SUMMARY
Objective
The objective of this study was to evaluate the association of urine clusterin/apolipoprotein J (Apo J) with the development and/or progression of diabetic kidney disease (DKD) in type 2 diabetes.
Materials and methods
159 type 2 diabetic patients and 20 non-diabetic subjects with estimated glomerular filtration rate (eGFR) ≥60 mL/min/1.73 m2 were enrolled. The baseline values of urine clusterin and tubular damage markers were measured. The primary outcome was the annual decline rate in eGFR and secondary outcomes were the development of chronic kidney disease (CKD) stage 3 or greater and the persistence/progression of albuminuria. The median follow-up duration of enrolled patients was 3.0 (1.0–5.9) years.
Results
Baseline clusterin levels in urine were significantly increased in type 2 diabetic subjects compared with those of non-diabetic subjects. The levels of urine clusterin had a significant correlation with urine tubular damage markers. A positive correlation between the annual rate of decline in eGFR and urine clusterin after adjusting for clinical confounding factors was detected. Multivariate analysis further indicated that urine clusterin correlated with the development of CKD stage 3 or greater and persistence/progression of albuminuria. In type 2 diabetic subjects with albuminuria, urine clusterin remained associated with the annual decline rate in eGFR and the progression of CKD stage.
Conclusions
Urine clusterin reflects tubular damage in the early-stage of DKD. The increase of urine clusterin along with albuminuria could be an independent predictive marker for the progression of DKD in type 2 diabetes.
Keywords: Albuminuria, Clusterin, Diabetic nephropathy, Glomerular filtration rate, Proteinuria
INTRODUCTION
The prevalence of diabetes is increasing worldwide and its complication of diabetic kidney disease (DKD) is a major global healthcare and socioeconomic burden, with serious effects for individual patients.1–3 DKD is clinically characterized by persistent albuminuria and a progressive decline in glomerular filtration rate (GFR) and is the leading cause of end-stage renal disease (ESRD).2 To improve the lives of people with DKD, identification of a new marker that can predict the development and progression of DKD at an early stage is urgently needed.
It is clear that the presence of albuminuria is considered to be an early sensitive marker of DKD.2 However, significant glomerular damage has already occurred when albuminuria is present.4 In addition, there is a limited value in the use of albuminuria because not everyone with DKD and reduced GFR has increased albuminuria and because the methodology for measuring albuminuria is not well standardized due to individual variability.2 Thus, the issue of whether albuminuria can be used as an early marker for DKD has been an outstanding subject in the field.
Over the past few years, significant advances have been made in identifying new diagnostic tools and non-invasive biomarkers for DKD, especially by using urine samples.5,6 Urine biomarkers in DKD patients can be categorized based on the pathogenic features of glomerular or tubular dysfunction.6 Although glomerular dysfunction has been thought to be a key factor for the development and/or progression of DKD, recent studies demonstrate that involvement of renal tubular damage is crucial in the pathogenesis of DKD.7,8 In clinical practice, the importance of tubular damage has been underestimated because of the limitation of sensitive tests for this in human subjects.9
Clusterin, also known as apolipoprotein J (Apo J), is a glycoprotein expressed ubiquitously in various metabolic tissues and body fluids.10 Clusterin is shown to be involved in the regulation of remodelling, lipid transport, complement inhibition, and apoptosis.11 Clusterin also plays an important role in cardiovascular-related diseases, including dyslipidaemia, atherosclerosis, obesity, and type 2 diabetes,12–14 all of which are pathogenic features of insulin resistance. In response to acute renal injury, including ischaemia/reperfusion injury, toxin-induced kidney injury, and unilateral ureteral obstruction, clusterin is rapidly induced in the kidney and urine.15 Interestingly, a higher level of clusterin is found in dedifferentiated tubular cells of the kidney.16 Thus, it is important to know that urine clusterin could be used as a tubular damage marker and is involved in development and/or progression of DKD.
In the current study, we evaluated the clinical implications of urine clusterin on the early development and/or progression of DKD in human subjects with type 2 diabetes. We also determined whether urine clusterin correlates with urine tubular makers and the annual rate of decline in eGFR in type 2 diabetic patients. Finally, we further evaluated whether urine clusterin can predict the persistence/progression of chronic kidney disease (CKD) stage and the persistence or progression of albuminuria.
SUBJECTS AND METHODS
Study population and design
A cohort of patients with type 2 diabetes mellitus and eGFR ≥60 mL/min/1.72 m2 was monitored for renal impairment through the Diabetic Kidney Disease Study [DKDS]) at Pusan National University Hospital in Busan, Korea. To identify early biological markers for DKD, type 2 diabetic patients were consecutively enrolled from outpatient clinics during two periods that were one year apart (1st DKDS cohort [from February 2010 to January 2011] and 2nd DKDS cohort [from February 2012 to February 2014]). The eligibility inclusion and exclusion criteria were described in a previous study17: To establish the early biomarkers for DKD, patients had relatively conserved renal function by meeting the following inclusion criteria: 1) eGFR ≥60 mL/min/1.73 m2, and serum creatinine <106 μmol/l, 2) stable renal function without a 2-fold elevation of serum creatinine for at least 5 months, and 3) no history of administration of renin-angiotensin system (RAS) inhibitors. Patients who had acute or chronic conditions that affected their renal function or urinary samples were excluded if they had: 1) a history of renal diseases other than diabetic nephropathy, 2) active urinary infection, 3) neoplastic, inflammatory disorders, 4) severe liver dysfunction, 5) uncontrolled thyroid disorders, 6) were pregnant, and 7) a recent history of acute myocardial infarction, stroke, or occlusive peripheral vascular disease. A total of 159 type 2 diabetic patients were enrolled. Age and sex matched non-diabetic subjects were randomly enrolled (n=20). They also fulfilled the following criteria: 1) no prior history of diabetes, renal disease or cardiovascular diseases, including hypertension, 2) fasting plasma glucose levels of <5.5 mmol/l after an overnight fasting, and 3) eGFR of ≥60 mL/min/1.73 m2 and serum creatinine <106 μmol/l. This study was performed in accordance with the Declaration of Helsinki and the protocol was approved by the Institutional Review Board of Pusan National University Hospital (20100024). Each patient provided written informed consent before enrollment. Data are available to all interested researchers on request to the Institutional Review Board of Pusan National University Hospital.
