Abstract
Purpose
To determine changes in quality of life measures when choroidal neovascularization (CNV) developed in the second eye of patients with initially unilateral neovascular age-related macular degeneration (AMD).
Methods
We analyzed responses to the 39-item National Eye Institute Visual Function Questionnaire (NEI-VFQ), 36-item Short Form Health Survey (SF-36), and Hospital Anxiety and Depression Scale (HADS) at baseline, and prior to and following second-eye CNV diagnosis in 92 participants enrolled in 2 Submacular Surgery Trials (SST). Paired t-tests for sample sizes over 30 and Wilcoxon signed-rank tests for sample sizes less than 30 were performed to compare scores.
Results
CNV development resulted in statistically and clinically significant changes in responses to 20 of 39 NEI-VFQ items, indicating visual function decline during a mean interval of 25 months. Little difference was noted between baseline scores and prior to CNV diagnosis, which averaged 8.9 months duration. Subscales demonstrated a statistically significant decline in general vision, near activities, distance activities, social functioning, role difficulties, dependency, and driving. There were minimal changes in the HADS and SF-36 scales.
Conclusion
CNV development in the second eye had a dramatic effect on visual functioning based on patient responses to the NEI-VFQ questionnaire. Our investigation is believed to be the first study using data collected prospectively to demonstrate vision-related quality of life changes that resulted from development of CNV in AMD patients.
Introduction
Age-related macular degeneration (AMD) is the leading cause of irreversible blindness in the developed world. Worldwide in 2020, an estimated 196 million people will suffer from AMD and 11.3 million people will suffer from advanced AMD [1]. Advanced AMD is defined as the presence of either geographic atrophy (GA) that involves the fovea (center of vision) or choroidal neovascularization (CNV), abnormal growth of blood vessels arising from the choroid. The burden of AMD is expected to rise with the aging of the population, from over 7.3 million cases in the United States in 2000 [2] to an expected 17.8 million cases expected by 2050 [3,4]. Non-neovascular AMD (NNVAMD) accounts for approximately 90% of all cases of AMD, but neovascular AMD (NVAMD) historically has accounted for the majority of visual impairment seen in this disease [5]. This ratio has changed somewhat since approval of anti-vascular endothelial growth factor (anti-VEGF) therapy for NVAMD. Treatment with intravitreal injections of anti-VEGF therapy results in improvement or stabilization of vision in over 90% of NVAMD patients [6]. Even with the profound impact of anti-VEGF therapy, CNV remains the leading cause of blindness due to AMD; furthermore, 7% of eyes treated for NVAMD become legally blind after 2 years [7,8].
Patients with NNVAMD are at risk for sudden progression to NVAMD, which occurs at a rate of 13% within 5 years [9]. Due to the aggressive nature of NVAMD, ophthalmologists regularly screen NNVAMD patients for development of CNV. The visual acuity (VA) at initiation of therapy is the best predictor for final visual acuity outcome at 1 and 2 years after therapy; thus early detection and treatment of NVAMD leads to improved visual prognoses [10]. Investigators of one study have demonstrated that, with early diagnosis and proper monitoring of NVAMD treatment, patients could avoid loss of 5 or more letters of visual acuity in affected eyes at one year after treatment initiation [11].
Deferral of therapy after onset of CNV has a negative effect on visual acuity outcome [12–14] because untreated CNV continues to grow at a mean growth rate of 10–18 μm per day [15,16]. One study showed that a delay in treatment of more than 28 days compared with 28 days or less resulted in a statistically significant greater percent of patients with at least 1 line loss of vision (p = 0.01) [17]. Another study demonstrated that with increasing time between onset of symptoms and initiation of treatment, visual acuities both at presentation and after treatment were markedly low [18]. For patients with mean symptom duration of 18 days, pre-treatment VA was 0.4 Snellen decimal and it improved to 0.49 after 2 anti-VEGF injections. For patients with mean symptom duration of 201 days, pre-treatment VA was 0.09 Snellen and it improved to 0.16 after treatment [18]. This difference in VA remained even after 1 year [19]. Thus, early detection of CNV is critical to optimize the visual outcomes of NVAMD patients.
