Abstract
Objective
Among critically ill patients with acute kidney injury, exposure to positive fluid balance (FB), compared with negative FB, has been associated with mortality and impaired renal recovery. However, its unclear whether positive and negative FB are associated with poor outcome compared to patients with even fluid balance (euvolemia). In this study, we examined the association between exposure to positive or negative FB, compared with even FB, on one-year mortality and renal recovery.
Design
Retrospective cohort study.
Setting
Eight medical-surgical ICUs at the University of Pittsburgh Medical Center, Pittsburgh, PA.
Patients
Critically ill patients admitted between July 2000 through October 2008.
Interventions
None
Measurements & Main Results
Among 18,084 patients, FB was categorized as negative (<0%); even (0 % – < 5%); or positive (≥5%). Following propensity matching, positive FB, compared with even or negative FB, was associated with increased mortality (30.3% vs. 21.1% vs. 22%, respectively, P<0.001). Using Gray’s model, negative compared with even FB, was associated with lower short-term mortality (adjusted hazard ratio range [AHR], 0.81, 95%CI, 0.68–0.96) but higher long-term mortality (AHR range, 1.16–1.22, P=0.004). Conversely, positive FB, was associated with higher mortality throughout one-year (AHR range, 1.30–1.92, P<0.001), which was attenuated in those who received RRT (Positive FB*RRT interaction AHR range, 0.43–0.89, P<0.001). Of patients receiving RRT, neither positive (adjusted odds ratio [AOR], 95% CI, 0.98, 0.68–1.4) nor negative (AOR, 0.81, 95% CI, 0.43–1.55) FB was associated with renal recovery.
Conclusions
Among critically ill patients, exposure to positive or negative FB, compared with even FB, was associated with higher one-year mortality. This mortality risk associated with positive FB, however, was attenuated by use of RRT. We found no association between FB and renal recovery.
Keywords: acute kidney injury, renal replacement therapy, dialysis, fluid balance, renal recovery, mortality
Introduction
Fluid homeostasis is frequently disrupted in critically ill patients. Especially, in those with acute kidney injury (AKI), positive or negative fluid balance (FB) is common. Although positive FB is reported in up to 40% of patients admitted to the intensive care unit (ICU)(1), the association between exposure to positive FB and long-term outcome is unclear. While some studies found a positive FB of >10% of body weight is associated with increased risk of short-term mortality (2–4), other studies found no such association (5). Prior studies were also confounded by indication bias for fluid administration (6, 7), included only a small number of patients (3), compared patients with positive FB to only negative FB (8), or did not control for premorbid conditions (e.g., chronic liver disease or heart failure) (9).
Negative FB is reported in up to 30% of critically ill patients (10) and is associated with lower risk-adjusted short-term mortality in some observational studies (11), whereas, other studies report no such association (5). Negative FB has also been advocated for early liberation from mechanical ventilation (5). However, observational studies indicate that exposure to negative FB may be associated with harm such as neurocognitive dysfunction (12). Moreover, these studies have used positive FB as a comparator instead of even FB, which is a physiological state. Whether exposure to negative FB, compared with even FB, is associated with long-term outcome, is unclear.
Among patients receiving renal replacement therapy (RRT), positive FB has been hypothesized to impair renal recovery by distortion of tissue architecture (13), predisposing to persistent dependence on RRT (14). Whereas, negative FB has been associated with earlier independence from RRT (11). However, no large study has examined the association between positive or negative FB, compared with even FB, on long-term renal recovery. In a large retrospective study of critically ill patients, we tested two hypotheses. First, we examined whether exposure to positive or negative FB, compared with even FB, is associated with risk-adjusted one-year mortality. Second, among patients receiving RRT, we examined whether positive or negative FB, compared with even FB, is associated with renal recovery.
Materials and Methods
Data Source
We conducted a retrospective study using a large academic medical center ICU database: the High-Density Intensive Care (HIDenIC) dataset. HIDenIC is a HIPAA compliant, limited dataset of adult patients admitted to ICUs at the University of Pittsburgh Medical Center, Pittsburgh, PA, details of which have been published elsewhere (15). The study population included adults admitted to medical, cardiac, abdominal transplant, cardiothoracic, surgical, neurovascular, neurotrauma, and trauma during an 8-year period (July 2000 through October 2008). More detailed description is in the supplement (S1). The University of Pittsburgh’s institutional review board approved the study.
Determination of Cumulative Fluid Balance
For each patient, we determined the cumulative FB expressed as percentage (%) from ICU admission until the end of the index ICU stay or until initiation of RRT, whichever was earlier, using the following equation (16):
Details regarding input and output fluids is described in the supplement (S2):
Definition of AKI
AKI was defined and classified according to the maximum Kidney Disease Improving Global Outcomes (KDIGO) criteria based on serum creatinine, urine output or both (17) during their entire length of index hospitalization (Supplement S3).
Outcomes
The primary outcome was time to mortality (censored at 1 year) from the index ICU admission, and renal recovery defined as alive and independent from RRT at one year. Dialysis dependence data was obtained from the United States Renal Data System (18), and mortality data from the Social Security Death Master File (19).