As a part of the DKDS, patients’ follow-up is still ongoing. Appropriate diabetes management, according to well-known standard guidelines, was provided by two endocrinologists and one nephrologist through their outpatient clinics. Of the 159 patients, 19 were excluded during follow-up for the following reasons: 12 were hospitalized for other severe acute and chronic diseases; 6 patients were diagnosed with malignancies, and 1 patient died of other causes during the follow-up period. Urine and blood samples for measurement of biological markers were collected at intervals of 12±1 (mean±SD) months during the follow-up period. Our primary outcome was the annual decline rate in eGFR during follow-up. The secondary outcomes were development of CKD stage 3 or greater and persistence/progression of albuminuria. To follow-up the renal outcomes, serum creatinine (for calculation of eGFR) and albumin-to-creatinine ratio (ACR) were measured using the same methods at intervals of 6±1 (mean±SD) months. For the analysis of this study, the patients were followed up until March 2016.
Metabolic parameter measurements and definitions
Random spot urine and blood samples were collected from each patient at their clinic visit. Medical histories and anthropometric measurements were also recorded the same day. The eGFR was estimated using the following equation derived from the Modification of Diet in Renal Disease (MDRD) formula: MDRD = 175 × (serum creatinine [μmol/L] × 0.0113L])−1.154 × (age in years)−0.203.18 A correction factor of 0.742 was used for females. The annual decline rate in eGFR was calculated as (eGFR at baseline – eGFR at last visit)/years of follow-up. CKD stage 3 or greater was defined as having eGFR <60 mL/min/1.73 m2 in two consecutive measurements from the last follow-up visit. Urinary albumin was measured through the latex turbidimetric immunoassay (Modular P800, Roche, Diagnostics, Mannhein, Germany). Total protein in the urine was measured using the turbidimetric method (Modular DP Hitachi, Roche), which utilizes a chemical reagent for precipitation. The lowest detectable level and the coefficient of variation in our laboratory were as follows: 20 mg/L and <0.48% for total proteinuria and 4 mg/L and <7.4% for albuminuria. The urine nonalbumin protein-to-creatinine ratio (NAPCR) was calculated by using total proteinuria and albuminuria: NAPCR=protein-to-creatinine ratio (PCR) – albumin-to-creatinine ratio (ACR).19 Albuminuria was measured based on spot urine samples and defined as A2 or A3 category (ACR ≥3 mg/mmol creatinine) according to Kidney Disease Improving Global Outcome (KDIGO).20 Several urine markers were measured using a commercial ELISA kit according to the manufacturer’s protocols: clusterin (Boster biological technology, Pleasanton, CA, USA), kidney injury molecule (KIM)-1 (R&D systems, Minneapolis, MN, USA), neurophil gelatinase-associated lipoprotein (NGAL) (R&D systems) and liver-type fatty acid-binding protein (LFABP) (CMIC Co. Ltd, Tokyo, Japan). Urine samples were centrifuged for 10 min at 3,000 rpm to remove particulate matter and stored at −70°C until analysis. All samples were analyzed with their duplicates. Values below the detection limit (20 ng/L for clusterin, 0.009 μg/L for KIM-1, 0.012 μg/L for NGAL and 3 μg/L for LFABP) were approximated using the mean value between zero and the lower limit of detection. The intra- and inter-assay coefficients of variation were as follows: for clusterin, 4.2–4.6% and 6.9–7.5%; for KIM-1, 3.9–4.4% and 6.0–7.8%; for NGAL, 3.1–4.4% and 5.6–7.9%; for LFABP, 9.6–11.9% and 3.5–7.9%. The data for urine markers were expressed as ratios of urine marker to urine creatinine (urine marker/Cr) to assess the hydration state and renal function of the patients.
Statistical analysis
Statistical analyses were performed using SPSS version 18.0 for windows (SPSS, Inc., Chicago, IL, USA). Type 2 diabetic patients were divided into quartile groups according to their baseline urine Clusterin/Cr levels. Data were presented as means±SD for normally distributed values and medians (interquartile range) for nonparametric values. Distributions of continuous variables were examined for skewness and kurtosis, and logarithm-transformed values of variables with non-Gaussian distribution were used for analyses. Geometric means are presented with 95% CI (confidence interval). Differences among the urine Clusterin/Cr quartile groups were analyzed by ANOVA, followed by the Bonferroni’s test for normally distributed values or the Kruskal–Wallis test for nonparametric values. Categorical variables were reported as frequencies and proportions. Pearson’s χ2 test was employed to analyze categorical data, when appropriate. Pearson correlation coefficient was used to test the correlation between individual continuous variables. Multivariate regression analyses with annual rates of decline in eGFR as the dependent variables and urine Clusterin/Cr as an independent variable were performed. Several models were conducted to adjust for confounding factors including age, sex, HbA1c, systolic BP, HDL cholesterol, duration of diabetes, and baseline eGFR. Multivariate Cox regression models for development for CKD 3 or greater and persistence of albuminuria, using an enter procedure, were conducted and included factors with a P value of <0.1 in the univariate analyses. A P value of <0.05 derived from the two-tailed Student’s t-test was considered to be of statistical significance.
