Abstract
Purpose
Cardiovascular disease is a major contributor to morbidity and mortality, and prevention relies on accurate identification of those at risk. Studies of the association between quality of life (QOL) and mortality and vascular events incompletely accounted for depression, cognitive status, social support, and functional status, all of which have an impact on vascular outcomes. We hypothesized that baseline QOL is independently associated with long-term mortality in a large, multi-ethnic urban cohort.
Methods
In the prospective, population-based Northern Manhattan Study, Spitzer QOL index (SQI, range 0–10, with ten signifying the highest QOL) was assessed at baseline. Participants were followed over a median 11 years for stroke, myocardial infarction (MI), and vascular and non-vascular death. Multivariable Cox proportional hazards regression estimated hazard ratio and 95% confidence interval (HR, 95% CI) for each outcome, with SQI as the main predictor, dichotomized at 10, adjusting for baseline demographics, vascular risk factors, history of cancer, social support, cognitive status, depression, and functional status.
Results
Among 3298 participants, mean age was 69.7 + 10.3 years; 1795 (54.5%) had SQI of 10. In fully adjusted models, SQI of 10 (compared to SQI <10) was associated with reduced risk of all-cause mortality (HR 0.80, 95% CI 0.72–0.90), vascular death (0.81, 0.69–0.97), non-vascular death (0.78, 0.67–0.91), and stroke or MI or death (0.82, 0.74–0.91). In fully adjusted competing risk models, there was no association with stroke (0.93, 0.74–1.17), MI (0.98, 0.75–1.28), and stroke or MI (1.03, 0.86–1.24). Results were consistent when SQI was analyzed continuously.
Conclusion
In this large population-based cohort, highest QOL was inversely associated with long-term mortality, vascular and non-vascular, independently of baseline primary vascular risk factors, social support, cognition, depression, and functional status. QOL was not associated with non-fatal vascular events.
Keywords: Quality of life, Patient-centered outcomes, Prospective, Cohort, Vascular outcomes
Introduction
Cardiovascular disease (CVD) accounts for >30% of deaths in the United States and the majority of disability [1]. It is responsible for one in four deaths worldwide [2]. The ability to accurately identify individuals at risk of cardiovascular morbidity and mortality would provide clinicians with information on prognosis and appropriate timing of preventative interventions. There is a vast literature about vascular risk factors and other “objective” factors being associated with cardiovascular disease outcomes, but less information is available about the impact of patient-centered outcomes, such as quality of life (QOL). As cardiovascular disease constitutes the majority of disease burden and cause-specific mortality in our society, it is important to study the association between baseline QOL and future vascular events and mortality.
The prognostic significance of patient-reported QOL on mortality has been previously examined in disease-specific groups such as diabetes [3], heart failure [4], ischemic heart disease [5, 6], and cancer [7]. Beyond mortality, QOL has been shown to predict readmission among patients who are chronically ill [8]. Population-based studies examining the relationship between baseline QOL and long-term vascular outcomes and mortality have been performed primarily in homogenous ethnic groups [9–12]. A study on 1739 participants free of CVD in Beijing demonstrated lower QOL, measured using the Chinese 35-Item QOL Instrument (QOL-35), was associated with increased risk of 10-year all-cause mortality [13]. Three separate studies on German [14], Scottish [15], and Canadian [16] populations reported all-cause mortality risk that were inversely related to QOL assessed using Short Form-12 (SF-12) [14, 15] and self-rated health (SRH) [14, 16]. Among 710 White Dutch men who rated themselves poorly on SRH, there was a higher risk of all-cause mortality and cancer mortality but not CVD mortality [17]. The inverse relationship was not seen when SRH was assessed among 1753 business executives of similar socioeconomic status in Finland; rather, a J-shaped relationship to mortality was seen; those who felt their health was “very good” or “poor,” compared to “fairly good,” had significant increases in all-cause mortality [18]. Generalization is limited due to the homogeneity of these studies, and the association of QOL and long-term mortality remains unclear in a multi-ethnic urban American population. Furthermore, studies examining non-fatal CVD events are relatively sparse with conflicting reports.
Prior population-based studies examining the prognostic ability of baseline QOL ratings have not controlled for depression, a major public health problem and important confounder [14, 15, 18–20]. Depression affects a person’s perceived QOL, and has been shown to independently impact morbidity and mortality. Adults with depression have a 28.9-year quality-adjusted life expectancy loss compared to those without depression—far exceeding life expectancy losses from other conditions such as stroke, heart disease, diabetes, hypertension, and smoking [21]. Analysis of the National Health and Nutrition Examination Survey (NHANES) data reported incremental decrease in quality-adjusted life years with increasing severity of depressive symptoms [22]. Comorbid depression among stable CAD patients led to diminished QOL and increased risk for future cardiac events [23]. Multiple studies have demonstrated that depression has an equal if not greater effect on QOL than most common medical conditions [21–23].
Functional status is another important confounder of QOL. In patients with CAD, adjustment for cardiac symptom severity had negligible effects on the association between SRH and major CVD events. When factoring in functional status, an approximate 50% reduction in hazard ratio magnitude was seen, SRH lost its significance as a predictor, and higher functional status was shown to be associated with reduced major CVD events [24]. In the Longitudinal Study on Aging, the number of ADL problems, not SRH, was associated with increased mortality among elderly African American males [25].
We sought to expand on previous studies and examine the prognostic ability of baseline QOL on mortality and non-fatal CVD events while controlling for depression and baseline functional status. We hypothesized that lower baseline QOL is associated with long-term mortality and vascular events, independently of medical risk factors and psychosocial and functional variables in a large, urban, population-based, race-ethnically diverse population.
Methods
The Northern Manhattan Study (NOMAS) population-based prospective cohort evaluates the effects of medical, socioeconomic, serum, and imaging risk factors on incident vascular disease and other outcomes in a multi-ethnic community. A total of 3298 participants were recruited by random digit dialing of published and unpublished telephone numbers between 1993 and 2001. Subjects were enrolled if they: (1) were >40 years of age; (2) lived in a pre-defined geographic area of northern Manhattan for >3 months in a household with a telephone; and (3) had no history of stroke. The telephone response rate was 91, 87% of eligible subjects indicated willingness to participate, and enrollment response rate was 75%. Race–ethnic differences were similar among responders and non-responders. The study was approved by the institutional review boards of Columbia University and the University of Miami, and informed consent was obtained from all participants. Further characteristics of the cohort have been previously reported [26–28].
