Abstract
Narcolepsy was first identified almost 130 years ago, but it was only 15 years ago that it was identified as a neurodegenerative disease linked to a loss of orexin neurons in the brain. It is unclear what causes the orexin neurons to die, but our strategy has been to place the gene for orexin into surrogate neurons in the validated mouse models of narcolepsy, and test whether it can block narcolepsy symptoms, such as cataplexy. In both the orexin knockout and the orexin-ataxin-3 mouse models of narcolepsy we have found that cataplexy can be blocked if the surrogate neurons are part of the circuit responsible for cataplexy. We have also determined that the orexin gene can be inserted into surrogate neurons in the amygdala to block emotion-induced cataplexy. Through the use of optogenetics we anticipate that it will be possible to preemptively block cataplexy.
Keywords: Narcolepsy, Sleep, Hypocretin, Viral Vectors
Gene therapy is being used to treat neurodegenerative diseases (for review see (Mandel et al., 2006), (Oehmig et al., 2004)). In a phase I study eight patients with mild Alzheimers were given ex-vivo NGF gene therapy and their rate of cognitive decline was slowed (Tuszynski et al., 2005). Currently, phase II trials are in progress. Motor functions were improved in six patients with Parkinson’s disease that were treated with AAV mediated delivery of aromatic l-amino acid decarboxylase (AADC) gene for synthesis of dopamine and serotonin (Muramatsu et al., 2010).
Genetic methods can be used to treat narcolepsy since the disease is linked to loss of the orexin neurons that are localized in only one place in the brain (Peyron et al., 2000, Thannickal et al., 2000). Therefore, it is feasible to test the hypothesis that orexin gene insertion into surviving neurons is able to decrease narcoleptic symptoms. We have focused on cataplexy, an important distinguishing symptom of narcolepsy, and demonstrated in two mouse models that cataplexy can be improved by orexin gene transfer (Liu et al., 2008, Liu et al., 2011). In this review, we discuss the use of genetically engineered methods as a neurobiological tool to understand the neural circuitry underlying narcoleptic symptoms. We recognize that pharmacological agents such as modafinil (Provigil) or sodium oxybate (Xyrem) are prescribed to patients with narcolepsy. We agree that the pharmacological approach is economical, easily distributed and accepted by patients. However, the current drugs of choice treat only some of the symptoms, and they lack specificity since they bathe the entire body. It is preferable to use pharmacological agents that act at specific sites and the gene transfer method can serve as a tool to identify such sites. We envision that with the aid of optogenetics and pharmacogenetics it will be feasible to identify the neurons that trigger the cataplexy attacks and then proceed to blocking the attacks from being triggered by emotions.
Orexins (hypocretins) and linkage with narcolepsy
The neuropeptides hypocretins (HCRT), also known as orexins, were linked to narcolepsy through forward (Lin et al., 1999) and reverse (Chemelli et al., 1999) genetic approaches. Subsequently, postmortem examination of brain tissue revealed loss of the orexin neurons in the narcoleptic patients (Peyron et al., 2000, Thannickal et al., 2000). It is now abundantly clear that narcolepsy is a neurodegenerative disease, since other markers that colocalize with HCRT are also absent in humans with narcolepsy (Blouin et al., 2005, Crocker et al., 2005).
Validated mouse models exist that clearly show that narcoleptic symptoms develop with deletion of the orexin peptide (orexin knockout) (Chemelli et al., 1999), the two orexin receptors (Kalogiannis et al., 2011), or the death of the orexin-containing neurons (Hara et al., 2001) (Tabuchi et al., 2014). In the newest mouse model (Tabuchi et al., 2014) the timing of the death of the orexin neurons can be controlled through the application of doxycycline. In the presence of doxycycline the orexin neurons remain intact, but once doxycycline administration (via drinking water) is stopped the diphtheria toxin accumulates in the orexin neurons, and within 7 days 80% of hypocretin neurons die. This model is best at mimicking the degeneration of the orexin neurons in human narcolepsy.
