related adverse events, bleeding and hemolysis. Limb ischemia requiring device explant was encountered in only 1 patient and severe bleeding in 2 patients. Severe hemolysis based on INTERMACS criteria occurred in 8% of cases, comparable to adult studies of Impella support for cardiogenic shock, where hemolysis rates have ranged from 7.5% to 62.5% (21,22). Overall adverse event rates in our population are similar to studies of Impella support in adult patients. In addition, device repositioning was required in almost 40% of patients, regardless of access method and location, indicating the achieving a stable implant remains an important issue in this population. Ongoing improvements in device design and novel techniques for vascular access as well as greater familiarity with percutaneous MCS strategies should drive improvements in adverse events and make these devices accessible to smaller patients.
The benefits of LV volume unloading with the Impella device include reduction in LV wall stress, improvement in coronary blood flow, and reduction in myocardial oxygen consumption(2,7,23). While no consistent hemodynamic data were obtained during the period of mechanical support in this multicenter cohort, nearly 40% of patients were able to be explanted for ventricular recovery, which suggests that the early utilization of temporary support such as Impella prior to the worsening of CGS and end-organ dysfunction may allow adequate time for recovery prior to consideration of a more permanent form of ventricular support. Patients with DCM were more likely to transition from Impella to another form of mechanical support, which may reflect a need for more durable support in this particular subset of patients. Additionally, the assessment of right heart function is important as adequate support of the systemic circulation with Impella is difficult in the setting of impaired RV function and may play a significant role in the need to transition to other support modalities. Impella devices were also used successfully to support high-risk arrhythmia ablation procedures, and to facilitate left-heart decompression in conjunction with ECMO, providing an alternative that may offer advantages over other percutaneous options(24).
In conclusion, in this multicenter retrospective study of Impella devices used for temporary systemic circulatory support in children as young as 4 years and as small as 15 kg, and for up to 51 days, preliminary findings regarding technical feasibility, clinical efficacy, and safety were encouraging. We recognize that these findings are limited not only by the retrospective study design but also by variable institutional participation. While these data cannot be generalized, they represent a solid advance in the collective experience with the Impella devices in pediatric patients. Ultimately, additional data will be necessary to understand the nuances and comparative benefits and risks of Impella in children and young adults, preferably through a prospective clinical trial. However, the evaluation of new circulatory support technologies in the pediatric population is a major challenge due to size and vulnerability of this population as well as the heterogeneity of disease. Thus, despite the limitations of these preliminary data, we are hopeful that they can serve as a foundation for further studies and evaluation of ongoing utilization in this high-risk population.
Acknowledgments
Data collection and management for this study were supported in part by a NIH Small Business Innovation Research Grant 5R44HL099192 to Abiomed, Inc. (PIs: Scott Corbett PhD, Sonya Bhavsar MSc, Noam Josephy MD, MSc, MBA)
References
- 1.Rihal CS, Naidu SS, Givertz MM, Szeto WY, Burke JA, Kapur NK, Kern M, Garratt KN, Goldstein JA, Dimas V, et al. 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care: Endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention. J Am Coll Cardiol. 2015;65(19):e7–e26. doi: 10.1016/j.jacc.2015.03.036. [DOI] [PubMed] [Google Scholar]
- 2.Naidu SS. Novel percutaneous cardiac assist devices: the science of and indications for hemodynamic support. Circulation. 2011;123(5):533–43. doi: 10.1161/CIRCULATIONAHA.110.945055. [DOI] [PubMed] [Google Scholar]
- 3.Bartoli CR, Rogers BD, Ionan CE, Pantalos GM. End-diastolic flow reversal limits the efficacy of pediatric intra-aortic balloon pump counterpulsation. J Thorac Cardiovasc Surg. 2014;147(5):1660–7. doi: 10.1016/j.jtcvs.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abiomed. Abiomed Impella therapy receives FDA approval for cardiogenic shock after heart attack or heart surgery. 2016 Apr 7; [press release] [Google Scholar]
- 5.FDA. SUMMARY OF SAFETY AND EFFECTIVENESS DATA (SSED) - HRPCI. http://www.accessdatafda.gov/cdrh_docs/pdf14/P140003S005B.pdf.
- 6.FDA. SUMMARY OF SAFETY AND EFFECTIVENESS DATA (SSED) - AMI/CS, PCCS. http://www.accessdatafda.gov/cdrh_docs/pdf14/P140003S005B.pdf.