Quality of life evaluation plays a key role in our understanding of the effect of NVAMD on patients. Finger et al. demonstrated that vision-related quality of life scores improved in patients with vision improvement after initiation of anti-VEGF therapy and decreased when vision declined [20]. The Submacular Surgery Trials (SST) were 3 randomized, multicenter clinical trials sponsored by the National Eye Institute primarily performed to determine whether surgical removal of subfoveal choroidal neovascularization (CNV) and associated hemorrhage in patients with AMD and other causes of CNV stabilized or improved vision and vision-related quality of life measures compared with observation. The SST Group has reported that changes in scores on the National Eye Institute Visual Function Questionnaire (NEI-VFQ) correlated with changes in visual acuity [21] and that participants with bilateral CNV at baseline had substantially worse scores at baseline [22] and during follow-up than those with unilateral CNV [23,24].
The goal of our analysis of the SST data was to investigate the changes in quality of life that occur with the development of CNV in second eyes of patients with NVAMD. We analyzed prospectively collected data from participants with unilateral subfoveal neovascular AMD who enrolled in the Submacular Surgery Trials (SST) and in whose contralateral eye (second or fellow eye) CNV developed during the course of the study to assess whether quality of life scores during the follow up period worsened with CNV development in the second eye.
Methods
The Submacular Surgery Trials (SST) were 3 randomized multicenter clinical trials sponsored by the National Eye Institute registered on ClinicalTrials.gov with identifier NCT00000150. SST participants were followed at scheduled examination and interview times for 2 to 4 years, depending on date of enrollment. We included data only from participants enrolled in the two randomized trials for AMD. The primary inclusion criterion for the analysis population was CNV development in the second eye during follow-up in the SST. The exclusion criteria were (1) CNV in both eyes at the time of SST enrollment and (2) CNV first observed at the last study examination because these patients would not have a subsequent interview from which scores could be compared to a pre-CNV score. The Data and Safety Monitoring Committee and the institutional review boards of the participating centers reviewed and approved the SST prior to initiation of patient enrollment in 1997. The current investigation was approved by the SST Archives Committee; a waiver of consent was granted by the Johns Hopkins University Institutional Review Board (IRB). The SST database and other SST documents, including the SST Manual of Procedures, are available from the Alan Mason Chesney Medical Archives of the Johns Hopkins School of Medicine [25].
SST methods and quality of life interviews have been well-described elsewhere [23,24,26,27]. In brief, participants were 50 years or older with untreated subfoveal CNV secondary to AMD in one eye, the “study eye”. Eligible patients could have CNV in one or both eyes, but only the eye that met defined eligibility criteria was deemed the study eye. Our analysis focuses on the development of CNV in the non-study second eye, or “fellow eye”, of those patients who did not have bilateral CNV at time of SST enrollment and had CNV diagnosed in the second eye during SST follow-up.
SST patients completed telephone interviews during which three quality of life instruments were administered by an interviewer: the 39-item version of the National Eye Institute Visual Function Questionnaire (NEI-VFQ) [28,29], the 36-item Short Form Health Survey (SF-36) [30, 31], and the 14-item Hospital Anxiety and Depression Scale (HADS) [32,33]. All SST participants completed the interview before randomization. Follow-up interviews were scheduled at 6, 12, and 24 months after randomization for all participants and at 36 and 48 months after randomization for participants who enrolled during the first two years of accrual. Clinical examinations were conducted on the same schedule. Interviews were conducted by trained interviewers at the SST Coordinating Center who were masked to the treatment assignment, study eye, and clinical data. For this study we evaluated only data from the interviews conducted at baseline, immediately prior to CNV diagnosis, and immediately following CNV diagnosis.
The NEI-VFQ assesses visual function and its impact on one’s life. The SST interview used a 39-item version of the NEI-VFQ that included the 25-item NEI-VFQ and the appendix of additional questions [34,35]. Item responses are used to create 11 subscales: general vision, near activities, distance activities, social functioning, mental health, role difficulties, dependency, driving, color vision, peripheral vision, and ocular pain. Subscale scores are derived from 1 to 6 items and range from 0 to 100. An NEI-VFQ overall score can be computed and also has a range of 0 to 100.
