Abstract
Hippocampal rhythms in clock gene expression, enzymatic activity, and long-term potentiation (LTP) are thought to underlie day-night differences in memory acquisition and recall. Glycogen synthase kinase 3-beta (GSK3β) is a known regulator of hippocampal function, and inhibitory phosphorylation of GSK3β exhibits region-specific differences over the light-dark cycle. Here, we sought to determine whether phosphorylation of both GSK3α and GSK3β isoforms have an endogenous circadian rhythm in specific areas of the hippocampus and whether chronic inhibition or activation alters the molecular clock and hippocampal plasticity (LTP). Results indicated a significant endogenous circadian rhythm in phosphorylation of GSK3β, but not GSK3α, in hippocampal CA1 extracts from mice housed in constant darkness for at least two weeks. To examine the importance of this rhythm, genetic and pharmacological strategies were used to disrupt the GSK3 activity rhythm by chronically activating or inhibiting GSK3. Chronic activation of both GSK3 isoforms in transgenic mice (GSK3-KI mice) diminished rhythmic BMAL1 expression. On the other hand, chronic treatment with a GSK3 inhibitor significantly shortened the molecular clock period of organotypic hippocampal PER2::LUC cultures. While WT mice exhibited higher LTP magnitude at night compared to day, the day-night difference in LTP magnitude remained with greater magnitude at both times of day in mice with chronic GSK3 activity. On the other hand, pharmacological GSK3 inhibition impaired day-night differences in LTP by blocking LTP selectively at night. Taken together, these results support the model that circadian rhythmicity of hippocampal GSK3β activation state regulates day/night differences in molecular clock periodicity and a major form of synaptic plasticity (LTP).
Introduction
In all mammalian cells, including neurons, the timing of the circadian clock is based on a delayed negative feedback loop resulting in rhythmic expression of core “clock genes”, in which the activators (CLOCK and BMAL1) are expressed in anti-phase to the suppressors (PERIOD and CRYPTOCHROME) (for review see Takahashi et al. (2008)). Rhythmic expression of clock genes exists in numerous brain regions such as the amygdala, olfactory bulb, cerebellum, and hippocampus, all of which are synchronized by a central clock in the suprachiasmatic (SCN) nucleus of the hypothalamus (see Dibner et al. (2010) for review). The molecular clock in the hippocampus is important for day-night differences in learning and memory, as well as rhythmic expression of enzymatic activity and some forms of synaptic plasticity (Chaudhury et al., 2005; Eckel-Mahan et al., 2008; Jilg et al., 2010; Yang et al., 2012). For example, arrhythmic Cryptochrome1/2 double knockout mice are unable to learn time-place associations (the ability to link food or reward with a particular place, as well as a specific time of day), an ability that is necessary for survival and reproduction (Van der Zee et al., 2008). In addition, Per2 mutants exhibit deficits in trace-fear conditioning, as well as synaptic plasticity (Wang et al., 2009). Finally, Bmal1−/− mice display initial hyperactivity and impaired intra- and inter-session habituation to a novel environment, suggesting a deficit in short- and long-term contextual memory formation (Kondratova et al., 2010). More recently, mice with forebrain-specific Bmal1 knockout were shown to have impaired Barnes maze performance and novel object location memory (Snider et al., 2016). These findings indicate that the circadian clock is involved in learning and memory processes, including synaptic plasticity. However, the clock-driven molecular mechanisms underlying diurnal differences in hippocampal plasticity and memory are not well understood.
A potential candidate regulator of day-night changes in hippocampal function is glycogen synthase kinase (GSK3), a serine/threonine kinase with two isoforms (GSK3α and GSK3β) that exhibit daily rhythms in phosphorylation (inactivation) in the brain (Besing et al., 2015; Iitaka et al., 2005; Iwahana et al., 2004; Kinoshita et al., 2012). Several lines of evidence suggest that GSK3 also modulates activity-dependent synaptic plasticity in the hippocampus (Peineau et al., 2008). After induction of long-term potentiation (LTP), phosphorylation of GSK3 (p-GSK3) is elevated, indicating that LTP is associated with GSK3 inactivation (Hooper et al., 2007; Peineau et al., 2007). Furthermore, overexpression of GSK-3β in mouse cortical and hippocampal neurons significantly decreases LTP magnitude which is reversed by treatment with lithium chloride, a known GSK3 inhibitor (Hooper et al., 2007). Another form of plasticity, long-term depression (LTD), is blocked by pharmacological GSK3 inhibition (Peineau et al., 2007). Lastly, overexpression or enhanced activation of GSK3 in rodents is associated with impaired Morris water maze performance, which is reversed by lithium chloride (Hernandez et al., 2002; Liu et al., 2003). These studies suggest that GSK3 may bi-directionally modulate two major forms of activity-dependent synaptic plasticity that are important for learning and memory processes.
Although p-GSK3β levels in hippocampus vary across the light-dark cycle (Kinoshita et al., 2012), it is not clear how these levels change in specific hippocampal subfields, whether phosphorylation of the GSK3α isoform shows daily variation, and/or whether these hippocampal oscillations are light-driven. These rhythms are likely to impact hippocampal function and plasticity. Therefore, we sought to test the hypothesis that the circadian variation in the phosphorylation state balance of GSK3α/β in hippocampus is critical for normal periodicity of the molecular clock as well as day/night differences in LTP. Through genetic and pharmacological alterations of GSK3 activity, we show that GSK3 activation modulates hippocampal function in a time-of-day dependent manner.