Statistical Analyses
We first examined the relationship between cumulative FB percentage and crude hospital mortality (Supplementary Figure E1). We defined negative, even, and positive FB as <0%, 0%-<5%, and ≥ 5%, respectively. For all analyses, patient characteristics were compared between those with negative or positive FB and even FB. Categorical variables were compared using Chi-squared test and continuous using one way analysis of variance and Kruskal-Wallis test.
We assessed the strength of association between FB and outcomes by conducting a three-way matched case-control study in which patients with negative or positive FB (cases) were matched with even FB (controls) in a 1:1:1 ratio using propensity scores (Supplement S4). We examined time to mortality censored at 1 year between the three FB groups using Kaplan Meier failure plots and compared using Log-rank test. We fitted Gray’s model (20) (supplement S5) to estimate risk-adjusted hazard ratios (AHR) for association between positive or negative FB, compared with even FB (reference category), and time to mortality censored at 1 year using five time nodes and six intervals, since Cox models failed proportionality assumptions for several covariates. We also fitted multivariable logistic regression and estimated risk-adjusted odds ratios (AOR) for mortality and renal recovery.
We adjusted for differences in age, race, baseline serum creatinine, body mass index, comorbidities, cardiac disease, heart failure, chronic liver disease and associated sequelae, admission for liver transplantation, malignancy, surgery, APACHE-III score, vasopressor use, mechanical ventilation use, suspected sepsis, and hypotensive index at ICU admission, presence of oliguria, AKI during hospitalization and use of RRT.
We performed three sensitivity analyses. First, we accounted for confounding due to undetected AKI caused by hemodilution by estimating FB-adjusted serum creatinine (21) (Supplement S6). Second, we varied the duration of FB calculation upto 72 hours and 7 days from ICU admission. Third, we used alternative threshold for defining FB as follows: negative (i.e, < −5%), even (−5% – 0%), and positive (≥0%). Statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC, USA) and Gray’s model analyses were performed using R 3.2.1, assuming statistical significance at P value <0.05.
Results
Patient Characteristics
Of 45,568 patients, we excluded patients with no available baseline weight (n=2,214), ICU duration ≤ 48hrs (n=18,032), death within 72hrs of ICU admission (n=663), chronic dialysis (n=2,386), admission for and those with history of renal transplantation (n=1,232), and serum creatinine ≥ 3.5 mgs/dl within 1 year of hospitalization (n=147), and missing data on FB (n=2,810). Of 18,084 patients who formed the analysis cohort, 15,229 patients (84%) developed AKI and 1,545 patients (8.5%) received RRT. The distribution of negative, even, and positive FB were, 26.6%, 28.2%, and 45.3%, respectively. Patients with positive and even FB were slightly older than those with negative FB (Table 1). Minor differences were noted among male sex, body mass index, and baseline serum creatinine between groups. History of cardiac disease and heart failure were more prevalent among patients with negative FB. Whereas, chronic liver disease, history of liver transplantation and admission for liver transplantation, multiple comorbidities, and malignancy were more prevalent in patients with positive FB.
Table 1.
Patient Characteristics by Fluid Balance
| Characteristic | No (%)
|
|||
|---|---|---|---|---|
| Negative FB (N=4,804) |
Even FB (N=5,096) |
Positive FB (N=8,184) |
P value | |
| Age, yrs, median (IQR) | 59 (47–71) | 60 (48–73) | 62 (49–74) | <0.001 |
| Male | 2,705 (56.3) | 2,968 (58.2) | 4,554 (55.7) | 0.0124 |
| Race | ||||
| Caucasian | 3,836 (79.8) | 4,010 (78.7) | 6,368 (77.8) | 0.084 |
| African-American | 335 (7) | 367 (7.2) | 641 (7.8) | |
| Other | 633 (13.2) | 719 (14.1) | 1,175 (14.4) | |
| BMI, kg/m2 median (IQR) | 26.6 (22.4–31.2) | 26.9 (22.8–31.6) | 25.1 (21.1–29.3) | <0.001 |
| Baseline serum creatinine, mg/dl, median (IQR) | 0.86 (0.7 – 1.05) | 0.9 (0.77 – 1.04) | 0.9 (0.7 – 1.06) | 0.0418 |
| History of hypertension | 1,512 (31.5) | 1,550 (30.4) | 2,606 (31.8) | 0.22 |
| History of diabetes | 814 (16.9) | 809 (15.9) | 1,402 (71.1) | 0.15 |
| History of cardiac disease | 979 (20.4) | 834 (16.4) | 1,329 (16.2) | <0.001 |
| History of vascular disease | 443 (9.2) | 450 (8.8) | 810 (9.9) | 0.10 |
| History of heart failure | 778 (16.2) | 617 (12.1) | 1,022 (12.5) | <0.001 |
| History of malignant neoplasms | 139 (2.9) | 177 (3.5) | 376 (4.6) | <0.001 |
| History of chronic liver disease | 352 (7.3) | 485 (9.5) | 1,188 (14.5) | <0.001 |
| History of sequela from liver disease | 283 (5.9) | 381 (7.5) | 999 (12.2) | <0.001 |
| History of liver transplantation | 95 (2) | 109 (2.1) | 285 (3.5) | <0.001 |
