Abstract
Electrophysiological recordings indicate that neurons which discharge maximally in association with distinct sleep-wake states are distributed through the brain, albeit in differing proportions. As studied using juxtacellular recording and labeling within the basal forebrain, four functional principal cell types are distinguished as: wake/paradoxical sleep (W/PS)-, slow wave sleep (SWS)-, W- and PS-max active. They are each comprised by both GABA and glutamate neurons, in addition to acetylcholine neurons belonging to the W/PS group. By their discharge profiles and interactions, the GABA and glutamate neurons of different groups are proposed to have the capacity to generate sleep-wake states with associated EEG and EMG activities, though to also be importantly regulated by neuromodulatory systems, each of which belong to one functional cell group.
Introduction
Over the last century the principal regions and chemical modulators of sleep-wake systems in the brain were identified through application of lesion, stimulation and pharmacological approaches in association with chemical neuroanatomical study (see for review, [1]). Yet, only recently have the principal cell types of these regions been distinguished using electrophysiological recordings of chemically identified neurons to fully characterize their discharge profiles and thereby understand how they can regulate sleep-wake states, as will be presented in this review (Figure 1).
Figure 1.
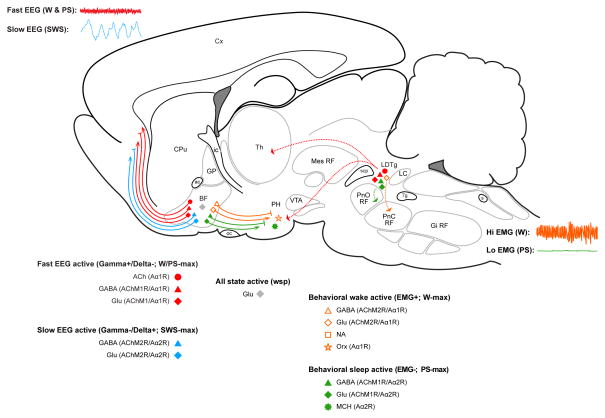
Schematic sagittal diagram of the rat brain showing principal cell types of the sleep-wake regulatory circuits. Three distinct sleep-wake states of wake (W), slow wave sleep (SWS) and paradoxical sleep (PS) are associated with distinct electroencephalogram (EEG, upper left) and electromyogram (EMG, lower right) activities which are in turn regulated by four functionally distinct cell groups according to their discharge profiles and major long projections: W/PS-max active (red), SWS-max active (blue), W-max active (orange) and PS-max active (green) in addition to state-indifferent wsp (gray) neurons, as recorded with the juxtacellular technique and fully characterized in the basal forebrain (BF) and also in the pontomesencephalic tegmentum and posterior hypothalamus (PH). Cells depicted were all identified according to their neurotransmitter. Based upon in vivo and in vitro pharmacological studies, the different neurons are assumed to bear particular receptors (R) for ACh (muscarinic, M) or NA (adrenergic, A), which are associated with excitation (AChM1R; Aα1R) or inhibition (AChM2R; Aα2R). Abbreviations: Abbreviations: 7g, genu 7th nerve; ac, anterior commissure; ACh, acetylcholine; BF, basal forebrain; CPu, caudate putamen; Cx, cortex; EEG, electroencephalogram; EMG, electromyogram; Gi RF, gigantocellular RF; Glu, glutamate; GP, globus pallidus; ic, internal capsule; LC, locus coeruleus nucleus; LDTg, laterodorsal tegmental nucleus; MCH, melanin concentrating hormone; Mes RF, mesencephalic RF; NA, noradrenaline; oc, optic chiasm; Orx, orexin,; PH, posterior hypothalamus; PnC RF, pontine, caudal part RF; PnO RF, pontine, oral part RF; PS, paradoxical sleep; RF, reticular formation; s, solitary tract; scp, superior cerebellar peduncle; SWS, slow wave sleep; Th, thalamus; VTA, ventral tegmental area; W, wake.
From early studies of the effects of lesions in humans and experimental animals (see for review [2]), the generation of sleep and wake states was attributed to different regions of the brain: sleep to the anterior hypothalamus, preoptic area and basal forebrain (BF) and wake to the posterior hypothalamus (PH) and brainstem reticular formation (RF) (Figure 1). Yet, within these regions, electrical stimulation could elicit different states depending upon the frequency of the stimulation: slow, eliciting slow wave electroencephalogram (EEG) activity with sleep and fast, eliciting fast EEG activity with wake along with elevated postural muscle electromyogram (EMG) (Figure 1), suggesting that the same neurons would drive different EEG activities and states or that different neurons within the same region would drive different EEG activities and states. In the thalamus, where specific sensory-motor relay and nonspecific projection neurons transmit inputs from the periphery and brain to the cerebral cortex, recording studies indicated that the same neurons would influence cortical activity and state by different patterns of slow vs. fast activity during naturally occurring slow wave sleep (SWS) and wake (W) (see for review, [3]). Yet within the brainstem, hypothalamus, preoptic area and BF areas, unit recording studies indicated that different neurons discharged more selectively during different states [3]. Accordingly in these regions, specific neuronal cell groups were thought to be responsible for the three major states of W, SWS and rapid eye movement sleep (REMS) or as was originally called in animals according to its essential character by Jouvet, paradoxical sleep (PS), as employed here (Figure 1). In the forebrain, neurons which discharged relatively selectively during SWS and/or PS, as sleep-active neurons, were recorded in the BF and preoptic area [4,5]. In the PH and brainstem RF, W-active neurons whose discharge was correlated with EEG or behavioral correlates of waking and EMG were recorded [6–8]. And in different regions of the brainstem, neurons which discharged relatively selectively during PS were identified (see for review, [3]).