The SF-36 items are indicators of self-reported physical and mental health. Items are aggregated to form 8 domains; domain scores can be combined to create physical and mental component summary scores that were calibrated by the developers to have mean values of 50 and standard deviations of 10 in the United States population [36].
The HADS is a clinical screening tool for anxiety and depression in outpatients that consists of 14 questions rated 0 to 3, with 7 questions relating to anxiety and 7 for depression. Scores for each scale range from 0 to 21, with patients whose scores are less than or equal to 7 classified as not a case of anxiety or depression, 8 to 10 a doubtful case, and more than 10 a definite case of anxiety or depression. All three questionnaires have been validated in several studies [30–35].
Data Acquisition and Analysis
Of patients with AMD enrolled in the SST, we selected those who had second eyes at risk of CNV development [37]. Among the patients at risk, we identified those in whom CNV developed in the second eye. We excluded from the analysis population those patients in whose second eye CNV had been diagnosed at the last SST examination and thus were unavailable for an interview after CNV diagnosis, to obtain the analysis population.
Data from interviews conducted at three time points were analyzed: baseline at entry to the SST, the scheduled interview immediately prior to CNV diagnosis, and the scheduled interview immediately following CNV diagnosis. Baseline interviews were completed by all participants. A high proportion (more than 90%) of the participants completed interviews at 6, 12, and 24 months after SST enrollment. More than half the participants were eligible for and completed 36-month interviews, but only about one third of the participants were eligible for and completed 48-month interviews.
Patient, study eye, and lesion baseline characteristics and interview data were recorded and included age, gender, race/ethnicity, current occupational status, evidence of CNV on fluorescein angiography (FA), CNV location relative to the fovea, CNV classification (classic versus occult CNV components), other components of the neovascular lesion, greatest diameter of neovascular lesion, visual acuity, and responses to the NEI-VFQ, SF-36, and HADS questionnaires. Visual acuity of each eye was measured separately after careful refraction and correction of refractive error. Best-corrected visual acuity of each eye was measured on a retroilluminated modified Bailey-Lovie chart (“ETDRS chart”) at a distance of either 2.0 meters or 0.5 meter, depending on the level of visual acuity of the eye. Visual acuity scores could range from 0 to 100 and were based on the participant’s distance from the chart and the number of letters read correctly on each line of the chart [35].
For each questionnaire measure (item, subscale, component summary score or overall score), two separate comparisons were made. One comparison was between the baseline scores and the scores immediately prior to CNV diagnosis to determine whether there were changes during the interval before CNV developed in the second eye that possibly represented aging or changes in other health conditions or that represented changes predictive of CNV. The second comparison was between the scores immediately prior to CNV diagnosis and the scores post CNV diagnosis.
Subgroup analysis was performed for patients based on the location relative to the fovea of second eye CNV at time of diagnosis, i.e., subfoveal, probably subfoveal or juxtafoveal, or extrafoveal. Subfoveal lesions are defined as those that extend under the center of the foveal avascular zone (FAZ). Juxtafoveal lesions are those that extend as close as 1 and 199 μm from the FAZ center, and extrafoveal lesions are located more than 200 μm from the FAZ center.
Statistical Methods
Each comparison was performed using a paired t-test for sample sizes of at least 30, where the distribution of scores could be assumed to be normally distributed. The Wilcoxon signed-rank test was performed when sample sizes were less than 30 or when distributions were observed to deviate markedly from normality. For each comparison, an unadjusted (nominal) p-value was calculated. The Bonferroni method was used to adjust the p-values for multiple comparisons of the same type within an interview instrument. Bonferroni-adjusted p-values less than 0.05 were judged to be statistically significant.
Multivariable linear regression models were used to analyze NEI-VFQ outcomes adjusting for change in SF-36 physical and mental health component summary scores as a proxy for change in medical co-morbidities [38,39]. For all participants in the analysis population, the model for each NEI-VFQ outcome included time (baseline versus before CNV diagnosis or before versus after CNV diagnosis) and the change in each SF-36 summary component score as explanatory variables. For subgroup analyses, the model included subgroup, time, a subgroup-by-time interaction term and the change in each SF-36 summary component score. Using a single model with an interaction term allowed comparison of subgroups for each outcome. The Bonferroni method again was used to adjust for multiple similar comparisons. All statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, NC, USA).