| Admission for liver transplantation | 149 (3.1) | 237 (4.7) | 651 (8) | <0.001 |
| Multiple comorbidities | 2,105 (43.8) | 2,139 (42) | 3,904 (47.7) | <0.001 |
| Surgical admission | 2,845 (59.2) | 3,089 (60.6) | 5,447 (66.6) | <0.001 |
| Medical Admission | 1,589 (33.1) | 1,625 (31.9) | 2,216 (27.1) | |
| Vasopressor use* | 1,195 (24.9) | 1,185 (23.3) | 2,616 (32) | <0.001 |
| Mechanical ventilation* | 2,640 (54.9) | 3,021 (59.3) | 5,981 (73.1) | <0.001 |
| Sepsis* | 583 (12.1) | 618 (12.1) | 1,467 (17.9) | <0.001 |
| APACHE III score, median (IQR) | 55 (39–74) | 58 (42–78) | 74 (55–94) | <0.001 |
| Hypotensive index, mmHg-hr, mean (SD)† | 7.27 (0.34) | 6.71 (0.31) | 9.98 (0.3) | <0.001 |
| AKI within 24hrs of ICU admission‡ | 1,322 (27.5) | 1,704 (33.4) | 2,529 (30.9) | <0.001 |
| Fluids administered in the first 24 hrs of ICU admission, L, median (IQR) | 2.71 (1.78 – 3.97) | 3.30 (2.32 – 4.65) | 4.29 (2.97 – 6.42) | <0.001 |
| Mean fluid balance during ICU stay, L, mean (SD) | −0.713 (0.01) | 0.639 (0.01) | 1.729 (0.02) | <0.001 |
| Fluid balance, L/day, median (IQR) | ||||
| At 72 hours | −0.966 (−2.546 – 0.726) | 1.968 (0.78 – 3.435) | 5.781 (3.42–8.903) | <0.001 |
| At 7 days | −1.775 (−3.771 – −0.561) | 1.862 (0.835 – 3.08) | 7.218 (4.711 – 11.147) | <0.001 |
Abbreviations: BMI – Body Mass Index; IQR - interquartile range; SD - standard deviation; APACHE, Acute Physiology and Chronic Health Evaluation; AKI, acute kidney injury
Missing data: Age (n=6); BMI (n=3151); type of admission (n=1273); 72 hour fluid balance (n=34); 7 day fluid balance (n=21); APACHE-III (n=31); reference serum creatinine (n=2); hypotensive index (n=164); fluids administered in first 24 hours (n=50).
At ICU admission
Hypotensive index defined as area under the curve for severity and duration of hypotension. Severity of hypotension was measured using a hypotensive index which integrates the duration and depth of systolic blood pressure (SBP) <90 mmHg in the first 24 hours after ICU admission. mmHg-hr is defined as a lowering of BP below 90 mm Hg over a time interval of 24 hours {i.e., a BP of 80 mmHg for 1 hour and BP ≥90 mmHg for 23 hours will result in 10 mmHg-hr ([90–80]*1=10); a BP of 85 mmHg for 10 hours and a BP ≥90 mmHg for 14 hours will result in 50 mmHg-hr ([90–85]*10=50) (25)}
AKI defined and classified according to the maximum Kidney Disease Improving Global Outcomes (KDIGO) criteria based on serum creatinine, urine output or both(17).
At ICU admission, patients with positive FB were likely to be under surgical services, have sepsis, be severely ill, have severe hypotension, and require vasopressors or mechanical ventilation. AKI within 24 hours of ICU admission was more prevalent among patients with even FB. Patients with positive FB, received more fluids in the first 24 hours of ICU admission, and had a mean positive balance of 1,729 mls per day. Patients with negative FB had a mean negative balance of 713 mls per day. At 72 hours and 7 days, patients with positive FB, had a median positive balance of 5.78 litres and 7.21 litres, whereas, patients in the negative FB had a median negative balance of 960 mls and 1.77 litres, respectively (Table 1).
Association Between FB and Mortality
Patients with positive FB were more likely to develop AKI, have oliguria, require RRT, have higher hospital length of stay, and increased mortality, compared with even or negative FB (Table 2; Figure 1). After propensity matching, 2,306 trios were generated, wherein patients with negative, even, and positive FB had similar characteristics except AKI on ICU admission (Table E1). Patients with positive FB had higher RRT requirement and mortality (Table E2), compared with even or negative FB (Figure E3).
Table 2.
Patient Outcomes by Fluid Balance
| Characteristic | No (%)
|
|||
|---|---|---|---|---|
| Negative FB (N=4,804) |
Even FB (N=5,096) |
Positive FB (N=8,184) |
P value | |
| AKI during hospitalization* | 3,626 (75.4) | 4,081 (80.1) | 7,522 (91.9) | <0.001 |
| AKI severity | ||||
| Stage 1 | 1,155 (24) | 1,044 (20.5) | 1,044 (12.8) | <0.001 |
| Stage 2 | 1,847 (38.5) | 2,218 (43.5) | 3,482 (42.6) | |
| Stage 3 | 624 (13) | 819 (16.1) | 2,996 (36.6) | |
| Oliguria during hospitalization§ | 3,096 (64.4) | 3,688 (72.4) | 7,074 (86.4) | <0.001 |
| RRT requirement | 89 (1.9) | 295 (5.8) | 1,161 (14.2) | <0.001 |
| Length of hospital stay, days, median (IQR) | 13 (8–22) | 12 (8–19) | 20 (12–33) | <0.001 |
| Mortality | ||||
| Hospital | 257 (5.4) | 454 (8.9) | 1,835 (22.4) | <0.001 |
| 1 year | 971 (20.2) | 1,120 (22) | 3,283 (40.1) | <0.001 |
Abbreviations: AKI – Acute Kidney Injury; RRT – Renal Replacement Therapy; FB – Fluid Balance; IQR – Interquartile range
AKI defined and classified according to the maximum Kidney Disease Improving Global Outcomes (KDIGO) criteria based on serum creatinine, urine output or both (17).