Pharmacological studies along with chemical neuroanatomical and lesion studies subsequently revealed the very important and ostensibly state-selective roles of neuromodulatory systems, notably the monoamine and acetylcholine (ACh) containing neurons, in sleep-wake states (see for review, [9]). Yet to fully understand the way in which each of these specific systems could actually regulate or modulate sleep-wake states, it was necessary to know the way in which the specific neurons discharged in relation to the sleep-wake states. With the discrete localization of the noradrenaline (NA) neurons in the locus coeruleus (LC) nucleus (Figure 1) for which extensive evidence indicated an important role in W, it was possible to record specifically from those NA neurons and learn that they discharge selectively during W, as W-active neurons, and become silent during sleep, to be off during PS [10,11].
On the other hand, the activity of ACh neurons could not be recorded with any certainty, since they lie intermingled with large numbers of noncholinergic, including GABA and glutamate (Glu), neurons in the BF [12] (Figure 1). Like the ACh neurons, BF GABA and Glu neurons project to the cerebral cortex [13]. Other GABA and Glu neurons project caudally to the PH and perhaps beyond [14]. Given this chemical and hodological, along with apparent functional heterogeneity of the BF cell population, it was essential to be able to record from chemically identified cells in order to determine the specific discharge properties and profiles of the ACh, GABA and Glu BF neurons.
Principal functional cell types and their neurotransmitters in the BF
By applying the technique of juxtacellular recording and labeling of neurons using micropipettes in naturally sleeping-waking head-fixed rats, we identified four functionally distinguishable principal sleep-wake cell types along with their neurotransmitters in the BF [15,16]. Each cell type was characterized according to the relationship of its average discharge rate to sleep-wake states and EEG and EMG activity (Figure 2).
Figure 2.
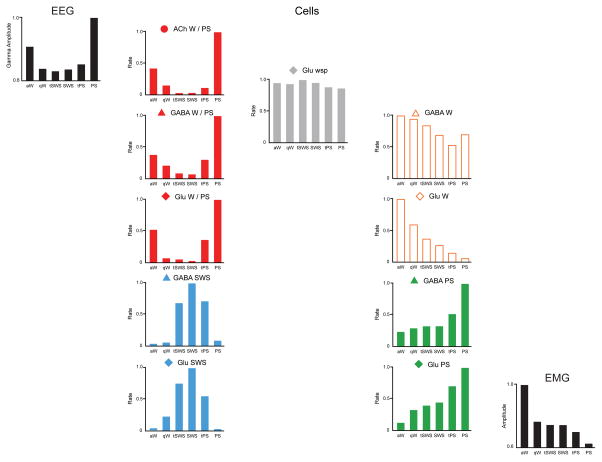
Discharge rates of principal cell types in BF across sleep-wake stages. Normalized average rates of firing shown with normalized average gamma (30 – 60 Hz) EEG activity and EMG activity across sleep wake stages of active or attentive wake (aW), quiet wake (qW), transition to SWS (tSWS), slow wave sleep (SWS), transition to PS (tPS) and paradoxical sleep (PS). Rates were taken from one exemplary neuron for each cell type [16]. Cells were recorded and filled with Neurobiotin using the juxtacellular technique for subsequent immunohistochemical identification of their neurotransmitter, as acetylcholine (ACh), GABA or (putative or identified) glutamate (Glu). They were classified into one of the four principal cell types (W/PS-max, SWS-max, W-max and PS-max) or state-indifferent (wsp) by statistical analysis of their rates in aW, SWS and PS.
The most populous functional cell type in the BF is represented by neurons which discharge in association with fast cortical activity during both W and PS, thus called W/PS-max active neurons (Figure 2). They represented almost half of all neurons recorded in the region and were comprised by 20% ACh, 30% GABA and the remaining 50% putative (or identified) Glu neurons. Based upon results from neuroanatomical (above) and physiological studies in anesthetized animals, most of these are assumed to have ascending projections to the cerebral cortex. Their discharge was positively correlated with gamma (30–60 Hz) EEG activity, which they are presumed to positively modulate in part by direct projections onto interneurons or principal cells in the cerebral cortex (Figure 1).
A much smaller functional cell type in the BF is represented by neurons which discharge in association with cortical slow waves during SWS and thus called SWS-max active neurons (Figure 2). They represented almost 20% of neurons recorded in the region and were comprised by a slight majority GABA and a bit less than half putative (or identified) Glu neurons. Also based upon other studies, most of these are assumed to have ascending projections to the cerebral cortex. Their discharge was negatively correlated with gamma activity and positively correlated with delta activity (0.5–4.0 Hz), either of which they may influence in part by direct projections to different cortical neurons (Figure 1).
A very small functional cell group in the BF is represented by neurons which discharge in association with behavioral arousal and high muscle tone during waking, thus called W-max active neurons (Figure 2). They represented only 10% of all neurons recorded in the BF and were comprised in the vast majority by Glu and few GABA neurons. Their discharge was positively correlated with postural muscle tone recorded on the EMG. From other studies, they are assumed to give rise primarily to descending projections to the PH and perhaps beyond by which they may indirectly influence behavior and muscle tone across the sleep-wake cycle (Figure 1).