Baseline evaluation
Participants underwent a comprehensive medical history, physical examination, medical record review, and fasting blood samples. Bilingual research assistants interviewed participants and collected data using standardized questions adapted from the Centers for Disease Control and Prevention Behavioral Risk Factor Surveillance System, as previously described [29]. Fasting total cholesterol was obtained using a Hitachi 705 automated spectrophotometer (Boehringer, Mannheim, Germany). Functional status was assessed by the Barthel Index (BI) [30, 31], which measures performance in ten activities of daily living (ADLs) and ranges from 0 to 100 in 5-point increments, with 100 indicating normal physical functioning.
QOL was assessed by the Spitzer QOL index (SQI) [32], a 10-point scale, with higher numbers denoting higher QOL. QOL was assessed in five domains—Activity, Daily Living, Health, Support, and Outlook—with a score of 0, 1, or 2 (highest) in each domain (supplemental). Prior research has established high internal consistency with a calculated Cronbach’s alpha of 0.78 [32, 33]. The SQI has previously been used in Spanish-speaking populations [34] and requires approximately 5 min to complete.
Follow-up
All participants were followed annually via phone to detect death, new neurological or cardiac symptoms and events, interval hospitalizations, cognitive function, and functional status via the BI. Ten percent of the cohort was brought in for in-person visits annually to exclude the possibility of missed events by telephone. Only two subjects were completely lost to follow-up after their baseline examination, and the average annual contact rate was 99%.
A positive screen for a cardiac or neurological event was followed by an in-person assessment to determine whether a vascular outcome had occurred. In addition, all admissions and discharges were screened for hospitalizations and outcomes that may not have been captured by telephone interview. Nearly 70% of vascular events led to hospitalizations at Columbia University Medical Center. Hospital records were reviewed to classify outcomes as previously reported [28]. Stroke included ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage, but not transient ischemic attack or venous sinus thrombosis. At least two stroke neurologists verified and classified all stroke cases. MI was defined by criteria adapted from the Cardiac Arrhythmia Suppression trial [35] and the Lipid Research Clinics Coronary Primary Prevention trial [36] requiring >2 of the following: (a) ischemic cardiac pain determined to be typical angina; (b) cardiac marker abnormalities (abnormal CK-MB fraction or troponin I values); and (c) ischemic EKG abnormalities. Diagnosis of MI was adjudicated by cardiologists independently after clinical data review.
Covariates
Analytic models were adjusted for the following variables: age, sex, race-ethnicity (self-reported as non-Hispanic white, non-Hispanic black, Hispanic, and other), hypercholesterolemia (defined as self-report of hypercholesterolemia, lipid lowering therapy use, or fasting total cholesterol level >240 mg/dL), self-reported cancer, diabetes mellitus (defined by self-report, fasting blood glucose level >126 mg/dL, or insulin/oral hypoglycemic use), hypertension (defined as a systolic blood pressure recording >140 mmHg or a diastolic blood pressure recording >90 mm Hg based on the average of two blood pressure measurements or the patient’s self-report of a history of hypertension or antihypertensive use), self-reported coronary artery disease or history of myocardial infarction (CAD), smoking (defined as either nonsmoker or smoker within the last year), alcohol use (with moderate alcohol use classified as 1 drink/month to 2 drinks/day), social variables [marital status, insurance status (classified as uninsured/Medicaid versus Medicare/private insurance), number of friends (individuals whom the participant knows well enough to visit in their homes)], cognitive factors [depression (defined as a Hamilton Depression scale score of >12) and performance on mini-mental state examination (analyzed as a continuous variable)], physical activity (assessed using a questionnaire adapted from the National Health Interview Survey and classified as any or none, as in previous research in this cohort) [37, 38], and functional status, measured by the BI.
Statistical analysis
Distributions of baseline characteristics were compared between those with SQI <10 versus SQI = 10, using a t-test for continuous variables and Chi-square for categorical variables. We chose the SQI cutoff of 10 because it was the median value and for clinical applicability of ideal versus suboptimal QOL scores. Distributions of the total SQI score were calculated, and the number of vascular events and deaths occurring during follow-up were counted.
Kaplan–Meier curves were created for each outcome separately and for composite outcomes, overall and stratified by SQI (10 vs. <10); differences between strata were tested by the log-rank test. Primary outcomes were all-cause mortality, non-vascular death, and vascular death, and secondary outcomes included stroke, MI, stroke or MI, composite outcome of stroke, MI, or vascular death, and composite outcome of stroke, MI, or all-cause death. Cox proportional hazards regression was performed for each primary and secondary outcome, with SQI as the main predictor dichotomized at 10. SQI was tested continuously as a secondary analysis. Univariate models were run first. Then, models were sequentially adjusted for baseline demographics (age, sex, race/ethnicity), vascular risk factors (diabetes, hypertension, CAD, and hypercholesterolemia), additional risk factors and social support (physical activity, moderate alcohol use, smoking, BMI, marital status, insurance status and number of friends), cancer and cognitive status (self-reported cancer, mini-mental state score, depression and anti-depressant usage), and functional status (BI).
To adjust for competing risks, Cox proportional hazards regression was performed for stroke and MI outcomes with death during the study period as a competing risk. For the composite outcome of stroke, MI, and vascular death, the competing risk was defined as non-vascular death. Subgroup analysis was performed among those with baseline SQI <10 to assess whether the effect of higher measured QOL is associated with outcomes even when the highest score of 10 is removed. Lastly, subgroup analysis among those with no baseline CAD was performed because one could argue that past CAD causes lower QOL, and in such scenarios, it could be the CAD which causes subsequent vascular events and mortality, not QOL.