RESULTS
Clinical and metabolic characteristic of study subjects
Table 1 summarizes the clinical and metabolic characteristics of non-diabetic and type 2 diabetes subjects according to the baseline urine clusterin quartiles. The subjects in the non-diabetic control group were well matched with type 2 diabetic patients by age, sex and body mass index (BMI). The mean age of type 2 diabetic patients was 56.9±10.7 years (range, 24–82 years), and there were 72 males and 87 females in this category. Age, sex, BMI, duration of diabetes, glycaemic control and dyslipidaemic status were not significantly different between the urine clusterin quartile groups of type 2 diabetes. The group with the highest urine clusterin level had a higher systolic blood pressure (SBP) (P=0.05). Urine clusterin positively correlated with SBP, diastolic blood pressure (DBP) and low-density lipoprotein (LDL) cholesterol at baseline (r=0.169, P=0.024; r=0.160, P=0.032; r=0.237, P=0.002, respectively) (Table 2). Subjects in both non-diabetic and type 2 diabetic groups showed well-conserved renal function, with eGFRs of 84.6±8.8 mL/min/1.73m2 and 93.1±29.3 mL/min/1.73 m2, respectively. The values of eGFR were not significantly different among the diabetic quartile groups according to urine clusterin at baseline (P=0.276). Urine clusterin levels were markedly elevated in type 2 diabetic subjects compared with those of the control (1.47 pg/mmol creatinine [95% CI, 4.47–13.99 pg/mmol creatinine] vs. 1.10 pg/mmol creatinine [95% CI, 1.06–2.36 pg/mmol creatinine], P=0.002). For clinical renal damage markers, there were significant differences among the urine clusterin quartile groups with respect to urine ACR, PCR and NAPCR (P=0.001, P<0.001 and P<0.001, respectively). All values of urine ACR, PCR and NAPCR in the Q4 group increased compared to those in the Q1 group, respectively (all P<0.001). Urine clusterin level notably correlated with urine ACR, PCR and NAPCR, respectively (r=0.369, P<0.001; r=0.398, P<0.001; r=0.403, P<0.001) (Table 2). More patients were administered antihypertensive medications in the highest urine clusterin quartile group than those in other groups (All P<0.05). In addition, diabetic retinopathy was more frequently observed in the Q4 group than in the Q1 group (P=0.004). Significant differences in urinary tubular markers including KIM-1, NGAL, LFABP were noted according to the urine clusterin levels (all P<0.001). The urine levels of KIM-1, NGAL and LFABP were greatly higher in the Q4 group than in the Q1 group (all P<0.001). Urine clusterin significantly correlated with other tubular markers (urine KIM-1, r=0.530; urine LFABP, r=0.439; urine NGAL, r=0.371; all P<0.001) (Table 3).
Table 1.
Baseline characteristics of laboratory and metabolic variables in patients with type 2 diabetes according to urine clusterin quartiles
| Nondiabetic control | Type 2 diabetic patients | P value | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Q1 | Q2 | Q3 | Q4 | |||
| Number | 20 | 40 | 40 | 40 | 39 | - |
| Range (pg/mg) | 1.2–35.4 | 0.1–2.5 | 2.6–12.2 | 12.3–51.1 | 51.3–2779.6 | - |
| Sex (male/female) | 10/10 | 20/20 | 11/29 | 26/14 | 15/24 | 0.06 |
| Age (years) | 55.6 ± 4.7 | 56.5 ± 10.6 | 55.4 ± 11.6 | 58.5 ± 11.7 | 59.3 ± 8.9 | 0.618 |
| BMI (kg/m2) | 23.4 ± 3.0 | 23.7 ± 3.4 | 23.4 ± 3.3 | 23.7 ± 3.9 | 23.3 ± 3.0 | 0.955 |
| Duration of diabetes (years) | - | 5.9 ± 5.7 | 8.7 ± 6.9 | 8.7 ± 7.1 | 8.5 ± 6.3 | 0.241 |
| SBP (mmHg) | 119 ± 15 | 124 ± 12 | 126 ± 14 | 121 ± 15 | 130 ± 17# | 0.050 |
| DBP (mmHg) | 74 ± 9 | 76 ± 8 | 78 ± 12 | 79 ± 10 | 80 ± 12 | 0.426 |
| HbA1c (%) | - | 7.4 ± 1.5 | 8.0 ± 1.9 | 8.4 ± 2.1 | 8.0 ± 2.0 | 0.106 |
| HbA1c (mmol/mol) | - | 56.9 ± 15.9 | 63.4 ± 20.7 | 67.7 ± 23.0 | 64.1 ± 21.9 | 0.106 |
| Total cholesterol (mmol/l) | 5.01 ± 0.85 | 4.56 ± 0.97 | 4.63 ± 0.91 | 4.62 ± 1.05 | 4.91 ± 1.16 | 0.602 |
| LDL cholesterol (mmol/l) | 3.31 ± 0.80 | 2.50 ± 0.76 | 2.87 ± 0.92 | 2.73 ± 0.90 | 3.07 ± 1.34 | 0.106 |
| Triglyceride (mmol/l)* | .68–1.92 | 1.03–2.97 | 1.08–2.49 | 0.87–2.14 | 1.28–3.84 | 0.269 |
| HDL cholesterol (mmol/l) | 1.42 ± 0.36 | 1.28 ± 0.39 | 1.25 ± 0.36 | 1.29 ± 0.47 | 1.29 ± 0.48 | 0.979 |
| CRP (mg/l)* | 0.3–0.9 | 0.2–1.3 | 0.4–1.9 | 0.2–1.6 | 0.3–1.7 | 0.151 |
| Uric acid (μmol/l) | 309 ± 59 | 291 ± 89 | 297 ± 77 | 268 ± 65 | 286 ± 119 | 0.553 |
| eGFR (mL/min/1.73 m2) | 84.6 ± 8.8 | 86.9 ± 16.7 | 98.2 ± 30.3 | 90.1 ± 23.8 | 97.0 ± 40.8 | 0.276 |
| Urine ACR (mg/mmol)* | - | 0.54–3.91 | 0.98–12.55 | 1.29–20.53 | 1.62–103.78† | 0.001 |
| Urine PCR (mg/mmol)* | - | 7.36–15.59 | 10.74–33.64 | 11.22–53.68 | 12.09–326.49†,§ | <0.001 |
| Urine NAPCR (mg/mmol)* | - | 7.09–13.03 | 9.27–19.91 | 8.19–21.54 | 96.8–139.76†,§,|| | <0.001 |
| Urine KIM-1/Cr (ng/mg)* | - | 0.6 (0.3–0.9) | 1.0 (0.5–1.8) | 1.2 (0.7–2.1)‡ | 2.7 (2.0–4.7)†,§,# | <0.001 |
| Urine NGAL/Cr (ng/mg)* | - | 4.2 (1.6–10.5) | 8.9 (4.2–17.0) | 13.0 (3.3–59.5)‡ | 26.4 (8.2 – 41.9)†,§ | <0.001 |
| Urine LFABP/Cr (ng/mg)* | - | 1.5 (1.2–3.1) | 1.9 (1.5–7.4) | 2.2 (1.5–5.6) | 6.5 (2.0–19.3)†,§,# | <0.001 |
| Antihypertensive medication, n (%) | - | 7 (17.5) | 5 (12.5) | 9 (23.1) | 20 (50.0)‡,§,# | 0.001 |
| Lipid lowering agents, n (%) | - | 17 (42.5) | 21 (52.5) | 12 (30.8) | 13 (39.6) | 0.169 |
| Diabetic retionopathy, n (%) | - | 7 (17.5) | 15 (37.5)‡ | 14 (35.9) | 19 (47.5)‡ | 0.040 |
Data are expressed as mean ± SD, number of patients (%), median (interquartile range), and geometric means (95% CI) unless otherwise indicated.
