Abstract
Purpose
Pancreatic neuroendocrine tumors (PanNETs) frequently utilize the Alternative Lengthening of Telomeres (ALT) pathway for telomere maintenance. ALT is strongly correlated with ATRX and DAXX alterations and a poor prognosis in primary PanNETs. Since fine needle aspiration (FNA) is a non-invasive way of sampling tumors, we evaluated whether we could accurately detect ALT and loss of ATRX/DAXX in a primary PanNET cohort of FNAs.
Experimental design
All pre-operative FNA cytology cases (2005–2016) with adequate remnant FNA cell block material were assessed for ALT by telomere-specific fluorescent in situ hybridization (FISH) and ATRX and DAXX protein expression by immunohistochemistry (IHC). For 21 patients undergoing tumor resection, the resected case was also assessed to determine the concordance between the FNA and surgical specimen.
Results
In the primary PanNET cohort of 65 FNAs, ALT was detected in 15 cases (23%). While all ATRX-negative and DAXX-negative tumors were ALT-positive, 3 of 14 (21%) ALT-positive cases did not show nuclear loss of either ATRX or DAXX. The ALT-positive tumors were associated with larger radiographic size (4.9 v. 2.4 cm, on average, p<0.05) and higher grade (p<0.05). Overall, there was 100% concordance in ALT status and ATRX/DAXX IHC results between the FNA and surgical specimen.
Conclusions
Both ALT and loss of ATRX/DAXX can be accurately performed on FNA specimens with adequate material. Since ALT is a fundamental mechanism of pathogenesis, the ability to determine ALT in small biospecimens has implications for the design of clinical trials.
Keywords: Pancreatic neuroendocrine tumors, ALT, ATRX, DAXX
INTRODUCTION
Pancreatic neuroendocrine tumors (PanNETs) are the second most common malignancy of the pancreas, and the 10-year survival rate of patients with this disease is only 40%1, 2. While PanNETs have an incidence of approximately 1 per 100,000 individuals per year, incidence in the United States has been increasing, perhaps due to better detection techniques3. In addition to prevalent mutations in MEN1, previous whole exome sequencing studies revealed that ~45% of PanNETs harbored mutually exclusive, inactivating mutations in either ATRX or DAXX4. While HIRA, the H3.3 chaperone, regulates localization of H3.3 to genes and regulatory regions (e.g. promoters and enhancers); H3.3 localization to telomeres appears to be independent of HIRA5. In contrast, the ATRX/DAXX complex deposits histone variant H3.3 in repetitive heterochromatic chromosomal regions, in particular at telomeres and pericentromeric regions5–7. We previously established an association between the somatic inactivating mutations in either ATRX or DAXX and the presence of the Alternative Lengthening of Telomeres (ALT) pathway, which is a telomerase-independent mechanism to maintain telomere lengths8. While most cancers maintain their telomeres through activation of the enzyme telomerase, telomere maintenance in ALT-positive cancers is mediated through homology-directed DNA repair9, 10.
Recent studies have demonstrated that, in comparison to ALT-negative and ATRX/DAXX intact tumors, ALT-positive and ATRX/DAXX-negative primary PanNETs tend to be larger in size, have a higher WHO grade, and are more likely to develop lymph node and distant metastases11–13. ALT is a critical mechanism of carcinogenesis in PanNETs, and as such, our ability to evaluate the status of these biomarkers will aid in the design of future clinical trials. For example, emerging data in other tumor types have demonstrated that ALT-positivity and/or ATRX loss confers in vitro sensitivity to radiation, as well as inhibition of topoisomerase and the DNA damage mediator, ATR14, 15. These early findings suggest that selected PanNETs may also share a similar sensitivity to these therapeutic strategies.
Because fine needle aspiration (FNA) is a non-invasive way of sampling tumors, we evaluated whether we could accurately detect the presence of ALT and ATRX/DAXX loss in FNAs from a cohort of primary PanNETs. For a subset of cases from patients undergoing tumor resection, we also evaluated the resected case to determine the concordance between the FNA and the surgical specimen.