Patients were classified to have developed oliguria according to the maximum Kidney Disease Improving Global Outcomes (KDIGO) criteria based on urine output (17).
Figure 1. Association between positive, even, and negative FB and mortality.
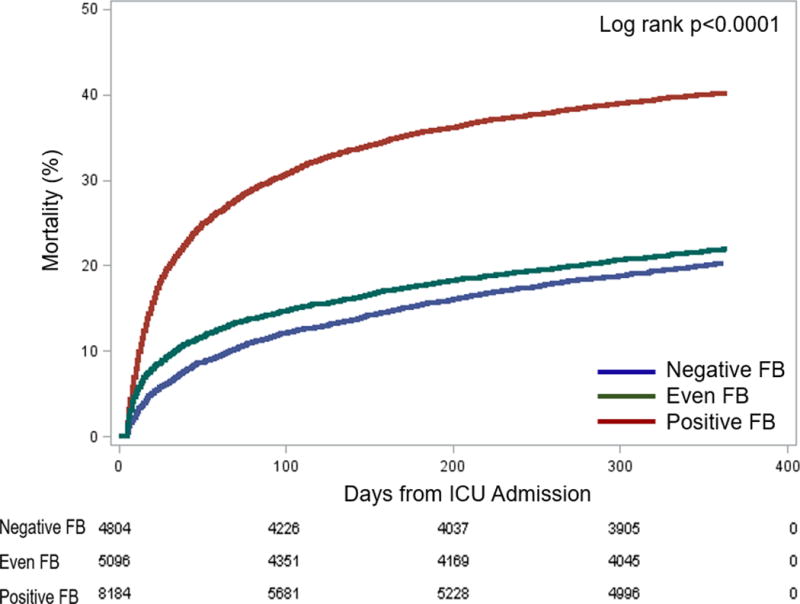
The Kaplan Meier failure plots by FB for probability of death over 1 year from ICU admission in the overall cohort. Red line represents positive FB (≥ 5%), green line represents even FB (0 – < 5%), and blue line represents negative FB (< 0%). The probability of death was highest in the positive FB and lowest in the negative FB, compared with even FB group (Log rank P<0.001).
Using logistic regression, positive FB, compared with even FB, was associated with 1-year mortality (AOR, 1.72, 95% CI, 1.55 – 1.92; Table E3). Using Gray’s model, positive FB was associated with highest risk of death within the first 178 days after ICU admission (AHR range,1.61–1.92). Subsequently, though this risk was attenuated (AHR, 1.30), the association persisted upto 365 days (P< 0.001; Table 3; Figure 2B). However, there was an interaction between positive FB and RRT such that patients with positive FB who received RRT had lower mortality (AHR range for interaction, 0.43–0.89, P value <0.001; Table E4, Figure E4).
Table 3.
Fluid Balance and Risk-adjusted One Year Mortality
| Population | Fluid Balance | Adjusted Hazard Ratio (95%CI) by Time Interval in Days$,* | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All subjects (n=18,084) |
5–11 | 11–21 | 21–42 | 42–88 | 88–178 | 178–365 | |||
|
|
|||||||||
| Negative vs Even | 0.81 (0.68–0.96) |
0.91 (0.78–1.06) |
1.01 (0.88–1.16) |
1.12 (0.98–1.28) |
1.16 (1.01–1.32) |
1.22 (1.05–1.42) |
0.004 | ||
| Positive vs Even | 1.68 (1.46–1.93) |
1.92 (1.69–2.17) |
1.86 (1.66–2.09) |
1.77 (1.58–1.98) |
1.61 (1.43–1.81) |
1.30 (1.14–1.49) |
<0.001 | ||
| Negative vs Positive | 0.48 (0.41–0.56) |
0.51 (0.49–0.58) |
0.56 (0.50–0.63) |
0.64 (0.57–0.72) |
0.73 (0.65–0.82) |
0.84 (0.74–0.97) |
<0.001 | ||
|
| |||||||||
| RRT subgroup (n=1,545) |
5–11 | 11–17 | 17–25 | 25–38 | 38–62 | 62–127 | 127–365 | ||
|
|
|||||||||
| Negative vs Even | 0.88 (0.52–1.50) |
0.99 (0.62–1.57) |
0.89 (0.58–1.39) |
0.93 (0.60–1.43) |
1.13 (0.72–1.76) |
1.10 (0.68–1.79) |
0.91 (0.52–1.59) |
0.889 | |
| Positive vs Even | 0.86 (0.65–1.14) |
0.95 (0.75–1.22) |
1.16 (0.92–1.46) |
1.07 (0.85–1.35) |
1.03 (0.80–1.31) |
1.19 (0.91–1.55) |
1.23 (0.90–1.68) |
0.230 | |
|
| |||||||||
| Non-RRT subgroup (n=16,539) |
5–11 | 11–22 | 22–47 | 47–100 | 100–192 | 192–365 | |||
|
|
|||||||||
| Negative vs Even | 0.82 (0.69–0.97) |
0.93 (0.80–1.08) |
1.04 (0.91–1.19) |
1.11 (0.97–1.27) |
1.14 (0.99–1.31) |
1.20 (1.03–1.40) |
0.01 | ||
| Positive vs Even | 1.62 (1.40–1.87) |
1.87 (1.65–2.12) |
1.83 (1.63–2.06) |
1.65 (1.47–1.86) |
1.51 (1.34–1.71) |
1.25 (1.08–1.44) |
<0.001 | ||
|