logarithm-transformed values were used for comparison. Comparison was performed among diabetic patients.
P < 0.001 vs. Q1;
P <0.05 vs. Q1;
P < 0.05 vs. Q2;
P < 0.001 vs. Q3.
P < 0.05 vs. Q3.
ACR, albumin-to-creatine; BMI, body mass index; CRP, C-reactive protein; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; KIM-1, kidney injury molecule-1; LFABP, liver-type fatty acid-binding protein; LDL, low-density lipoprotein; NAPCR, nonalbumin protein-to-creatinine ratio; NGAL, neutrophil gelatinase-associated lipocalin; PCR, protein-to-creatinine ratio; SBP, systolic blood pressure.
Table 2.
Correlations between urine clusterin and other clinical variables
| Variable | Urine Clusterin/Cr* | |
|---|---|---|
|
| ||
| r | P value | |
| Age | 0.038 | 0.616 |
| BMI | −0.062 | 0.421 |
| Systolic BP | 0.169 | 0.024 |
| Diastolic BP | 0.160 | 0.032 |
| HbA1c | 0.129 | 0.106 |
| Total cholesterol | −0.075 | 0.324 |
| LDL cholesterol | 0.237 | 0.002 |
| Triglyceride* | 0.062 | 0.416 |
| HDL cholesterol | 0.057 | 0.448 |
| CRP* | 0.065 | 0.410 |
| Uric acid | −0.026 | 0.735 |
| eGFR | 0.078 | 0.300 |
| Urine ACR* | 0.369 | <0.001 |
| Urine PCR* | 0.398 | <0.001 |
| Urine NAPCR* | 0.403 | <0.001 |
ACR, albumin-to-creatine; BMI, body mass index; Cr, creatinine; CRP, C-reactive protein; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NAPCR, nonalbumin protein-to-creatinine ratio; PCR, protein-to-creatinine ratio; SBP, systolic blood pressure.
logarithm-transformed values were used for comparison.
Table 3.
Correlations between urine clusterin and other urine tubular markers.
| Urine Clusterin/Cr* | Urine KIM-1/Cr* | Urine LFABP/Cr* | Urine NGAL/Cr* | |
|---|---|---|---|---|
| Urine Clusterin/Cr* | – | 0.530† | 0.439† | 0.371† |
| Urine KIM-1/Cr* | 0.530† | – | 0.504† | 0.614† |
| Urine LFABP/Cr* | 0.439† | 0.504† | – | 0.459† |
| Urine NGAL/Cr* | 0.371† | 0.614† | 0.459† | – |
Logarithm-transformed values were used for analysis.
P < 0.001
Cr, creatinine; KIM-1, kidney injury molecule-1; LFABP, liver-type fatty acid binding protein; NGAL, neutrophil gelatinase-associated lipocalin.
Association of the annual rate of decline in eGFR with urine clusterin levels
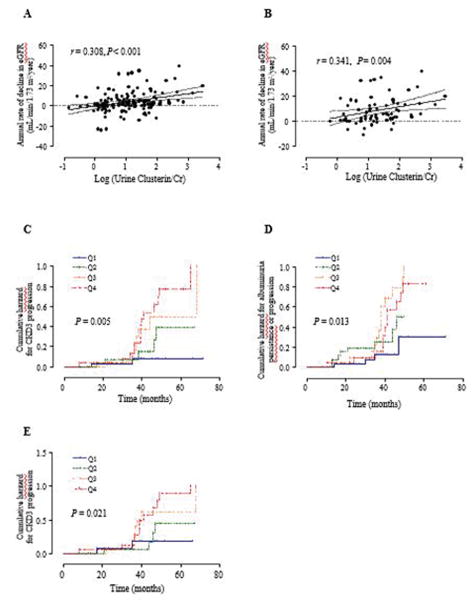
The median follow-up duration was 3.0 years (range, 1.0–5.9 years). In all diabetic patients, the median annual rate of decline in eGFR was 3.4 mL/min/1.73m2/year during the follow-up period. The annual rate of decline in eGFR positively correlated with baseline urine clusterin in univariate analysis (standard β=0.308, P<0.001) (Fig. 1A and Table 4). After adjusting for age, sex and clinical confounding factors including HbA1c, SBP, HDL cholesterol and duration of diabetes, urine clusterin level significantly associated with annual rate of decline in eGFR (standard β=0.283, P=0.004, Table 4). Finally, urine clusterin showed positive correlations with annual rate of decline in eGFR in the final model after adjusting for baseline eGFR (standard β=0.258, P=0.007). To verify the role of urine clusterin on renal function in the patients along with aluminuria, we also analyzed patients with albuminuria (A2 and A3, ACR ≥30 mg/g). Urine clusterin still correlated with the annual rate of decline in eGFR (standard β=0.341, P=0.004) in this sub-group analysis (Table 4 and Fig. 1B). The positive correlation between urine clusterin and the annual rate of decline in eGFR remained in the final model after adjusting for several clinical factors and baseline eGFR (standard β=0.322, P=0.0015).
Figure 1.