MATERIALS AND METHODS
Case Selection
After approval from the Institutional Review Board for a waived consent retrospective data review, the pathology database at the Johns Hopkins Medical Institutions was searched for all pre-operative FNA cytology cases between 2005 and 2016. All cases were acquired either under trans-abdominal or endoscopic ultrasound-guided FNA with on-site evaluation of adequacy. Each identified case was reviewed and the diagnosis was confirmed. Cases in which the cell block hematoxylin and eosin (H&E) or subsequent levels contained more than 100 cells were included; cases with fewer cells did not contain sufficient material for the appropriate biomarker analysis.
ATRX and DAXX Immunohistochemistry
Immunohistochemistry (IHC) was performed on formalin-fixed, paraffin embedded tissue sections from cell block preparations from FNA specimens or surgical resection specimens. Immunolabeling for ATRX was performed using an anti-ATRX (HPA001906, Sigma-Aldrich, 1:100) antibody. The labeling was performed in the Ventana Benchmark XT autostainer using CC1 antigen retrieval buffer for 30 minutes at 95°C, incubation with primary antibody for 32 minutes, and detection with Ventana ultraView Universal DAB. In contrast immunolabeling for DAXX was performed as previously described8, 13 using an anti-DAXX (HPA008736, Sigma-Aldrich, 1:100) antibody. Briefly, tissue sections were deparaffinized, hydrated in xylene, and serially diluted in ethanol. For antigen retrieval, sections were steamed with citrate buffer for 30 min and then blocked against endogenous peroxidase activity with dual endogenous enzyme blocking agent (Dako) for 10 min. Sections were incubated with primary antibody (1:100 dilution) for 2 hours at room temperature followed by secondary antibody (Leica Microsystems) for 30 min and detected with 3,30-diaminobenzidine (Sigma-Aldrich). Sections were counterstained with hematoxylin, rehydrated, and mounted. Only nuclear labeling was evaluated for ATRX and DAXX. Cases were scored as negative for ATRX or DAXX if nuclear expression was completely lost (despite retaining cytoplasmic expression) and adequate internal positive controls were present.
Telomere-specific Fluorescence in Situ Hybridization (FISH)
As previously described8, 13, 16, deparaffinized slides were hydrated, steamed for 25 minutes in citrate buffer (Vector Laboratories, Burlingame, CA), dehydrated, and hybridized with a Cy3-labeled peptide nucleic acid (PNA) probe complementary to the mammalian guanine-rich telomere repeat sequence (N-terminus to C-terminus; Cy3-CCCTAACCCTAACCCTAA). As a positive control for hybridization efficiency, an Alexafluor-488 labeled PNA probe specific to human centromeric DNA repeats for the CENP-B binding sequence (N-terminus to C-terminus; Alexafluor-488-ATTCGTTGGAAACGGGA) was included in the hybridization solution. Following post-hybridization washes, the slides were counterstained with DAPI. Slides were imaged with a Nikon 50i epifluorescence microscope equipped with X-Cite series 120 illuminator (EXFO Photonics Solutions Inc., Ontario, CA) and appropriate fluorescence excitation/emission filters. Grayscale images were captured using Nikon NIS-Elements software and an attached Photometrics CoolsnapEZ digital camera. Images were pseudo-colored and merged.
Characteristics of ALT-positive tumors in fixed tissue specimens include dramatic cell-to-cell telomere length heterogeneity and the presence of large, ultra-bright nuclear foci of telomere FISH signals marking ALT-associated telomeric DNA in interphase nuclei8, 17. As such, cases were visually assessed and classified as ALT-positive if ultra-bright nuclear foci of telomere FISH signals were present in the cancer cells. The total intensities of individual ALT-associated, ultra-bright nuclear foci have been previously measured to be >10-fold higher than that of mean integrated signal intensities for individual non-neoplastic cells in the same cases8, 13, 17.