The orexin neurons are localized only in the perifornical and lateral hypothalamus (Peyron et al., 1998) area from where they project widely throughout the CNS, with especially heavy innervation to neurons implicated in arousal. Therefore, it is not surprising that sleep attacks and sleepiness occur with loss of the orexin neurons. Cataplexy is another symptom of narcolepsy that is usually triggered by strong emotions (Aldrich, 1991). The orexin neurons project to the pontine areas implicated in maintaining muscle tone during waking (Peyron et al., 1998), and loss of the orexin innervation destabilizes the circuit.
Since the underlying neurons that make and secrete orexin have died, it is necessary to identify surrogates that will accomplish this task. The important question is to identify suitable surrogate neurons. The orexin neurons project to the entire brain and spinal cord but it is not known which target site controls some or all of the narcoleptic symptoms (Peyron et al., 1998). Replacing the orexin could restore normal sleep-wake function, but which function? Narcolepsy is characterized by excessive daytime sleepiness, sleep fragmentation, sleep attacks, SOREMPs (sleep-onset REM periods), and cataplexy. Would transfer of the orexin gene rescue all of these, or are some more sensitive and easily affected by the presence of the peptide?
When we began our studies (Liu et al., 2008, Liu et al., 2011) we considered these issues and began by first placing the orexin gene in surrogate neurons of the lateral hypothalamus since the orexin neurons are localized only in this region from where they innervate various targets.
Orexin gene transfer into surrogate neurons in the hypothalamus and pons
Our initial approach was to use the surviving neurons in the perifornical area as surrogates because they are likely to have the same neuronal connectivity as the neurons that died. Therefore, we first tested our hypothesis in the orexin knockout mice (Liu et al., 2008). In these mice only the orexin gene has been deleted but the underlying network is intact compared to the hypocretinataxin mice where the underlying neurons have died. We used a herpes-simplex virus-1 (HSV-1) to transfer the gene for orexin into surrogate neurons (Liu et al., 2008). Our in-vitro and in-vivo tests demonstrated that the virus successfully transferred the orexin gene into neurons. Moreover, an ELISA assay confirmed that the peptide was detected in the CSF of mice given the gene transfer. During the four day life-span of the vector the incidence of cataplexy declined by 60%, and the levels of REM sleep during the second half of night were similar to levels in wild-type mice indicating that narcoleptic sleep-wake behavior in orexin knockout mice can be improved by targeted gene transfer.
In the next study (Liu et al., 2011) we used the orexin-ataxin-3 mice and a recombinant adenoassociated (rAAV) virus inserted the orexin gene into lateral hypothalamic neurons. The advantage of the rAAV was that it increased the lifespan of expression of HCRT to at least three weeks, compared to the four-day active cycle of the HSV-1 that was used in our initial study. We also inserted the orexin gene into neurons containing melanin concentrating hormone. The MCH neurons lie alongside the orexin neurons and project to the same targets as the orexin neurons. However, they are a separate population (Peyron et al., 1998). The MCH neurons are active during sleep (Hassani et al., 2009) whereas the orexin neurons are active in waking (Lee et al., 2005, Mileykovskiy et al., 2005). Thus, the MCH neurons promote sleep whereas the orexin neurons promote arousal. Optogenetic activation of the MCH neurons induces sleep in both mice (Konadhode et al., 2013) and rats (Blanco-Centurion et al., 2016) indicating that these neurons may induce sleep across mammals. Optogenetic activation of the orexin neurons induces arousal (Carter and De Lecea, 2011). In the orexin-ataxin-3 mice the orexin neurons die while the MCH neurons are still present (Hara et al., 2001). Therefore, we hypothesized that placing the orexin gene into the MCH neurons should block narcoleptic symptoms. However, we found that insertion of the orexin gene into MCH neurons did not affect cataplexy whereas insertion of orexin gene into other adjacent neurons significantly decreased cataplexy. We were initially puzzled why placing the orexin gene into MCH neurons did not work. However, the MCH neurons are not active in waking and therefore cannot release orexin to block cataplexy. We also found that using the striatal neurons as surrogates had no effect since these neurons are not part of the sleep network. Therefore, from this study we concluded that one cannot simply place the orexin gene anywhere in the brain; for it to be effective the orexin gene has to be placed in a neuron that is connected to the circuit regulating cataplexy, and the neuron also has to be active during cataplexy.
