Abstract
Objective
Respiratory muscle weakness frequently develops during mechanical ventilation, although in children there are limited data about its prevalence and whether it is associated with extubation outcomes. We sought to identify risk factors for pediatric extubation failure, with specific attention to respiratory muscle strength.
Design
Secondary analysis of prospectively collected data
Setting
Tertiary care pediatric ICU
Patients
409 mechanically ventilated children
Interventions
Respiratory measurements using esophageal manometry and respiratory inductance plethysmography were made pre-extubation during airway occlusion and on CPAP of 5 and PS of 10/ above PEEP 5 cmH20, as well as 5 and 60 minutes post-extubation.
Measurements and Main Results
Thirty-four patients (8.3%) were re-intubated within 48 hours of extubation. Re-intubation risk factors included lower maximum airway pressure during airway occlusion (aPiMax), longer length of ventilation, post-extubation upper airway obstruction (UAO), high respiratory effort post-extubation (Pressure Rate Product (PRP), Pressure Time Product, Tension Time Index) and high post-extubation Phase Angle. Nearly 35% of children had diminished respiratory muscle strength (aPiMax ≤ 30 cmH20) at the time of extubation, and were nearly three times more likely to be re-intubated than those with preserved strength (aPiMax >30 cmH20; 14% vs. 5.5%, p=0.006). Re-intubation rates exceeded 20% when children with low aPiMax had moderately elevated effort after extubation (PRP > 500), while children with preserved aPiMax had re-intubation rates > 20% only when post-extubation effort was very high (PRP > 1000). When children developed post-extubation UAO, re-intubation rates were 47.4% for those with low aPiMax compared to 15.4% for those with preserved aPiMax (p=0.02). Multivariable risk factors for re-intubation included acute neurologic disease, lower aPiMax, post-extubation UAO, higher pre-extubation PEEP, higher post-extubation PRP, and lower height.
Conclusions
Neuromuscular weakness at the time of extubation was common in children and was independently associated with re-intubation, particularly when post-extubation effort was high.
Keywords: Airway Extubation, Work of Breathing, pediatrics, ventilator weaning, respiratory muscles
Introduction
Extubation failure is associated with longer ICU length of stay and higher mortality (1–3). Previous pediatric studies have had limited success identifying pre-extubation risk factors for extubation failure such as direct measures of respiratory load or effort (3–21). This may be because children deemed clinically ready to extubate have oftentimes resolved their respiratory disease, with low respiratory effort at extubation. Moreover, nearly half of pediatric extubation failures are related to post-extubation upper airway obstruction (UAO), which may be difficult to predict pre-extubation, particularly for children with uncuffed endotracheal tubes (8).
It is becoming increasingly recognized that ventilator induced neuromuscular (particularly diaphragm) weakness is common amongst mechanically ventilated adults (22–24), and contributes to prolonged weaning, extubation failure, and higher mortality (14, 16, 22, 24–28); although many of these weak patients do not fail extubation (16, 22, 25, 28, 29). In children, the relationship between respiratory muscle strength and extubation failure is understudied, but is of crucial importance given maturational changes to respiratory mechanics and diaphragm histology which occur throughout infancy.
We sought to characterize risk factors for pediatric extubation failure, with particular attention to measures of respiratory muscle strength before extubation, and measures of respiratory load and effort both before and after extubation. We hypothesized that children with respiratory muscle weakness at the time of extubation would be more likely to be re-intubated, particularly if they have high load after extubation, such as from post-extubation UAO.
Methods
Secondary analysis of data gathered from a prospective cohort study (8, 30), including mechanically ventilated children in the pediatric or cardiothoracic ICU at Children’s Hospital Los Angeles from July 2012–April 2015. Inclusion criteria: >37 weeks gestational age - 18 years, intubated ≥ 12 hours with planned extubation from 7 am to 5 pm Monday–Friday. Exclusion criteria: contraindication to an esophageal catheter or Respiratory Inductance Plethysmography (RIP) bands. Informed consent was obtained from the parent/guardian, and the study was approved by the Institutional Review Board (CCI-11-00210).
Study Protocol
Pre-extubation, we placed an age appropriate esophageal balloon catheter (Carefusion, Avea SmartCath 6,7, or 8Fr), RIP bands (Viasys Healthcare, Respiband Plus, Hoechberg, Germany or Nox Medical, Reykjavik, Iceland), and a self-calibrating pneumotachometer (Viasys Variflex 51000-40094). The study protocol is summarized in Digital Supplementary Material (DSM) Figure 1. The clinical team decided when to extubate, most frequently after passage of a spontaneous breathing trial (SBT) on CPAP of 5 cmH20. After the decision to extubate, we switched the patient to Pressure Support 10/PEEP 5 cmH2O (PS) for 5 minutes followed by CPAP alone at 5 cmH2O (CPAP) for 5 minutes, during which time we recorded data from esophageal manometry, spirometry, and RIP. We measured vital signs and extubation readiness parameters (tidal volume, rapid shallow breathing index) on CPAP of 5 cmH20. Subsequently, we placed the child on standardized ventilator settings to calculate respiratory system compliance and endotracheal tube cuff leak percentages. Compliance measurements were only used for analysis when the difference between inspiratory and expiratory tidal volume was < 20%. Immediately before extubation, we performed a standardized airway occlusion maneuver (Negative Inspiratory Force (NIF)) for a minimum of 3 (average 5) consecutive breaths, measuring esophageal (ePiMax) and airway pressure (aPiMax). aPiMax was measured using the 42-NS60 NIF meter (Instrumentation Industries, Bethel Park, PA). After extubation, we removed the spirometer but recorded esophageal manometry and RIP data during 5 minutes of steady state breathing, 5 and 60 minutes post-extubation. We followed clinical outcomes such as re-intubation within 48 hours of extubation, therapies to treat UAO, and duration of non-invasive respiratory support (NRS) after extubation (High Flow Nasal Cannula, nasal or oro-nasal CPAP or BiPAP) (DSM Figure 1). The clinical team controlled the use and management of NRS after extubation.