Materials and Methods
Animals
Male, wild-type (WT) C57-BL6/J mice (generated within our colony or purchased from Jackson Laboratories, Bar Harbor, ME, USA) were age matched to male, GSK3α/β21A/21A/9A/9A (GSK3-KI) mice (2–5 months old) backcrossed for at least 10 generations to C57BL/6 mice. GSK3-KI mice have serine-alanine substitutions (S9 and S21) on both GSK3 isoforms, rendering GSK3 de-phosphorylated and constitutively active (McManus et al., 2005). Both mutations are necessary to disrupt circadian activity rhythms (Paul et al., 2012). Male, Per2Luc+/− knock-in mice (16–60-d-old) backcrossed to C57BL/6 for 12 generations (Yoo et al., 2004) were used to measure PER2-driven luminescence. In general, animals were group-housed in a 12:12 light/dark (LD) cycle with the exception of animals used for western blot analysis of rhythms in constant darkness (DD). These animals were housed individually in cages equipped with running wheels (Coulbourn Instruments, Whitehall, PA, USA), and wheel-running activity was recorded and analyzed using ClockLab software (Actimetrics, Wilmette, IL). Mice were housed in DD for at least 14 days before being sacrificed via cervical dislocation and rapid decapitation at circadian times (CTs) determined by locomotor activity rhythms (where CT12 equals activity onset and the beginning of subjective night). All animals had access to food and water ad libitum and were handled in accordance with the University of Alabama at Birmingham (UAB) Institutional Animal Care and Use Committee (IACUC) and National Institutes of Health (NIH) guidelines.
Protein extraction and western blotting
Protein extraction and western blotting were carried out as previously described (Besing et al., 2015). Coronal slices containing the hippocampus (600 μm) were cut on a vibroslicer (Campden 7000SMZ, World Precision Instruments, Lafayette, IN) at ~4°C in HBSS (14175-103 Gibco, Carlsbad, CA). Sections were homogenized in 1% SDS with mini complete protease inhibitors (Roche, Indianapolis, IN). Samples were run on 7.5% SDS-PAGE polyacrylamide gels at 10 μg/well and transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA). The membranes were blocked in 5% milk TBST (150 mM NaCl, Tris-base 20 mM, 1% Tween 20). The membranes were then incubated overnight with primary antibodies against BMAL1 (Sigma, 1:1000), p-GSK3β (Cell Signaling, 1:750), p-GSK3α (Cell Signaling, 1:400), GSK3β (Cell Signaling, 1:2000), β-Actin (Millipore, 1:50,000), or α-Tubulin (Sigma, 1:4000). The following day, membranes were incubated with either Anti-Rabbit IgG secondary (Jackson Immuno Research, 1:10,000). For densitometry, normalization included two steps. First, for each blot/time course (4–6 replicates per ZT), both bands for the protein of interest and the loading control protein at each ZT time were divided by the bands for the positive control sample, which was a single sample of whole brain homogenate that was included on each blot (allowing comparison between blots). Second, for each sample, the normalized protein of interest was divided by the normalized loading control for each sample (i.e., p-GSK3α/β and BMAL1 were normalized to total GSK3α/β and α-Tubulin loading controls, respectively; as in (Franklin et al., 2013; Sahar et al., 2010)). Western blots were quantified using Image J and analyzed as in (Brendza et al., 2002; Paul et al., 2012).
Bioluminescence and Organotypic Slice Culture Procedures
Using cervical dislocation and rapid decapitation, Per2Luc+/− knock-in mice were killed between 3–8 h after lights on, or Zeitgeber Time (ZT) 3–8 (where ZT 12 refers to lights off). Coronal slices containing the hippocampus (400 μm) were cut on a vibroslicer (Campden 7000smz; World Precision Instruments, Inc.) at ~4°C in HBSS (14175-103; Gibco, Carlsbad, CA) supplemented with 25 U/ml streptomycin/penicillin, NaHCO3 (7.5%; Sigma, St. Louis, MO), and 1.0 M HEPES. Hippocampus tissue was then transferred to culture membranes (Millipore, Billerica, MA) in 35-mm culture dishes with 1.0 ml of DMEM (90-013-PB; Cellgro, Manassas, VA) supplemented with 4.5 g/L glucose, 1.0 M HEPES, 25 U/ml penicillin/streptomycin, 2% B27, and 0.1 mM beetle luciferin (Promega, Madison, WI). PER2::LUC levels from hippocampal slice cultures were detected from bioluminescence measurements with a LumiCycle (Actimetrics, Wilmette, IL) that was maintained at 36°C in a standard incubator as in (Besing et al., 2012; Filiano et al., 2013; Gamble et al., 2007). For GSK3 inhibitor treatments, after one full cycle, explants were treated with either 10 μl of 20 mM CHIR-99021 (CHIR; final concentration of 20 μM; Stemgent) or vehicle (10 μl DMSO, 0.04%) into the original media chronically. Data were analyzed by using LumiCycle data analysis software (Actimetrics, Wilmette, IL). For each selection of data, the baseline shift was removed by fitting a polynomial curve with an order of one less than the number of cycles. After determining a goodness of fit, data with at least 80% of the variance accounted for by the curve was used for analysis. To calculate period changes, four full cycles after each media change were used to determine the period.
Hippocampal slice preparation and electrophysiological recordings
Mice were euthanized using cervical dislocation followed by rapid decapitation. Brains were removed and placed in ice-cold “high-sucrose” artificial cerebrospinal fluid (aCSF) [in mM: 85 NaCl, 2.5 KCl, 4 MgSO4, 0.5 CaCl2, 1.25 NaH2PO4, 25 NaHCO3, 25 glucose and 75 sucrose saturated in 95% O2 and 5% CO2]. Coronal slices (400 μM) of hippocampus were prepared using a vibratome (Leica, VT1000P) and then transferred into a chamber containing standard aCSF [in mM: 119 NaCl, 2.5 KCl, 1.3 MgSO4, 2.5 CaCl2, 1 NaH2PO4, 26 NaHCO3, 11 glucose saturated in 95% O2 and 5% CO2] and stored at 28°C where slices remained until recording. For daytime recordings, brain slices were prepared at ZT 1 and recorded from ZT 2–10. For nighttime recordings, brain slices were prepared at ZT 12 and recorded from ZT 13–21. Experiments comparing different drug treatments, genotypes, or time of day were interleaved with one another. Field excitatory postsynaptic potentials (fEPSPs) were recorded from slices in a submersion recording chamber which was continuously perfused at 3–5 ml/min with aCSF warmed to 26–29°C. In order to stimulate Schaffer collateral axons, a bipolar stimulating electrode was placed in stratum radiatum within 200–300 μm of a recording electrode. Baseline fEPSPs were obtained by delivering a 0.1Hz stimulation to elicit fEPSPs of 0.35–0.5 mV amplitude for 20 min. LTP was induced by delivering a high-frequency (100 Hz) stimulus of 2 trains (0.5 s duration; 15 s interval). It is important to note that this weaker stimulation protocol was used in order to avoid masking a day/night difference in LTP magnitude, and as such, this stimulation protocol elicited a decrementing potentiation likely because weak stimulation does not induce stable, protein synthesis dependent LTP (Reymann and Frey, 2007). This strategy is similar to that used in a previous study that demonstrated a day/night difference in LTP magnitude in mice (Chaudhury et al., 2005). Slices were perfused with either CHIR-99021 (2.0 μM; Stemgent) or vehicle (DMSO; 0.04%) for at least 20 min prior to high frequency stimulation (HFS) and for the duration of the recordings.