Results
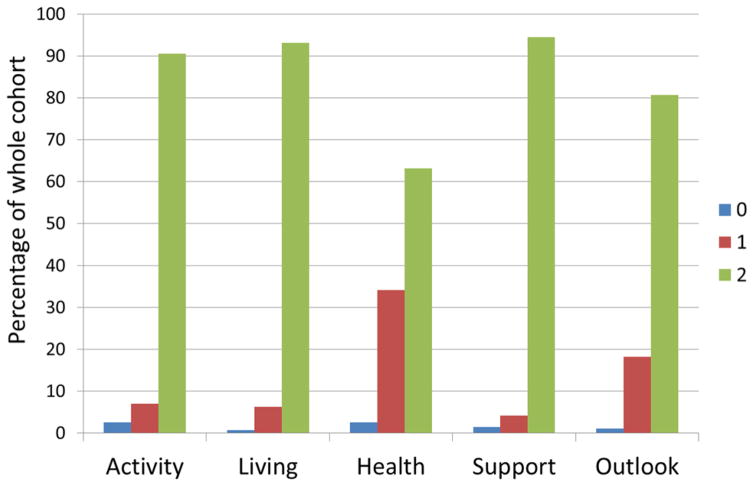
Table 1 shows baseline characteristics in the entire cohort (n = 3298) and compares frequencies among those with baseline SQI <10 (n = 1502) versus SQI = 10 (n = 1796). Compared to individuals with SQI <10, those with SQI = 10 were slightly younger, more often male, with lower BMI and higher educational attainment. Individuals with SQI = 10 were more likely to have Medicare or private insurance, be married, have a wider social network of friends, and have higher mini-mental status scores, and lower Hamilton depression scale scores. Those with SQI < 10 had more vascular risk factors such as diabetes, CAD, atrial fibrillation, smoking, alcohol consumption, and lower levels of physical activity. Figure 1 shows distributions of SQI responses, stratified by domain, among all participants. Mean SQI was 9.1 (SD 1.3). Over a median of 11 years of follow-up, there were 265 first MIs (definite and probable), 369 first strokes, and 1615 deaths, 626 (38.8%) vascular, 826 (51.1%) non-vascular, and 143 (8.9%) unknown.
Table 1.
Baseline characteristics of study population
| Variable | Entire cohort | Individuals with QOL score <10 | Individuals with QOL score of 10 | p value |
|---|---|---|---|---|
| Number of participants, No. (%) | 3298 (100) | 1502 (45.5) | 1796 (54.5) | |
| Demographics | ||||
| Age, mean (SD), y | 69.7 (10.3) | 70.4 (10.8) | 69.1 (9.8) | 0.0004 |
| Male, no. (%) | 1227 (37.2) | 468 (31.2) | 759 (42.3) | <0.0001 |
| Race-ethnicity | 0.4 | |||
| Non-hispanic white, No. (%) | 690 (20.9) | 333 (22.2) | 357 (19.9) | |
| Non-hispanic black, No. (%) | 803 (24.4) | 353 (23.5) | 450 (25.1) | |
| Hispanic, No. (%) | 1728 (52.4) | 779 (51.8) | 949 (52.8) | |
| Other, No. (%) | 77 (2.3) | 37 (2.5) | 40 (2.2) | |
| Received at least high school education, No. (%) | 1511 (45.8) | 658 (43.8) | 853 (47.5) | 0.035 |
| Marital status, No. (%) married | 1042 (31.6) | 415 (27.6) | 627 (34.9) | <0.0001 |
| Health insurance, No. (%) | <0.0001 | |||
| Medicaid or no insurance | 1435 (43.8) | 726 (48.8) | 709 (39.7) | |
| Medicare or private insurance | 1841 (56.2) | 763 (51.2) | 1078 (60.3) | |
| Vascular risk factors, No. (%) | ||||
| Hypertension | 2429 (73.6) | 1119 (74.5) | 1310 (72.9) | 0.3 |
| Alcohol consumption | <0.0001 | |||
| Never drank | 821 (24.9) | 411 (27.4) | 410 (22.8) | |
| Past drinker | 799 (24.2) | 399 (26.5) | 400 (22.3) | |
| Light drinker | 421 (12.8) | 201 (13.4) | 220 (12.3) | |
| Moderate drinker | 1086 (32.9) | 418 (27.8) | 668 (37.2) | |
| Intermediate drinker | 120 (3.6) | 51 (3.4) | 69 (3.8) | |
| Heavy drinker | 51 (1.6) | 22 (1.5) | 29 (1.6) | |
| Physical activity | ||||
| None | 1389 (42.1) | 701 (46.7) | 688 (38.3) | <0.0001 |
| Any | 1909 (57.9) | 801 (53.3) | 1108 (61.7) | |
| Diabetes mellitus | 716 (21.8) | 362 (24.2) | 354 (19.7) | 0.002 |
| Smoking | 0.014 | |||
| Never | 1545 (46.9) | 722 (48.1) | 823 (45.8) | |
| Former | 1249 (37.9) | 531 (35.4) | 718 (40) | |
| Current | 502 (15.2) | 248 (16.5) | 254 (14.2) | |
| Body mass index, mean (SD), kg/m2 | 27.8 (5.5) | 28.1 (6) | 27.6 (5) | 0.005 |
| Hypercholesterolemia | 2050 (62.2) | 925 (61.6) | 1125 (62.6) | 0.5 |
| History of atrial fibrillation | 143 (4.3) | 80 (5.3) | 63 (3.5) | 0.01 |
| History of coronary heart disease | 704 (21.4) | 388 (25.8) | 316 (17.6) | <0.0001 |
| Other medical conditions, No. (%) | ||||
| Hamilton depression scale score, mean (SD) | 3.2 (3.8) | 4.9 (4.6) | 1.7 (2.2) | <0.0001 |
| Hamilton depression score >12 | 138 (4.2) | 131 (8.7) | 7 (0.4) | <0.0001 |
| Taking anti-depressant medication | 203 (7.8) | 146 (11.4) | 57 (4.3) | <0.0001 |
| Mini-mental state score, mean (SD) | 26.0 (3.8) | 25.4 (4.1) | 26.5 (3.4) | <0.0001 |
| Social variables, No. (%) | ||||
| Number of people known well enough to visit with in their homes | <0.0001 | |||
| None | 130 (3.9) | 74 (4.9) | 56 (3.1) | |
| 1 or 2 | 367 (11.1) | 199 (13.3) | 168 (9.4) | |
| 3 or 4 | 653 (19.8) | 289 (19.3) | 364 (20.3) | |
| 5 or more | 2145 (65.1) | 937 (62.5) | 1208 (67.2) | |
Fig. 1.