Post processing Analysis
We post-processed all files to compute a variety of respiratory parameters (DSM Table 1). We filtered artifacts, and obtained breath by breath calculations for each parameter, using the median value over a minimum of 30 calm, steady state breaths for analysis. From esophageal pressure we computed Pressure Rate Product (PRP), Pressure Time Product (PTP), Inspiratory Time (Ti), Total Respiratory Cycle Time (Ttot), and change in esophageal pressure (Pi). From RIP we computed Phase Angle (PA). During airway occlusion, we computed maximum change in airway pressure (aPiMax) and esophageal pressure (ePiMax). Using ePiMax as well as Pi, we computed Pi/ePiMax, and Tension Time Index (TTI) on each study condition (DSM Table 1). We calibrated RIP flow during airway occlusion, to assess for inspiratory flow limitation after extubation to characterize post-extubation upper airway UAO, as previously published (8, 31). For analysis, we labeled patients as having UAO when inspiratory flow limitation was new after extubation with an increase in Pressure Rate Product (PRP) of at least 50% over values before extubation on CPAP, and when they received a UAO specific intervention such as racemic epinephrine, heliox, or corticosteroids. We further classified UAO as subglottic if an airway maneuver did not reduce PRP by at least 50% (8).
Hardware and Software
Sensors were attached to the Bicore II device (CareFusion, Houten, The Netherlands), and data was post-processed using a custom module we developed in Polybench (Applied Biosignals GmbH, Weener, Germany), along with a customized software module from VivoSense® (Vivonetics, San Diego, CA, USA).
Analysis
The primary outcome was re-intubation within 48 hours. The secondary outcomes were prolonged use (> 48 hours) of non-invasive respiratory support (NRS) after extubation, and re-intubation within 7 days (for those who were on NRS for > 48 hours). We hypothesized that patients with low PiMax would be more likely to fail extubation, particularly when respiratory load was high after extubation (such as from UAO or persistent respiratory disease). We stratified patients into 3 groups: re-intubation within 48 hours, prolonged use of NRS, or no extubation failure. We report descriptive statistics, stratified by each of these groups using median (interquartile range) or number (percent). We analyzed continuous variables with Kruskall Wallis Analysis of Variance (ANOVA), with multiple comparisons based on mean ranks between subgroups. We analyzed categorical variables with observed minus expected Chi-squared test, with pairwise multiple comparisons using Chi-squared or Fisher Exact tests with p<0.025 considered significant (32). We created multivariable logistic regression models for the outcomes of re-intubation and prolonged NRS. For the subgroup on NRS after extubation, we also created a multivariable model examining re-intubation within 7 days. We considered variables for inclusion if they had a univariate association (p<0.2) with the outcome measure, retaining variables which remained statistically significant (p<.0.05). For the model, we selected variables which were highly correlated with one another based on a correlation matrix (such as PRP, PTP, Pi/PiMax, TTI) retaining the variable with the highest univariate association with the outcome, to avoid issues of co-linearity. We report Area under the Receiver Operating Characteristic (ROC) plot to assess discrimination, and the Hosmer-Lemeshow test to assess model calibration, with p>0.1 considered adequately calibrated. We performed statistical analysis in Statistica 10 (Dell, Tulsa, Oklahoma) and Stata 10 (Stata-Corp, College Station, Texas).
Results
Of 1159 eligible patients, 409 were included. Median age was 5 months (IQR 1, 16), and approximately half were post-op cardiac surgery. Previous publications have detailed reasons for non-enrollment (8, 30), the most frequent of which was extubation on a night or weekend. Of the 409 patients, 34 were re-intubated within 48 hours (8.3%) and 42 (10.3%) were on NRS for > 48 hours post-extubation. Eight of these 42 patients on NRS for > 48 hours were subsequently re-intubated (range 49–134 hours after the initial extubation). Nine patients were re-intubated before the 60 minute assessment was completed. Clinicians determined extubation readiness with a SBT on CPAP alone for 367 (89.7%) patients, on PS/PEEP for 39 (9.5%) patients, and on a low rate for 3 patients (0.7%). We present descriptive statistics in Table 1 (and DSM Tables 2–4).
Table 1.
Selected univariate risk factors for extubation failure, patient demographics. For all variables considered, please see Digital Supplementary Materials. All p values represent overall effect from ANOVA or Chi-squared. For multiple comparisons, | indicates difference between no failure and re-intubated, * difference between no failure and NRS > 48 hrs.