In the third study (Blanco-Centurion C, 2012), the orexin gene was placed in the dorsal pons, an area that has a rich history in regulating REM sleep and motor control. The results indicated that orexin gene transfer into the pontine neurons was also effective in decreasing cataplexy. We also found that it increased length of waking bouts at night, albeit modestly. In our pontine study we did not target the orexin into phenotypic specific neurons in the pons. However, another group combined optogenetics and DREADD to provide a better cellular resolution of the phenotype of neurons in the pons involved in cataplexy (Hasegawa et al., 2014). They showed that the orexin input onto serotonin neurons reduces cataplexy, while orexin input onto LC neurons maintains waking (Hasegawa et al., 2014).
Other groups have shown that there are brain sites where waking can be improved. For instance, one group injected the orexin gene into more ventral regions of the lateral hypothalamus in orexin-ataxin-3 mice and it modestly improved waking but not cataplexy (Kantor et al., 2013). Another group (Mochizuki et al., 2011) used mice with a mutation in the hypocretin-2 receptor (orexin-2R). Canine narcolepsy is linked to a mutation in the hypocretin-2 receptor (Lin et al., 1999) and mice with deletion of this receptor also show narcoleptic symptoms (Kalogiannis et al., 2011). Mochizuki et al., (Mochizuki et al., 2011) normalized the hypocretin-2 receptor in the posterior hypothalamus, including the histamine neurons in the tuberomammillary nucleus and found that it increased the length of waking bouts. They concluded that the hypocretin-2 receptor on the histamine neurons regulated waking. New evidence supports this conclusion (Bastianini et al., 2015).
The amygdala and emotion-induced cataplexy
Cataplexy is a sudden episode of muscle tone loss (muscle atoina) during wakefulness lasting from a few seconds to minutes. Normally, muscle atonia occurs only during REM sleep. However, in narcolepsy, muscle atonia occurs during wakefulness due to the loss of orexin or its receptors. Cataplexy in humans and canines is triggered by strong emotions such as laughter (humans) and food (canines). In mice, certain sugary foods or predator odor also triggers cataplexy (Clark EL, et al., 2009; Morawska M, et al., 2011). This suggests that the limbic system can trigger cataplexy. Indeed, cataplexy-active neurons have been found in the amygdala of narcoleptic dogs. Neurons in central nucleus of the amygdala (CeA) and basolateral amygdala (BLA) increase discharge prior to and during cataplexy (Gulyani S, et al., 2002). Lesion of the amygdala reduces cataplexy induced by positive emotions (Burgess CR, et al., 2013). Based on this evidence we began to identify the amygdala circuit triggering cataplexy. In the first study, we found that orexin gene transfer into amygdala (mostly CeA and BLA) significantly decreased predator odor-induced cataplexy attacks (Liu M, et al., 2016). This suggests that amygdala is a key structure integrating emotions and cataplexy. Introducing orexin into amygdala circuits probably reduces cataplexy by antagonizing amygdala’s inhibition on muscle tone (Fig. 1). However, amygdala neurons project to many brain regions and it is important to identify the circuit underlying emotion-induced cataplexy.
Fig. 1. A neural circuit model of narcolepsy and our strategy to pre-emptively block cataplexy from being triggered by emotions.