Statistical Analysis
Data were summarized using descriptive statistics (count and frequency for categorical variables; mean and range for continuous variables). Fisher’s exact tests were used to compare the distribution of categorical variables between groups. Student’s t-tests were used to test the difference in means between groups. Disease-specific survival for each patient was calculated as the length of time from the date of diagnosis to the date of last follow-up or death. Patients who died due to causes other than their PanNET disease were censored at their time of death. We estimated survival curves using the Kaplan-Meier method, and differences in the survival distributions between groups were tested using log-rank tests.
For cases not undergoing resection, stage was estimated (“estimated stage”) based on radiographic size and the presence or absence of metastases. An estimated stage 1A was defined as tumors with radiographic size <2cm and no synchronous or metachronous metastasis. One case fit these criteria but was called pT2 on resection and thus is considered stage 1B. Similarly, we estimated tumor grade (“estimated grade”) based on the Ki67 proliferation index as observed on the cell block material when a tumor was not resected, except in two instances in which the material on cell block was overtly high grade. If Ki67 had not been previously performed on a specimen, we did not estimate tumor grade for that case. The Ki67 proliferation index was estimated as a percentage of neoplastic cells demonstrating nuclear positivity for Ki67 under light microscopy without digital device assistance.
We fit univariate and multivariate Cox proportional hazards models to quantify the hazard ratios associated with covariates of interest and death. Two-tailed p-values of less than 0.05 were considered statistically significant. All analyses were performed using R v. 3.3.0.
RESULTS
Clinicopathologic Characteristics
The clinicopathologic characteristics of the 65 patients included in the study are shown in Table 1. The mean age at the time of diagnosis was 59 years (range: 23–84 years) and 51% of patients were women. The mean radiographic size of tumors at the time of diagnosis was 3 cm (range: 0.4–14.5 cm) and 55% of tumors were located in the pancreatic body or tail. The tumor grade was known or was estimated in a total of 53 neoplasms; 70% of these cases were classified as grade 1. The remaining 16 cases were grade 2 or higher. Of the 65 patients, 29 (45%) underwent surgical resection. There was excellent correlation between gross size and radiographic size at the time of diagnosis (r=0.87). Fourteen (22%) patients presented with unresectable disease and succumbed within one year of the FNA diagnosis; 5 of these patients received chemotherapy or radiation. In contrast, 10 (15%) patients did not have subsequent follow-up records at our institution or documentation of death in the social security index. At the time this study was completed, 14 (22%) patients were not surgically resected and continued to undergo surveillance. Almost 90% of patients had a non-functional PanNET and 11% of patients had an established functional PanNET (n=7). Based on radiologic tumor size and presence of metastasis, the tumor stage was known or estimated as stage 1A (n=37, 60%) or stage 1B or higher (n=24, 40%).
Table 1.
Clinicopathologic characteristics based on ALT pathway activation and loss of DAXX/ATRX expression.