For each VFQ subscale or SF-36 component summary score for which the unadjusted p-value for the prior to and following CNV comparison was not statistically significant, the power was calculated using a type I error of 0.05 and the observed mean difference, standard deviation of the difference, and sample size. A power of 80% or higher was considered acceptable.
Results
Description of analysis population
Of the 790 participants with AMD who enrolled in the SST, 380 had unilateral CNV at baseline and therefore were at risk of CNV development in the second eye. Of the 380 patients at risk, CNV developed in the second eye of 98 patients during SST follow up.
For 26 of the patients, the baseline interview was the only one available prior to CNV diagnosis. Thus of the 92 patients eligible for inclusion, 66 patients completed interviews from baseline to prior to CNV diagnosis. A total of 75 patients had interviews before and after CNV diagnosis and were included in this second comparison.
Baseline demographic and visual acuity characteristics of all 98 patients in whom CNV developed in the second eye, the 92 patients with new second eye CNV diagnosed before the end of the SST follow-up, and the 3 subgroups based on incident CNV location are summarized in Table 1. The mean age was 76 years (standard deviation 5.7 years) and most patients (90%) had retired from work. Although slightly more women experienced second eye CNV, some small subgroups contained more men. All but one patient in whose second eye CNV developed were Caucasian (99%). For all 92, the mean number of months from baseline interviews to those prior to CNV diagnosis was 8.9 months. The mean number of months from baseline interviews to those following CNV diagnosis was 25 months. For the 66 patients with CNV for whom the interview prior to CNV diagnosis was not baseline, the mean number of months from baseline interviews to those prior to CNV diagnosis was 12.5. For the 77 patients with CNV with interviews after CNV diagnosis, the mean number of months from baseline interviews to those following CNV diagnosis was 25.0 months, and the mean number of months from before to after diagnosis was 17.8.
Table 1.
Baseline Sociodemographic and Visual Acuity Characteristics of SST Participants in Whom CNV Developed in the Second Eye
| Characteristic | All CNV (n=98) |
CNV diagnosed before last SST follow-up | |||
|---|---|---|---|---|---|
| Total CNV (n=92) |
Subfoveal CNV (n=42) |
Probably subfoveal or juxtafoveal CNV (n=17) |
Extrafoveal CNV (n=30) |
||
| Age, years, mean (std dev) | 76.3 (5.7) | 76.3 (5.8) | 76.2 (5.7) | 76.4 (6.3) | 76.5 (6.1) |
| Gender, n (%) | |||||
| Men | 46 (47) | 44 (48) | 14 (33) | 12 (71) | 17 (57) |
| Women | 52 (53) | 48 (52) | 28 (67) | 5 (29) | 13 (43) |
| Current occupational status, n (%) | |||||
| Retired | 88 (90) | 83 (90) | 36 (86) | 17 (100) | 27 (90) |
| Employed | 8 (8) | 8 (9) | 6 (14) | 0 (0) | 2 (7) |
| Other | 2 (2) | 1 (1) | 0 (0) | 0 (0) | 1 (3) |
| Visual acuity at baseline | |||||
| Fellow eye | |||||
| Visual acuity score, mean (std dev) | 93.3 (7.1) | 93.2 (7.2) | 92.1 (6.6) | 94.1 (8.8) | 93.7 (7.4) |
| Snellen equivalent, n (%) | |||||
| 20/20 or better | 39 (40) | 36 (39) | 11 (26) | 8 (47) | 16 (53) |
| 20/25–20/40 | 50 (51) | 47 (51) | 28 (67) | 7 (41) | 10 (33) |
| 20/50–20/80 | 8 (8) | 8 (9) | 3 (7) | 1 (6) | 4 (13) |
| 20/100–20/160 | 1 (1) | 1 (1) | 0 (0) | 1 (6) | 0 (0) |
| 20/200 or worse | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Study eye | |||||
| Visual acuity score, mean (std dev) | 44.6 (17.5) | 44.8 (17.6) | 46.4 (16.4) | 45.8 (19.1) | 41.5 (18.6) |
| Snellen equivalent, n (%) | |||||
| 20/50–20/80 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 20/100–20/160 | 45 (46) | 43 (47) | 21 (50) | 8 (47) | 12 (40) |
| 20/200 or worse | 53 (54) | 49 (53) | 21 (50) | 9 (53) | 18 (60) |
Abbreviations: SST = Submacular Surgery Trials; CNV = choroidal neovascularization; st dev = standard deviation
As expected, the visual acuity in the SST study eyes, all of which had CNV, was poor at baseline. Also as expected, visual acuity in the fellow eyes free of CNV at baseline was good, with 91% of patients having visual acuity of 20/40 or better.