Distributions of domain-specific scores of quality of life
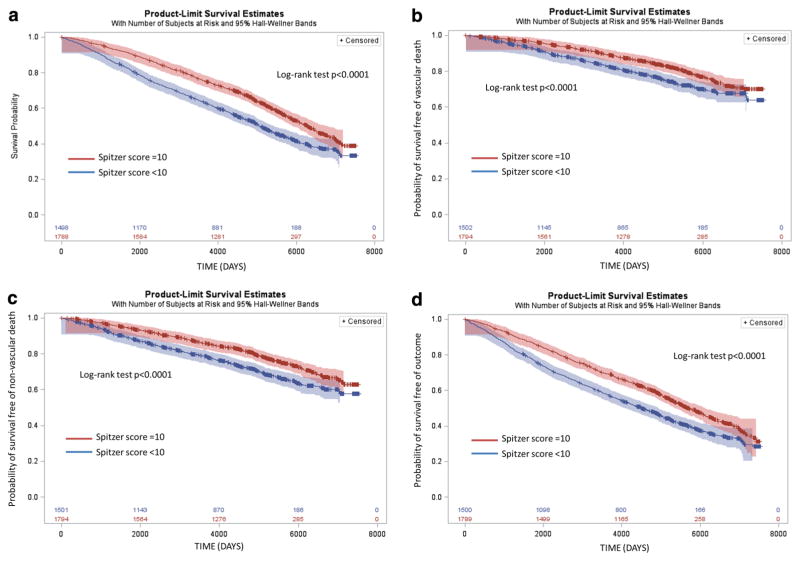
The association between baseline SQI dichotomized at 10 and vascular events and mortality occurring during follow-up was tested in unadjusted models and models adjusting for demographics, medical, social, cognitive, and cancer factors and baseline functional status (Table 2). In fully adjusted models, SQI of 10 (compared to SQI <10) was significantly associated with lower risk for all primary outcomes: all-cause mortality (HR 0.80, 95% CI 0.72–0.90), vascular death (HR 0.81, 0.69–0.97), and non-vascular death (HR 0.78, 0.67–0.91). Results were similar when baseline SQI was tested as a continuous variable (Table 2). In unadjusted and adjusted models, higher SQI was associated with a lower risk for all primary outcomes. Figure 2 shows the Kaplan–Meier curves among those with SQI <10 and SQI = 10 for cumulative risks of all-cause mortality, vascular death, non-vascular death, and stroke or MI or death. Cumulative risk probabilities were higher among those with SQI <10 (compared to SQI = 10; all log-rank p< 0.0001). The greatest divergence in the Kaplan–Meier curves occurred over the first 3–4 years. In a sub-analysis of follow-up limited to 3.5 years, the association between SQI of 10 and mortality was more robust with lower risk for all-cause mortality (HR 0.66, 0.51–0.85), vascular death (HR 0.65, 0.44–0.96), and non-vascular death (HR 0.66, 0.48–0.91).
Table 2.
The association between dichotomized and continuous quality of life score and vascular outcomes, in unadjusted and adjusted models
| SQI = 10 versus SQI <10 | SQI continuous, per point increase | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | Hazard ratio | 95% CI | p value | Hazard ratio | 95% CI | p value |
| Primary outcomes | ||||||
| All-cause mortality | ||||||
| Unadjusted model | 0.69 | 0.62–0.76 | <0.0001 | 0.81 | 0.78–0.83 | <0.0001 |
| Model A | 0.71 | 0.64–0.78 | <0.0001 | 0.83 | 0.81–0.86 | <0.0001 |
| Model B | 0.75 | 0.67–0.85 | <0.0001 | 0.87 | 0.83–0.91 | <0.0001 |
| Model C | 0.80 | 0.72–0.90 | <0.0001 | 0.89 | 0.85–0.94 | <0.0001 |
| Vascular death | ||||||
| Unadjusted model | 0.69 | 0.59–0.80 | <0.0001 | 0.81 | 0.77–0.86 | <0.0001 |
| Model A | 0.72 | 0.62–0.85 | <0.0001 | 0.85 | 0.80–0.89 | <0.0001 |
| Model B | 0.76 | 0.63–0.91 | 0.0031 | 0.88 | 0.82–0.94 | 0.0002 |
| Model C | 0.81 | 0.69–0.97 | 0.018 | 0.91 | 0.84–0.98 | 0.017 |
| Non-vascular death | ||||||
| Unadjusted model | 0.68 | 0.59–0.78 | <0.0001 | 0.79 | 0.76–0.83 | <0.0001 |
| Model A | 0.68 | 0.59–0.78 | <0.0001 | 0.81 | 0.77–0.85 | <0.0001 |
| Model B | 0.74 | 0.63–0.87 | 0.0003 | 0.85 | 0.80–0.89 | <0.0001 |
| Model C | 0.78 | 0.67–0.91 | 0.001 | 0.87 | 0.81–0.93 | <0.0001 |
| Secondary outcomes | ||||||
| Stroke | ||||||
| Unadjusted model | 0.86 | 0.70–1.05 | 0.14 | 0.92 | 0.84–0.99 | 0.03 |
| Model A | 0.88 | 0.72–1.09 | 0.25 | 0.94 | 0.86–1.02 | 0.14 |
| Model B | 0.88 | 0.70–1.11 | 0.29 | 0.94 | 0.85–1.04 | 0.22 |
| Model C | 0.87 | 0.69–1.08 | 0.21 | 0.91 | 0.82–1.02 | 0.09 |
| Competing risk modela | 0.93 | 0.74–1.17 | 0.54 | |||
| Myocardial infarction | ||||||
| Unadjusted model | 0.85 | 0.67–1.07 | 0.17 | 0.91 | 0.83–1.01 | 0.06 |
| Model A | 0.89 | 0.69–1.13 | 0.33 | 0.94 | 0.86–1.04 | 0.25 |
| Model B | 0.83 | 0.63–1.10 | 0.19 | 0.91 | 0.82–1.02 | 0.12 |
| Model C | 0.90 | 0.70–1.17 | 0.43 | 0.98 | 0.87–1.11 | 0.82 |
| Competing risk modela | 0.98 | 0.75–1.28 | 0.88 | |||
| Stroke or myocardial infarction | ||||||
| Unadjusted model | 0.85 | 0.72–0.99 | 0.05 | 0.90 | 0.85–0.96 | 0.0015 |
| Model A | 0.88 | 0.74–1.04 | 0.13 | 0.93 | 0.87–0.98 | 0.02 |
| Model B | 0.87 | 0.72–1.05 | 0.15 | 0.92 | 0.85–0.99 | 0.04 |
| Model C | 0.89 | 0.75–1.06 | 0.19 | 0.95 | 0.88–1.02 | 0.17 |
| Competing Risk Modela | 1.03 | 0.86–1.24 | 0.72 | |||
| Stroke, myocardial infarction, or vascular death | ||||||
| Unadjusted model | 0.75 | 0.66–0.85 | <0.0001 | 0.84 | 0.81–0.88 | <0.0001 |
| Model A | 0.78 | 0.69–0.89 | 0.0002 | 0.87 | 0.83–0.91 | <0.0001 |
| Model B | 0.80 | 0.69–0.92 | 0.0024 | 0.88 | 0.83–0.93 | <0.0001 |
| Model C | 0.83 | 0.73–0.96 | 0.0097 | 0.90 | 0.85–0.96 | 0.0014 |
| Competing risk modelb | 0.89 | 0.78–1.03 | 0.13 | |||
| Stroke, myocardial infarction, or all-cause mortality | ||||||
| Unadjusted model | 0.72 | 0.66–0.79 | <0.0001 | 0.82 | 0.80–0.85 | <0.0001 |
| Model A | 0.75 | 0.68–0.82 | <0.0001 | 0.85 | 0.82–0.88 | <0.0001 |
| Model B | 0.77 | 0.69–0.86 | <0.0001 | 0.87 | 0.83–0.90 | <0.0001 |
| Model C | 0.82 | 0.74–0.91 | 0.0002 | 0.89 | 0.85–0.94 | <0.0001 |