Statistical Analysis
All statistical comparisons were made in SPSS software (version 22), and alpha (Type I error) was set at 0.05. For analysis of the western blot experiments, significant rhythmicity was determined using cosinor analysis. A non-linear regression was run using the following equation: F(t) = Mesor + A * Cos((2πt/T) + Acrophase), where Mesor refers to the midline estimating statistic of rhythm, and A is the amplitude (peak-to-trough difference), T is the period (set to a constant of 24 h in the absence of multiple cycles), and Acrophase, which refers to the time of the cosine maximum. A linearized cosinor function [F(t) = Mesor + A*Cos(2πt/24) + B*Sin(2πt/24)] was used to determine statistical significance of the regression, yielding the same R2 values as the nonlinear regression. For rhythms that did not fit a cosine function (Fig. 1A), the rhythmicity was determined using the non-parametric JTK-Cycle test in R (Hughes et al., 2010). All other experiments were analyzed using independent t-tests, mixed model ANOVAs, or two-way ANOVA, as appropriate. Least significance difference (LSD) tests were used for post-hoc analysis.
Figure 1.
Rhythmic phosphorylation of GSK3β, but not GSK3α persists in constant darkness in CA1 of the hippocampus. The hippocampus (area CA1) was sampled over a 24-h period from mice housed in an LD cycle (A) or a DD cycle for two weeks (B). A,B, Quantification (top; n = 22 or 28/time course, respectively; mean ± SEM per ZT or CT bin, respectively) and representative western blots (bottom) of the p-GSK3β to total GSK3β ratio. C, Quantification (top; n = 27/time course; mean ± SEM per CT bin) and representative western blots (bottom) of p-GSK3α to total GSK3α ratio. Lines indicate the significant cosinor function fit.
Results
Circadian regulation of GSK3α/β inactivation in area CA1 of hippocampus
Expression of core clock genes oscillates in nearly every cell, including those in hippocampus (Jilg et al., 2010). Thus, the molecular clock may also drive phosphorylation of GSK3 in hippocampus. Therefore, we quantified the phosphorylated/total GSK3β ratio every 4 hours over a 24-h period in a light:dark (LD) cycle. A statistically significant rhythm was evident for area CA1 (n = 23/time course; cosinor analysis, F(2, 20) = 8.38, p = 0.002; JTK Cycle test, p = 0.0086; Fig. 1A), but not for the dentate gyrus (n = 22/time course; F(2, 19) = 1.98, p = 0.17; data not shown). We next tested the hypothesis that the 24-h variation of GSK3 phosphorylation (for both GSK3α and GSK3β isoforms) in area CA1 persists in constant conditions. The CA1 regions of mice housed in constant dark (DD) for 2 weeks were isolated across a 24-h period, and cosinor non-linear regression analysis of p-GSK3β levels revealed a significant 24-h rhythm where phosphorylation is increased during the subjective day and decreased during subjective night, corresponding to higher GSK3 activation during the night compared to day (n = 28/time course; R2 = 0.21, F(2, 25) = 3.37 p = 0.05; Fig. 1B). Interestingly, the p-GSK3β rhythm was delayed compared to the primary circadian clock in the SCN (Besing et al., 2015) and peaked in the middle of the subjective day under DD conditions (mesor, 0.84 ± .08; amplitude, 0.29 ± 0.11; phase, CT 5.74 ± 1.67; Fig. 1A). In contrast to GSK3β, quantification of the p-GSK3α/total GSK3α ratio in CA1 did not reveal a significant rhythm (n = 27/time course; cosinor nonlinear regression analysis, R2 = 0.035, F(2, 24) = 0.43, p = 0.65; Fig. 1C), such that rhythmic phosphorylation of GSK3 in area CA1 was specific to the GSK3β isoform only. Similar to the SCN (Besing et al., 2015), total GSK3α and GSK3β levels in hippocampus were stable across the 24-h day (R.C. Besing, unpublished observations).
GSK3 regulates the molecular circadian clock in hippocampus
In order to examine whether the rhythm of GSK3 inactivation is necessary for normal periodicity of the molecular clock in the hippocampus, we investigated the effects of chronic GSK3 inhibition using a real-time, read out of the molecular clock via the widely used Period2 luciferase (mPer2Luc+/−) mouse model (Wang et al., 2009; Yoo et al., 2004). Because the PER2::LUC hippocampal cultures live for several days (Wang et al., 2009), period length can be determined. Therefore, we chronically applied a selective GSK3 inhibitor to organotypic tissue cultures of the hippocampus from adult, mPer2Luc+/− reporter mice. We found that application of the GSK3 inhibitor (20 μM CHIR) shortened the period length by over an hour compared to that of vehicle-treated cultures (n = 21/group; t(40) = −7.27; p < 0.0001; Fig. 2A,B). Additionally, we saw no difference in amplitude of PER2::LUC rhythms between the vehicle and CHIR (t(40) = 0.18, p = 0.86; Fig. 2B); however, variability in explant size and health may have prevented our ability to accurately measure amplitude. Thus, these results suggest that the inhibitory rhythm of GSK3 impacts the period length such that chronic inhibition speeds up the molecular clock in the hippocampus.
Figure 2.
GSK3 inhibition shortens the period of the molecular clock. PER2::LUC organotypic Organotypic hippocampal cultures were chronically treated with vehicle (black line or bars) or CHIR-99021 (20 μM; dashed line or white bars). A, Representative bioluminescent traces for each treatment condition. B, Bar graph (n = 21/ group; mean ± SEM) of period length (left) and amplitude (right). * p < 0.05.