Change in visual acuity relative to CNV diagnosis
There was minimal change in visual acuity in the fellow eyes prior to CNV diagnosis with 90% of patients having 20/40 or better visual acuity. However, after CNV diagnosis, visual acuity dramatically declined in the fellow eye, with only 29% of patients having 20/40 or better vision and 32% with 20/200 or worse vision (Table 2).
Table 2.
Visual Acuity During Follow-up of SST Participants in Whom CNV Developed in the Second Eye
| Characteristic | All CNV | CNV diagnosed before last SST follow-up | |||
|---|---|---|---|---|---|
| Total CNV | Subfoveal CNV | Probably subfoveal or juxtafoveal CNV | Extrafoveal CNV | ||
| Visual acuity prior to CNV diagnosis | (n=93) | (n=90) | (n=41) | (n=17) | (n=29) |
| Fellow eye | |||||
| Visual acuity score, mean (std dev) | 93.2 (6.8) | 93.1 (6.9) | 91.1 (7.7) | 95.0 (6.0) | 94.6 (5.9) |
| Snellen equivalent, n (%) | |||||
| 20/20 or better | 33 (35) | 32 (36) | 9 (22) | 8 (47) | 15 (52) |
| 20/25–20/40 | 51 (55) | 49 (54) | 26 (63) | 8 (47) | 12 (41) |
| 20/50–20/80 | 9 (10) | 9 (10) | 6 (15) | 1 (6) | 2 (7) |
| 20/100–20/160 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 20/200 or worse | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Study eye | |||||
| Visual acuity score, mean (std dev) | 35.9 (16.6) | 35.9 (16.7) | 37.5 (18.0) | 34.9 (17.1) | 34.5 (15.4) |
| Snellen equivalent, n (%) | |||||
| 20/20 or better | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 20/25–20/40 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 20/50–20/80 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 20/100–20/160 | 17 (18) | 17 (19) | 10 (24) | 3 (18) | 4 (14) |
| 20/200 or worse | 76 (82) | 73 (81) | 31 (76) | 14 (82) | 25 (86) |
| Characteristic | All CNV | CNV with diagnosis before 48 months | |||
| Total CNV | Subfoveal CNV | Probably subfoveal or juxtafoveal CNV | Extrafoveal CNV | ||
| Visual acuity following CNV diagnosis | (n=69) | (n=69) | (n=29) | (n=14) | (n=26) |
| Fellow eye | |||||
| Visual acuity score, mean (std dev) | 64.8 (22.7) | 64.8 (22.7) | 60.6 (21.7) | 64.8 (26.3) | 69.5 (21.6) |
| Snellen equivalent, n (%) | |||||
| 20/20 or better | 6 (9) | 6 (9) | 1 (3) | 1 (7) | 4 (15) |
| 20/25–20/40 | 14 (20) | 14 (20) | 6 (21) | 3 (21) | 5 (19) |
| 20/50–20/80 | 12 (17) | 12 (17) | 4 (14) | 4 (29) | 4 (15) |
| 20/100–20/160 | 15 (22) | 15 (22) | 5 (17) | 2 (14) | 8 (31) |
| 20/200 or worse | 22 (32) | 22 (32) | 13 (45) | 4 (29) | 5 (19) |
| Study eye | |||||
| Visual acuity score, mean (std dev) | 37.3 (16.2) | 37.3 (16.2) | 39.6 (16.2) | 36.0 (19.5) | 35.5 (14.8) |
| Snellen equivalent, n (%) | |||||
| 20/20 or better | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 20/25–20/40 | 1 (1) | 1 (1) | 1 (3) | 0 (0) | 0 (0) |
| 20/50–20/80 | 2 (3) | 2 (3) | 0 (0) | 1 (7) | 1 (4) |
| 20/100–20/160 | 9 (13) | 9 (13) | 6 (21) | 2 (14) | 1 (4) |
| 20/200 or worse | 57 (83) | 57 (83) | 22 (76) | 11 (79) | 24 (92) |
Abbreviations: SST = Submacular Surgery Trials; CNV = choroidal neovascularization; st dev = standard deviation
Characteristics of incident CNV are summarized in Table 3. Of the 92 patients with incident second eye CNV diagnosed before the 48-month study visit, the neovascular lesion of 42 was subfoveal, 17 probably subfoveal or juxtafoveal, and 30 extrafoveal. Most patients (88%) had primarily occult CNV with a mean dimension of the lesion of 3663 μm The subfoveal CNV subgroup had larger lesions with a mean of 4632 μm whereas the extrafoveal CNV subgroup had lesions with a mean diameter of 2509 μm.