Model A: adjusted for age, male sex, race and ethnicity, diabetes, hypertension, coronary artery disease and hypercholesterolemia
Model B: as in Model A with additional adjustment for physical activity, moderate alcohol use, smoking, BMI, marital status, insurance status, number of friends, mini-mental status score, depression, cancer and anti-depressant use Model C: as in Model B with additional adjustment for Barthel index score
Competing risk model: As in Model C, with death as competing risk
Competing risk model: As in Model C, with non-vascular death as competing risk
Fig. 2.
Kaplan–Meier curves showing cumulative risk of outcomes, stratified by Spitzer score. a All-cause mortality. b Vascular death c Non-vascular death. d Stroke, MI, or all-cause death
For secondary outcomes, SQI of 10 (compared to SQI <10) was significantly associated with lower composite risk of stroke, MI, or all-cause mortality (HR 0.82, 0.74–0.91). In fully adjusted competing risk models, SQI of 10 (compared to SQI <10) was not associated with risk of stroke (HR 0.93, 95% CI 0.74–1.17), MI (HR 0.98, 0.75–1.28), stroke or MI (HR 1.03, 0.86–1.24), and composite of stroke, MI, or vascular death (HR 0.89, 0.78–1.03). Similar magnitude and direction of association were seen among all secondary outcomes when SQI was analyzed as a continuous measurement.
Over half the participants had a baseline SQI = 10. In the subgroup analysis limited to those with baseline SQI <10 (n = 1502), the results were consistent when SQI was analyzed continuously. Namely, there was a reduced hazard of all-cause mortality (HR 0.87, 0.82–0.92), vascular death (HR 0.88, 0.80–0.97), non-vascular death (HR 0.84, 0.77–0.91), and stroke, MI, or all-cause mortality (HR 0.87, 0.83–0.93) but not with stroke (HR 0.96, 0.83–1.11), MI (HR 1.00, 0.84–1.18), or stroke or MI (HR 0.95, 0.85–1.06).
In the subgroup analysis of participants without baseline CAD (n = 2594), in a fully adjusted model, there was no association between SQI = 10 and MI (0.93, 0.80–1.07) but there were associations with lower hazards of stroke or MI (0.91, 0.83–0.99) and stroke, MI or all-cause death (0.85, 0.81–0.89).
Discussion
In this large, population-based, prospective cohort study, we found that higher baseline QOL were associated with reduced hazards of mortality, vascular and non-vascular, over more than 10 years of follow-up. Those with higher scores on SQI, a validated generic QOL scale, had fewer medical conditions, were more often male, had greater social support, less depression, and higher cognitive scores. However, the associations between SQI and mortality were independent of baseline demographics, medical and vascular risk factors, depression, social factors, and functional status. Those with the highest SQI score had approximately 20% reduced mortality compared to those with lower SQI scores, in a fully adjusted model. Results were consistent when SQI was analyzed as a continuous variable as well as dichotomized at 10. Significant associations between baseline SQI score and outcomes were maintained in subgroup analyses among those with SQI <10 and among those without baseline CAD.
Prior population-based studies, mostly of homogenous groups in other countries, with no control for depression and functional status, have generally shown an inverse relationship between QOL or SRH and mortality [13–16]. In the American population, a prospective study of African Americans over two decades ago reported poor SRH was an independent predictor of mortality for women, but not for men [25]. Among 2166 community-dwelling elderly Medicare recipients in Utah, those with the lowest scores on SF-12 had 6× higher mortality [39]. More recently, a study of 4677 participants using cross-sectional data from NHANES demonstrated SRH was inversely associated with all-cause mortality and CVD mortality after controlling for 10-year atherosclerotic cardiovascular disease risk; there was no gender difference seen [19]. The population consisted of 77% non-Hispanic Whites and only 9% non-Hispanic Black and 6% Hispanic patients. In our analysis, we confirmed a similar relationship of lower QOL and increased mortality risk in a multi-ethnic urban cohort comprised mostly Hispanic and non-Hispanic Blacks with high prevalence of vascular risk factors. In the models adjusting for depression and anti-depressant usage, we found only slight attenuation of hazards; the overall predictive ability of baseline QOL and mortality outcomes remained strong. The same was true in the fully adjusted model where we controlled for baseline functional status.