We recently reported that chronic GSK3 activation significantly impacts the amplitude of the molecular clock (i.e., BMAL1) in the SCN (Besing et al., 2015), using a transgenic mouse model in which serine-alanine mutations render both isoforms of GSK3 constitutively active (GSK3-KI) but at endogenous levels (McManus et al., 2005; Paul et al., 2012). These mice show disrupted circadian behavior and SCN firing rate rhythms, which did not occur in the single, isoform mutations (Paul et al., 2012). Therefore, we hypothesized that chronic GSK3 activation may also dampen BMAL1 expression rhythms in the hippocampus. Double transgenic GSK3-KI mice or WT control mice were housed in DD for at least 2 weeks, and BMAL1 expression from area CA1 was quantified over a 24-h period. Cosinor analysis revealed a significant BMAL1 expression rhythm in area CA1 from WT mice (n = 26/time course; R2 = 0.28, F(2, 23) = 4.40, p = 0.02) with peak BMAL1 expression in the subjective day (mesor, 0.73 ± 0.07; amplitude, 0.29 ± 0.10; phase, 4.77 ± 1.39; Fig. 3A,B). In GSK3-KI mice, however, BMAL1 expression failed to exhibit a significant 24-h rhythm in CA1 (n = 27/time course; R2 = 0.14, F(2, 24) = 1.90, p = 0.17 Fig. 3A,C), suggesting that the GSK3 phosphorylation rhythm in area CA1 is necessary for normal rhythmic expression of BMAL1. There was no significant difference in overall BMAL1 expression between the WT and GSK3-KI mice (mean ± SEM, WT: 0.72 ± 0.08; KI, 0.75 ± 0.08; t(51) = −0.31, p = 0.76).
Figure 3.
BMAL1 expression is arrhythmic in mice with chronic GSK3 activation. The hippocampus (area CA1) was sampled over a 24-h period from WT and GSK3-KI mice housed in a DD cycle for two weeks. A, Representative western blots of BMAL1, re-blotted for loading control. B, Quantification (mean ± SEM per CT bin) of BMAL1 expression (normalized to loading control) in WT mice (n = 26/time course). C, Quantification (mean ± SEM per CT bin) of BMAL1 expression (normalized to loading control) in GSK3-KI mice (n = 27/time course).
Chronic GSK3 activation results in elevated LTP at the CA3-CA1 Schaffer collateral pathway
Given that GSK3 is an important modulator of hippocampal plasticity (Hooper et al., 2007; Peineau et al., 2007), and its activation dynamically changes over the 24-h day in area CA1 (Fig. 1), we examined whether this p-GSK3 rhythm is critical for normal synaptic plasticity rhythms at the CA3-CA1 Schaffer collateral pathway in the hippocampus. In order to answer this question, we first measured LTP magnitude during the day and night from GSK3-KI and WT mice. WT mice exhibited a significant day/night difference with LTP magnitude being greater during the night, consistent with previous studies (Chaudhury et al., 2005). Surprisingly, GSK3-KI mice exhibited the same day/night difference (enhanced at night) as WT mice, but had an overall increase in LTP magnitude at both times of day (n = 10–19/group; mixed model ANOVA, main effect of post-HFS time, F(2,150) = 6.17, p = 0.003; main effect of time of day, F(1,150) = 21.86, p = 0.000006; main effect of genotype, F(1,150) = 6.17, p < 0.000001; no other interactions were significant; Fig. 4A–C).
Figure 4.
Chronic GSK3 activation facilitates LTP in area CA1of hippocampus. LTP was induced with HFS at CA3-CA1 synapses during the projected day (ZT 2–10) or night (ZT 13–21). A, HFS-induced LTP in slices from WT mice (day, open symbols, n = 15; night, closed symbols, n = 19; mean ± SEM). B, HFS-induced LTP in slices from GSK3-KI mice (day, open symbols, n = 10; night, closed symbols, n = 11; mean ± SEM). C, Summary of averaged data (over time) from A,B (mean ± SEM) during the day (white, D) or night (black, N). Mixed model ANOVA indicated main effects of post-HFS time, time of day, and genotype (p < 0.05 for each).
GSK3 inhibition reduces LTP magnitude selectively at night
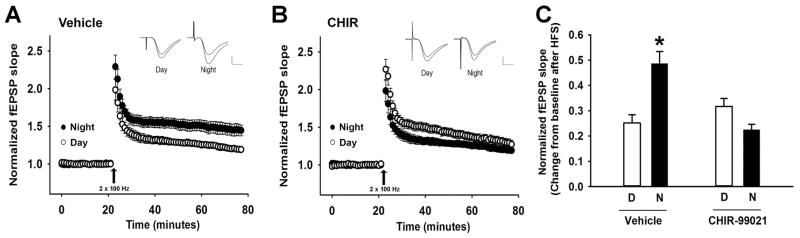
Given that chronic activation of GSK3 increased LTP magnitude at both day and night recording times (Fig. 4), we sought to examine whether acute inhibition of GSK3 would suppress LTP at both times of day. Therefore, we applied a GSK3 inhibitor to hippocampal slices and recorded LTP at CA3-CA1 synapses during the day (projected ZT 2–10) and night (projected ZT 13–21). Intriguingly, chronic bath application of CHIR (2 μM) resulted in a phase-specific suppression in LTP magnitude compared to vehicle (n = 9–12/group; mixed model ANOVA, main effect of time of day, F(1, 102) = 4.43, p = 0.04; main effect of drug, F(1, 102) = 8.15, p = 0.01; time of day x drug interaction, F(1, 102) = 22.77, p = 0.000006; Fig. 5A–C). During the night, slices treated with CHIR exhibited a significantly lower LTP magnitude compared to vehicle slices (pairwise comparisons, vehicle day vs. vehicle night, p = 0.02; drug night vs. vehicle night, p = 0.01), while during the day both treatment groups exhibited similar LTP magnitude (p = 0.78; Fig. 5C).
Figure 5.