Table 3.
Characteristics of CNV that Developed in the Second Eye of SST Participants
| CNV with diagnosis before last SST follow-up | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All CNV | Total CNV | Subfoveal CNV | Probably subfoveal or juxtafoveal CNV | Extrafoveal CNV | ||||||
| Characteristic | N | Statistic | N | Statistic | N | Statistic | N | Statistic | N | Statistic |
| CNV location, n (%) | 94 | 89 | 42 | 17 | 30 | |||||
| Subfoveal | 44 (47) | 42 (47) | 42 (100) | 0 (0) | 0 (0) | |||||
| Probably subfoveal | 8 (9) | 7 (8) | 0 (0) | 7 (41) | 0 (0) | |||||
| Juxtafoveal | 11 (12) | 10 (11) | 0 (0) | 10 (59) | 0 (0) | |||||
| Extrafoveal | 31 (33) | 30 (34) | 0 (0) | 0 (0) | 30 (100) | |||||
| Classic CNV as percent of new lesion, n (%) | 96 | 90 | 42 | 16 | 30 | |||||
| No classic CNV | 69 (72) | 67 (74) | 26 (62) | 14 (88) | 26 (87) | |||||
| Questionable classic CNV | 1 (1) | 1 (1) | 1 (2) | 0 (0) | 0 (0) | |||||
| < 50% classic | 14 (15) | 12 (13) | 9 (21) | 1 (6) | 1 (3) | |||||
| ≥ 50% classic | 12 (12) | 10 (11) | 6 (14) | 1 (6) | 3 (10) | |||||
| Occult CNV present in new lesion, n (%) | 95 | 89 | 42 | 16 | 30 | |||||
| Yes | 81 (85) | 78 (88) | 36 (86) | 15 (94) | 27 (90) | |||||
| Questionable | 1 (1) | 1 (1) | 1 (2) | 0 (0) | 0 (0) | |||||
| No | 13 (14) | 10 (11) | 5 (12) | 1 (6) | 3 (10) | |||||
| Greatest diameter of lesion, μm, mean (std dev) | 92 | 3673 (1870) | 86 | 3663 (1824) | 42 | 4632 (1826) | 16 | 3141 (1337) | 28 | 2509 (1189) |
Abbreviations: SST = Submacular Surgery Trials; CNV = choroidal neovascularization; std dev = standard deviation
National Eye Institute Visual Function Questionnaire (NEI-VFQ)
Comparison of baseline responses with those prior to CNV diagnosis demonstrated no statistically important change over time in any individual NEI-VFQ item (Supplemental Table 1) or in any subscale score from multivariable linear regression models that used changes in SF-36 physical and mental health component summary scores as a proxy for change in medical comorbidities (Table 4). However, comparison of scores before and after CNV diagnosis in the second eye demonstrated statistically and clinically significant worse vision-related function as reflected in responses to 20 of 39 individual items after adjusting for change in scores on the two SF-36 component summary scales and accounting for multiple comparisons (Supplemental Table 1). These items included limitation of activities due to eye sight (staying at home due to sight, difficulty going out to events, accomplishing less due to eyesight, relying too much on others due to poor sight, being limited in ability to do things, not driving, and not leaving home alone due to sight), perception of changes in vision (rating of eyesight) and difficulty performing activities of daily living (finding items on a crowded shelf, figuring out bills, recognizing people, participating in sports, watching TV, and reading street signs, small print, and newspapers).