| Variable | All Patients | No Extubation Failure | Re-intubated | NRS > 48 hours | p |
|---|---|---|---|---|---|
| 409 | 333 (81.4%) | 34 (8.3%) | 42 (10.3%) | ||
| Age (months) | 5 (1,16) | 5 (1,19) | 2.5 (1,6) | 4.5 (2,12) | 0.16 |
| Genetic Disorder | 67 (16.4%) | 48 (14.4%) * | 6 (17.6%) | 13 (31%) * | 0.04 |
| Length of intubation (hrs) | 99 (34, 192) | 94 (31,178) | * | 139 (86,214) | | 169 (70,376) * | 0.001 |
| Respiratory System Compliance (ml/cmH2O/kg) | 0.59 (0.48,0.72) | 0.60 (0.50, 0.72) | 0.56 (0.47,0.67) | 0.51 (0.42,0.70) | 0.09 |
| PEEP at Extubation (cmH2O) | 5 (5,5) | 5 (5,5) | 5 (5,5) | 5 (5,5) | 0.14 |
| Intubation for Neurologic Disease | 38 (9.3%) | 30 (9%) | 7 (21%) | 1 (2.4%) | 0.02 |
| During Airway Occlusion (NIF) | |||||
| aPi Max (Airway, cmH2O) | 40 (30,50) | 40 (30, 50) | | 30 (25,40) | | 35 (28,48) | 0.003 |
| ePi Max (Esophageal, cmH2O) | 34.5 (22.8,51.3) | 35.5 (23,52.5) | 28.5 (22.4,43.5) | 33.5 (20.2,50) | 0.18 |
| CPAP 5 cmH2O | |||||
| Phase Angle (PA) | 30 (20,50) | 30 (20,45) | 27.5 (15,40) | 32.5 (25,60) | 0.04 |
| Rapid Shallow Breathing Index (bpm/ml/kg) | 5.5 (3.2,8.3) | 5.4 (3.2,8.2) | 6.4 (3.9,7.2) | 6.9 (4.0,10.9) | 0.07 |
| Respiratory Rate | 36 (25,48) | 35 (25,46)* | 39 (30,46) | 42.5 (27,60)* | 0.03 |
| Post- Extubation | |||||
| Upper Airway Obstruction (all) | 100 (25%) | 60 (18%) |* | 20 (59%)| | 20 (48%)* | <0.0001 |
| Subglottic UAO | 49 (12%) | 27 (8.1%) | | 14 (41%) | | 8 (19%) | <0.0001 |
| 5 min Post Extubation | |||||
| Phase Angle (PA) | 45 (25, 88) | 40 (22,75) |* | 70 (45,140) | | 60 (35,150) * | 0.0001 |
| Pressure Rate Product (PRP) | 300 (180,500) | 300 (170,450) |* | 500 (360,1300) | | 388 (200,750) * | <0.0001 |
| Pressure Time Product (PTP) | 135 (84,220) | 129 (82,207) | | 224 (125,454)| | 190 (101,260) | 0.0004 |
| Inspiratory Time (sec) (Ti) | 0.49 (0.38,0.63) | 0.50 (0.39,0.65) | 0.44 (0.37,0.57) | 0.43 (0.35,0.57) | 0.02 |
| Respiratory cycle time (sec) (Ttot) | 1.46 (1.05,2.0) | 1.51 (1.10, 2.04) | 1.34 (0.92,1.18) | 1.26 (0.85,1.92) | 0.01 |
| Ti/Ttot | 0.37 (0.30,0.44) | 0.36 (0.30,0.41) | 0.37 (0.30,0.46) | 0.36 (0.31,0.43) | 0.22 |
| Tension Time Index (TTI) | 0.036 (0.02,0.068) | 0.033 (0.02,0.057) | | 0.07 (0.04,0.16) | | 0.04 (0.03,0.07) | 0.0001 |
| Pi/PiMax | 0.19 (0.11,0.34) | 0.15 (0.09,0.24) | | 0.46 (0.27,0.78) | | 0.24 (0.14,0.43) | <0.0001 |
Re-intubation by 48 hours
There was slight age dependence to aPiMax and ePiMax, with neonates having the lowest values (Table 5, DSM). Pre-extubation, re-intubation risk factors included lower aPiMax, and longer length of ventilation. Post-extubation, UAO, and high PRP, PTP, TTI or Phase Angle 5 or 60 minutes after extubation (all p<0.05) were associated with re-intubation. Non-significant trends were noted for higher pre-extubation PEEP, lower height, and intubation for neurologic disease (Table 1, DSM Tables 2–4).
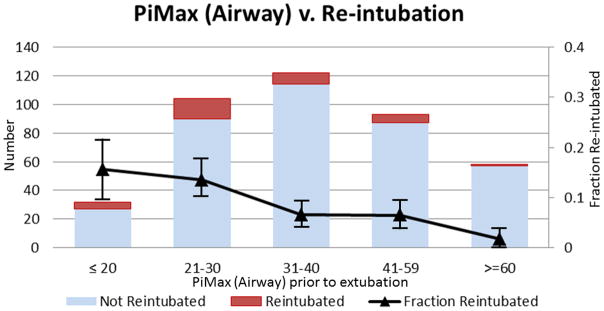
There was a dose response relationship between lower aPiMax and re-intubation (Figure 1a). The re-intubation rate was 14% for those with aPiMax ≤ 30 cmH20 (19/136), compared to 5.5% for those with aPiMax > 30 cmH20 (15/273) with a univariate odds ratio of 2.8 (1.37, 5.69). Low aPiMax alone was a fair marker of re-intubation risk (AUC 0.66 (0.57, 0.75), Table 2). While aPiMax was slightly lower for neonates, the trend for lower aPiMax amongst those who failed extubation was consistent when stratifying by age (Figure 2 DSM). There were no clear associations between pre-extubation respiratory effort (PRP, PTP, TTI) and re-intubation (DSM Table 3), with pre-extubation respiratory effort generally close to population norms. However, post-extubation, PRP, PTP, TTI, Pi/Pimax, and Phase Angle were all associated with re-intubation, with Pi/PiMax and PRP having the highest discrimination ability (Table 2, DSM Table 4). There was a dose response relationship with higher PRP after extubation and re-intubation (Figure 3 DSM). The re-intubation rate was 17.4% for those with PRP > 500 (16/92), compared to 5.7% for those with PRP ≤ 500 (18/317), with a univariate odds ratio of 3.5 (1.7, 7.2).
Figure 1.