| |||||||||
| Non-AKI subgroup (n=6,098) |
5–13 | 13–42 | 42–90 | 90–160 | 160–260 | 260–365 | |||
|
|
|||||||||
| Negative vs Even | 1.00 (0.73–1.36) |
1.00 (0.77–1.31) |
1.24 (0.97–1.59) |
1.41 (1.1–1.79) |
1.31 (1.02–1.69) |
1.10 (0.82–1.48) |
0.064 | ||
| Positive vs Even | 1.62 (1.20–2.18) |
1.49 (1.15–1.93) |
1.41 (1.10–1.80) |
1.47 (1.14–1.88) |
1.24 (0.95–1.62) |
1.06 (0.78–1.45) |
0.002 | ||
Shown are adjusted hazard ratios (AHR) estimated from Gray’s model (20) for association between FB and mortality for each time interval. Models for all subjects, non-RRT, and non-AKI subgroups included six time intervals and five time nodes. Whereas, RRT subgroup included seven time intervals and six time nodes. For each of the above models, the default timing of nodes is chosen by the statistical program based on number of observations within each time interval. A hazard ratio < 1 suggests that FB is associated with lower mortality and a hazard ratio > 1 suggests FB is associated with higher mortality. P values reported are for the ranges of hazard ratios from the model.
Adjusted for age, race, baseline serum creatinine, body mass index, comorbidities, cardiac disease, heart failure, liver disease and associated sequelae, liver transplant, malignancy, surgery, admission APACHE-III score, vasopressor use, mechanical ventilation use, suspected sepsis, and hypotensive index, oliguria, acute kidney injury stratified according KDIGO guidelines (17) and RRT use (except in the RRT subgroup)
Figure 2. Association between FB and risk-adjusted mortality using Gray’s model.
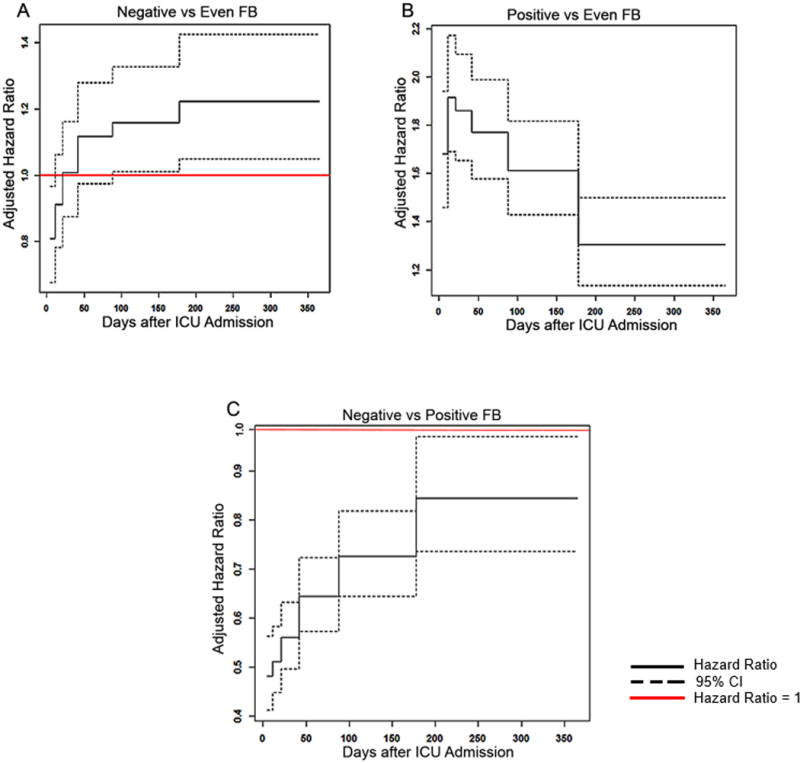
Figure shows varying adjusted hazard ratios with 95% CIs for risk of death over 365 days estimated from Gray's model using five time nodes and six intervals. A hazard ratio < 1 suggests that FB is associated with lower mortality and a hazard ratio > 1 suggests FB is associated with higher mortality. Models were adjusted for age, race, baseline serum creatinine, body mass index, comorbidities, cardiac disease, heart failure, liver disease and associated sequelae, liver transplantation, malignancy, surgery, admission APACHE III score, vasopressor use, mechanical ventilation use, suspected sepsis, and hypotensive index, oliguria, AKI and RRT use.
Negative FB as compared with even FB, is associated with decreased risk for death in the first 21 days after ICU admission, however subsequently, the risk of death is significantly higher from 88 days up to one year (A). Positive FB as compared with even FB, is associated with increased risk of death over one year. This risk was much higher early on and is relatively less from 178 days after ICU admission till the remainder of the year (B). Negative FB as compared with positive FB, was associated with decreased risk of death over the 1 year with the lowest risk upto 11 days after ICU admission (C).