Another relatively small functional group of neurons are those which discharge maximally during PS, called PS-max neurons (Figure 2). These represented a bit more than 20% of all neurons recorded in the BF and were comprised in the majority (>60%) of GABA and the remainder putative (or identified) Glu neurons. These neurons increased their discharge during sleep and particularly PS, when muscle tone is minimal or absent. Their discharge was negatively correlated with EMG. Through other studies, they are assumed to give rise primarily to descending projections to the PH and perhaps beyond, by which they could indirectly influence behavior and muscle tone across the sleep-wake cycle (Figure 1).
Finally, another small cell group is represented by those neurons which discharge at the same rate across all states and are thus state-indifferent and called wsp (for W/SWS/PS equivalent) neurons (Figure 2). They represented a very small proportion of neurons recorded (< 5%), though could be underrepresented by selection in these studies. All such wsp recorded neurons were putative (or identified) Glu.
We would thus consider there to be four basic functional sleep-wake regulatory cell types and an additional state-indifferent group of cells in the BF. They each appear to give rise to local collaterals through which they may influence the other cell types, in addition to their primary long ascending or descending projections [16]. Particularly with the use of micropipettes (which have minimal bias for the size of cells recorded), these four functional cell types have also been found by other investigators in the BF and the adjacent preoptic area [4,17–19]. In fact as here, within no one region or nucleus were all neurons found to be sleep-active in the BF or preoptic area. These findings would support results from c-Fos studies which found that all nuclei of the BF and preoptic area contained wake-active and sleep-active neurons, and that both wake-active and sleep-active neurons included GABA and presumed Glu neurons, albeit in differing proportions [20]. The four principal functional cell types recorded by us in the BF would thus appear to represent the basic functional cell types of the sleep-wake regulatory circuits distributed through the BF and preoptic area, areas and nuclei which many lesion studies suggested were primarily involved in generating sleep (including the ventrolateral preoptic nucleus, which is portrayed as the sleep center [17,18,21]). It would instead appear that functionally different cell types are codistributed through these regions, albeit with varying concentrations or predominance. It should also be pointed out that no one of these functional cell types is comprised by neurons utilizing one particular neurotransmitter or modulator, and all are comprised by both GABA and Glu cells, albeit with varying proportions. On the other hand, all ACh BF cells are W/PS-max active neurons and can thus be identified with the function of cortical activation during W and PS.
It was also surprising to learn that in the PH, which from early work was considered to be a wake center, the same four principal functional cell types have been recorded, especially when using micropipettes, with a significant proportion of sleep-max, particularly PS-max active cells [19,22]. Although not sampled in an exhaustive manner using the juxtacellular technique, we also found all four functional cell types in the PH including a significant number of GABA (and putative or identified Glu) sleep-max, particularly PS-max active cells [23].
Principal cell types in the pontomesencephalic tegmentum
The principal cell types identified in the BF have also been identified by us in the cholinergic cell area of the pontomesencephalic tegmentum using juxtacellular recording and identification [24] (Figure 1). As in the BF, the W/PS-max active cells which discharge in association with fast gamma cortical activity represented about half of all cells in the laterodorsal/sublaterodorsal/medial pedunculopontine tegmental (LDT/SubLDT/mPPT) nuclei and were comprised by equal proportions of ACh, GABA and Glu neurons. From neuroanatomical studies, these neurons are assumed to give rise to important ascending pathways as part of the ascending reticular activating system to relays in the thalamus, hypothalamus and BF, as well as to other neurons in the RF. The other important group representing near 25% of cells was comprised by GABA and more numerous Glu PS-max active neurons whose discharge is negatively correlated with EMG activity and which are assumed to give rise to important projections into the brainstem RF and possibly spinal cord to influence EMG activity indirectly. Finally, a significant number of W-max active cells, whose discharge was positively correlated with EMG, represented almost 20% of recorded cells, were identified entirely as Glu containing and also assumed to give rise to projections into the brainstem RF and possibly spinal cord to influence behavior and EMG activity indirectly. Using micropipettes for recording, other researchers have also found W/PS-, W- and PS-max active neurons in the region of the ACh neurons in the pontomesencephalic tegmentum (see for review, [19]).