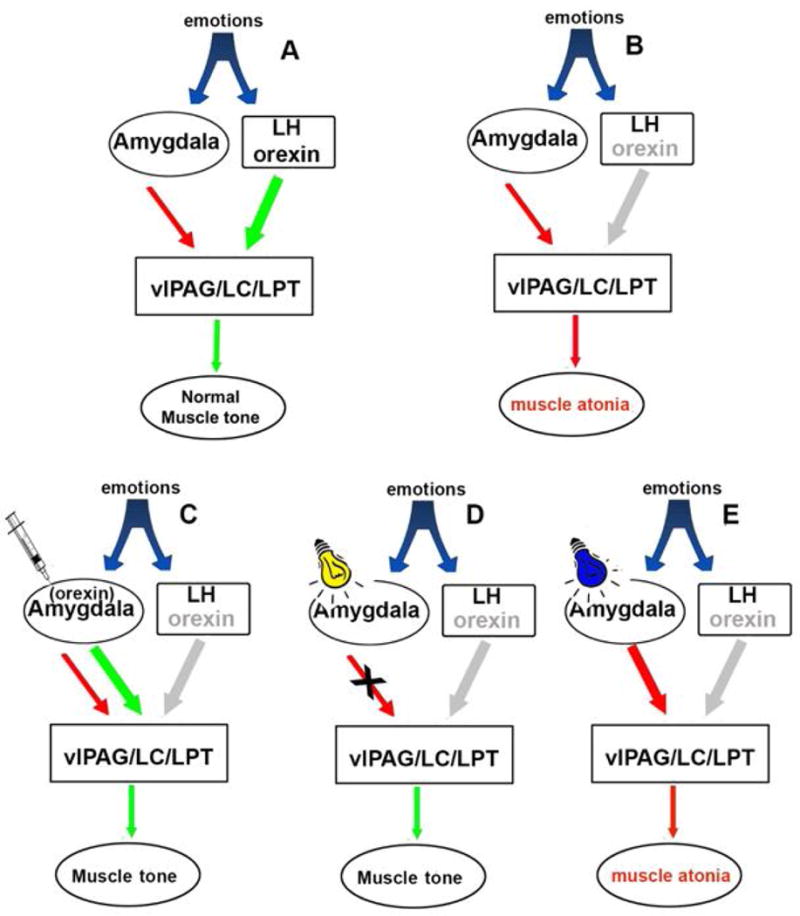
Normal muscle tone is regulated by the activity of pontine neurons (Brooks and Peever, 2008). During emotions the orexin excitatory signal from the lateral hypothalamus neutralizes the inhibitory signal from the amygdala and maintains the activity of pontine neurons (A). In narcoleptic mice the orexin excitatory signal from the lateral hypothalamus is absent. Therefore, the inhibitory signal from the amygdala overwhelms and inhibits pontine neurons controlling muscle tone, resulting in muscle atonia during waking (i.e. cataplexy) (B). When we insert orexin gene into surrogate neurons in the amygdala (C) or block the inhibitory input (D), the pons will be disinhibited and be able to maintain muscle tone. But if we excite the amygdala GABA pathway there will be more inhibitory input to pons that will exacerbate cataplexy (E).