Unlike mortality outcomes, results from studies looking at non-fatal CVD events are less robust and uniform. Lower QOL in a Beijing cohort, measured by QOL-35, predicted subsequent risk for incident MI but not stroke [11]. In Danish women, but not men, low SRH predicted higher risk of ischemic heart disease [40]. A recent cohort study from Iran reported no association between QOL, measured by WHOQOL-BREF, and ischemic heart disease although there was a paradoxical relationship between higher QOL and stroke incidence [41]. In UK studies comprised of Brit-ish White Caucasians, those in the bottom quartile of SF-36 physical component summary scores had double the risk of incident coronary heart disease [9] and stroke [10]. The Chinese and UK cohorts had fairly low rates of CVD events on follow-up, likely because the participants were relatively healthy, middle-aged, and all free of CVD at baseline. We found that poor QOL, measured by SQI, was not significantly associated with increased risk of subsequent non-fatal CVD events.
The reason for discrepancies among non-fatal CVD outcomes is unclear but may be due to a combination of several explanations. First, the NOMAS population has a higher prevalence of vascular risk factors, and this may increase the overall risk. It is known that patients with CAD are at higher risk of subsequent CVD and mortality. Patients with CAD who had poor SRH have significantly higher risk for all-cause mortality, CAD-related mortality, major CVD events (MI, CABG, PCI, death), as well as shorter times to these events [24, 42, 43]. To account for this potential confounder, we performed subgroup analysis among those without baseline CAD, and there was a marginally lower risk for stroke or MI. Second, most studies to date, which have not adjusted for depression and baseline functional status, show physical functioning and independence [11, 13] domains to be the main driver for subsequent CVD events; the predictive ability of baseline QOL may have been attenuated once we controlled for these factors. For example, in our analysis of stroke or MI outcomes with SQI analyzed continuously, significance was gradually lost from the unadjusted model to subsequent models adjusting for depression and functional status. Lastly, it is possible that the QOL instrument we used is not as sensitive as the QOL-35 or SF-36 for non-fatal CVD event prediction. Future directions can include testing a different measurement, such as SF-36 or SF-12, in our cohort to see if the findings can be replicated.
QOL scales reflect a composite measure of the complex, intricate, and multidimensional nature of a person’s life, incorporating biological, emotional, and social dimensions. These subjective experiences are only directly accessible by individuals themselves, which may make the subjective measure of QOL a more accurate predictor of risk compared to diagnoses of medical conditions. Traditional health profiles classify medical and vascular risk factors as primarily dichotomous variables. However, each disease has a wide spectrum of severity that affects each individual differently. Individual differences in frailty, a measure of decreased physical reserve rather than manifest disability, have been associated with vascular outcomes and mortality in prior studies [44, 45]. As individuals experience various mental and physical stresses over the life course, they accumulate multi-system deficits reflected as frailty. Eventually a critical point is reached of diminished resilience where the ability to deal with further stress is impaired and any further disruption to the system will require a longer time to re-establish equilibrium [46]. Low QOL has been associated with development of frailty [18]. QOL may be a surrogate marker of a person’s frailty and resilience, reflecting the cumulative toll each individual condition or risk factor has on the person. QOL may reflect a patient’s attitude on health such that patients with lower QOL may be more neglectful of preventative measures and maintaining a healthy lifestyle. Low QOL may also reflect inadequate treatment regimens for existing medical conditions. Regardless of the ultimate mechanism, QOL is an easily measured subjective factor that is a strong predictor of mortality outcomes.
Strengths of this study include its population-based design and random sampling technique thus allowing for generalizability to an urban, multi-ethnic population. There was excellent annual follow-up and thorough identification of vascular events and mortality, which allowed accurate estimation of the effect of QOL on these outcomes. We improved on prior studies by additionally controlling for depression and functional status.
Our study is limited in that SQI was administered once at baseline, and thus we could not control for changes in QOL over time when calculating risk models. Changes in self-assessed QOL over time have been shown to predict subsequent mortality [47]. However, QOL in Canadians prospectively measured by SF-36 has been demonstrated to be relatively stable over 5 years [48]. Similarly, QOL and SRH in the US population have been demonstrated to be relatively stable, and decrements large enough to increase mortality risk occurred infrequently at 2.4% [20]. Our goal was to determine if baseline assessment alone was adequate to determine future risk and to this end the predictive ability appears appropriate. A second limitation was our choice to use SQI which limits comparison to other studies. The SQI was chosen during the initial design of the study as it had only ten questions, making it less time consuming to obtain during clinic visits. There are other QOL instruments available currently and this can be an area of further research to see if alternate instruments yield similar findings in this cohort.
In summary, we found that lower baseline QOL, assessed by SQI, was independently associated with future mortality, both vascular and non-vascular. Clinicians may be able to use this powerful and easily assessed screening tool to identify patients at risk. We did not find any association with non-fatal CVD events. Further research would clarify whether QOL adds incremental value to formal risk prediction scores, and whether interventions targeted specifically to improving QOL (targeted to individual well-being, social support, and ADL performance) would also translate into improved vascular outcomes and reduced mortality.