GSK3 inhibition reduces LTP magnitude in a phase-specific manner. LTP was induced with HFS at CA3-CA1 synapses during the projected day (ZT 2–10) or night (ZT 13–21). A, HFS-induced LTP in slices treated with vehicle (day, open symbols, n = 15; night, closed symbols, n = 19; mean ± SEM). B, HFS-induced LTP in slices treated with chronic bath application of 2 μM CHIR-99021 (day, open symbols, n = 10; night, closed symbols, n = 11; mean ± SEM). C, Summary of averaged data (over time) from A,B (mean ± SEM) during the day (white, D) or night (black, N). Mixed model ANOVA indicated main effects of post-HFS time, time of day, and genotype (p < 0.05 for each) and a significant time of day x drug interaction (p < 0.05). * Significantly different from all other groups, p < 0.05.
Discussion
The circadian molecular clock is important for hippocampal processes such as spatial memory, time-place association, contextual memory, and synaptic plasticity (Jager et al., 2014; Snider et al., 2016; Van der Zee et al., 2008; Wang et al., 2009); however, the underlying mechanisms of this circadian regulation is less clear. Hippocampal processes such as spatial learning and memory as well as synaptic plasticity are dependent upon activation of GSK3 (Hernandez et al., 2002; Hooper et al., 2007; Liu et al., 2003; Peineau et al., 2007). Thus, the present study sought to define the role of temporal regulation of GSK3 activation in hippocampal function. Here, we demonstrate three key findings. First, our study showed that GSK3β is rhythmically inactivated in the CA1 region of hippocampus in both a light/dark cycle and in constant dark conditions. Second, we found that GSK3 serves as a modulator of the hippocampal molecular clock such that chronic GSK3 activation disrupted BMAL1 rhythms while chronic GSK3 inhibition shortened PER2 periodicity. Third, our results that GSK3 inhibition disrupted day-night differences in LTP suggest that GSK3 modulates dynamic hippocampal function over the 24-h day.
Our results show that GSK3 phosphorylation is temporally regulated in the CA1 region of hippocampus. This result is consistent with rhythmic phosphorylation of GSK3 in the SCN (Besing et al., 2015; Fuentealba et al., 2004; Iitaka et al., 2005; Iwahana et al., 2004). Unlike the SCN, however, only phosphorylation of GSK3β (and not GSK3α) exhibited a circadian rhythm in area CA1 of hippocampus (present study and Besing et al. (2015)). Moreover, our analysis of mice maintained in constant darkness showed that 24-h variation in phosphorylation of GSK3β in area CA1 was endogenously driven with a phase that peaked ~3 hours later than in the SCN (Besing et al., 2015). While phosphorylation of GSK3β did not vary over the day-night cycle in the dentate gyrus, it is possible that rhythmicity would be observed in other subfields, such as area CA3, an important area of future investigation. This 24-h rhythm in GSK3β phosphorylation in area CA1 may be due to rhythmic expression and/or phosphorylation of Akt/AKT, the kinase which is primarily responsible for phosphorylating and inhibiting GSK3 (Hur and Zhou, 2010). A significant circadian rhythm of Akt expression or AKT phosphorylation has been observed in the SCN (Panda et al., 2002) and heart (Durgan and Young, 2010), respectively. Given the critical importance of GSK3 in hippocampal plasticity (Hooper et al., 2007; Peineau et al., 2007), we hypothesized that this rhythm could also be important for the dynamic changes in LTP magnitude across the 24-h day.
In order to determine the importance of the GSK3 activation rhythm, we used genetic and pharmacological strategies to disrupt this rhythm by chronically activating or inhibiting GSK3, respectively. First, our results show that inactivation-resistant GSK3 mice (GSK3-KI mice) had disrupted BMAL1 rhythmicity such that BMAL1 expression no longer exhibited a significant 24-h rhythm in area CA1. This finding is consistent with studies investigating GSK3 regulation of the molecular clock in vitro or more recently, in the SCN (Besing et al., 2015; Sahar et al., 2010). In particular, GSK3 may reduce BMAL1 expression indirectly through transcriptional repression via REVERBα or directly through proteosomal degradation (Sahar et al., 2010; Valnegri et al., 2011; Yin et al., 2006). Surprisingly, we found that overall BMAL1 levels in area CA1 were unaltered by chronic GSK3 activation. Possible explanations include temporal regulation of GSK3 intracellular localization or other compensatory mechanisms. Second, we found that chronic GSK3 inhibition shortened the cycle of the hippocampal molecular clock, consistent with evidence in other mammalian cell types and brain areas (Besing et al., 2015; Hirota et al., 2008; Li et al., 2012). Although it is well documented that the GSK3 inhibitor lithium lengthens the period of circadian rhythms (Abe et al., 2000; Dokucu et al., 2005; Hirota et al., 2008; Iwahana et al., 2007; LeSauter and Silver, 1993; Li et al., 2012; Mason and Biello, 1992; Mohawk et al., 2009; Noguchi et al., 2016; Osland et al., 2011), our results with a more selective GSK3 inhibitor suggest that lithium-induced effects may be due to other nonspecific targets such as inositol phosphatase inhibition (Quiroz et al., 2004).
In addition to molecular clock function, we also investigated the effects of chronic GSK3 activation in regulating day-night differences in a major form of synaptic plasticity (LTP). Surprisingly, GSK3-KI mice continued to show day-night differences in the LTP magnitude at CA3-CA1 synapses. Compared to wild-type mice, GSK3-KI mice exhibited heightened LTP magnitude at both times of day. This result contrasts with prior work showing that constitutive activation of the GSK3α subunit (but not the GSK3β subunit) reduces LTP and long-term depression and that constitutive activation of both subunits (as in GSK3-KI mice) does not impact LTP magnitude (Polter et al., 2010; Shahab et al., 2014). One limitation of these studies and ours is the possibility of developmental and/or compensatory mechanisms in this whole body GSK3-KI mouse model. Moreover, there are methodological differences among prior studies and the present work that could contribute to the variability in outcomes. These differences include the electrophysiology stimulation protocols, the use of only day-time recordings, as well as the mixed background strain of the mice in both of the prior studies (C57BL/6 x Balb/c; McManus et al. (2005)). In the present study, all mice were backcrossed at least 10 generations to C57BL/6J in order to avoid the documented effect of mouse strain on LTP magnitude (Gerlai, 2002; Jones et al., 2001; Nguyen et al., 2000). Finally, it is possible that the slice preparation procedure itself re-synchronizes the hippocampal clock network in GSK3-KI mice and thus, any arrhythmicity potentially observed in vivo would be lost in this “de-afferented” and stimulated state. In subsequent experiments, in vitro manipulations avoided this confound with potential slice preparation resetting.