It is now feasible to identify and manipulate this circuit because new axonal tracers have been developed that match anatomy and function of specific projection in the brain. These new tools involve transsynaptic retrograde tracers, such as wheat-germ agglutinin-Cre and Cre-loxp techniques. For example, we can express the orexin gene (or any other gene, such as the gene channelrhodopsin-2) into neurons that project only to specific sites in the brain. We have demonstrated feasibility of such an approach by injecting rAAV vectors with Cre-inducible expression of orexin gene (AAV-DIO-orexin) into CeA, and retrograde tracing AAV vectors expressing Cre recombinase (AAV-mCherry-WGA-Cre) into pons. The expressed Cre recombinase will cross the synapsis and be retrogradely transported along the axon to the neuronal soma in CeA. Injection of the rAAV-DIO-orexin into the CeA will induce orexin expression in the Cre-expressing neuron (Fig. 2). We have found that orexin gene transfer into CeA neurons projecting specifically to the pons significantly inhibits predator-odor induced cataplexy in narcoleptic mice (Fig. 2). The technique can be further refined so that the orexin (or ChR2) is expressed in phenotype specific neurons projecting to the pons. For instance, effects of GABAergic projections from CeA to various pons structures (ventrolateral periaqueductal gray, locus, dorsal raphe, etc.) on cataplexy can be tested with novel retrograde tracer canine adenovirus-2 (CAV-2) vector and narcoleptic VGAT-IRES-Cre transgenic mice.
Fig 2.
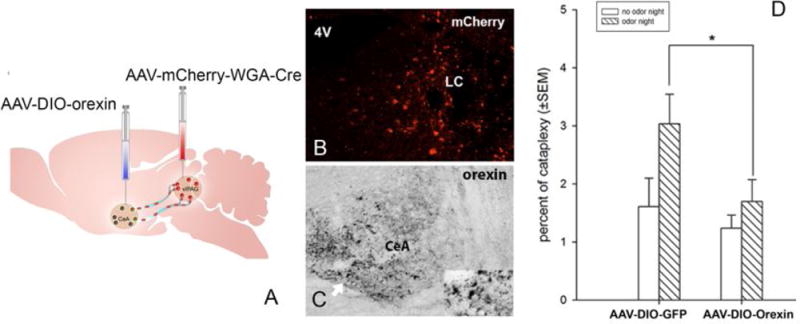
Illustration of WGA-Cre circuit mapping tool and preliminary behavioral data that placing orexin into a specific circuit reduces cataplexy. Photo A illustrates that injection of AAV-mCherry-WGA-Cre into pons and AAV-DIO-orexin injection into the amygdala can identify CeA neurons projecting to the pons. WGA-Cre (red dots) transports retrogradely (trans-synaptic) to neuronal somata in amygdala, where Cre drives orexin expression (green). Photo B depicts extent of injection of WGA-Cre-mCherry in the vlPAG/LC area three weeks after injection. Photo C depicts neurons containing Cre-driven Orexin expression in the CeA. Figure D summarizes the behavioral data that Cre-driven orexin expression in CeA neurons projecting to the pons significantly suppresses predator odor-induced cataplexy (N=7/each group) (*=p<0.05).
Conclusions
New tools such as optogenetics and pharmacogenetics are helping to refine our understanding of the neural circuitry responsible for wake, non-REM and REM sleep (Weber and Dan, 2016). The sleep disorder, narcolepsy, has revealed that this circuit is destabilized resulting in sleep attacks, excessive sleepiness and cataplexy, when the orexin peptide or its receptors are removed. The orexin gene transfer method is demonstrating how to stabilize the circuit and restore normal sleep-wake behavior.
Highlights.
Narcolepsy is linked to a specific loss of neurons containing the neuropeptide orexin. Narcolepsy has no cure currently.
Orexin gene transfer has been used to rescure cataplexy and sleepiness in narcoleptic animal models.
Diessecting the circuitry linking emotion and muscle atonia is crucial for preventing and treating cataplexy.