Acknowledgments
Funding This work was supported by grants from the National Institute of Neurological Disorders and Stroke (R01 NS48134, MSVE; R37 29993, RLS/MSVE).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11136-017-1567-8) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of interest None of the authors has a financial relationship or conflict of interest relevant to the topic of the manuscript.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Executive summary: Heart disease and stroke statistics-2016 update: A report from the american heart association. Circulation. 2016;133:447–454. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McEwen LN, Kim C, Haan MN, et al. Are health-related quality-of-life and self-rated health associated with mortality? Insights from Translating Research Into Action for Diabetes (TRIAD) Primary Care Diabetes. 2009;3:37–42. doi: 10.1016/j.pcd.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez-Artalejo F, Guallar-Castillon P, Pascual CR, et al. Health-related quality of life as a predictor of hospital readmission and death among patients with heart failure. Archives of Internal Medicine. 2005;165:1274–1279. doi: 10.1001/archinte.165.11.1274. [DOI] [PubMed] [Google Scholar]
- 5.Hansen TB, Thygesen LC, Zwisler AD, et al. Self-reported health-related quality of life predicts 5-year mortality and hospital readmissions in patients with ischaemic heart disease. European Journal of Preventive Cardiology. 2015;22:882–889. doi: 10.1177/2047487314535682. [DOI] [PubMed] [Google Scholar]
- 6.Hofer S, Benzer W, Oldridge N. Change in health-related quality of life in patients with coronary artery disease predicts 4-year mortality. International Journal of Cardiology. 2014;174:7–12. doi: 10.1016/j.ijcard.2014.03.144. [DOI] [PubMed] [Google Scholar]
- 7.Gotay CC, Kawamoto CT, Bottomley A, Efficace F. The prognostic significance of patient-reported outcomes in cancer clinical trials. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 2008;26:1355–1363. doi: 10.1200/JCO.2007.13.3439. [DOI] [PubMed] [Google Scholar]
- 8.Pearson S, Stewart S, Rubenach S. Is health-related quality of life among older, chronically ill patients associated with unplanned readmission to hospital? Australian and New Zealand Journal of Medicine. 1999;29:701–6. doi: 10.1111/j.1445-5994.1999.tb01618.x. [DOI] [PubMed] [Google Scholar]
- 9.Myint PK, Luben RN, Surtees PG, Wainwright NW, Wareham NJ, Khaw KT. Physical functional health predicts the incidence of coronary heart disease in the European Prospective Investigation into Cancer-Norfolk prospective population-based study. International Journal of Epidemiology. 2010;39:996–1003. doi: 10.1093/ije/dyq061. [DOI] [PubMed] [Google Scholar]
- 10.Myint PK, Surtees PG, Wainwright NW, et al. Physical health-related quality of life predicts stroke in the EPIC-Norfolk. Neurology. 2007;69:2243–2248. doi: 10.1212/01.wnl.0000296010.21252.78. [DOI] [PubMed] [Google Scholar]
- 11.Xie G, Zou H, Myint PK, et al. Baseline overall health-related quality of life predicts the 10-year incidence of cardiovascular events in a Chinese population. Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation. 2016;25:363–371. doi: 10.1007/s11136-015-1066-8. [DOI] [PubMed] [Google Scholar]
- 12.Munoz MA, Subirana I, Elosua R, et al. Utility of a short quality of life questionnaire to predict cardiovascular events. International Journal of Cardiology. 2011;151:392–394. doi: 10.1016/j.ijcard.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Xie G, Laskowitz DT, Turner EL, et al. Baseline health-related quality of life and 10-year all-cause mortality among 1739 Chinese adults. PloS ONE. 2014;9:e101527. doi: 10.1371/journal.pone.0101527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haring R, Feng YS, Moock J, et al. Self-perceived quality of life predicts mortality risk better than a multi-bio-marker panel, but the combination of both does best. BMC Medical Research Methodology. 2011;11:103. doi: 10.1186/1471-2288-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ul-Haq Z, Mackay DF, Pell JP. Association between physical and mental health-related quality of life and adverse outcomes; a retrospective cohort study of 5,272 Scottish adults. BMC Public Health. 2014;14:1197. doi: 10.1186/1471-2458-14-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan MS, Berthelot JM, Feeny D, McFarland BH, Khan S, Orpana H. The predictive validity of health-related quality of life measures: mortality in a longitudinal population-based study. Quality of Life Research : An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation. 2007;16:1539–1546. doi: 10.1007/s11136-007-9256-7. [DOI] [PubMed] [Google Scholar]
- 17.Giltay EJ, Vollaard AM, Kromhout D. Self-rated health and physician-rated health as independent predictors of mortality in elderly men. Age and Ageing. 2012;41:165–171. doi: 10.1093/ageing/afr161. [DOI] [PubMed] [Google Scholar]
- 18.Huohvanainen E, Strandberg AY, Stenholm S, Pitkala KH, Tilvis RS, Strandberg TE. Association of self-rated health in midlife with mortality and old age frailty: A 26-year follow-up of initially healthy men. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2016;71:923–928. doi: 10.1093/gerona/glv311. [DOI] [PubMed] [Google Scholar]
- 19.Barger SD, Cribbet MR, Muldoon MF. Participant-reported health status predicts cardiovascular and all-cause mortality independent of established and nontraditional biomarkers: Evidence from a representative US sample. Journal of the American Heart Association. 2016;5(9):e003741. doi: 10.1161/JAHA.116.003741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jerant A, Tancredi DJ, Franks P. Mortality prediction by quality-adjusted life year compatible health measures: findings in a nationally representative US sample. Medical Care. 2011;49:443–450. doi: 10.1097/MLR.0b013e318206c231. [DOI] [PubMed] [Google Scholar]