Single regression analysis of the annual rate of decline in eGFR using urine clusterin in patients with all type 2 diabetic patients (A) and those with albuminuria (B). Cumulative incidences of CKD stage 3 or greater (eGFR <60mL/min/1.73 m2) (C) and persistence/progression for albuminuria (D) using the Kaplan-Meier method and the log-rank test in all type 2 diabetic patients according to urine Clusterin/Cr quartile (Q1 to Q4). Cumulative incidences of CKD stage 3 or greater (eGFR <60mL/min/1.73 m2) in those with albuminuria (E) according to urine Clusterin/Cr quartile. Logarithm-transformed value of urine Clusterin/Cr was used for analysis. Cr, creatinine; eGFR, estimated glomerular filtration rate. CKD, chronic kidney disease; Q, quartile.
Table 4.
Multivariate regression of the annual rate of decline in eGFR as a dependent variable in type 2 diabetic patients
| Model | All (n=159) | Albuminuria (n=87) | ||
|---|---|---|---|---|
|
|
|
|||
| Standard β | P value | Standard β | P value | |
| Model 1 | 0.308 | <0.001 | 0.341 | 0.004 |
| Model 2 | 0.305 | <0.001 | 0.328 | 0.005 |
| Model 3 | 0.283 | 0.004 | 0.344 | 0.010 |
| Model 4 | 0.258 | 0.007 | 0.322 | 0.015 |
Model 1, crude; Model 2, adjusted for age and sex; Model 3, adjust for significant clinical parameters for eGFR decline including HbA1c, SBP, HDL cholesterol and duration of diabetes; Model 4, adjusted for baseline eGFR. eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; SBP, systolic blood pressure.
Association of development of CKD 3 or greater and persistence/progression of albuminuria with urine clusterin levels
Of 159 patients, CKD progressed to stage 3 or greater in 34 (21.4%) patients during the 3-year follow-up. There was a significant difference in the cumulative incidence of CKD 3 or greater (eGFR <60 mL/min/1.73m2) in patients according to urine clusterin quartile groups (P=0.032, Table 5 and Fig. 1C). In the multivariate analysis including clinical factors for renal impairment, the two groups with the higher range of urine clusterin (Q3 and Q4) showed a higher cumulative incidence of CKD stage 3 or greater than the lowest quartile group (Q3 vs. Q1: HR, 10.10, 95% CI = 1.18–86.86, P=0.035; Q4 vs. Q1: HR, 11.76, 95% CI=1.43–96.96, P=0.022). Meanwhile, the cumulative incidence of albuminuria persistence or progression was different in the patients according to urine clusterin quartiles (P=0.013, Table 5 and Fig. 1D). Finally, urine clusterin was associated with albuminuria persistence/progression (Q3 vs. Q1: HR, 9.94; 95% CI=2.02–48.87; P=0.005). When we analyzed clusterin levels of patients in the albuminuria group, there was a marked difference in the cumulative incidence of CKD3 or greater in patients according to urine clusterin quartile groups (P=0.021, Fig. 1E). In addition, the highest quartile group of urine clusterin (Q4) showed a higher cumulative incidence of CKD stage 3 or greater than that of the lowest quartile group (Q4 vs. Q1: HR, 4.85, 95% CI=1.08–21.74, P=0.039).
Table 5.
Univariate and multivariate analysis for development of CKD 3 or greater (eGFR <60 mL/min/1.73 m2) and persistence/progression for albuminuria in the patients with type 2 diabetes.
| Development of CKD 3 or greater | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Univariate analysis | Multivariate analysis | |||||
|
|
|
|||||
| Log-rank statistics | df | P | HR | 95% CI | P | |
| Sex, male | 2.97 | 1 | 0.085 | - | - | - |
| Age, ≥65 years | 0.21 | 1 | 0.644 | - | - | - |
| BMI, ≥25 kg/m2 | 0.71 | 1 | 0.400 | - | - | - |
| Duration of diabetes, ≥10 years | 5.37 | 1 | 0.020 | 1.28 | 0.55–3.01 | 0.571 |
| HbA1c, ≥7% (53 mmol/mol) | 0.40 | 1 | 0.528 | - | - | - |
| LDC cholesterol, ≥2.6mmol/l | 0.11 | 1 | 0.746 | - | - | - |
| HDL cholesterol, ≤1.0 (M), ≤1.3mmol/l (F) | 3.28 | 1 | 0.070 | 1.98 | 0.73–5.40 | 0.183 |
| Triglycerides, ≥3.0mmol/l | 1.29 | 1 | 0.257 | - | - | - |
| eGFR, <90 mL/min/1.73 m2 | 4.85 | 1 | 0.028 | 2.81 | 1.10–7.17 | 0.030 |
| Antihypetensive medication, yes | 7.25 | 1 | 0.007 | 2.48 | 0.93–6.57 | 0.069 |
| Lipid lowering agents, yes | 2.64 | 1 | 0.105 | - | - | - |
| Urine Clusterin/Cr, pg/mg | 12.77 | 3 | 0.005 | 0.032 | ||
| Q1 | 1.00 | |||||
| Q2 | 3.35 | 0.34–32.83 | 0.299 | |||
| Q3 | 10.10 | 1.18–86.86 | 0.035 | |||
| Q4 | 11.76 | 1.43–96.96 | 0.022 | |||
|
| ||||||
| Persistence/progression for albuminuria | ||||||
|
| ||||||
| Univariate analysis | Multivariate analysis | |||||
|
|
|
|||||
| Log-rank statistics | df | P | HR | 95% CI | P | |
|
| ||||||
| Sex, male | 0.18 | 1 | 0.668 | - | - | - |
| Age, ≥65 years | 2.84 | 1 | 0.092 | 5.78 | 1.28–26.22 | 0.023 |
| BMI, ≥25 kg/m2 | 0.65 | 1 | 0.419 | - | - | - |
| Duration of diabetes, ≥10 years | 6.19 | 1 | 0.013 | 2.29 | 1.01–5.21 | 0.047 |
| HbA1c, ≥7% (53 mmol/mol) | 4.05 | 1 | 0.044 | 1.55 | 0.60–4.01 | 0.362 |
| LDC cholesterol, ≥2.6mmol/l | 1.16 | 1 | 0.281 | - | - | - |
| HDL cholesterol, ≤1.0 (M), ≤1.3mmol/l (F) | 0.76 | 1 | 0.382 | - | - | - |
| Triglycerides, ≥3.0mmol/l | 2.37 | 1 | 0.123 | - | - | - |
| eGFR, <90 mL/min/1.73 m2 | 2.42 | 1 | 0.120 | - | - | - |
| Antihypetensive medication, yes | 0.83 | 1 | 0.361 | - | - | - |
| Lipid lowering agents, yes | 4.54 | 1 | 0.033 | 0.47 | 0.20–1.08 | 0.075 |
| Urine Clusterin/Cr, pg/mg | 10.81 | 3 | 0.013 | 0.032 | ||
| Q1 | 1.00 | |||||
| Q2 | 4.75 | 0.94–23.91 | 0.059 | |||
| Q3 | 9.94 | 2.02–48.87 | 0.005 | |||
| Q4 | 4.73 | 0.95–23.57 | 0.058 | |||
BMI, body mass index; CI, coefficient interval; CKD, chronic kidney disease; Cr, creatinine; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; HR, hazard ratio; LDL, low-density lipoprotein; Q, quartile.