Acknowledgments
This work was supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research Development (BLR&D), and NIH grants NS052287, NS079940, NS098541, R01NS096151.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldrich MS. The neurobiology of narcolepsy. TINS. 1991;14:235–239. doi: 10.1016/0166-2236(91)90121-a. [DOI] [PubMed] [Google Scholar]
-
*.Bastianini S, Silvani A, Berteotti C, Lo Martire V, Cohen G, Ohtsu H, Lin JS, Zoccoli G. Histamine Transmission Modulates the Phenotype of Murine Narcolepsy Caused by Orexin Neuron Deficiency. PLoS One. 2015;10:e0140520. doi: 10.1371/journal.pone.0140520. [DOI] [PMC free article] [PubMed] [Google Scholar];
• Mice lacking both orexin and histamine signalling display narcoleptic behavior
• Lack of histamine signalling alone does not result in narcoleptic signs
• Lack of histamine produced obesity
- Blanco-Centurion CLM, Konadhode R, Pelluru D, Shiromani P. Orexin gene transfer into dorsolateral pons decreases cataplexy in orexin knockout mice. Sleep. 2012;1:31–40. doi: 10.5665/sleep.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
*.Blanco-Centurion C, Liu M, Konadhode RP, Zhang X, Pelluru D, van den Pol AN, Shiromani PJ. Optogenetic activation of melanin-concentrating hormone neurons increases non-rapid eye movement and rapid eye movement sleep during the night in rats. Eur J Neurosci. 2016;44:2846–2857. doi: 10.1111/ejn.13410. [DOI] [PMC free article] [PubMed] [Google Scholar];
• Optogenetic activation of MCH neurons at night increased both REM and NREM sleep
• Optogenetic activation of MCH neurons during day cycle increase REM sleep
• MCH neurons drive sleep in mammals
- Blouin AM, Thannickal TC, Worley PF, Baraban JM, Reti IM, Siegel JM. Narp immunostaining of human hypocretin (orexin) neurons: loss in narcolepsy. Neurology. 2005;65:1189–1192. doi: 10.1212/01.wnl.0000175219.01544.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks PL, Peever JH. Unraveling the mechanisms of REM sleep atonia. Sleep. 2008;31:1492–1497. doi: 10.1093/sleep/31.11.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, De Lecea L. Optogenetic investigation of neural circuits in vivo. Trends MolMed. 2011;17:197–206. doi: 10.1016/j.molmed.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Crocker A, Espana RA, Papadopoulou M, Saper CB, Faraco J, Sakurai T, Honda M, Mignot E, Scammell TE. Concomitant loss of dynorphin, NARP, and orexin in narcolepsy. Neurology. 2005;65:1184–1188. doi: 10.1212/01.wnl.0000168173.71940.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
-
**.Hasegawa E, Yanagisawa M, Sakurai T, Mieda M. Orexin neurons suppress narcolepsy via 2 distinct efferent pathways. J Clin Invest. 2014;124:604–616. doi: 10.1172/JCI71017. [DOI] [PMC free article] [PubMed] [Google Scholar];
• Combining optogenetics and chemogenetics to elucidate pontine neurons triggering narcolepsy
• Orexin receptor restoration in dorsal raphe inhibits cataplexy-like episodes
• Orexin receptor restoration in locus coeruleus consolidates fragmented wakefulness
- Hassani OK, Lee MG, Jones BE. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. PNAS. 2009;106:2418–2422. doi: 10.1073/pnas.0811400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalogiannis M, Hsu E, Willie JT, Chemelli RM, Kisanuki YY, Yanagisawa M, Leonard CS. Cholinergic modulation of narcoleptic attacks in double orexin receptor knockout mice. PLoS One. 2011;13:e18697. doi: 10.1371/journal.pone.0018697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor S, Mochizuki T, Lops SN, Ko B, Clain E, Clark E, Yamamoto M, Scammell TE. Orexin gene therapy restores the timing and maintenance of wakefulness in narcoleptic mice. Sleep. 2013;36:1129–1138. doi: 10.5665/sleep.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konadhode RR, Pelluru D, Blanco-Centurion C, Zayachkivsky A, Liu M, Uhde T, Glen WB, Jr, Van den Pol AN, Mulholland PJ, Shiromani PJ. Optogenetic Stimulation of MCH Neurons Increases Sleep. J Neurosci. 2013;33:10257–10263. doi: 10.1523/JNEUROSCI.1225-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Liu M, Blanco-Centurion C, Konadhode R, Begum S, Pelluru D, Gerashchenko D, Sakurai T, Yanagisawa M, van den Pol AN, Shiromani PJ. Orexin gene transfer into zona incerta neurons suppresses muscle paralysis in narcoleptic mice. J Neurosci. 2011;31:6028–6040. doi: 10.1523/JNEUROSCI.6069-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