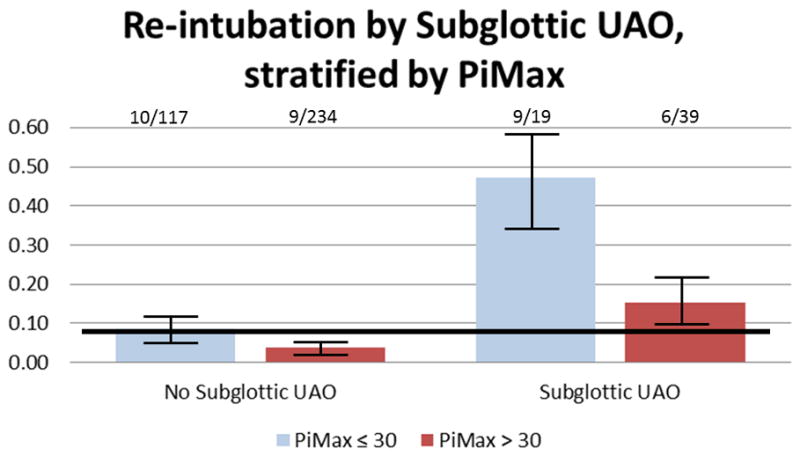
(a) There is a dose response relationship between lower aPiMax and re-intubation (test of trend p=0.01). Those with aPiMax ≤ 30 cmH20 were 2.8 (1.37, 5.69) times more likely to be re-intubated than those with aPiMax > 30 cmH20. (b) For children with maintained respiratory muscle capacity (PiMax >30 cmH20), re-intubation rates do not exceed the population average unless effort is very high (PRP > 1000). For children with diminished respiratory muscle capacity (PiMax ≤ 30 cmH20), re-intubation rates are always higher than the population average, and they become accelerated as effort increases. (c) When children with maintained respiratory muscle capacity (PiMax >30 cmH20) have subglottic UAO after extubation, their re-intubation rates are nearly double the population average (15.4% vs 8.3%). However, when children with diminished respiratory muscle capacity (PiMax ≤ 30 cmH20) have subglottic UAO after extubation, their re-intubation rates are 5.7 times the population average (47.4% vs. 8.3%). Solid line is average re-intubation rate. Numbers above bar represent re-intubated/total in each category. Bars represent +/− 1 standard error from the re-intubation rate.
Table 2.
Univariate odds ratios and Areas Under the Curve (AUC) of the Receiver Operating Characteristic (ROC) Plot with respective 95% Confidence interval on outcome of re-intubation.
| Parameter | OR (95%CI) | AUC ROC (95%CI) |
|---|---|---|
| PiMax (Airway) (per 1) | 0.95 (0.92,0.98) | 0.659 (0.567,0.751) |
| 5 min PRP (per 100) | 1.16 (1.09,1.24) | 0.741 (0.652,0.830) |
| 5 min PTP (per 30) | 1.10 (1.05,1.16) | 0.676 (0.572,0.779) |
| 5 min TTI (per 0.01) | 1.01 (1.0,1.03) | 0.718 (0.625,0.811) |
| 5 min Pi/Pmax (per 0.01) | 1.014 (1.007,1.021) | 0.743 (0.684,0.838) |
| 5 min Phase Angle (per 10) | 1.1 (1.033,1.183) | 0.659 (0.566, 0.752) |
| Rapid Shallow Breathing Index (RSBI) pre-extubation | 1.01 (0.93,1.11) | 0.534 (0.443,0.624) |
The combination of high post-extubation effort in a patient with diminished respiratory muscle strength imparted the highest re-intubation risk (Figure 1b). For children with aPiMax ≤ 30 cmH2O, re-intubation rates were always higher than the population average, and they accelerated as post-extubation effort increased (PRP>500). For children with aPiMax >30 cmH2O re-intubation rates did not exceed the population average unless effort was very high (PRP > 1000). Post-extubation subglottic UAO was the most common reason for high respiratory effort, and when children with aPiMax ≤ 30 cmH2O had subglottic UAO after extubation, their re-intubation rate was 47.4% (9/19), 5.7 times higher than the population average (34/409 (8.3%)) and 3 times higher than children with aPiMax > 30 with subglottic UAO (6/39 (15.3%) p=0.02, Figure 1c)). Multivariable risk factors for re-intubation include primary intubation for neurologic disease, lower aPiMax, post-extubation UAO, higher pre-extubation PEEP, higher post-extubation PRP, and lower height (Table 3, discrimination: AUC 0.823, calibration: HL p=0.16).
Table 3.
Multivariable model identifying risk factors associated with re-intubation
| Parameter | OR (95%CI) | p |
|---|---|---|
| Intubated for Neurologic Disease | 4.6 (1.5,14.0) | 0.007 |
| aPiMax (Airway, per 1) | 0.94 (0.90,0.98) | 0.001 |
| UAO post extubation | 2.47 (1.09,5.59) | 0.05 |
| PEEP at extubation (per 1) | 2.46 (1.09,5.58) | 0.03 |
| PRP 5minutes post extubation (per 1) | 1.001 (1.0006,1.002) | <0.0001 |
| Height (per 1 cm) | 0.98 (0.96,0.999) | 0.045 |
Post-extubation NRS
Overall, 161/409 (39.4%) patients were placed on NRS within 48 hours of extubation, 92 of which were electively extubated to NRS. Patients electively extubated to NRS were younger, were intubated longer, had higher pre-extubation respiratory rate, heart rate, RSBI, PRP, phase angle, and lower pre-extubation oxygen saturation and compliance compared to those who were not extubated to NRS. Factors associated with unplanned use of NRS included shorter height, longer length of intubation, higher pre-extubation respiratory rate and RSBI, lower pre-extubation saturation, and post-extubation UAO (DSM Table 6).