| All Cases | ALT-negative | ALT-positive | p-value | ATRX/DAXX-negative | ATRX/DAXX-positive | p-value | |
|---|---|---|---|---|---|---|---|
| N | 65 | 50 | 15 | 11 | 50 | ||
| Gender | |||||||
| Female | 33 | 25 | 8 | 1.00 | 6 | 23 | 0.74 |
| Male | 32 | 25 | 7 | 5 | 27 | ||
| Race | |||||||
| White | 51 | 38 | 13 | 1.00 | 10 | 38 | 0.72 |
| Black | 7 | 6 | 1 | 0 | 6 | ||
| Other | 7 | 6 | 1 | 1 | 6 | ||
| Mean Age (Range), years | 58.6 (23–84) | 58.6 (23–84) | 58.7 (43–82) | 0.99 | 60.5 (44–82) | 58.12 (23–84) | 0.57 |
| Mean Radiographic Tumor Size (Range), cm | 2.98 (0.4–14.5) | 2.35 (0.49–6.30) | 4.92 (0.4–14.5) | 0.024 | 4.84 (0.4–10.5) | 2.32 (0.49–6.3) | 0.020 |
| Functional | |||||||
| No | 58 | 44 | 14 | 1.00 | 11 | 44 | 0.58 |
| Yes | 7 | 6 | 1 | 0 | 6 | ||
| Location+ | |||||||
| Head, neck, or uncinate | 29 | 24 | 5 | 0.38 | 5 | 23 | 1.00 |
| Body and tail | 35 | 25 | 10 | 6 | 26 | ||
| Resection Grade | |||||||
| 1 | 20 | 17 | 3 | 0.036 | 3 | 17 | 0.18 |
| 2 | 7 | 3 | 4 | 4 | 3 | ||
| 3 | 2 | 2 | 0 | 0 | 2 | ||
| Estimated Grade* | |||||||
| 1 | 34 | 30 | 4 | 0.012 | 3 | 29 | 0.024 |
| 1–2 | 3 | 1 | 2 | 2 | 1 | ||
| 2 | 10 | 5 | 5 | 4 | 6 | ||
| 2–3 | 1 | 1 | 0 | 0 | 1 | ||
| 3 | 5 | 5 | 0 | 0 | 5 | ||
| Synchronous Metastases+ | |||||||
| Absent | 47 | 38 | 9 | 0.20 | 6 | 39 | 0.12 |
| Present | 17 | 11 | 6 | 5 | 10 | ||
| Metachronous Metastases+ | |||||||
| Absent | 61 | 47 | 14 | 1.00 | 11 | 47 | 1.00 |
| Present | 2 | 2 | 0 | 0 | 2 | ||
| ALT | |||||||
| Negative | 0 | 47 | <0.0001 | ||||
| Positive | 11 | 3 |
Estimated grade includes specimens in which tumor grade was estimated based on the Ki67 proliferation in cell block material when the tumor was not surgically resected.
Sufficient data was not available in some instances to definitively determine the tumor location (1 case), synchronous metastases (1 case), and metachronous metastases (2 cases).
Expression of ATRX and DAXX Correlates with ALT Activation
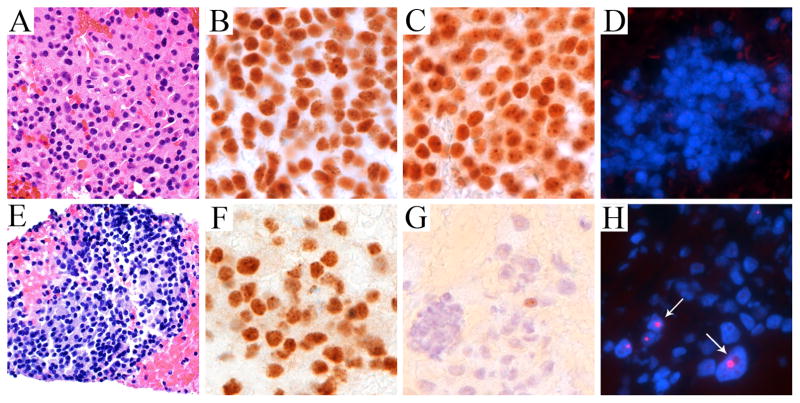
The results of immunolabeling for ATRX and DAXX, and of the telomere-specific FISH for ALT are shown in Figure 1. Four specimens contained only enough residual material for FISH; in these cases, the expression of ATRX and DAXX could not be assessed. Loss of nuclear expression of ATRX was identified in 6 FNA samples (10%), and loss of nuclear expression of DAXX was identified in 5 additional FNAs (8%). All cases of ATRX loss were associated with intact DAXX labeling and vice versa, so that 11 cases in total had loss of nuclear expression of ATRX or DAXX (18%). ALT was detected in 15 cases (23%). While all ATRX-negative and DAXX-negative tumors were ALT-positive, we did identify 3 of 14 (21%) ALT-positive cases in which both ATRX and DAXX remained intact. These three cases represent smaller tumors (≤ 3.0 cm), while one patient had MEN-1 syndrome and another patient had a synchronous metastasis. Of these cases, one tumor was resected (grade 2), another tumor was predicted to be grade 1 based on FNA material, and the third tumor did not have a Ki67 index to allow grade prediction. Within the entire cohort, ALT-positive tumors were associated with larger radiographic size (4.9 v. 2.4 cm, on average, p<0.05) and higher grade (p<0.05). Similarly, ATRX/DAXX-negative tumors were associated with larger radiographic size (4.8 v. 2.3 cm, on average, p<0.05) and higher known or estimated grade (p<0.05). We did not observe significant associations between presence of ALT or ATRX/DAXX loss and patients’ age, gender, race, tumor functionality, location, or stage (Table 1).