- 7.Burkhoff D, Sayer G, Doshi D, Uriel N. Hemodynamics of Mechanical Circulatory Support. J Am Coll Cardiol. 2015;66(23):2663–74. doi: 10.1016/j.jacc.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 8.Kapur NK, Zisa DC. Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) fails to solve the haemodynamic support equation in cardiogenic shock. EuroIntervention. 2016;11(12):1337–9. doi: 10.4244/EIJV11I12A261. [DOI] [PubMed] [Google Scholar]
- 9.Dimas VV, Murthy R, Guleserian KJ. Utilization of the Impella 2. 5 micro-axial pump in children for acute circulatory support. Catheter Cardiovasc Interv. 2014;83(2):261–2. doi: 10.1002/ccd.25042. [DOI] [PubMed] [Google Scholar]
- 10.Andrade JG, Al-Saloos H, Jeewa A, Sandor GG, Cheung A. Facilitated cardiac recovery in fulminant myocarditis: pediatric use of the Impella LP 5. 0 pump. J Heart Lung Transplant. 2010;29(1):96–7. doi: 10.1016/j.healun.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Hollander SA, Reinhartz O, Chin C, Yeh J, Maeda K, Mallidi H, Bernstein D, Rosenthal D. Use of the Impella 5. 0 as a bridge from ECMO to implantation of the HeartMate II left ventricular assist device in a pediatric patient. Pediatr Transplant. 2012;16(2):205–6. doi: 10.1111/j.1399-3046.2011.01578.x. [DOI] [PubMed] [Google Scholar]
- 12.Vanderpluym CJ, Fynn-Thompson F, Blume ED. Ventricular assist devices in children: progress with an orphan device application. Circulation. 2014;129(14):1530–7. doi: 10.1161/CIRCULATIONAHA.113.005574. [DOI] [PubMed] [Google Scholar]
- 13.Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367(14):1287–96. doi: 10.1056/NEJMoa1208410. [DOI] [PubMed] [Google Scholar]
- 14.Sjauw KD, Engstrom AE, Vis MM, van der Schaaf RJ, Baan J, Jr, Koch KT, de Winter RJ, Piek JJ, Tijssen JG, Henriques JP. A systematic review and meta-analysis of intra-aortic balloon pump therapy in ST-elevation myocardial infarction: should we change the guidelines? Eur Heart J. 2009;30(4):459–68. doi: 10.1093/eurheartj/ehn602. [DOI] [PubMed] [Google Scholar]
- 15.Seyfarth M, Sibbing D, Bauer I, Frohlich G, Bott-Flugel L, Byrne R, Dirschinger J, Kastrati A, Schomig A. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008;52(19):1584–8. doi: 10.1016/j.jacc.2008.05.065. [DOI] [PubMed] [Google Scholar]
- 16.Premarket Assessment of Pediatric Medical Devices - Guidelines for Industry and Food and Drug Administration Staff. Issued March 24, 2014. http://www.fda.gov/RegulatoryInformation/Guidances/ucm089740.htm.
- 17.Rosenthal DN, Almond CS, Jaquiss RD, Peyton CE, Auerbach SR, Morales DR, Epstein DJ, Cantor RS, Kormos RL, Naftel DC, et al. Adverse events in children implanted with ventricular assist devices in the United States: Data from the Pediatric Interagency Registry for Mechanical Circulatory Support (PediMACS) J Heart Lung Transplant. 2016;35(5):569–77. doi: 10.1016/j.healun.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blume ED, Rosenthal DN, Rossano JW, Baldwin JT, Eghtesady P, Morales DL, Cantor RS, Conway J, Lorts A, Almond CS, et al. Outcomes of children implanted with ventricular assist devices in the United States: First analysis of the Pediatric Interagency Registry for Mechanical Circulatory Support (PediMACS) J Heart Lung Transplant. 2016;35(5):578–84. doi: 10.1016/j.healun.2016.01.1227. [DOI] [PubMed] [Google Scholar]
- 19.Murthy R, Brenes J, Dimas VV, Guleserian KJ. Ringed polytetrafluoroethylene (Gore-Tex) tunneled “chimney” graft for pediatric use of Impella 2. 5 axial flow pump. J Thorac Cardiovasc Surg. 2014;147(4):1421–2. doi: 10.1016/j.jtcvs.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 20.Lemaire A, Anderson MB, Lee LY, Scholz P, Prendergast T, Goodman A, Lozano AM, Spotnitz A, Batsides G. The Impella device for acute mechanical circulatory support in patients in cardiogenic shock. Ann Thorac Surg. 2014;97(1):133–8. doi: 10.1016/j.athoracsur.2013.07.053. [DOI] [PubMed] [Google Scholar]
- 21.Badiye AP, Hernandez GA, Novoa I, Chaparro SV. Incidence of Hemolysis in Patients with Cardiogenic Shock Treated with Impella Percutaneous Left Ventricular Assist Device. ASAIO J. 2016;62(1):11–4. doi: 10.1097/MAT.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 22.Lauten A, Engstrom AE, Jung C, Empen K, Erne P, Cook S, Windecker S, Bergmann MW, Klingenberg R, Luscher TF, et al. Percutaneous left-ventricular support with the Impella-2. 5-assist device in acute cardiogenic shock: results of the Impella-EUROSHOCK-registry. Circ Heart Fail. 2013;6(1):23–30. doi: 10.1161/CIRCHEARTFAILURE.112.967224. [DOI] [PubMed] [Google Scholar]
- 23.Kapur NK, Qiao X, Paruchuri V, Morine KJ, Syed W, Dow S, Shah N, Pandian N, Karas RH. Mechanical Pre-Conditioning With Acute Circulatory Support Before Reperfusion Limits Infarct Size in Acute Myocardial Infarction. JACC Heart Fail. 2015;3(11):873–82. doi: 10.1016/j.jchf.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Eastaugh LJ, Thiagarajan RR, Darst JR, McElhinney DB, Lock JE, Marshall AC. Percutaneous left atrial decompression in patients supported with extracorporeal membrane oxygenation for cardiac disease. Pediatr Crit Care Med. 2015;16(1):59–65. doi: 10.1097/PCC.0000000000000276. [DOI] [PubMed] [Google Scholar]