Modeling the activity and interaction of principal cell types across sleep-wake states
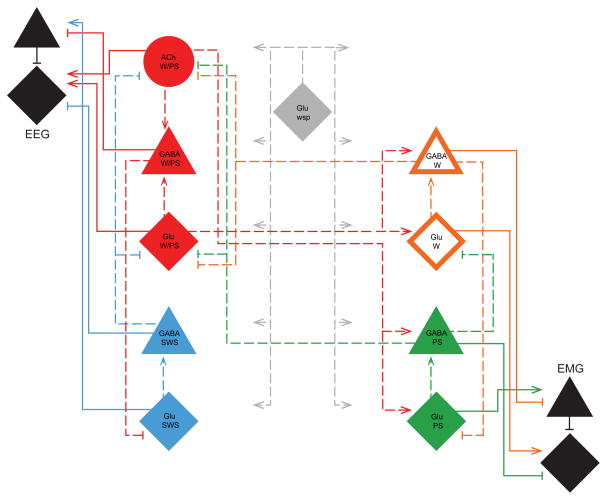
Given the reciprocal profiles of discharge of the principal functional cell types in the BF across sleep-wake states, it appeared that these profiles could be generated by interactions between the four principal functional cell groups and their constituent ACh, GABA and Glu neurons. With Cordova, Naqib and Pack, we sought to test the most simple models of interaction between these cells that could simulate their discharge profiles and with those, the changes in the EEG and EMG activities that underlie the three principal states of W, SWS and PS [25] (Figure 3). Using principles of a dynamical systems model previously applied by Tamakawa et al. [19], we entered the normalized discharge rates across sleep-wake stages of representative identified cell types recorded in the BF [16] (Figure 2). The model began with the finding cum principle that GABA and Glu neurons constitute each principal functional cell type, in addition to ACh neurons which also comprise the W/PS-max cell type in the BF. ACh neurons were incorporated just like Glu neurons, as excitatory neurons and GABA as inhibitory neurons. We thus assumed that the excitatory neuron of each pair (or set) could excite the inhibitory neuron of that functional pair, whose output would go to the excitatory neuron of the reciprocally related pair. In addition, the Glu state-indifferent wsp cell type would provide a continual excitatory input to all neurons and serve as a continuous driving force for the model. The W/PS-max active and SWS-max active neurons would together form an EEG module whose output would be to the cerebral cortex onto interneurons and principal cells, which would generate fast gamma activity. The W-max active and PS-max active neurons would together form an EMG module whose (ultimate though indirect) output would be to the motor nuclei (of brainstem or spinal cord) onto interneurons and motor neurons, which would generate EMG activity. Based upon a mutual inhibition by the opposing groups of each module, oscillations in activity could be generated which are similar to those occurring naturally across sleep-wake states. A more complex interaction must occur between these modules in the generation of three states and most notably PS. For this, a reciprocal interaction between the EEG and EMG modules was applied, with however the excitatory output of Glu neurons to the W-max and the excitatory output of ACh neurons only to the PS-max cells, according to evidence that ACh differentially modulates W vs. PS active neurons (see below). In this manner, the discharge profiles of the principal cell types across the sleep-wake states could be simulated along with an output of changing gamma EEG activity (as principal cell output) and EMG activity (as motor neuron output) to generate three distinct sleep-wake states together with transitional stages reflecting the oscillatory nature of the activity modulation and sleep-wake cycle.
Figure 3.
Model of the principal cell types and their connections in sleep-wake regulatory circuits within the BF. Based upon the normalized firing rates of the exemplary principal cell types, W/PS, SWS, W and PS along with wsp and of the EEG gamma and EMG activities (Figure 2), the most parsimonious set of connections were applied in designing a dynamical systems model (with 14 elements) that would simulate the cells’ discharge profiles with EEG and EMG activities (as reflected in principal cortical or principal motor neuron output) across sleep-wake stages. Connections ending with arrows are excitatory and those ending with bars are inhibitory. Abbreviations: Cx, cortex; IN, interneuron; P, principal neuron; SC, spinal cord.
Activity and roles of neuromodulatory systems
Glu and GABA neurons distributed through the core of the forebrain and brainstem are assumed to compose the effector neurons of EEG and EMG changes that underlie sleep-wake states and to have the capacity to generate these states by their different discharge profiles and interactions. Neuromodulatory systems nonetheless play important if not critical roles in modulating the activity of the Glu and GABA neurons. Whereas in our parsimonious model, we incorporated only excitatory output of ACh neurons including that to cortical principal and PS-max active neurons (Figure 3), there is considerable pharmacological evidence to indicate a critical role of inhibitory as well as excitatory cholinergic output through different muscarinic (M) receptors (Rs) (Figure 1). Thus ACh likely has an excitatory action upon W/PS-max and PS-max active neurons through AChM1Rs and an inhibitory action upon W-max active neurons through AChM2Rs, as has been suggested by pharmacological studies showing differential effects of ACh on different BF neurons [26] and brainstem neurons [27] and the induction of PS by local injections of the cholinergic agonist, carbachol, in the BF or brainstem which depends importantly upon AChM2Rs [28,29].
Moreover, the role of NA LC neurons, which are known as typical W-max active neurons [11] is executed through differential actions upon different neurons in the BF and preoptic area, where NA excites W/PS-active ACh and likely other GABA and Glu W/PS neurons through adrenergic α1 receptors (Aα1Rs) and inhibits sleep-active noncholinergic, including GABA neurons, through Aα2Rs [20,26,30–32] (Figure 1). Moreover, from early pharmacological studies (see for review [33]), it is known that ACh can only elicit PS under the conditions that the monoamines are depleted and thus the NA and other monoamine neurons inoperative or silent.
A key role of orexin (Orx or hypocretin) in the promotion of waking with muscle tone has been well known since the discovery that it is the peptide which is missing in narcolepsy with cataplexy (see for review [34]). With juxtacellular recording and identification, we established that the Orx neurons discharge maximally during W and stop firing during SWS and PS, as W-max active neurons [35] (Figure 1). Innervating and exciting all other arousal systems, including importantly the NA LC neurons [36], by diffuse projections throughout the brain and spinal cord, the Orx neurons thus play a key role in stimulating and maintaining arousal. It is likely that only with their silence during sleep or absence as in narcolepsy with cataplexy that ACh neurons can elicit PS with muscle atonia.
Using the juxtacellular technique, we were also able to record from identified melanin concentrating hormone (MCH) neurons, which are intermingled with the Orx neurons in the PH and found that they discharged in a manner reciprocal to that of the Orx neurons, selectively during sleep and most actively during PS, as PS-max active neurons [37] (Figure 1). They are also inhibited by NA In contrast to the Orx neurons, which are primarily excited by NA [38]. Through inhibitory actions on diffusely distributed targets, including importantly the NA LC neurons [39], MCH neurons could promote sleep.