Twenty-one of these 161 patients on NRS were re-intubated within 48 hours of extubation, 8 were re-intubated between 48 hours and 7 days, and 42 remained on NRS for > 48 hours post-extubation (prolonged NRS). Patients with planned extubation to NRS had a higher 7 day re-intubation rate than those not on NRS. Patients with unplanned NRS were more likely to be re-intubated at 48 hours and within 7 days (DSM Table 6).
Prolonged post-extubation NRS
Univariate risk factors for prolonged NRS included genetic disorders, longer length of intubation, higher pre-extubation phase angle on PS or CPAP, faster pre-extubation respiratory rate, post-extubation UAO, and higher PRP 5 and 60 minutes post-extubation (Table 1, DSM Tables 2–4). Non-significant trends were noted for lower pre-extubation PEEP, lower respiratory system compliance, higher pre-extubation rapid shallow breathing index (RSBI), and intubation for shock. Variables which retained an independent relationship with prolonged NRS included genetic disorders, longer length of intubation, higher pre-extubation respiratory rate, lower pre-extubation PEEP, higher phase angle on CPAP prior to extubation, and post-extubation UAO (Table 7 DSM, discrimination: AUC 0.815, calibration: HL p=0.14). Planned extubation to NRS was not independently associated with re-intubation within 48 hours (p= 0.12), re-intubation within 7 days (p=0.84), or prolonged NRS (p=0.17). Independent risk factors for re-intubation within 7 days for those on NRS (n=161) were lower aPiMax (p=0.01) and higher PRP at 60 min (p=0.01).
Discussion
We found that respiratory muscle weakness is a major contributor to pediatric extubation failure, with an important interaction between respiratory effort (or load) and strength. Children with preserved respiratory strength can tolerate relatively high effort after extubation, as their likelihood of re-intubation only increases over the general population when effort is extremely high. In contrast, children with impaired respiratory strength are more likely to fail extubation even when effort is only moderately increased.
Ventilator induced diaphragm dysfunction is present in up to 50% of mechanically ventilated adults, and leads to impaired weaning and extubation failure (23–25, 27, 28, 33–35). While many adult studies use diaphragm ultrasound (23, 27, 28, 36, 37), we used a direct measure of strength (aPiMax), and found that that respiratory muscle weakness is nearly as common in children (35%). Maximum airway (aPiMax) or esophageal (ePiMax) pressure measurements during airway occlusion are regarded as gold standard measures of respiratory muscle strength in adults (38). If maximal voluntary efforts cannot be guaranteed (14, 38–42), twitch stimulation of the phrenic nerve is sometimes substituted (38, 43), although this technique may have high variability and limited reproducibility in children (44–49). In addition, diaphragm strength can be isolated from the intercostal muscles by using two simultaneous pressure transducers (to calculate trans-diaphragmatic pressure) (38, 50), although this is not practical for repeated use in children and no double-balloon catheters are commercially available for infants.
While previous studies have demonstrated inconsistent results, we found that systematic assessment of aPiMax and ePiMax are clinically relevant in children. In our protocol, a trained provider ensured the patient was at end-exhalation and that the airway remained occluded for at minimum 3 but most of the time 5 consecutive breaths (42). We tried to minimize the effects of sedation by ensuring that the patient was as close to extubation as possible (median GCS was 11T at extubation). We reviewed recordings in real time to gauge patient effort, re-doing maneuvers when necessary. We found that aPiMax had the strongest association with re-intubation, with a similar trend with ePiMax. While most argue ePiMax is the best measure, we found more artifacts in esophageal compared to airway pressure during airway occlusion, particularly for young children (42, 51) (DSM Table 5).
In addition, we found many measures of respiratory system effort (PRP, PTP), strength (PiMax), or a combination of effort, strength, or endurance (Pi/PiMax, TTI) measured just after extubation have a similar ability to discriminate the risk of re-intubation in children. Pi/PiMax and PRP 5 minutes after extubation appear to have the strongest discrimination ability. Interestingly TTI had lower discrimination ability than Pi/PiMax, which was somewhat unexpected since TTI measures effort, capacity, and endurance (over time) together. This may be because the Ti/Ttot component of TTI was not associated with extubation failure (DSM Table 2,3). Furthermore, while PTP is often thought of as the best measure to capture inspiratory effort (because it integrates the area under the signal), our data highlight that PRP performs equally well, if not better. This may be due to variability in the calculations, particularly when inspiratory times are short for young infants. Our respiratory cycle time measurements were based on esophageal manometry, to enable fair comparison pre and post-extubation. This highlights potential sources of error with PTP and TTI calculations (38).
Other risk factors for re-intubation included neurologic disease, higher PEEP, post-extubation UAO, and lower height. Post-extubation UAO is a surrogate for increased load (and thereby effort), so it is not surprising that it is associated with re-intubation. Neurologic disease is a known risk factor for extubation failure, corroborating previous investigations (2, 7). Lower height is likely a surrogate for age or pulmonary development, re-iterating the youngest children are highest risk for re-intubation. Higher PEEP at extubation likely represents persistent respiratory disease or lower airway obstruction, although it is important to note only 3 re-intubated patients had a PEEP > 5 cmH20, implying this may not be clinically relevant.
Interestingly, other than post-extubation UAO, risk factors for prolonged use of NRS were different than risk factors for re-intubation. We did not have a specific protocol for weaning NRS, so perhaps it is not surprising that high respiratory rate, genetic disorders, longer length of intubation and thoraco-abdominal asynchrony (phase angle) were associated with prolonged use of NRS, as clinicians may be unlikely to wean NRS aggressively on these patients. Moreover, many of these same factors were associated with planned extubation to NRS, although our study did not demonstrate that pre-emptive extubation to NRS was associated with extubation outcome. Lower PEEP was also associated with prolonged NRS, with 9 of the 42 patients being on a PEEP of 3 or 4 cmH2O, many of whom post-op cardiac surgery (analysis not shown). Patients with single ventricle physiology who are status post Glenn or Fontan procedures are often on lower PEEP levels, and are preferentially extubated early to NRS to promote pulmonary blood flow.