Pharmacological inhibition of GSK3 has been shown to block NMDAR-dependent LTD but not LTP (Peineau et al., 2007). Because levels of de-phosphorylated (active) GSK3 are different during the day and night (Fig. 1), we investigated whether GSK3 inhibition has time-of-day-specific effects on a major form of synaptic plasticity (LTP). In order to acutely manipulate GSK3 activation, we utilized pharmacological GSK3 inhibitors to assess LTP in CA3-CA1 synapses during the day and night. We found that in the presence of GSK3 inhibition, LTP magnitude was decreased selectively at night (when nocturnal mice are active, and GSK3 phosphorylation or inhibition is lowest) with no effect during the day, consistent with reports from other day-time LTP experiments (Peineau et al., 2007). This outcome reveals the requirement of GSK3 activation for normal LTP magnitude specific only to the night. Given this result, it will be equally important for future studies to investigate whether rhythmic GSK3 activity influences day-night differences in LTD, which remain to be demonstrated in hippocampal slices.
Altogether, our results point to the importance of assessing plasticity at different times of the day when animals are typically active or inactive. Indeed, cognitive function is influenced by both time-of-day and sleep homeostasis (Wright et al., 2012). In addition to cognition, hippocampal synaptic plasticity also varies at different times of the day (Fig. 4,6; Chaudhury et al. (2005)), suggesting that the homeostatic control of plasticity in hippocampus (Surmeier and Foehring, 2004) may be regulated by the circadian clock. Our understanding of the rhythmicity of GSK3 activation and how it modulates synaptic plasticity may provide insight into chronotherapeutic approaches in medication administration (Dallmann et al., 2014). This treatment approach could be important for several neurological and neuropsychiatric diseases given that overactive GSK3 signaling has been implicated in depression, bipolar disorder, and Alzheimer’s disease (Li and Jope, 2010; Machado-Vieira et al., 2010). In all three of these disorders, patients typically suffer from some form of circadian disruption (Foster and Wulff, 2005; Hope et al., 1998; Wulff et al., 2010).
Acknowledgments
This work was supported by National Institutes of Health Grants R01NS082413 to K.L.G, F31NS086282 to J.R.P, F31NS084683 to LMH, and T32NS061788 – Training Program in the Neurobiology of Cognition and Cognitive Disorders. The authors would like to thank Daniel Mount for help with animal maintenance, Dr. Martin Young for discussing the organization of the paper, and Drs. Norman Ruby and Robert Deacon for technical assistance and advice with behavioral paradigms.
Footnotes
Conflict of interest: The authors declare no competing financial interests.
References
- Abe M, Herzog ED, Block GD. Lithium lengthens the circadian period of individual suprachiasmatic nucleus neurons. Neuroreport. 2000;11(14):3261–4. doi: 10.1097/00001756-200009280-00042. [DOI] [PubMed] [Google Scholar]
- Besing RC, Hablitz LM, Paul JR, Johnson RL, Prosser RA, Gamble KL. Neuropeptide Y-induced phase shifts of PER2::LUC rhythms are mediated by long-term suppression of neuronal excitability in a phase-specific manner. Chronobiol Int. 2012;29(2):91–102. doi: 10.3109/07420528.2011.649382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besing RC, Paul JR, Hablitz LM, Rogers CO, Johnson RL, Young ME, Gamble KL. Circadian rhythmicity of active GSK3 isoforms modulates molecular clock gene rhythms in the suprachiasmatic nucleus. J Biol Rhythms. 2015;30(2):155–60. doi: 10.1177/0748730415573167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendza RP, Bales KR, Paul SM, Holtzman DM. Role of apoE/Abeta interactions in Alzheimer’s disease: insights from transgenic mouse models. Mol Psychiatry. 2002;7(2):132–5. doi: 10.1038/sj.mp.4001006. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Wang LM, Colwell CS. Circadian regulation of hippocampal long-term potentiation. J Biol Rhythms. 2005;20(3):225–36. doi: 10.1177/0748730405276352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallmann R, Brown SA, Gachon F. Chronopharmacology: new insights and therapeutic implications. Annu Rev Pharmacol Toxicol. 2014;54:339–61. doi: 10.1146/annurev-pharmtox-011613-135923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–49. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- Dokucu ME, Yu L, Taghert PH. Lithium- and valproate-induced alterations in circadian locomotor behavior in Drosophila. Neuropsychopharmacology. 2005;30(12):2216–24. doi: 10.1038/sj.npp.1300764. [DOI] [PubMed] [Google Scholar]
- Durgan DJ, Young ME. The cardiomyocyte circadian clock: emerging roles in health and disease. Circ Res. 2010;106(4):647–58. doi: 10.1161/CIRCRESAHA.109.209957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel-Mahan KL, Phan T, Han S, Wang H, Chan GC, Scheiner ZS, Storm DR. Circadian oscillation of hippocampal MAPK activity and cAmp: implications for memory persistence. Nat Neurosci. 2008;11(9):1074–82. doi: 10.1038/nn.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiano AN, Millender-Swain T, Johnson R, Jr, Young ME, Gamble KL, Bailey SM. Chronic Ethanol Consumption Disrupts the Core Molecular Clock and Diurnal Rhythms of Metabolic Genes in the Liver without Affecting the Suprachiasmatic Nucleus. PLoS One. 2013;8(8):e71684. doi: 10.1371/journal.pone.0071684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster RG, Wulff K. The rhythm of rest and excess. Nat Rev Neurosci. 2005;6(5):407–14. doi: 10.1038/nrn1670. [DOI] [PubMed] [Google Scholar]