New information from optogenetic and chemogenetic manipulation of chemically specific cell groups
Applying newly available techniques based upon genetic tagging and optical or pharmacological manipulation of specific cells, it has recently become possible to selectively stimulate specific cell groups and thus test their role in sleep-wake regulation. Particularly for the neuromodulatory systems, these approaches have thus substantiated many of the theories concerning the roles of sleep-wake regulatory cell groups. Applying optogenetics, selective activation of the Orx neurons was shown to promote waking [40], whereas that of MCH neurons was shown to promote SWS and PS [41–43], corroborating the roles of these W-max and PS-max active cells, respectively (Figure 1). Even more potently, it was shown that selective activation of NA LC W-active neurons rapidly elicited waking with prominent behavioral arousal [44] (Figure 1).
With regard to the ACh neurons, photo-stimulation of these in the BF and pontomesencephalic tegmentum has been associated with elicitation of cortical activation and a transition from slow wave EEG in SWS to fast EEG with either W or PS [45–48], thus confirming the role of ACh neurons in stimulating cortical activation by their discharge during both W and PS (Figure 1).
Demonstration of the roles of the Glu and GABA presumed sleep-wake effector neurons has proven more problematic, given the functional heterogeneity and intermingling of these cell types in multiple regions (Figure 1). Thus stimulation of all Glu or GABA neurons in any region would have opposing and/or diverse influences. Yet, photo- or chemo-stimulation of genetically tagged Glu or GABA, like ACh, BF neurons commonly evoked cortical activation [47,49], likely reflecting the fact that these groups are all comprised in the majority by W/PS-max active neurons. Selective tagging of a subset of the GABA neurons as has been possible in the BF for the parvalbumin neurons, which comprise the vast majority of the cortically projecting GABA neurons [50], revealed that their photo-stimulation elicits cortical activation with gamma activity [47,51]. Yet, it is possible that cortically projecting SWS-max active GABA BF neurons also contain parvalbumin, in which case the effect of stimulating parvalbumin like GABA BF neurons would likely also be determined by the predominance of the most numerous W/PS-max active GABA neurons in that region. In this regard, recording of GABA along with Glu and ACh photo-tagged neurons using an optrode in BF mainly revealed cells of the predominant W/PS-max active type though did find some somatostatin neurons which discharged maximally during SWS and which when stimulated elicited SWS [47]. However, even the somatostatin GABA neurons were found to be heterogeneous in their discharge profiles in this study. We would thus assume that the effect of photo-stimulation or chemo-stimulation in the BF can provide coherent effects when involving ACh neurons, given their functional homogeneity, whereas the effect of stimulating the functionally heterogeneous GABA or Glu cell populations would likely reflect the role of the predominant cell type, which in the BF is W/PS-max active. Similarly, in the PH, photo-stimulation and chemo-stimulation of GABA neurons has elicited awakening [52,53], again presumably reflecting the effect of stimulating the predominant cell type of what are functionally heterogeneous GABA neurons in that area as well.
In the brainstem, photo-stimulation of GABA and Glu neurons would also have the same problem as in the BF, yet has revealed certain prominent roles of GABA or Glu neurons in SWS, PS and/or muscle atonia, corresponding presumably to the predominant functional cell type in those areas [54–56].
Conclusions
In summary, research over the past century has progressively revealed the discharge profiles of neurons in different regions along with their different projections and different neurotransmitters or neuromodulators by which they regulate sleep-wake states. Four principal functional cell types have been distinguished through the core of the forebrain and brainstem, W/PS-max, SWS-max, W-max and PS-max active, which are each comprised by both GABA and Glu neurons of differing proportions in different regions and which through their activities and interactions constitute the effector neurons of sleep-wake state generation. Acting in part upon GABA and Glu effector neurons, neuromodulatory systems, including importantly ACh, NA, Orx and MCH neurons, each constitute one principal functional cell type and accordingly selectively promote different states with their EEG and EMG correlated activities. With increasingly specific genetic tagging of cell types according to both neurotransmitter and receptor [57] and calcium imaging [58] along with photo manipulation of the tagged cells, it is possible that future studies will reveal the full image of the differential distributions and activities of constituent cells of the principal functional cell types along with their interactions to further our understanding of sleep-wake regulatory circuits and the generation of sleep-wake states.
Highlights.
Four functional cell types are distinguished by firing rate across sleep-wake states.
Each functional cell type is comprised by both GABA and glutamate neurons.
GABA and glutamate neurons of the four types are co-distributed through the brain.
They have the capacity to regulate sleep-wake states with EEG and EMG activity.
They are regulated in turn by functionally homogeneous neuromodulatory cell types.