Our study has limitations. First, we performed measurements generally after patients had passed an SBT. This likely explains why effort of breathing measures did not predict extubation failure prior to extubation, because these patients had near complete resolution of their respiratory disease, and as previously shown effort of breathing during the SBT is often lower than it is after extubation (30), or even at ICU discharge (25). Second, we obtained a snap-shot of respiratory muscle strength with PiMax, and did not measure endurance. Most patients in our ICUs undergo a 2 hour SBT on CPAP, which may be a better measure of endurance, but it would be important to perform a similar study during initial SBTs, to see if these parameters identify patients who fail the SBT. Interestingly, the change in TTI from 5min to 60 minutes post extubation, which may measure endurance, was not associated with re-intubation or extubation failure (analysis not shown). Third, for analysis we defined weakness as aPiMax ≤ 30 cmH20 for all patients; although aPiMax is lower for neonates. Moreover, the performance of the esophageal catheter may differ or some respiratory reflexes may be more pronounced in neonates. While PiMax values are lower for neonates, the pattern of lower aPiMax amongst those who are re-intubated holds across all age strata, reinforcing these methods appear to work across all age groups. Fourth, airway occlusion was for an average of 5 breaths, and we did not routinely do 8–10 breaths allowing exhalation, as others have done (42) to be closer to residual volume. Our measurements occurred closer to functional residual capacity (38); nevertheless, our findings show low PiMax is associated with outcome. Finally, because we did not study patients on nights and weekends, many eligible patients were not enrolled. The characteristics of the included population are similar to the population which was not enrolled, as we have detailed in previous publications (8) (30). Moreover, some may believe that generalizability to the general PICU is limited because nearly half of the patients were post-op cardiac surgery. However, presence of cardiac surgery was not significant in multivariable models, implying these findings are generalizable to both cardiac and non-cardiac patients. Certainly replication of these findings in a multi-center study would be the best way to determine generalizability.
In conclusion, impaired respiratory system strength, measured by low aPiMax at the time of extubation, is a significant risk factor for re-intubation in children, particularly when respiratory effort after extubation is high such as with post-extubation UAO. These findings stress the importance of monitoring respiratory effort after extubation, and using more aggressive UAO management or prevention strategies for patients with low pre-extubation PiMax. This group with low PiMax should be the focus of future UAO prevention or treatment studies. Future research should also focus on strategies to preserve children’s respiratory system strength during mechanical ventilation.
Supplementary Material
Acknowledgments
Sources of Support
National Institutes of Health/NICHD 1K23HL103785
Los Angeles Basin Clinical Translational Science Institute
Footnotes
Work Performed at Children’s Hospital Los Angeles
Copyright form disclosure: Dr. Khemani’s institution received funding from National Institutes of Health (NIH)/National Institute of Child Health and Human Development (NICHD) 1K23HL103785 and from Los Angeles Basin Clinical Translational Science Institute. He received support for article research from the NIH. Dr. Flink received funding from CareFusion, and disclosed having a patent issued: Method, system and software for assessing extubation failure (WO 2015184187 A3). The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Edmunds S, Weiss I, Harrison R. Extubation failure in a large pediatric ICU population. Chest. 2001;119(3):897–900. doi: 10.1378/chest.119.3.897. [DOI] [PubMed] [Google Scholar]
- 2.Kurachek SC, Newth CJ, Quasney MW, et al. Extubation failure in pediatric intensive care: a multiple-center study of risk factors and outcomes. Crit Care Med. 2003;31(11):2657–2664. doi: 10.1097/01.CCM.0000094228.90557.85. [DOI] [PubMed] [Google Scholar]
- 3.Newth CJ, Venkataraman S, Willson DF, et al. Weaning and extubation readiness in pediatric patients. Pediatr Crit Care Med. 2009;10(1):1–11. doi: 10.1097/PCC.0b013e318193724d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mikhno A, Ennett CM. Prediction of extubation failure for neonates with respiratory distress syndrome using the MIMIC-II clinical database. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:5094–5097. doi: 10.1109/EMBC.2012.6347139. [DOI] [PubMed] [Google Scholar]
- 5.Mhanna MJ, Zamel YB, Tichy CM, et al. The “air leak” test around the endotracheal tube, as a predictor of postextubation stridor, is age dependent in children. Crit Care Med. 2002;30(12):2639–2643. doi: 10.1097/00003246-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Wratney AT, Benjamin DK, Jr, Slonim AD, et al. The endotracheal tube air leak test does not predict extubation outcome in critically ill pediatric patients. Pediatr Crit Care Med. 2008;9(5):490–496. doi: 10.1097/PCC.0b013e3181849901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harel Y, Vardi A, Quigley R, et al. Extubation failure due to post-extubation stridor is better correlated with neurologic impairment than with upper airway lesions in critically ill pediatric patients. Int J Pediatr Otorhinolaryngol. 1997;39(2):147–158. doi: 10.1016/s0165-5876(97)01488-2. [DOI] [PubMed] [Google Scholar]