DISCUSSION
The current study clearly provides new evidence of a relationship between urine clusterin and tubular damage markers, and identifies urine clusterin as a decisive indicator for tubular damage in type 2 diabetic subjects. We also show that urine clusterin is linked to CKD stage and albuminuria as an indicator of progression of DKD in the early stages of nephropathy in type 2 diabetic patients (eGFR 60 mL/min/1.73 m2).
Knowledge of urine clusterin’s action in the development of acute and chronic kidney disease remains incomplete. It has been reported that increased clusterin gene expression in obstructed rabbit kidneys is related to a decrease in renal concentrating ability and GFR levels.21 In this model, an elevation of clusterin protein in obstructed kidneys and of urinary clusterin excretion was also observed.21 Experimental evidence with obese insulin-resistant animals also demonstrated that clusterin mRNA in the kidney positively correlated with urine protein excretion and negatively correlated with creatinine clearance,22 suggesting that urine clusterin is involved in the renal injury of CKD. Given that clusterin expression was greatly up-regulated in the tubular epithelium of dilated, convoluted proximal tubules,22 it is conceivable that urine clusterin is a decisive indicator of tubular abnormalities. Together, these observations raised the possibility that clusterin functions as a potential marker during renal injury in both acute and chronic kidney disease.
It is likely that increased levels of clusterin in urine are more closely associated with tubular damage than with glomerular damage. For example, de novo clusterin synthesis was elevated in the renal tubular epithelium after renal injury, and then excreted in the urine.23 In addition, induction of clusterin primarily occurred in the tubular epithelial cell but not in the glomerulus of the kidneys of the nephrotic animal model, and ultimately precedes the development of tubular disease.16 Moreover, urine clusterin showed a better diagnostic performance compared with serum blood urea nitrogen (BUN) and creatinine for detecting proximal tubular injury, whereas urine total protein, cystatin C and β2-microglobulin displayed a better diagnostic performance for detecting glomerular injury.24 These observations, combined with our findings that urine clusterin correlates with tubular damage markers in type 2 diabetic subjects, suggest that elevation of urine clusterin could be a tubular damage marker in DKD. Among two models for kidney injury leading to CKD, the “fibrosis hypothesis” suggests that a variety of initial kidney insults result in tubular injury, eliciting further inflammation and damage to the tubulointerstitium that proceeds to CKD.25 In this study, the finding that clusterin correlated with stage 3 CKD or progression of albuminuria might reinforce that at this stage tubular damage may be more evident.
We propose the hypothesis that up-regulation of urine clusterin along with albuminuria may reflect proteinuria-induced renal injury/apoptosis. In support of this, an in vitro study demonstrates that treatment of cultured mouse proximal tubule cells with albumin leads to increased clusterin levels in the media, which in turn inhibits the NF-kB signalling pathway. As a result, albumin-stimulated apoptosis in tubule cells is increased.26 Although albuminuria has been thought to be a sensitive marker for DKD, there is a concern about the pathogenic nature of DKD’s progression. Interestingly, diabetic patients who have albuminuria sometimes showspontaneous remission to normal albumin levels while others progress kidney failure during the course of a long-term follow-up.26,27 Thus, it appears that the use of only albuminuria as a predictor of progression of the disease could provide incorrect information in DKD patients. Combined with information from urine clusterin, however, the progression of DKD can correctly be predicted in diabetic patients.
It has been reported that glomerular clusterin is variably expressed in human membranous nephritis, a major cause of nephrotic syndrome; it has also emerged as a factor influencing proteinuria and was associated with a reduction of proteinuria after a follow-up of 1.5 years.28,29 In this regard, mice lacking clusterin developed a progressive glomerulopathy characterized by the deposition of immune complexes in the mesangium.30 These results support the notion that clusterin may have a protective role for CKD and can modify immune complex metabolism and disposal. However, it is uncertain whether this glomerular clusterin is correlated to the emergence and increase of urine clusterin in the development and/or progression of DKD.
Evidence revealed that RAS may affect renal clusterin expression during the renal injury.31–33 Clusterin attenuates angiotensin II-induced renal fibrosis through the inhibition of NF-κB signalling and the downregulation of angiotensin II type I receptor (AT1R) signalling, indicating that there is a link between clusterin and angiotensin II signalling in the context of renal disease.34 A study with the nephrectomized rat model demonstrated that nephrectomy caused a progressive increase in clusterin mRNA levels in the remnant kidney and that an angiotensin-converting enzyme (ACE) inhibitor prevented the injury-induced increase in clusterin mRNA and proteinuria.32 Collectively, these data suggest that the induction of clusterin is sensitive to renal injury, along with proteinuria, and is modulated by the renin-angiotensin system. In this respect, we exclude the possibility that urine clusterin levels in our patients are reflected in the renin-angiotensin system as the patients taking RAS inhibitors had a sufficient washout period for these drugs before the study.