Acknowledgments
The research presented was funded by grants from NIH (MH-60119) and CIHR (MOP 13458, 82762 and130502). I would particularly like to thank Chris Cordova with Faisal Naqib, Oum Hassani and Chris Pack who worked upon the simulation and model of BF neurons in regulating sleep-wake states. I also thank Napoleon Soberanis for his assistance with the schematic figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jones BE. Neurobiology of waking and sleeping. Handb Clin Neurol. 2011;98:131–149. doi: 10.1016/B978-0-444-52006-7.00009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moruzzi G. The sleep-waking cycle. Ergeb Physiol. 1972;64:1–165. doi: 10.1007/3-540-05462-6_1. [DOI] [PubMed] [Google Scholar]
- 3.Steriade M, Hobson JA. Neuronal activity during the sleep-waking cycle. Prog Neurobiol. 1976;6:155–376. [PubMed] [Google Scholar]
- 4.Szymusiak R, Alam N, Steininger TL, McGinty D. Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res. 1998;803:178–188. doi: 10.1016/s0006-8993(98)00631-3. [DOI] [PubMed] [Google Scholar]
- 5.Szymusiak R, McGinty D. Sleep-related neuronal discharge in the basal forebrain of cats. Brain Res. 1986;370:82–92. doi: 10.1016/0006-8993(86)91107-8. [DOI] [PubMed] [Google Scholar]
- 6.Siegel JM. Behavioral functions of the reticular formation. Brain Res. 1979;180:69–105. doi: 10.1016/0165-0173(79)90017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steininger TL, Alam MN, Gong H, Szymusiak R, McGinty D. Sleep-waking discharge of neurons in the posterior lateral hypothalamus of the albino rat. Brain Res. 1999;840:138–147. doi: 10.1016/s0006-8993(99)01648-0. [DOI] [PubMed] [Google Scholar]
- 8.Steriade M, Oakson G, Ropert N. Firing rates and patterns of midbrain reticular neurons during steady and transitional states of the sleep-waking cycle. Exp Brain Res. 1982;46:37–51. doi: 10.1007/BF00238096. [DOI] [PubMed] [Google Scholar]
- 9.Jouvet M. The role of monoamines and acetylcholine-containing neurons in the regulation of the sleep-waking cycle. Ergeb Physiol. 1972;64:165–307. doi: 10.1007/3-540-05462-6_2. [DOI] [PubMed] [Google Scholar]
- 10.Hobson JA, McCarley RW, Wyzinski PW. Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science. 1975;189:55–58. doi: 10.1126/science.1094539. [DOI] [PubMed] [Google Scholar]
- 11.Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gritti I, Henny P, Galloni F, Mainville L, Mariotti M, Jones BE. Stereological estimates of the basal forebrain cell population in the rat, including neurons containing choline acetyltransferase, glutamic acid decarboxylase or phosphate-activated glutaminase and colocalizing vesicular glutamate transporters. Neuroscience. 2006;143:1051–1064. doi: 10.1016/j.neuroscience.2006.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henny P, Jones BE. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. Eur J Neurosci. 2008;27:654–670. doi: 10.1111/j.1460-9568.2008.06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henny P, Jones BE. Vesicular glutamate (VGluT), GABA (VGAT), and acetylcholine (VAChT) transporters in basal forebrain axon terminals innervating the lateral hypothalamus. J Comp Neurol. 2006;496:453–467. doi: 10.1002/cne.20928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee MG, Hassani OK, Alonso A, Jones BE. Cholinergic basal forebrain neurons burst with theta during waking and paradoxical sleep. J Neurosci. 2005;25:4365–4369. doi: 10.1523/JNEUROSCI.0178-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassani OK, Lee MG, Henny P, Jones BE. Discharge profiles of identified GABAergic in comparison to cholinergic and putative glutamatergic basal forebrain neurons across the sleep-wake cycle. J Neurosci. 2009;29:11828–11840. doi: 10.1523/JNEUROSCI.1259-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi K, Lin JS, Sakai K. Characterization and mapping of sleep-waking specific neurons in the basal forebrain and preoptic hypothalamus in mice. Neuroscience. 2009;161:269–292. doi: 10.1016/j.neuroscience.2009.02.075. [DOI] [PubMed] [Google Scholar]
- 18.Sakai K. Sleep-waking discharge profiles of median preoptic and surrounding neurons in mice. Neuroscience. 2011;182:144–161. doi: 10.1016/j.neuroscience.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Tamakawa Y, Karashima A, Koyama Y, Katayama N, Nakao M. A quartet neural system model orchestrating sleep and wakefulness mechanisms. J Neurophysiol. 2006;95:2055–2069. doi: 10.1152/jn.00575.2005. [DOI] [PubMed] [Google Scholar]
- 20.Modirrousta M, Mainville L, Jones BE. GABAergic neurons with alpha2-adrenergic receptors in basal forebrain and preoptic area express c-Fos during sleep. Neuroscience. 2004;129:803–810. doi: 10.1016/j.neuroscience.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 21.Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- 22.Koyama Y, Takahashi K, Kodama T, Kayama Y. State-dependent activity of neurons in the perifornical hypothalamic area during sleep and waking. Neuroscience. 2003;119:1209–1219. doi: 10.1016/s0306-4522(03)00173-8. [DOI] [PubMed] [Google Scholar]