Association between positive FB and mortality was also present among the subgroup of patients who never received RRT (Table 3; Figure E5D) and those who never developed AKI (Figure E5F). Nevertheless, this risk was absent among the subgroup of patients who received RRT (Figure E5B; Table 3). We found no difference in mortality between those who received continuous renal replacement therapy (CRRT) vs. intermittent hemodialysis (IHD) (AOR, 1.35, 95%CI, 0.97–1.87, P=0.0706).
Negative FB, as compared with even FB, was not associated with mortality using logisitic regression (AOR, 1.067, 95% CI, 0.94 – 1.21; Table E3). However, using Gray’s model, negative FB had variable association with mortality. Up to 11 days following ICU admission, negative FB was associated with lower mortality (AHR, 0.81, 95%CI, 0.68–0.96; Figure 2A; Table 3). After 88 days, negative FB was associated with higher mortality that persisted upto 365 days (AHR, 1.16–1.22; Figure 2A; Table 3). This variable association was also present among the subgroup of patients who never received RRT (Table 3, Figure E5C). Whereas, patients with negative FB when compared with positive FB, had lower mortality throughout 365 days (AHR range, 0.48 – 0.84; Figure 2C; Table 3).
Association Between FB and Renal Recovery in Patients Receiving RRT
Of patients receiving RRT (n=1,545), the distribution of negative, even, and positive FB before initiation of RRT were, 5.8%, 19.1%, and 75.1%, respectively. Minor differences were noted in race, body mass index, hypotensive index, baseline serum creatinine and estimated glomerular filtration rate (eGFR), and RRT modality (Table E5). Negative FB was more prevalent in patients with history of cardiac disease, heart failure, and multiple comorbidities. Patients with positive FB were more likely to have history of chronic liver disease, were admitted under surgical service, and for liver transplantation, and receive CRRT.
Patients with positive FB received more fluids in the first 24 hours of ICU admission; were severely ill and more likely to receive mechanical ventilation; were a median 10 litres positive at 7 days after ICU admission, and had higher hospital length of stay. There was delayed initiation of RRT among patients with positive FB. Vasopressor use was more common among patients with even FB. There were no differences in the distribution of mortality or renal recovery between the three FB groups (Table E5). Of the subgroup of 585 survivors (37.9%) at 1 year, 462 (79%) were independent of RRT.
After propensity matching, there was no difference in renal recovery (Table E2). Using logisitic regression, neither negative (AOR, 0.81, 95% CI, 0.43–1.6) nor positive FB (AOR, 0.98, 95% CI, 0.68 – 1.4), was associated with renal recovery (Table E6). We also found no association between FB and renal recovery among survivors (n=585) at one year (positive FB AOR, 0.92 [0.48 – 1.74]; negative FB AOR, 0.68 [0.26 – 1.77]; Table E6).
Sensitivity Analyses for Association Between FB and Mortality
When risk of AKI was re-defined using FB-adjusted serum creatinine (21), positive FB was still associated with higher risk of death (AHR range, 1.40 – 1.67, P<0.001; Table E7), whereas, negative FB had variable association (AHR range, 0.77 – 1.22, P=0.019). While exposure to negative or positive FB upto 72 hours was not associated with mortality, prolonged exposure to positive FB upto 7 days was associated with risk of death (AHR range, 1.26 – 1.50, P< 0.001). Negative FB, however, was associated with lower risk initially (AHR, 0.82) and higher risk (AHR 1.14–1.18 Table E6) subsequently. After re-defining negative, even, and positive FB as < −5%, −5 to <0%, and ≥0% FB, respectively, we found that negative FB was associated with higher risk of death only after 42 days (AHR range 1.21–1.36). Whereas, positive FB was associated with mortality up to 178 days (AHR range 1.23–1.64, Table E6).
Discussion
Among critically ill patients, positive compared with even and negative FB, was associated with higher risk of death persisting up to one year. Whereas, negative FB compared with even FB, had variable association: Early on after ICU admission it was associated with lower risk, subsequently however, negative FB was associated with higher risk of death that persisted up to one year. Among patients receiving RRT, neither negative nor positive FB was associated with renal recovery.
Our study addresses several knowledge gaps not addressed by prior literature. First, previous studies that found association between positive FB and mortality have only used negative FB (8) as a comparator population resulting in over estimation of the benefit of negative FB (or harm associated with positive FB) in critically ill patients. Whereas, our study is the first to compare patients with positive to that of even FB comprising nearly one-third of ICU patients. Although our finding suggests that positive FB is associated with mortality, our risk estimates for positive FB and mortality were somewhat modest compared to patients with even FB.
Second, we used propensity matching to account for indication bias for fluid administration, and also extensively adjusted for severity of illness, severity of hypotension, as well as other variables to account for residual confounding, and performed sensitivity analyses which other studies have not addressed. Our finding suggests that positive FB per se is associated with long-term risk of death independent of severity of illness. Third, we found a significant interaction between positive FB and RRT, such that, patients with positive FB who received RRT had lower mortality compared to patients with even FB (Figure E4). Taken together these findings suggest that once fluid overload has occurred, mechanical fluid removal, may lower long-term risk of death.