Figure 1. Representative ALT-negative and ALT-positive pancreatic neuroendocrine tumors detected on fine needle aspirates.
H&E (A, E), ATRX (B, F), DAXX with retained nuclear expression (C) and loss of nuclear expression (G), and telomere-specific FISH to detect ALT (D, H). Arrows highlight cells with ultra-bright telomeric DNA foci indicative of ALT (DAPI nuclear counterstain). All images were taken at × 400 original magnification.
Correlation with Surgical Resection Specimens
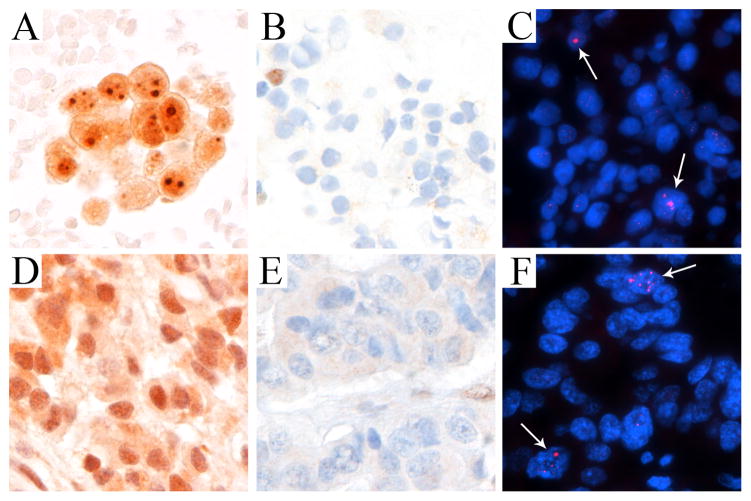
Among 29 surgical resection specimens, 20 could be evaluated by IHC for ATRX and DAXX and 21 could be evaluated for ALT by telomere-specific FISH. Overall, there was 100% (21/21) concordance for telomere-specific FISH for ALT on cytology and surgical pathology; 16 negative specimens were called negative on resection while 5 positive specimens were called positive on resection. In comparison, we also found 100% concordance between ATRX/DAXX IHC on cytology and surgical pathology. Specifically, ATRX showed 100% (20/20) concordance between the two specimen types; 3 negative specimens were called negative/heterogeneous on resection while 17 positive specimens were called positive on resection (one not evaluated on resection). Additionally, DAXX showed 100% (20/20) concordance; 2 negative specimens were called negative on resection while 18 positive specimens were called positive on resection (one not evaluated on resection). An example of the concordance between the FNA and corresponding resected case is shown in Figure 2.
Figure 2. Concordance of a pancreatic neuroendocrine tumor detected on a fine needle aspirate (A–C) and on the resected specimen (D–F).
DAXX with retained nuclear expression (A, D), ATRX with loss of nuclear expression (B, E), and telomere-specific FISH to detect ALT (C, F). Arrows highlight cells with ultra-bright telomeric DNA foci indicative of ALT (DAPI nuclear counterstain). All images were taken at × 400 original magnification.