- 23.Hassani OK, Henny P, Lee MG, Jones BE. GABAergic neurons intermingled with orexin and MCH neurons in the lateral hypothalamus discharge maximally during sleep. Eur J Neurosci. 2010;32:448–457. doi: 10.1111/j.1460-9568.2010.07295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boucetta S, Cisse Y, Mainville L, Morales M, Jones BE. Discharge profiles across the sleep-waking cycle of identified cholinergic, GABAergic, and glutamatergic neurons in the pontomesencephalic tegmentum of the rat. J Neurosci. 2014;34:4708–4727. doi: 10.1523/JNEUROSCI.2617-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cordova CA, Hassani OK, Lee MG, Naqib F, Pack CC, Jones BE. Modeling sleep-wake activity profiles of cholinergic, GABAergic and glutamateric basal forebrain cell groups. Society for Neuroscience On-line Abstracts. 2010 doi:108.15/MMM57. [Google Scholar]
- 26.Fort P, Khateb A, Serafin M, Muhlethaler M, Jones BE. Pharmacological characterization and differentiation of non-cholinergic nucleus basalis neurons in vitro. NeuroReport. 1998;9:1–5. doi: 10.1097/00001756-199801050-00013. [DOI] [PubMed] [Google Scholar]
- 27.Greene RW, Carpenter DO. Actions of neurotransmitters on pontine medial reticular formation neurons of the cat. J Neurophysiol. 1985;54:520–531. doi: 10.1152/jn.1985.54.3.520. [DOI] [PubMed] [Google Scholar]
- 28.Reid MS, Nishino S, Tafti M, Siegel JM, Dement WC, Mignot E. Neuropharmacological characterization of basal forebrain cholinergic stimulated cataplexy in narcoleptic canines. Exp Neurol. 1998;151:89–104. doi: 10.1006/exnr.1998.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reid MS, Tafti M, Nishino S, Siegel JM, Dement WC, Mignot E. Cholinergic regulation of cataplexy in canine narcolepsy in the pontine reticular formation is mediated by M2 muscarinic receptors. Sleep. 1994;17:424–435. [PMC free article] [PubMed] [Google Scholar]
- 30.Fort P, Khateb A, Pegna A, Muhlethaler M, Jones BE. Noradrenergic modulation of cholinergic nucleus basalis neurons demonstrated by in vitro pharmacological and immunohistochemical evidence in the guinea pig brain. Eur J Neurosci. 1995;7:1502–1511. doi: 10.1111/j.1460-9568.1995.tb01145.x. [DOI] [PubMed] [Google Scholar]
- 31.Gallopin T, Fort P, Eggermann E, Cauli B, Luppi PH, Rossier J, Audinat E, Muhlethaler M, Serafin M. Identification of sleep-promoting neurons in vitro. Nature. 2000;404:992–995. doi: 10.1038/35010109. [DOI] [PubMed] [Google Scholar]
- 32.Manns ID, Lee MG, Modirrousta M, Hou YP, Jones BE. Alpha 2 adrenergic receptors on GABAergic, putative sleep-promoting basal forebrain neurons. Eur J Neurosci. 2003;18:723–727. doi: 10.1046/j.1460-9568.2003.02788.x. [DOI] [PubMed] [Google Scholar]
- 33.Jones BE. Paradoxical sleep and its chemical/structural substrates in the brain. Neuroscience. 1991;40:637–656. doi: 10.1016/0306-4522(91)90002-6. [DOI] [PubMed] [Google Scholar]
- 34.Nishino S, Mignot E. Narcolepsy and cataplexy. Handb Clin Neurol. 2011;99:783–814. doi: 10.1016/B978-0-444-52007-4.00007-2. [DOI] [PubMed] [Google Scholar]
- 35.Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, van den Pol AN. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415:145–159. [PubMed] [Google Scholar]
- 37.Hassani OK, Lee MG, Jones BE. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. Proc Natl Acad Sci U S A. 2009;106:2418–2422. doi: 10.1073/pnas.0811400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bayer L, Eggermann E, Serafin M, Grivel J, Machard D, Muhlethaler M, Jones BE. Opposite effects of noradrenaline and acetylcholine upon hypocretin/orexin versus melanin concentrating hormone neurons in rat hypothalamic slices. Neuroscience. 2005;130:807–811. doi: 10.1016/j.neuroscience.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 39.Del Cid-Pellitero E, Jones BE. Immunohistochemical evidence for synaptic release of GABA from melanin-concentrating hormone containing varicosities in the locus coeruleus. Neuroscience. 2012;223:269–276. doi: 10.1016/j.neuroscience.2012.07.072. [DOI] [PubMed] [Google Scholar]
- 40.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jego S, Glasgow SD, Herrera CG, Ekstrand M, Reed SJ, Boyce R, Friedman J, Burdakov D, Adamantidis AR. Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat Neurosci. 2013;16:1637–1643. doi: 10.1038/nn.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konadhode RR, Pelluru D, Blanco-Centurion C, Zayachkivsky A, Liu M, Uhde T, Glen WB, Jr, van den Pol AN, Mulholland PJ, Shiromani PJ. Optogenetic stimulation of MCH neurons increases sleep. J Neurosci. 2013;33:10257–10263. doi: 10.1523/JNEUROSCI.1225-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsunematsu T, Ueno T, Tabuchi S, Inutsuka A, Tanaka KF, Hasuwa H, Kilduff TS, Terao A, Yamanaka A. Optogenetic manipulation of activity and temporally controlled cell-specific ablation reveal a role for MCH neurons in sleep/wake regulation. J Neurosci. 2014;34:6896–6909. doi: 10.1523/JNEUROSCI.5344-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, Deisseroth K, de Lecea L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–1533. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **45.Han Y, Shi YF, Xi W, Zhou R, Tan ZB, Wang H, Li XM, Chen Z, Feng G, Luo M, et al. Selective activation of cholinergic basal forebrain neurons induces immediate sleep-wake transitions. Curr Biol. 2014;24:693–698. doi: 10.1016/j.cub.2014.02.011. Genetically defined ACh BF neurons were tagged with ChR2 for photo-activation in freely moving mice. Stimulation during SWS sleep elicited cortical activation and waking or REM sleep, leading to the conclusion that selective activation of cholinergic BF neurons is sufficeint to suppress SWS and promote W and REM sleep. [DOI] [PubMed] [Google Scholar]