- 8.Khemani RG, Hotz J, Morzov R, et al. Evaluating Risk Factors for Pediatric Post-extubation Upper Airway Obstruction Using a Physiology-based Tool. Am J Respir Crit Care Med. 2016;193(2):198–209. doi: 10.1164/rccm.201506-1064OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bousso A, Ejzenberg B, Ventura AM, et al. Evaluation of the dead space to tidal volume ratio as a predictor of extubation failure. Jornal de Pediatria. 2006;82(5):347–353. doi: 10.2223/JPED.1520. [DOI] [PubMed] [Google Scholar]
- 10.Farias JA, Alia I, Retta A, et al. An evaluation of extubation failure predictors in mechanically ventilated infants and children. Intensive Care Med. 2002;28(6):752–757. doi: 10.1007/s00134-002-1306-6. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson LP, Walsh BK, Munhall D, et al. A spontaneous breathing trial with pressure support overestimates readiness for extubation in children. Pediatr Crit Care Med. 2011;12(6):e330–335. doi: 10.1097/PCC.0b013e3182231220. [DOI] [PubMed] [Google Scholar]
- 12.Fontela PS, Piva JP, Garcia PC, et al. Risk factors for extubation failure in mechanically ventilated pediatric patients. Pediatr Crit Care Med. 2005;6(2):166–170. doi: 10.1097/01.PCC.0000154922.65189.48. [DOI] [PubMed] [Google Scholar]
- 13.Foronda FK, Troster EJ, Farias JA, et al. The impact of daily evaluation and spontaneous breathing test on the duration of pediatric mechanical ventilation: a randomized controlled trial. Crit Care Med. 2011;39(11):2526–2533. doi: 10.1097/CCM.0b013e3182257520. [DOI] [PubMed] [Google Scholar]
- 14.Harikumar G, Egberongbe Y, Nadel S, et al. Tension-time index as a predictor of extubation outcome in ventilated children. Am J Respir Crit Care Med. 2009;180(10):982–988. doi: 10.1164/rccm.200811-1725OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manczur TI, Greenough A, Pryor D, et al. Comparison of predictors of extubation from mechanical ventilation in children. Pediatr Crit Care Med. 2000;1(1):28–32. doi: 10.1097/00130478-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Manczur TI, Greenough A, Pryor D, et al. Assessment of respiratory drive and muscle function in the pediatric intensive care unit and prediction of extubation failure. Pediatr Crit Care Med. 2000;1(2):124–126. doi: 10.1097/00130478-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Riou Y, Chaari W, Leteurtre S, et al. Predictive value of the physiological deadspace/tidal volume ratio in the weaning process of mechanical ventilation in children. Jornal de Pediatria. 2012;88(3):217–221. doi: 10.2223/JPED.2190. [DOI] [PubMed] [Google Scholar]
- 18.Saikia B, Kumar N, Sreenivas V. Prediction of extubation failure in newborns, infants and children: brief report of a prospective (blinded) cohort study at a tertiary care paediatric centre in India. Springerplus. 2015;4:827. doi: 10.1186/s40064-015-1607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venkataraman ST, Khan N, Brown A. Validation of predictors of extubation success and failure in mechanically ventilated infants and children. Crit Care Med. 2000;28(8):2991–2996. doi: 10.1097/00003246-200008000-00051. [DOI] [PubMed] [Google Scholar]
- 20.Currie A, Patel DS, Rafferty GF, et al. Prediction of extubation outcome in infants using the tension time index. Archives of Disease in Childhood Fetal & Neonatal Edition. 2011;96(4):F265–269. doi: 10.1136/adc.2010.191015. [DOI] [PubMed] [Google Scholar]
- 21.Dimitriou G, Greenough A, Endo A, et al. Prediction of extubation failure in preterm infants. Archives of Disease in Childhood Fetal & Neonatal Edition. 2002;86(1):F32–35. doi: 10.1136/fn.86.1.F32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolf GK, Walsh BK, Green ML, et al. Electrical activity of the diaphragm during extubation readiness testing in critically ill children. Pediatr Crit Care Med. 2011;12(6):e220–224. doi: 10.1097/PCC.0b013e3181fe28fc. [DOI] [PubMed] [Google Scholar]
- 23.Goligher EC, Fan E, Herridge MS, et al. Evolution of Diaphragm Thickness during Mechanical Ventilation. Impact of Inspiratory Effort Am J Respir Crit Care Med. 2015;192(9):1080–1088. doi: 10.1164/rccm.201503-0620OC. [DOI] [PubMed] [Google Scholar]
- 24.Supinski GS, Callahan LA. Diaphragm weakness in mechanically ventilated critically ill patients. Crit Care. 2013;17(3):R120. doi: 10.1186/cc12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emeriaud G, Larouche A, Ducharme-Crevier L, et al. Evolution of inspiratory diaphragm activity in children over the course of the PICU stay. Intensive Care Med. 2014;40(11):1718–1726. doi: 10.1007/s00134-014-3431-4. [DOI] [PubMed] [Google Scholar]
- 26.Hooijman PE, Beishuizen A, Witt CC, et al. Diaphragm muscle fiber weakness and ubiquitin-proteasome activation in critically ill patients. Am J Respir Crit Care Med. 2015;191(10):1126–1138. doi: 10.1164/rccm.201412-2214OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matamis D, Soilemezi E, Tsagourias M, et al. Sonographic evaluation of the diaphragm in critically ill patients. Technique and clinical applications Intensive Care Med. 2013;39(5):801–810. doi: 10.1007/s00134-013-2823-1. [DOI] [PubMed] [Google Scholar]