- 21.Jia H, Zack MM, Thompson WW, Crosby AE, Gottesman II. Impact of depression on quality-adjusted life expectancy (QALE) directly as well as indirectly through suicide. Social Psychiatry and Psychiatric Epidemiology. 2015;50:939–949. doi: 10.1007/s00127-015-1019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia H, Lubetkin EI. Incremental decreases in quality-adjusted life years (QALY) associated with higher levels of depressive symptoms for U.S. Adults aged 65 years and older. Health and Quality of Life Outcomes. 2017;15:9. doi: 10.1186/s12955-016-0582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stafford L, Berk M, Reddy P, Jackson HJ. Comorbid depression and health-related quality of life in patients with coronary artery disease. Journal of Psychosomatic Research. 2007;62:401–410. doi: 10.1016/j.jpsychores.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Rutledge T, Linke SE, Johnson BD, et al. Self-rated versus objective health indicators as predictors of major cardiovascular events: the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation. Psychosomatic Medicine. 2010;72:549–555. doi: 10.1097/PSY.0b013e3181dc0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onawola RS, LaVeist TA. Subjective health status as a determinant of mortality among African-American elders. Journal of the National Medical Association. 1998;90:754–758. [PMC free article] [PubMed] [Google Scholar]
- 26.Elkind MS, Sciacca R, Boden-Albala B, Rundek T, Paik MC, Sacco RL. Moderate alcohol consumption reduces risk of ischemic stroke: The Northern Manhattan Study. Stroke; A Journal of Cerebral Circulation. 2006;37:13–19. doi: 10.1161/01.STR.0000195048.86810.5b. [DOI] [PubMed] [Google Scholar]
- 27.Willey JZ, Paik MC, Sacco R, Elkind MS, Boden-Albala B. Social determinants of physical inactivity in the Northern Manhattan Study (NOMAS) Journal of Community Health. 2010;35(6):602–608. doi: 10.1007/s10900-010-9249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sacco RL, Anand K, Lee HS, et al. Homocysteine and the risk of ischemic stroke in a triethnic cohort: the NOrthern MAnhattan Study. Stroke; A Journal of Cerebral Circulation. 2004;35:2263–2269. doi: 10.1161/01.STR.0000142374.33919.92. [DOI] [PubMed] [Google Scholar]
- 29.Gentry EM, Kalsbeek WD, Hogelin GC, et al. The behavioral risk factor surveys: II. Design, methods, and estimates from combined state data. American Journal of Preventive Medicine. 1985;1:9–14. [PubMed] [Google Scholar]
- 30.Mahoney FI, Barthel DW. Functional evaluation: The Barthel Index. Maryland State Medical Journal. 1965;14:61–65. [PubMed] [Google Scholar]
- 31.Granger CV, Dewis LS, Peters NC, Sherwood CC, Barrett JE. Stroke rehabilitation: Analysis of repeated Barthel index measures. Archives of Physical Medicine and Rehabilitation. 1979;60:14–17. [PubMed] [Google Scholar]
- 32.Spitzer WO, Dobson AJ, Hall J, et al. Measuring the quality of life of cancer patients: A concise QL-index for use by physicians. Journal of Chronic Diseases. 1981;34:585–597. doi: 10.1016/0021-9681(81)90058-8. [DOI] [PubMed] [Google Scholar]
- 33.Addington-Hall JM, MacDonald LD, Anderson HR. Can the Spitzer Quality of Life Index help to reduce prognostic uncertainty in terminal care? British Journal of Cancer. 1990;62:695–699. doi: 10.1038/bjc.1990.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dapueto JJ, Francolino C, Servente L, et al. Evaluation of the functional assessment of cancer therapy-general (FACT-G) Spanish Version 4 in South America: Classic psycho-metric and item response theory analyses. Health and Quality of Life Outcomes. 2003;1:32. doi: 10.1186/1477-7525-1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Echt DS, Liebson PR, Mitchell LB, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. The New England Journal of Medicine. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 36.Schaefer EJ, Lamon-Fava S, Jenner JL, et al. Lipoprotein(a) levels and risk of coronary heart disease in men. The lipid Research Clinics Coronary Primary Prevention Trial. Jama. 1994;271:999–1003. doi: 10.1001/jama.1994.03510370051031. [DOI] [PubMed] [Google Scholar]
- 37.Sacco RL, Gan R, Boden-Albala B, et al. Leisure-time physical activity and ischemic stroke risk: The Northern Manhattan Stroke Study. Stroke; A Journal of Cerebral Circulation. 1998;29:380–387. doi: 10.1161/01.str.29.2.380. [DOI] [PubMed] [Google Scholar]
- 38.Willey JZ, Paik MC, Sacco R, Elkind MS, Boden-Albala B. Social determinants of physical inactivity in the Northern Manhattan Study (NOMAS) Journal of Community Health. 2010;35:602–608. doi: 10.1007/s10900-010-9249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dorr DA, Jones SS, Burns L, et al. Use of health-related, quality-of-life metrics to predict mortality and hospitalizations in community-dwelling seniors. Journal of the American Geriatrics Society. 2006;54:667–673. doi: 10.1111/j.1532-5415.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- 40.Mikkelsen SS, Mortensen EL, Flensborg-Madsen T. A prospective cohort study of quality of life and ischemic heart disease. Scandinavian Journal of Public Health. 2014;42:60–66. doi: 10.1177/1403494813504504. [DOI] [PubMed] [Google Scholar]
- 41.Dadjou Y, Kermani-Alghoraishi M, Sadeghi M, et al. The impact of health-related quality of life on the incidence of ischaemic heart disease and stroke; a cohort study in an Iranian population. Acta Cardiologica. 2016;71:221–226. doi: 10.2143/AC.71.2.3141853. [DOI] [PubMed] [Google Scholar]
- 42.Olson KL, Stiefel M, Ross C, et al. Self-rated health among patients with coronary artery disease enrolled in a cardiovascular risk reduction service. Population Health Management. 2016;19:24–30. doi: 10.1089/pop.2014.0178. [DOI] [PubMed] [Google Scholar]
- 43.Bosworth HB, Siegler IC, Brummett BH, et al. The association between self-rated health and mortality in a well-characterized sample of coronary artery disease patients. Medical Care. 1999;37:1226–1236. doi: 10.1097/00005650-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 44.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 45.Klein BE, Klein R, Knudtson MD, Lee KE. Frailty, morbidity and survival. Archives of Gerontology and Geriatrics. 2005;41:141–149. doi: 10.1016/j.archger.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 46.Varadhan R, Seplaki CL, Xue QL, Bandeen-Roche K, Fried LP. Stimulus-response paradigm for characterizing the loss of resilience in homeostatic regulation associated with frailty. Mechanisms of Ageing and Development. 2008;129:666–670. doi: 10.1016/j.mad.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kroenke CH, Kubzansky LD, Adler N, Kawachi I. Prospective change in health-related quality of life and subsequent mortality among middle-aged and older women. American Journal of Public Health. 2008;98:2085–2091. doi: 10.2105/AJPH.2007.114041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hopman WM, Berger C, Joseph L, et al. The natural progression of health-related quality of life: results of a five-year prospective study of SF-36 scores in a normative population. Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation. 2006;15:527–536. doi: 10.1007/s11136-005-2096-4. [DOI] [PubMed] [Google Scholar]