Fourth, using Gray’s modeling approach to delineate the varying hazard of death associated with exposure to positive or negative FB at various time intervals, we found that negative FB, compared with even FB, was associated with lower mortality only in the short-term but increased mortality in the long-term. This finding is in contrast with the propensity-matched analyses, which may have been underpowered to detect small differences in mortality due to small sample size. Moreover, the potential early benefit associated with negative FB might have attenuated any harm driving the association towards null in the propensity-matched analyses and the multivariable logistic regression models.
We believe that the short-term mortality benefit associated with negative FB might be due to ICU care since most patients remain on organ support. However, the mechanism behind increased long-term risk are unknown and not previously reported in the literature. Also, prior studies only compared patients with negative FB to that of positive FB and has over-estimated the mortality benefit associated with negative FB (11). Our study challenges the exisiting paradigm and suggests that negative FB is relatively “beneficial” only when compared to patients with positive FB, which is a pathological state. Whereas, negative FB is associated with harm when compared to patients with even FB, which may represent a physiologic state.
Our study finding has implications for clinical care. For instance, negative FB is currently advocated for liberating patients early from mechanical ventilator (5). However, the long-term consequences of such recommendations are unclear. Nevertheless, our study supports other studies that found evidence of harm associated with negative FB. For instance, in the Fluid and Catheter Treatment Trial (FACTT), patients randomized to conservative fluid management strategy, though liberated from mechanical ventilator earlier, had a higher risk of neurocognitive dysfunction at one year than those randomized to liberal fluid management strategy (12). Thus, our study suggests further research is needed to disentangle the relative benefit and harm associated with negative FB.
Fifth, in our study, both negative and positive FB prior to initiation of RRT were not associated with renal recovery. Our findings differ from prior studies that showed fluid overload at dialysis initiation is associated with dialysis dependence at hospital discharge (22), and at 1 year (23). These conflicting results underline a need for better understanding of the impact of FB on long-term renal recovery.
Our study has important limitations. First, given the observational nature of the study, it is not possible to make causal inferences between FB and outcomes. Second, we only calculated FB from ICU admission since FB charting in the emergency department or ward are known to be unreliable (24) which, could have resulted in misclassification of FB. Nevertheless, this misclassification is non-differential and is only likely to bias the results towards the null hypothesis. Third, we do not know whether patients in the even FB category represent euvolemia. Nevertheless, we chose 0%–5% FB as euvolemia because most patients who receive fluids in the first 24 hrs of ICU admission are presumed to have unaccounted hypovolemia. Nevetheless, our sensitivity analysis shows that even when euvolemia was defined −5% to 0%, association between negative or positive FB on outcomes were unchanged. Fourth, being a retrospective study, we were unable to account for any measurement errors related to weight. Fifth, we treated all variables with constant hazard in the Grays model, which might have introduced residual confounding due to time-varying nature of some of the variables (e.g., severity of illness). Sixth, being a single centre study, our study may not be generalizable to other ICU populations. Nevertheless, our ICU patient population included a variety of medical and surgical patients typical of academic medical center ICU population.
Conclusions
In this large study, we found patients with positive FB had higher one year risk-adjusted mortality compared to patients with even FB. This mortality risk associated with positive FB was attenuated by use of RRT. Whereas, patients with negative FB had variable association with mortality. Early after ICU admission, negative FB was associated with decreased risk of death. Later on, however, it was associated with higher mortality that persisted up to one year. Among patients receiving RRT, FB was not associated with renal recovery.
Supplementary Material
Acknowledgments
We thank Daniel G Winger and Li Wang from the University of Pittsburgh Clinical and Translational Science Institute for assistance with the statistical models.
Source of Support: This project was partially supported by the National Institutes of Health through Grant Number UL1-TR-000005 awarded to Univeristy of Pittsburgh’s Clinical and Translational Science Institute.
Footnotes
Author Contributions: Drs. Murugan and Balakumar had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Murugan, Balakumar
Acquisition of data: Kellum, Clermont
Analysis and interpretation of data: Murugan, Balakumar, Clermont, Palevsky, Kellum, Sileanu
Drafting of the manuscript: Murugan, Balakumar
Critical revision of the manuscript for important intellectual content: Kellum, Palevsky, Clermont, Murugan
Statistical analysis: Balakumar, Sileanu
Administrative, technical, or material support: Kellum, Balakumar, Murugan, Sileanu
Study supervision: Murugan, Kellum
Copyright form disclosure: Dr. Murugan received support for article research from the National Institutes of Health (NIH). Dr. Sileanu disclosed work for hire. Dr. Palevsky disclosed government work, and he received funding from Complexa, Stealth Biotherapeutics, and Baxter. Dr. Clermont received funding from Astute Medical.
Supplemental digital content is available for this article.