Survival Outcomes and Prognostic Factors
The mean follow-up time in this cohort was 21 months (median = 10.2 months, range = 0–132 months). A total of 12 patients died during the follow-up period, of which 11 were from disease-specific causes. Estimated disease-specific survival rates at 1-year and 2-years were 82% (95% CI: 72–94%) and 78% (95% CI: 67–92%), respectively. We assessed the prognostic significance of clinical and pathologic covariates by comparing the survival distributions between groups of interest using log-rank tests and quantified effects using univariate Cox proportional hazards models. As expected, we found that higher-grade tumors, advanced primary tumor (pT) stage, overall stage (stage 1B or higher vs. 1A), perineural invasion, and lymph node and synchronous metastases were each associated with decreased survival (Table 2). Surgical resection was associated with a survival advantage (HR: 0.091 95% CI: 0.01–0.71). In this cohort, ALT status and ATRX/DAXX labeling were not prognostic of survival or among the subset of patients undergoing surgical resection. Multivariate analyses were limited by the small number of observed deaths in our cohort.
Table 2.
Univariate Cox proportional hazards model.
| Patient/Tumor Characteristics | No. of Patients/No. of Deaths | HR (95%CI) | p-value |
|---|---|---|---|
| Gender (male vs. female) | 65/11 | 0.72 (0.22–2.38) | 0.59 |
| Age (years) | 65/11 | 1.03 (0.98–1.07) | 0.27 |
| Functional (yes vs. no/unknown) | 65/11 | 0 (0-Inf) | 1.00 |
| Location (head/neck/unc vs. body/tail) | 64/10 | 1.15 (0.33–3.98) | 0.83 |
| Radiologic Tumor Size (>=2cm vs. <2cm) | 62/10 | 6.01 (0.76–47.51) | 0.089 |
| Resection Grade (2 or 3 vs. 1) | 30/3 | 4.79 (0.43–53.19) | 0.20 |
| Predicted Grade (>=2 vs. <2) | 53/9 | 9.45 (1.96–45.61) | 0.0052 |
| LVI (present vs. absent) | 26/1 | Inf (0-Inf) | 1 |
| Perineural Invasion (present vs. absent) | 26/1 | Inf (0-Inf) | 1 |
| ALT (positive vs. negative) | 65/11 | 0.79 (0.17–3.69) | 0.767 |
| ATRX/DAXX (either negative vs. both positive) | 61/9 | 0.70 (0.09–5.69) | 0.737 |
| Treatment (surgery vs. other) | 58/11 | 0.09 (0.01–0.71) | 0.022 |
| DAXX (negative vs. positive) | 62/10 | 0.43 (0.05–3.58) | 0.43 |
| ATRX (negative vs. positive) | 61/9 | 0 (0-Inf) | 1.00 |
| Synchronous Metastasis (Yes vs. No) | 64/10 | 29.59 (3.74–233.90) | 0.0013 |
| Metachronous Metastasis (Yes vs. No) | 63/9 | 0 (0-Inf) | 1.00 |
Abbreviations: LVI, lymphovascular invasion; unc, uncinate; CI, confidence interval; No., number
DISCUSSION
FNA is an important, minimally invasive method for obtaining diagnostic material from patients presenting with a pancreatic mass. The material is often sufficient for ancillary molecular testing, which is becoming increasingly important to guide pre- or non-surgical management in a variety of patients - including patients with unresectable tumors, those planning for neoadjuvant therapy, and those undergoing surveillance of small tumors as part of conservative management. While the evaluation of ALT among PanNET resection specimens has previously been described11–13, this is the first study to examine the use of telomere-specific FISH to determine ALT status on FNA specimens. Analysis of ALT on FNA specimens may help clinicians identify which small, low-grade tumors should be resected or monitored through surveillance. Importantly, because telomere maintenance through ALT activation is a fundamental mechanism of carcinogenesis, identifying ALT-positive PanNETs will become increasingly important in the design of clinical trials for new targeted therapies, including small molecules that may target the ALT pathway14, 15.