- 28.DiNino E, Gartman EJ, Sethi JM, et al. Diaphragm ultrasound as a predictor of successful extubation from mechanical ventilation. Thorax. 2014;69(5):423–427. doi: 10.1136/thoraxjnl-2013-204111. [DOI] [PubMed] [Google Scholar]
- 29.Kallio M, Peltoniemi O, Anttila E, et al. Electrical activity of the diaphragm during neurally adjusted ventilatory assist in pediatric patients. Pediatr Pulmonol. 2015;50(9):925–931. doi: 10.1002/ppul.23084. [DOI] [PubMed] [Google Scholar]
- 30.Khemani RG, Hotz J, Morzov R, et al. Pediatric extubation readiness tests should not use pressure support. Intensive Care Med. 2016;42(8):1214–1222. doi: 10.1007/s00134-016-4387-3. [DOI] [PubMed] [Google Scholar]
- 31.Khemani R, Flink R, Hotz J, et al. Respiratory inductance plethysmography calibration for pediatric upper airway obstruction: an animal model. Pediatr Res. 2015;77(1–1):75–83. doi: 10.1038/pr.2014.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armitage P, Berry G, Matthews J. Statistical Methods in Medical Research. 4. Oxford; 2002. [Google Scholar]
- 33.Daniel Martin A, Smith BK, Gabrielli A. Mechanical ventilation, diaphragm weakness and weaning: a rehabilitation perspective. Respir Physiolo Neurobiol. 2013;189(2):377–383. doi: 10.1016/j.resp.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Mussi R, Spadaro S, Mirabella L, et al. Impact of prolonged assisted ventilation on diaphragmatic efficiency: NAVA versus PSV. Crit Care. 2016;20(1):1. doi: 10.1186/s13054-015-1178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heunks LM, Doorduin J, van der Hoeven JG. Monitoring and preventing diaphragm injury. Curr Opin Crit Care. 2015;21(1):34–41. doi: 10.1097/MCC.0000000000000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baldwin CE, Paratz JD, Bersten AD. Diaphragm and peripheral muscle thickness on ultrasound: intra-rater reliability and variability of a methodology using non-standard recumbent positions. Respirology. 2011;16(7):1136–1143. doi: 10.1111/j.1440-1843.2011.02005.x. [DOI] [PubMed] [Google Scholar]
- 37.Vivier E, Mekontso Dessap A, Dimassi S, et al. Diaphragm ultrasonography to estimate the work of breathing during non-invasive ventilation. Intensive Care Med. 2012;38(5):796–803. doi: 10.1007/s00134-012-2547-7. [DOI] [PubMed] [Google Scholar]
- 38.ATS, ERS. ATS/ERS Statement on Respiratory Muscle Testing. American Journal of Respiratory and Critical Care Medicine. 2002;166(4):518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 39.Doorduin J, van Hees HW, van der Hoeven JG, et al. Monitoring of the respiratory muscles in the critically ill. Am J Respir Crit Care Med. 2013;187(1):20–27. doi: 10.1164/rccm.201206-1117CP. [DOI] [PubMed] [Google Scholar]
- 40.Gozal D, Shoseyov D, Keens TG. Inspiratory pressures with CO2 stimulation and weaning from mechanical ventilation in children. Am Rev Respir Dis. 1993;147(2):256–261. doi: 10.1164/ajrccm/147.2.256. [DOI] [PubMed] [Google Scholar]
- 41.Wen AS, Woo MS, Keens TG. How many maneuvers are required to measure maximal inspiratory pressure accurately. Chest. 1997;111(3):802–807. doi: 10.1378/chest.111.3.802. [DOI] [PubMed] [Google Scholar]
- 42.Harikumar G, Moxham J, Greenough A, et al. Measurement of maximal inspiratory pressure in ventilated children. Pediatr Pulmonol. 2008;43(11):1085–1091. doi: 10.1002/ppul.20905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watson AC, Hughes PD, Louise Harris M, et al. Measurement of twitch transdiaphragmatic, esophageal, and endotracheal tube pressure with bilateral anterolateral magnetic phrenic nerve stimulation in patients in the intensive care unit. Crit Care Med. 2001;29(7):1325–1331. doi: 10.1097/00003246-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Dimitriou G, Greenough A, Moxham J, et al. Influence of maturation on infant diaphragm function assessed by magnetic stimulation of phrenic nerves. Pediatr Pulmonol. 2003;35(1):17–22. doi: 10.1002/ppul.10209. [DOI] [PubMed] [Google Scholar]
- 45.Luo YM, Hart N, Mustfa N, et al. Reproducibility of twitch and sniff transdiaphragmatic pressures. Respir Physiolo Neurobiol. 2002;132(3):301–306. doi: 10.1016/s1569-9048(02)00115-5. [DOI] [PubMed] [Google Scholar]
- 46.Man WD, Luo YM, Mustfa N, et al. Postprandial effects on twitch transdiaphragmatic pressure. Eur Respir J. 2002;20(3):577–580. doi: 10.1183/09031936.02.00302702. [DOI] [PubMed] [Google Scholar]
- 47.Polkey MI, Kyroussis D, Hamnegard CH, et al. Paired phrenic nerve stimuli for the detection of diaphragm fatigue in humans. Eur Respir J. 1997;10(8):1859–1864. doi: 10.1183/09031936.97.10081859. [DOI] [PubMed] [Google Scholar]
- 48.Rafferty GF, Greenough A, Dimitriou G, et al. Assessment of neonatal diaphragm function using magnetic stimulation of the phrenic nerves. Am J Respir Crit Care Med. 2000;162(6):2337–2340. doi: 10.1164/ajrccm.162.6.2004019. [DOI] [PubMed] [Google Scholar]
- 49.Rafferty GF, Mustfa N, Man WD, et al. Twitch airway pressure elicited by magnetic phrenic nerve stimulation in anesthetized healthy children. Pediatr Pulmonol. 2005;40(2):141–147. doi: 10.1002/ppul.20241. [DOI] [PubMed] [Google Scholar]
- 50.Benditt JO. Esophageal and gastric pressure measurements. Respir Care. 2005;50(1):68–75. [PubMed] [Google Scholar]
- 51.Steier J, Kaul S, Seymour J, et al. The value of multiple tests of respiratory muscle strength. Thorax. 2007;62(11):975–980. doi: 10.1136/thx.2006.072884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.