- Franklin AV, King MK, Palomo V, Martinez A, McMahon LL, Jope RS. Glycogen Synthase Kinase-3 Inhibitors Reverse Deficits in Long-term Potentiation and Cognition in Fragile X Mice. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba RA, Farias G, Scheu J, Bronfman M, Marzolo MP, Inestrosa NC. Signal transduction during amyloid-beta-peptide neurotoxicity: role in Alzheimer disease. Brain Res Brain Res Rev. 2004;47(1–3):275–89. doi: 10.1016/j.brainresrev.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Gamble KL, Allen GC, Zhou T, McMahon DG. Gastrin-releasing peptide mediates light-like resetting of the suprachiasmatic nucleus circadian pacemaker through cAMP response element-binding protein and Per1 activation. J Neurosci. 2007;27(44):12078–87. doi: 10.1523/JNEUROSCI.1109-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R. Hippocampal LTP and memory in mouse strains: is there evidence for a causal relationship? Hippocampus. 2002;12(5):657–66. doi: 10.1002/hipo.10101. [DOI] [PubMed] [Google Scholar]
- Hernandez F, Borrell J, Guaza C, Avila J, Lucas JJ. Spatial learning deficit in transgenic mice that conditionally over-express GSK-3beta in the brain but do not form tau filaments. J Neurochem. 2002;83(6):1529–33. doi: 10.1046/j.1471-4159.2002.01269.x. [DOI] [PubMed] [Google Scholar]
- Hirota T, Lewis WG, Liu AC, Lee JW, Schultz PG, Kay SA. A chemical biology approach reveals period shortening of the mammalian circadian clock by specific inhibition of GSK-3beta. Proc Natl Acad Sci U S A. 2008;105(52):20746–51. doi: 10.1073/pnas.0811410106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper C, Markevich V, Plattner F, Killick R, Schofield E, Engel T, Hernandez F, Anderton B, Rosenblum K, Bliss T, et al. Glycogen synthase kinase-3 inhibition is integral to long-term potentiation. Eur J Neurosci. 2007;25(1):81–6. doi: 10.1111/j.1460-9568.2006.05245.x. [DOI] [PubMed] [Google Scholar]
- Hope T, Keene J, Gedling K, Fairburn CG, Jacoby R. Predictors of institutionalization for people with dementia living at home with a carer. Int J Geriatr Psychiatry. 1998;13(10):682–90. doi: 10.1002/(sici)1099-1166(1998100)13:10<682::aid-gps847>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Hughes ME, Hogenesch JB, Kornacker K. JTK_CYCLE: an efficient nonparametric algorithm for detecting rhythmic components in genome-scale data sets. J Biol Rhythms. 2010;25(5):372–80. doi: 10.1177/0748730410379711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur EM, Zhou FQ. GSK3 signalling in neural development. Nat Rev Neurosci. 2010;11(8):539–51. doi: 10.1038/nrn2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iitaka C, Miyazaki K, Akaike T, Ishida N. A role for glycogen synthase kinase-3beta in the mammalian circadian clock. J Biol Chem. 2005;280(33):29397–402. doi: 10.1074/jbc.M503526200. [DOI] [PubMed] [Google Scholar]
- Iwahana E, Akiyama M, Miyakawa K, Uchida A, Kasahara J, Fukunaga K, Hamada T, Shibata S. Effect of lithium on the circadian rhythms of locomotor activity and glycogen synthase kinase-3 protein expression in the mouse suprachiasmatic nuclei. Eur J Neurosci. 2004;19(8):2281–7. doi: 10.1111/j.0953-816X.2004.03322.x. [DOI] [PubMed] [Google Scholar]
- Iwahana E, Hamada T, Uchida A, Shibata S. Differential effect of lithium on the circadian oscillator in young and old hamsters. Biochem Biophys Res Commun. 2007;354(3):752–6. doi: 10.1016/j.bbrc.2007.01.042. [DOI] [PubMed] [Google Scholar]
- Jager J, O’Brien WT, Manlove J, Krizman EN, Fang B, Gerhart-Hines Z, Robinson MB, Klein PS, Lazar MA. Behavioral changes and dopaminergic dysregulation in mice lacking the nuclear receptor Rev-erbalpha. Mol Endocrinol. 2014;28(4):490–8. doi: 10.1210/me.2013-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilg A, Lesny S, Peruzki N, Schwegler H, Selbach O, Dehghani F, Stehle JH. Temporal dynamics of mouse hippocampal clock gene expression support memory processing. Hippocampus. 2010;20(3):377–88. doi: 10.1002/hipo.20637. [DOI] [PubMed] [Google Scholar]
- Jones MW, Peckham HM, Errington ML, Bliss TV, Routtenberg A. Synaptic plasticity in the hippocampus of awake C57BL/6 and DBA/2 mice: interstrain differences and parallels with behavior. Hippocampus. 2001;11(4):391–6. doi: 10.1002/hipo.1053. [DOI] [PubMed] [Google Scholar]
- Kinoshita C, Miyazaki K, Ishida N. Chronic stress affects PERIOD2 expression through glycogen synthase kinase-3beta phosphorylation in the central clock. Neuroreport. 2012;23(2):98–102. doi: 10.1097/WNR.0b013e32834e7ec2. [DOI] [PubMed] [Google Scholar]
- Kondratova AA, Dubrovsky YV, Antoch MP, Kondratov RV. Circadian clock proteins control adaptation to novel environment and memory formation. Aging (Albany NY) 2010;2(5):285–97. doi: 10.18632/aging.100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSauter J, Silver R. Lithium lengthens the period of circadian rhythms in lesioned hamsters bearing SCN grafts. Biol Psychiatry. 1993;34(1–2):75–83. doi: 10.1016/0006-3223(93)90259-g. [DOI] [PubMed] [Google Scholar]
- Li J, Lu WQ, Beesley S, Loudon AS, Meng QJ. Lithium impacts on the amplitude and period of the molecular circadian clockwork. PLoS One. 2012;7(3):e33292. doi: 10.1371/journal.pone.0033292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Jope RS. Is glycogen synthase kinase-3 a central modulator in mood regulation? Neuropsychopharmacology. 2010;35(11):2143–54. doi: 10.1038/npp.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SJ, Zhang AH, Li HL, Wang Q, Deng HM, Netzer WJ, Xu H, Wang JZ. Overactivation of glycogen synthase kinase-3 by inhibition of phosphoinositol-3 kinase and protein kinase C leads to hyperphosphorylation of tau and impairment of spatial memory. J Neurochem. 2003;87(6):1333–44. doi: 10.1046/j.1471-4159.2003.02070.x. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Salvadore G, DiazGranados N, Ibrahim L, Latov D, Wheeler-Castillo C, Baumann J, Henter ID, Zarate CA., Jr New therapeutic targets for mood disorders. ScientificWorldJournal. 2010;10:713–26. doi: 10.1100/tsw.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R, Biello SM. A neurophysiological study of a lithium-sensitive phosphoinositide system in the hamster suprachiasmatic (SCN) biological clock in vitro. Neurosci Lett. 1992;144(1–2):135–8. doi: 10.1016/0304-3940(92)90734-o. [DOI] [PubMed] [Google Scholar]
- McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, Alessi DR. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 2005;24(8):1571–83. doi: 10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohawk JA, Miranda-Anaya M, Tataroglu O, Menaker M. Lithium and genetic inhibition of GSK3beta enhance the effect of methamphetamine on circadian rhythms in the mouse. Behav Pharmacol. 2009;20(2):174–83. doi: 10.1097/FBP.0b013e32832a8f43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PV, Abel T, Kandel ER, Bourtchouladze R. Strain-dependent differences in LTP and hippocampus-dependent memory in inbred mice. Learn Mem. 2000;7(3):170–9. doi: 10.1101/lm.7.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Lo K, Diemer T, Welsh DK. Lithium effects on circadian rhythms in fibroblasts and suprachiasmatic nucleus slices from Cry knockout mice. Neurosci Lett. 2016;619:49–53. doi: 10.1016/j.neulet.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osland TM, Ferno J, Havik B, Heuch I, Ruoff P, Laerum OD, Steen VM. Lithium differentially affects clock gene expression in serum-shocked NIH–3T3 cells. J Psychopharmacol. 2011;25(7):924–33. doi: 10.1177/0269881110379508. [DOI] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109(3):307–20. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Paul JR, Johnson RL, Jope RS, Gamble KL. Disruption of circadian rhythmicity and suprachiasmatic action potential frequency in a mouse model with constitutive activation of glycogen synthase kinase 3. Neuroscience. 2012;226:1–9. doi: 10.1016/j.neuroscience.2012.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peineau S, Bradley C, Taghibiglou C, Doherty A, Bortolotto ZA, Wang YT, Collingridge GL. The role of GSK-3 in synaptic plasticity. Br J Pharmacol. 2008;153(Suppl 1):S428–37. doi: 10.1038/bjp.2008.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, Lo E, Wu D, Saule E, Bouschet T, et al. LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron. 2007;53(5):703–17. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Polter A, Beurel E, Yang S, Garner R, Song L, Miller CA, Sweatt JD, McMahon L, Bartolucci AA, Li X, et al. Deficiency in the inhibitory serine-phosphorylation of glycogen synthase kinase-3 increases sensitivity to mood disturbances. Neuropsychopharmacology. 2010;35(8):1761–74. doi: 10.1038/npp.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz JA, Gould TD, Manji HK. Molecular effects of lithium. Mol Interv. 2004;4(5):259–72. doi: 10.1124/mi.4.5.6. [DOI] [PubMed] [Google Scholar]
- Reymann KG, Frey JU. The late maintenance of hippocampal LTP: requirements, phases, ‘synaptic tagging’, ‘late-associativity’ and implications. Neuropharmacology. 2007;52(1):24–40. doi: 10.1016/j.neuropharm.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Sahar S, Zocchi L, Kinoshita C, Borrelli E, Sassone-Corsi P. Regulation of BMAL1 protein stability and circadian function by GSK3beta-mediated phosphorylation. PLoS One. 2010;5(1):e8561. doi: 10.1371/journal.pone.0008561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahab L, Plattner F, Irvine EE, Cummings DM, Edwards FA. Dynamic range of GSK3alpha not GSK3beta is essential for bidirectional synaptic plasticity at hippocampal CA3-CA1 synapses. Hippocampus. 2014;24(12):1413–6. doi: 10.1002/hipo.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider KH, Dziema H, Aten S, Loeser J, Norona FE, Hoyt K, Obrietan K. Modulation of learning and memory by the targeted deletion of the circadian clock gene Bmal1 in forebrain circuits. Behav Brain Res. 2016;308:222–35. doi: 10.1016/j.bbr.2016.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Foehring R. A mechanism for homeostatic plasticity. Nat Neurosci. 2004;7(7):691–2. doi: 10.1038/nn0704-691. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9(10):764–75. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valnegri P, Khelfaoui M, Dorseuil O, Bassani S, Lagneaux C, Gianfelice A, Benfante R, Chelly J, Billuart P, Sala C, et al. A circadian clock in hippocampus is regulated by interaction between oligophrenin-1 and Rev-erbalpha. Nat Neurosci. 2011;14(10):1293–301. doi: 10.1038/nn.2911. [DOI] [PubMed] [Google Scholar]
- Van der Zee EA, Havekes R, Barf RP, Hut RA, Nijholt IM, Jacobs EH, Gerkema MP. Circadian time-place learning in mice depends on Cry genes. Curr Biol. 2008;18(11):844–8. doi: 10.1016/j.cub.2008.04.077. [DOI] [PubMed] [Google Scholar]
- Wang LM, Dragich JM, Kudo T, Odom IH, Welsh DK, O’Dell TJ, Colwell CS. Expression of the circadian clock gene Period2 in the hippocampus: possible implications for synaptic plasticity and learned behaviour. ASN Neuro. 2009;1(3) doi: 10.1042/AN20090020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KP, Lowry CA, Lebourgeois MK. Circadian and wakefulness-sleep modulation of cognition in humans. Front Mol Neurosci. 2012;5:50. doi: 10.3389/fnmol.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff K, Gatti S, Wettstein JG, Foster RG. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci. 2010;11(8):589–99. doi: 10.1038/nrn2868. [DOI] [PubMed] [Google Scholar]
- Yang Z, Zhang W, Wang M, Ruan D, Chen J. Effects of daytime, night and sleep pressure on long-term depression in the hippocampus in vivo. Neurosci Lett. 2012;511(2):106–9. doi: 10.1016/j.neulet.2012.01.050. [DOI] [PubMed] [Google Scholar]
- Yin L, Wang J, Klein PS, Lazar MA. Nuclear receptor Rev-erbalpha is a critical lithium-sensitive component of the circadian clock. Science. 2006;311(5763):1002–5. doi: 10.1126/science.1121613. [DOI] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101(15):5339–46. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]