One limitation of FNA is that the small sampling may be discordant with the corresponding larger surgical resection. FNA may sample only certain areas of heterogeneous tumors, causing results that may differ from what is seen in the final resection specimens. For instance, Ki67 proliferation indices may be discordant in PanNETs18, 19. Here, we found no discordance between 21 FNA and resection specimen pairs for ALT analysis. In addition, we found no discordance between 20 FNA and resection specimen pairs for ATRX and DAXX IHC. However, a previous study of resected specimens identified PanNET cases with intratumoral heterogeneity by observing heterogeneous staining patterns of ALT and loss of ATRX or DAXX, suggesting the potential for misclassifying the status of the tumor based on the FNA biomarker results alone11. Finally, while validation studies are needed, telomere-specific FISH for ALT may be a more ideal assay since 21% (3 of 14 cases) of the ALT-positive tumors maintained nuclear expression of ATRX and DAXX, suggesting that nuclear protein expression may not always mirror the mutational status of the gene (e.g. missense mutations)8, 11, 12.
In summary, we have found that both ATRX/DAXX IHC and telomere-specific FISH can be accurately performed on FNA specimens to reliably assess ALT status, as long as the cell block material used for analysis is adequate. In many instances, dedicated passes for cell block preparations are already performed once tumor cells are identified during on site evaluation. This practice allows for the performance of ancillary studies, including neuroendocrine markers (e.g. synaptophysin and chromogranin) and Ki67 analysis. As a result, we conclude that ATRX/DAXX IHC and telomere-specific FISH can be reliably performed in FNA specimens to determine ALT status early in the diagnostic evaluation and this testing may be useful in the design of clinical trials.
Acknowledgments
Funding for this study was provided by NIH grant CA62924 and the Sol Goldman Pancreatic Cancer Research Center.
Footnotes
Disclosure of potential conflicts of interest: No potential conflicts of interest were disclosed.
Author Contributions:
Christopher J. VandenBussche: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Writing – review and editing, Supervision, Project administration
Derek B. Allison: Methodology, Validation, Formal analysis, Investigation, Writing – review and editing
Mindy K. Graham: Validation, Investigation, Writing – review and editing
Vivek Charu: Methodology, Formal analysis, Writing – original draft, Writing – review and editing, Visualization
Anne Marie Lennon: Conceptualization, Resources, Writing – review and editing
Christopher L. Wolfgang: Formal analysis, Resources, Writing – review and editing
Ralph H. Hruban: Conceptualization, Writing – review and editing
Christopher M. Heaphy: Conceptualization, Methodology, Investigation, Resources, Writing – original draft, Writing – review and editing, Visualization, Supervision, Project administration
References
- 1.Ekeblad S, Skogseid B, Dunder K, Oberg K, Eriksson B. Prognostic factors and survival in 324 patients with pancreatic endocrine tumor treated at a single institution. Clin Cancer Res. 2008;14(23):7798–803. doi: 10.1158/1078-0432.CCR-08-0734. [DOI] [PubMed] [Google Scholar]
- 2.Halfdanarson TR, Rubin J, Farnell MB, Grant CS, Petersen GM. Pancreatic endocrine neoplasms: epidemiology and prognosis of pancreatic endocrine tumors. Endocr Relat Cancer. 2008;15(2):409–27. doi: 10.1677/ERC-07-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hallet J, Law CH, Cukier M, Saskin R, Liu N, Singh S. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015;121(4):589–97. doi: 10.1002/cncr.29099. [DOI] [PubMed] [Google Scholar]