- 46.Irmak SO, de Lecea L. Basal forebrain cholinergic modulation of sleep transitions. Sleep. 2014;37:1941–1951. doi: 10.5665/sleep.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **47.Xu M, Chung S, Zhang S, Zhong P, Ma C, Chang WC, Weissbourd B, Sakai N, Luo L, Nishino S, et al. Basal forebrain circuit for sleep-wake control. Nat Neurosci. 2015;18:1641–1647. doi: 10.1038/nn.4143. Genetically defined ACh, Glu, parvalbumin-GABA and somatostatin-GABA BF neurons were tagged with ChR2 for photo-activation and recording using an optrode in naturally sleeping-waking head-fixed mice. Whereas most ACh, Glu and parvalbumin neurons were W-REM type cells and their stimulation elicited waking with cortical activation, some somatostatin neurons discharged maximally during SWS and their stimulation elicited SWS sleep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Dort CJ, Zachs DP, Kenny JD, Zheng S, Goldblum RR, Gelwan NA, Ramos DM, Nolan MA, Wang K, Weng FJ, et al. Optogenetic activation of cholinergic neurons in the PPT or LDT induces REM sleep. Proc Natl Acad Sci U S A. 2015;112:584–589. doi: 10.1073/pnas.1423136112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *49.Anaclet C, Pedersen NP, Ferrari LL, Venner A, Bass CE, Arrigoni E, Fuller PM. Basal forebrain control of wakefulness and cortical rhythms. Nat Commun. 2015;6:8744. doi: 10.1038/ncomms9744. Using chemogenetic systems targeted toward cholinergic, glutamateric and GABAergic BF neurons, chemo-activation of the cholinergic and glutamatergic cells was found to influence the EEG by suppressing slow activity, whereas chemo-activation of the GABAergic neurons was found to stimulate high frequency EEG along with sustained wakefulness in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gritti I, Manns ID, Mainville L, Jones BE. Parvalbumin, calbindin, or calretinin in cortically projecting and GABAergic, cholinergic, or glutamatergic basal forebrain neurons of the rat. J Comp Neurol. 2003;458:11–31. doi: 10.1002/cne.10505. [DOI] [PubMed] [Google Scholar]
- 51.Kim T, Thankachan S, McKenna JT, McNally JM, Yang C, Choi JH, Chen L, Kocsis B, Deisseroth K, Strecker RE, et al. Cortically projecting basal forebrain parvalbumin neurons regulate cortical gamma band oscillations. Proc Natl Acad Sci U S A. 2015;112:3535–3540. doi: 10.1073/pnas.1413625112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herrera CG, Cadavieco MC, Jego S, Ponomarenko A, Korotkova T, Adamantidis A. Hypothalamic feedforward inhibition of thalamocortical network controls arousal and consciousness. Nat Neurosci. 2016;19:290–298. doi: 10.1038/nn.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Venner A, Anaclet C, Broadhurst RY, Saper CB, Fuller PM. A Novel Population of Wake-Promoting GABAergic Neurons in the Ventral Lateral Hypothalamus. Curr Biol. 2016;26:2137–2143. doi: 10.1016/j.cub.2016.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anaclet C, Lin JS, Vetrivelan R, Krenzer M, Vong L, Fuller PM, Lu J. Identification and characterization of a sleep-active cell group in the rostral medullary brainstem. J Neurosci. 2012;32:17970–17976. doi: 10.1523/JNEUROSCI.0620-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krenzer M, Anaclet C, Vetrivelan R, Wang N, Vong L, Lowell BB, Fuller PM, Lu J. Brainstem and spinal cord circuitry regulating REM sleep and muscle atonia. PLoS One. 2011;6:e24998. doi: 10.1371/journal.pone.0024998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weber F, Chung S, Beier KT, Xu M, Luo L, Dan Y. Control of REM sleep by ventral medulla GABAergic neurons. Nature. 2015;526:435–438. doi: 10.1038/nature14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *57.Bonnavion P, Jackson AC, Carter ME, de Lecea L. Antagonistic interplay between hypocretin and leptin in the lateral hypothalamus regulates stress responses. Nat Commun. 2015;6:6266. doi: 10.1038/ncomms7266. This study employed genetic tagging of cells bearing a particular receptor (for leptin) and thus opens the possibility of examining functionally selective cell groups according to their neuromodulatory receptors, as can be applied to the study of principal functional cell types in sleep-wake regulatory systems. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **58.Cox J, Pinto L, Dan Y. Calcium imaging of sleep-wake related neuronal activity in the dorsal pons. Nat Commun. 2016;7:10763. doi: 10.1038/ncomms10763. This study is one of the first to apply calcium imaging to sleep-wake regulatory neurons within the deep, central core structures of the brain in head-fixed mice. With cell type specific microendoscopic calcium imaging in the dorsal pons, whereas ACh neurons appeared to be active during W and REM, Glu and GABA neurons appeared to comprise respectively clusters of REM-max or W-max active neurons (in ways which appear to differ from our juxtacellular recording results from the LDT/SubLDT region) [DOI] [PMC free article] [PubMed] [Google Scholar]