The potential limitations of the current study come from the fact that urine clusterin is also increased by acute renal injury such as ischaemia/reperfusion injury, toxicant-induced kidney injury, and unilateral ureteral obstruction,15,24 all of which are often found in diabetic patients. Thus, if urine clusterin is applied as a biomarker of renal injury in the clinical setting, it is difficult to discriminate between acute renal injury and chronic kidney disease. Given that increased levels of urine clusterin after acute injury can decline,36 it would be important to know the kinetics of the short-term changes in the context of acute damage, which could provide an important clue to overcome this limitation. Even though we excluded patients with several acute illnesses which might affect the natural course of DKD at baseline and during the follow-up period, it is unlikely that our subjects could distinguish between AKI and DKD.
The cohort size is relatively small and the follow-up period was short. As the development and progression of DKD has a variable course and requires a longer time frame, a large cohort study will be needed to further confirm the concept of this study. As the MDRD equation is less accurate, especially in a cohort of patients, at levels above 60 mL/min/1.73 m2, it might lead to misdiagnosis or misclassification of CKD in individuals with mild renal insufficiency.35 Measured GFR using urinary or plasma clearance of exogenous filtration markers, rather than eGFR, could have provided the best measure of renal decline. The concentration of urine clusterin was derived from a single point urine sample. Serial samples during the natural course of type 2 diabetes could help to further elucidate the role of clusterin on the pathophysiological mechanisms involved in the development and progression of DKD.
In conclusion, an increase in urine clusterin is considered to be an important indicator of irreversible renal tubular injury, which is linked to the progressive decline of renal function in the early stage of DKD. In a clinical setting, increased levels of urine clusterin and albuminuria in diabetic patients may be informative in predicting the progression of CKD. The clinical use of urine clusterin as a diagnostic and therapeutic target in the development and progression of DKD will require further investigation.
Acknowledgments
Funding
This work was supported by grants from the National Institutes of Health (R01DK111529 to Y.B.K.), the American Diabetes Association (7-12-BS-094 to Y.B.K.), and a Biomedical Research Institute grant (2010-3 to S.H.S) from Pusan National University Hospital. M.C.K. is a recipient of a postdoctoral fellowship award from the American Diabetes Association (1-17-PDF-146).
Abbreviations
- ACR
albumin-to creatinine ratio
- ACE
angiotensin-converting enzyme
- AT1R
angiotensin II type I receptor
- BMI
body mass index
- CKD
chronic kidney disease
- DKD
diabetic kidney disease
- DBP
diastolic blood pressure
- ESRD
end-stage renal disease
- eGFR
estimated glomerular filtration rate
- HDL
high-density lipoprotein
- KDIGO
Kidney Disease Improving Global Outcome
- KIM
kidney injury molecule
- LDL
low-density lipoprotein
- LFABP
liver-type fatty acid-binding protein
- MDRD
Modification of Diet in Renal Disease
- NAPCR
nonalbumin protein-to-creatinine ratio
- NGAL
neurophil gelatinase-associated lipoprotein
- PCR
protein-to-creatinine ratio
- Q
quartile
- RAS
renin-angiotensin system
- SBP
systolic blood pressure
Footnotes
Disclosure statement: The authors have nothing to disclose.
Author contribution
S.S.K and I.J.K. designed the study. S.H.S., J.H.K., Y.K.J., B.H.K., M.C.K., S.H.H., M.C., S.W.C., and S.S.K. performed most of metabolic parameters measurements. S.S.K. and Y.B.K. analyzed all data and interpreted experimental data. Y.K.K. and I.J.K. provided conceptual advice and contributed to the data analysis. S.S.K. and Y.B.K. wrote the manuscript. I.J.K. and Y.B.K. are the guarantors of this work and, as such, had full access to all the data in this work and will take responsibility for the integrity of the data and the accuracy of the data analysis.
Potential conflicts of interest
All authors declare that they have no conflicts of interest.
References
- 1.de Boer IH, Rue TC, Hall YN, et al. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305:2532–2539. doi: 10.1001/jama.2011.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care. 2014;37:2864–2883. doi: 10.2337/dc14-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park CW. Diabetic kidney disease: from epidemiology to clinical perspectives. Diabetes Metab J. 2014;38:252–260. doi: 10.4093/dmj.2014.38.4.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barratt J, Topham P. Urine proteomics: the present and future of measuring urinary protein components in disease. CMAJ. 2007;177:361–368. doi: 10.1503/cmaj.061590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamijo-Ikemori A, Sugaya T, Kimura K. Novel urinary biomarkers in early diabetic kidney disease. Curr Diab Rep. 2014;14:513. doi: 10.1007/s11892-014-0513-1. [DOI] [PubMed] [Google Scholar]
- 6.Matheson A, Willcox MD, Flanagan J, et al. Urinary biomarkers involved in type 2 diabetes: a review. Diabetes Metab Res Rev. 2010;26:150–171. doi: 10.1002/dmrr.1068. [DOI] [PubMed] [Google Scholar]
- 7.Thomas MC, Burns WC, Cooper ME. Tubular changes in early diabetic nephropathy. Adv Chronic Kidney Dis. 2005;12:177–186. doi: 10.1053/j.ackd.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Nauta FL, Boertien WE, Bakker SJ, et al. Glomerular and tubular damage markers are elevated in patients with diabetes. Diabetes Care. 2011;34:975–981. doi: 10.2337/dc10-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas MC, Burns WC, Cooper ME. Tubular changes in early diabetic nephropathy. Adv Chronic Kidney Dis. 2005;12:177–186. doi: 10.1053/j.ackd.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Trougakos IP, Gonos ES. Clusterin/apolipoprotein J in human aging and cancer. Int J Biochem Cell Biol. 2002;34:1430–1448. doi: 10.1016/s1357-2725(02)00041-9. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg ME, Silkensen J. Clusterin: physiologic and pathophysiologic considerations. Int J Biochem Cell Biol. 1995;27:633–645. doi: 10.1016/1357-2725(95)00027-m. [DOI] [PubMed] [Google Scholar]