Financial Disclosure and Conflicts of Interest: The authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Lowell JA, Schifferdecker C, Driscoll DF, et al. Postoperative fluid overload: not a benign problem. Critical care medicine. 1990;18(7):728–733. doi: 10.1097/00003246-199007000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Vaara ST, Korhonen AM, Kaukonen KM, et al. Fluid overload is associated with an increased risk for 90-day mortality in critically ill patients with renal replacement therapy: data from the prospective FINNAKI study. Crit Care. 2012;16(5):R197. doi: 10.1186/cc11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fülöp T, Pathak MB, Schmidt DW, et al. Volume-related weight gain and subsequent mortality in acute renal failure patients treated with continuous renal replacement therapy. ASAIO journal (American Society for Artificial Internal Organs: 1992) 2010;56(4):333. doi: 10.1097/MAT.0b013e3181de35e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein SL, Currier H, Graf JM, et al. Outcome in children receiving continuous venovenous hemofiltration. Pediatrics. 2001;107(6):1309–1312. doi: 10.1542/peds.107.6.1309. [DOI] [PubMed] [Google Scholar]
- 5.National Heart L, Blood Institute Acute Respiratory Distress Syndrome Clinical Trials N. Wiedemann HP, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 6.Lee J, Louw E, Niemi M, et al. Association between fluid balance and survival in critically ill patients. Journal of internal medicine. 2015;277(4):468–477. doi: 10.1111/joim.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murugan R, Kellum JA. Fluid balance and outcome in acute kidney injury: is fluid really the best medicine? Critical care medicine. 2012;40(6):1970. doi: 10.1097/CCM.0b013e31824e1a1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Payen D, de Pont AC, Sakr Y, et al. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;12(3):R74. doi: 10.1186/cc6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang N, Jiang L, Zhu B, et al. Fluid balance and mortality in critically ill patients with acute kidney injury: a multicenter prospective epidemiological study. Critical Care. 2015;19(1):1–11. doi: 10.1186/s13054-015-1085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alsous F, Khamiees M, DeGirolamo A, et al. Negative fluid balance predicts survival in patients with septic shock: a retrospective pilot study. CHEST Journal. 2000;117(6):1749–1754. doi: 10.1378/chest.117.6.1749. [DOI] [PubMed] [Google Scholar]
- 11.Bellomo R, Cass A, Cole L, et al. An observational study fluid balance and patient outcomes in the Randomized Evaluation of Normal vs. Augmented Level of Replacement Therapy trial Critical care medicine. 2012;40(6):1753. doi: 10.1097/CCM.0b013e318246b9c6. [DOI] [PubMed] [Google Scholar]
- 12.Mikkelsen ME, Christie JD, Lanken PN, et al. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. American journal of respiratory and critical care medicine. 2012;185(12):1307–1315. doi: 10.1164/rccm.201111-2025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prowle JR, Echeverri JE, Ligabo EV, et al. Fluid balance and acute kidney injury. Nat Rev Nephrol. 2010;6(2):107–115. doi: 10.1038/nrneph.2009.213. [DOI] [PubMed] [Google Scholar]
- 14.Wald R, Quinn RR, Luo J, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. Jama. 2009;302(11):1179–1185. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 15.Kellum JA, Sileanu FE, Murugan R, et al. Classifying AKI by Urine Output versus Serum Creatinine Level. Journal of the American Society of Nephrology: JASN. 2015 doi: 10.1681/ASN.2014070724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selewski DT, Cornell TT, Lombel RM, et al. Weight-based determination of fluid overload status and mortality in pediatric intensive care unit patients requiring continuous renal replacement therapy. Intensive Care Med. 2011;37(7):1166–1173. doi: 10.1007/s00134-011-2231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eknoyan G, Lameire N, Eckardt K, et al. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury Kidney international Supplements. 2012;2:1–138. [Google Scholar]
- 18.Saran R, Li Y, Robinson B, et al. US Renal Data System 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2015;65(6 Suppl 1):A7. doi: 10.1053/j.ajkd.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill ME, Rosenwaike I. Social Security Administration's Death Master File: The Completeness of Death Reporting at Older Ages, The. Soc Sec Bull. 2001;64:45. [PubMed] [Google Scholar]
- 20.Kasal J, Jovanovic Z, Clermont G, et al. Comparison of Cox and Gray’s survival models in severe sepsis*. Critical care medicine. 2004;32(3):700–707. doi: 10.1097/01.ccm.0000114819.37569.4b. [DOI] [PubMed] [Google Scholar]
- 21.Liu KD, Thompson BT, Ancukiewicz M, et al. Acute kidney injury in patients with acute lung injury: impact of fluid accumulation on classification of acute kidney injury and associated outcomes. Critical care medicine. 2011;39(12):2665. doi: 10.1097/CCM.0b013e318228234b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouchard J, Soroko SB, Chertow GM, et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney international. 2009;76(4):422–427. doi: 10.1038/ki.2009.159. [DOI] [PubMed] [Google Scholar]
- 23.Heung M, Wolfgram DF, Kommareddi M, et al. Fluid overload at initiation of renal replacement therapy is associated with lack of renal recovery in patients with acute kidney injury. Nephrology Dialysis Transplantation. 2011:gfr470. doi: 10.1093/ndt/gfr470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wise LC, Mersch J, Racioppi J, et al. Evaluating the reliability and utility of cumulative intake and output. Journal of nursing care quality. 2000;14(3):37–42. doi: 10.1097/00001786-200004000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Kane-Gill SL, Sileanu FE, Murugan R, et al. Risk Factors for Acute Kidney Injury in Older Adults With Critical Illness: A Retrospective Cohort Study. American Journal of Kidney Diseases. 2014 doi: 10.1053/j.ajkd.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