- 4.Jiao Y, Shi C, Edil BH, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331(6021):1199–203. doi: 10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg AD, Banaszynski LA, Noh KM, et al. Distinct factors control histone variant H3. 3 localization at specific genomic regions. Cell. 2010;140(5):678–91. doi: 10.1016/j.cell.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drane P, Ouararhni K, Depaux A, Shuaib M, Hamiche A. The death-associated protein DAXX is a novel histone chaperone involved in the replication-independent deposition of H3. 3. Genes Dev. 2010;24(12):1253–65. doi: 10.1101/gad.566910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong LH, Ren H, Williams E, et al. Histone H3. 3 incorporation provides a unique and functionally essential telomeric chromatin in embryonic stem cells. Genome Res. 2009;19(3):404–14. doi: 10.1101/gr.084947.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heaphy CM, de Wilde RF, Jiao Y, et al. Altered telomeres in tumors with ATRX and DAXX mutations. Science. 2011;333(6041):425. doi: 10.1126/science.1207313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho NW, Dilley RL, Lampson MA, Greenberg RA. Interchromosomal homology searches drive directional ALT telomere movement and synapsis. Cell. 2014;159(1):108–21. doi: 10.1016/j.cell.2014.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dilley RL, Verma P, Cho NW, Winters HD, Wondisford AR, Greenberg RA. Break-induced telomere synthesis underlies alternative telomere maintenance. Nature. 2016;539(7627):54–58. doi: 10.1038/nature20099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singhi AD, Liu TC, Roncaioli JL, et al. Alternative Lengthening of Telomeres and Loss of DAXX/ATRX Expression Predicts Metastatic Disease and Poor Survival in Patients with Pancreatic Neuroendocrine Tumors. Clin Cancer Res. 2017;23(2):600–09. doi: 10.1158/1078-0432.CCR-16-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marinoni I, Kurrer AS, Vassella E, et al. Loss of DAXX and ATRX are associated with chromosome instability and reduced survival of patients with pancreatic neuroendocrine tumors. Gastroenterology. 2014;146(2):453–60. e5. doi: 10.1053/j.gastro.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Kim JY, Brosnan-Cashman JA, An S, et al. Alternative lengthening of telomeres in primary pancreatic neuroendocrine neoplasms is associated with aggressive clinical behavior and poor survival. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-16-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flynn RL, Cox KE, Jeitany M, et al. Alternative lengthening of telomeres renders cancer cells hypersensitive to ATR inhibitors. Science. 2015;347(6219):273–7. doi: 10.1126/science.1257216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koschmann C, Calinescu AA, Nunez FJ, et al. ATRX loss promotes tumor growth and impairs nonhomologous end joining DNA repair in glioma. Sci Transl Med. 2016;8(328):328ra28. doi: 10.1126/scitranslmed.aac8228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cesare AJ, Heaphy CM, O’Sullivan RJ. Visualization of Telomere Integrity and Function In Vitro and In Vivo Using Immunofluorescence Techniques. Curr Protoc Cytom. 2015;73:12 40 1–31. doi: 10.1002/0471142956.cy1240s73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heaphy CM, Subhawong AP, Hong SM, et al. Prevalence of the alternative lengthening of telomeres telomere maintenance mechanism in human cancer subtypes. Am J Pathol. 2011;179(4):1608–15. doi: 10.1016/j.ajpath.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farrell JM, Pang JC, Kim GE, Tabatabai ZL. Pancreatic neuroendocrine tumors: accurate grading with Ki-67 index on fine-needle aspiration specimens using the WHO 2010/ENETS criteria. Cancer Cytopathol. 2014;122(10):770–8. doi: 10.1002/cncy.21457. [DOI] [PubMed] [Google Scholar]
- 19.Hasegawa T, Yamao K, Hijioka S, et al. Evaluation of Ki-67 index in EUS-FNA specimens for the assessment of malignancy risk in pancreatic neuroendocrine tumors. Endoscopy. 2014;46(1):32–8. doi: 10.1055/s-0033-1344958. [DOI] [PubMed] [Google Scholar]