- 12.Park S, Mathis KW, Lee IK. The physiological roles of apolipoprotein J/clusterin in metabolic and cardiovascular diseases. Rev Endocr Metab Disord. 2014;15:45–53. doi: 10.1007/s11154-013-9275-3. [DOI] [PubMed] [Google Scholar]
- 13.Won JC, Park CY, Oh SW, et al. Plasma clusterin (ApoJ) levels are associated with adiposity and systemic inflammation. PLoS One. 2014;9:e103351. doi: 10.1371/journal.pone.0103351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoofnagle AN, Wu M, Gosmanova AK, et al. Low clusterin levels in high-density lipoprotein associate with insulin resistance, obesity, and dyslipoproteinemia. Arterioscler Thromb Vasc Biol. 2010;30:2528–2534. doi: 10.1161/ATVBAHA.110.212894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol. 2008;48:463–493. doi: 10.1146/annurev.pharmtox.48.113006.094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Correa-Rotter R, Ibarra-Rubio ME, Schwochau G, et al. Induction of clusterin in tubules of nephrotic rats. J Am Soc Nephrol. 1998;9:33–37. doi: 10.1681/ASN.V9133. [DOI] [PubMed] [Google Scholar]
- 17.Kim SS, Song SH, Kim IJ, et al. Clinical implication of urinary tubular markers in the early stage of nephropathy with type 2 diabetic patients. Diabetes Res Clin Pract. 2012;97:251–257. doi: 10.1016/j.diabres.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 18.Myers GL, Miller WG, Coresh J, et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006;52:5–18. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- 19.Kim SS, Song SH, Kim IJ, et al. Urinary cystatin C and tubular proteinuria predict progression of diabetic nephropathy. Diabetes Care. 2013;36:656–661. doi: 10.2337/dc12-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.KDIGO. 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2013;3:1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 21.Schlegel PN, Matthews GJ, Cichon Z, et al. Clusterin production in the obstructed rabbit kidney: correlations with loss of renal function. J Am Soc Nephrol. 1992;3:1163–1171. doi: 10.1681/ASN.V351163. [DOI] [PubMed] [Google Scholar]
- 22.Laping NJ, Olson BA, Day JR, et al. The age-related increase in renal clusterin mRNA is accelerated in obese Zucker rats. J Am Soc Nephrol. 1998;9:38–45. doi: 10.1681/ASN.V9138. [DOI] [PubMed] [Google Scholar]
- 23.Ishii A, Sakai Y, Nakamura A. Molecular pathological evaluation of clusterin in a rat model of unilateral ureteral obstruction as a possible biomarker of nephrotoxicity. Toxicol Pathol. 2007;35:376–382. doi: 10.1080/01926230701230320. [DOI] [PubMed] [Google Scholar]
- 24.Dieterle F, Perentes E, Cordier A. Urinary clusterin, cystatin C, beta2-microglobulin and total protein as markers to detect drug-induced kidney injury. Nat Biotechnol. 2010;28:463–469. doi: 10.1038/nbt.1622. [DOI] [PubMed] [Google Scholar]
- 25.Hodgkins KS, Schnaper HW. Tubulointerstitial injury and the progression of chronic kidney disease. Pediatr Nephrol. 2012;27:901–909. doi: 10.1007/s00467-011-1992-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takase O, Minto AW, Puri TS, et al. Inhibition of NF-kappaB-dependent Bcl-xL expression by clusterin promotes albumin-induced tubular cell apoptosis. Kidney Int. 2008;73:567–577. doi: 10.1038/sj.ki.5002563. [DOI] [PubMed] [Google Scholar]
- 27.de Boer IH, Rue TC, Cleary PA, et al. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch Intern Med. 2011;171:412–420. doi: 10.1001/archinternmed.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gall MA, Hougaard P, Borch-Johnsen K, et al. Risk factors for development of incipient and overt diabetic nephropathy in patients with non-insulin dependent diabetes mellitus: prospective, observational study. BMJ. 1997;314:783–788. doi: 10.1136/bmj.314.7083.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rastaldi MP, Candiano G, Musante L, et al. Glomerular clusterin is associated with PKC-alpha/beta regulation and good outcome of membranous glomerulonephritis in humans. Kidney Int. 2006;70:477–485. doi: 10.1038/sj.ki.5001563. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg ME, Girton R, Finkel D, et al. Apolipoprotein J/clusterin prevents a progressive glomerulopathy of aging. Mol Cell Biol. 2002;22:1893–1902. doi: 10.1128/MCB.22.6.1893-1902.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoo KH, Thornhill BA, Chevalier RL. Angiotensin stimulates TGF-beta1 and clusterin in the hydronephrotic neonatal rat kidney. Am J Physiol Regul Integr Comp Physiol. 2000;278:R640–R645. doi: 10.1152/ajpregu.2000.278.3.R640. [DOI] [PubMed] [Google Scholar]
- 32.Chung KH, Gomez RA, Chevalier RL. Regulation of renal growth factors and clusterin by AT1 receptors during neonatal ureteral obstruction. Am J Physiol. 1995;268:F1117–F1123. doi: 10.1152/ajprenal.1995.268.6.F1117. [DOI] [PubMed] [Google Scholar]
- 33.Olson BA, Ali SM, Contino LC, et al. Angiotensin-converting enzyme inhibition alters clusterin mRNA expression in the kidney following renal mass reduction. Pharmacology. 1998;57:13–19. doi: 10.1159/000028221. [DOI] [PubMed] [Google Scholar]
- 34.Jung GS, Jeon JH, Jung YA, et al. Clusterin/apolipoprotein J attenuates angiotensin II-induced renal fibrosis. PLoS One. 2014;9:e105635. doi: 10.1371/journal.pone.0105635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Froissart M1, Rossert J, Jacquot C, et al. Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol. 2005;16:763–773. doi: 10.1681/ASN.2004070549. [DOI] [PubMed] [Google Scholar]
- 36.Vinken P, Starckx S, Barale-Thomas E, et al. Tissue Kim-1 and urinary clusterin as early indicators of cisplatin-induced acute kidney injury in rats. Toxicol Pathol. 2012;40:1049–1062. doi: 10.1177/0192623312444765. [DOI] [PubMed] [Google Scholar]