**.Liu M, Blanco-Centurion C, Konadhode RR, Luan L, Shiromani PJ. Orexin gene transfer into the amygdala suppresses both spontaneous and emotion-induced cataplexy in orexin knockout mice. Eur J Neurosci. 2016;43:681–8. doi: 10.1111/ejn.13158. [DOI] [PMC free article] [PubMed] [Google Scholar];
• Aversive odor triggers cataplexy in narcoleptic mice
• First evidence that orexin gene insertion into amygdala blocks emotion-induced cataplexy
• Brain circuitry links emotion and cataplexy
- Liu M, Thankachan S, Kaur S, Begum S, Blanco-Centurion C, Sakurai T, Yanagisawa M, Neve R, Shiromani PJ. Orexin (hypocretin) gene transfer diminishes narcoleptic sleep behavior in mice. Eur J Neurosci. 2008;28:1382–1393. doi: 10.1111/j.1460-9568.2008.06446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel RJ, Manfredsson FP, Foust KD, Rising A, Reimsnider S, Nash K, Burger C. Recombinant adeno-associated viral vectors as therapeutic agents to treat neurological disorders. Molecular therapy : the journal of the American Society of Gene Therapy. 2006;13:463–483. doi: 10.1016/j.ymthe.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki T, Arrigoni E, Marcus JN, Clark EL, Yamamoto M, Honer M, Borroni E, Lowell BB, Elmquist JK, Scammell TE. Orexin receptor 2 expression in the posterior hypothalamus rescues sleepiness in narcoleptic mice. PNAS. 2011;108:4471–4476. doi: 10.1073/pnas.1012456108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu S, Fujimoto K, Kato S, Mizukami H, Asari S, Ikeguchi K, Kawakami T, Urabe M, Kume A, Sato T, Watanabe E, Ozawa K, Nakano I. A phase I study of aromatic L-amino acid decarboxylase gene therapy for Parkinson’s disease. Molecular therapy : the journal of the American Society of Gene Therapy. 2010;18:1731–1735. doi: 10.1038/mt.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehmig A, Fraefel C, Breakefield XO. Update on herpesvirus amplicon vectors. Molecular therapy : Eur J Neurosci. 2004;10:630–643. doi: 10.1016/j.ymthe.2004.06.641. [DOI] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nature medicine. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
**.Tabuchi S, Tsunematsu T, Black SW, Tominaga M, Maruyama M, Takagi K, Minokoshi Y, Sakurai T, Kilduff TS, Yamanaka A. Conditional ablation of orexin/hypocretin neurons: a new mouse model for the study of narcolepsy and orexin system function. J Neurosci : the official journal of the Society for Neuroscience. 2014;34:6495–6509. doi: 10.1523/JNEUROSCI.0073-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar];
• A new animal model of narcolepsy that uses Tet-On methodology to kill orexin neurons
• Timing of the loss of the orexin neurons mimics human narcolepsy
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski MH, Thal L, Pay M, Salmon DP, U HS, Bakay R, Patel P, Blesch A, Vahlsing HL, Ho G, Tong G, Potkin SG, Fallon J, Hansen L, Mufson EJ, Kordower JH, Gall C, Conner J. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nature medicine. 2005;11:551–555. doi: 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
-
*.Weber F, Dan Y. Circuit-based interrogation of sleep control. Nature. 2016;538:51–59. doi: 10.1038/nature19773. [DOI] [PubMed] [Google Scholar];
• Review paper describing how new neuroscience tools have helped identify the neural circuitry underlying sleep