Table 4.
NEI-VFQ Scores for SST Participants and 3 Subgroups (Subfoveal, Probably Subfoveal or Juxtafoveal, and Extrafoveal CNV) in Whom CNV Developed in the Second Eye Before the last SST examination, Comparing Scores from (1) Baseline to prior to CNV diagnosis, and (2) Prior to and following CNV diagnosis
| Comparison of mean NEI-VFQ scores adjusted for Changes in SF-36 component scores | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline to prior to CNV diagnosis | Prior to and following diagnosis of CNV | ||||||||||
| Subgroup and NEI-VFQ scale | N | Baseline | Prior | P-value* | Adjusted p-value† | N | Prior | Following | P-value* | Adjusted p-value† | Power for NSR |
| Total CNV | |||||||||||
| NEI-VFQ subscale | |||||||||||
| General vision | 66 | 60.9 | 60.0 | 0.72 | 1.00 | 75 | 60.9 | 48.1 | < 0.0001 | < 0.001 | |
| Near activities | 66 | 68.2 | 67.7 | 0.86 | 1.00 | 75 | 68.2 | 46.8 | < 0.0001 | < 0.001 | |
| Distance activities | 66 | 72.3 | 71.1 | 0.69 | 1.00 | 75 | 71.8 | 53.8 | < 0.0001 | < 0.001 | |
| Social functioning | 66 | 88.5 | 85.9 | 0.29 | 1.00 | 75 | 85.6 | 75.1 | 0.0004 | 0.005 | |
| Mental health | 66 | 68.4 | 67.4 | 0.79 | 1.00 | 75 | 66.8 | 57.8 | 0.009 | 0.11 | |
| Role difficulties | 66 | 74.3 | 71.9 | 0.50 | 1.00 | 75 | 70.8 | 52.5 | < 0.0001 | < 0.001 | |
| Dependency | 66 | 84.1 | 78.8 | 0.17 | 1.00 | 75 | 80.8 | 60.7 | < 0.0001 | < 0.001 | |
| Driving | 59 | 63.0 | 58.9 | 0.37 | 1.00 | 66 | 59.4 | 20.2 | < 0.0001 | < 0.001 | |
| Color vision | 65 | 87.5 | 89.8 | 0.46 | 1.00 | 74 | 86.8 | 78.4 | 0.03 | 0.33 | |
| Peripheral vision | 66 | 77.3 | 79.5 | 0.56 | 1.00 | 75 | 77.0 | 72.0 | 0.15 | 1.00 | 0.56 |
| Ocular pain | 66 | 84.7 | 89.9 | 0.06 | 1.00 | 75 | 84.6 | 88.4 | 0.20 | 1.00 | 0.36 |
| VFQ-39 overall score | 66 | 75.4 | 74.7 | 0.76 | — | 75 | 74.0 | 60.0 | < 0.0001 | — | |
| Subfoveal CNV | |||||||||||
| NEI-VFQ subscale | |||||||||||
| General vision | 34 | 60.7 | 59.3 | 0.68 | 1.00 | 30 | 61.3 | 48.7 | 0.004 | 0.04 | |
| Near activities | 34 | 66.3 | 64.9 | 0.72 | 1.00 | 30 | 66.1 | 43.2 | < 0.0001 | < 0.001 | |
| Distance activities | 34 | 70.4 | 68.3 | 0.62 | 1.00 | 30 | 70.3 | 49.7 | < 0.0001 | < 0.001 | |
| Social functioning | 34 | 87.6 | 87.0 | 0.87 | 1.00 | 30 | 88.7 | 76.3 | 0.007 | 0.09 | |
| Mental health | 34 | 66.0 | 64.8 | 0.82 | 1.00 | 30 | 65.6 | 50.9 | 0.006 | 0.07 | |
| Role difficulties | 34 | 71.3 | 67.7 | 0.45 | 1.00 | 30 | 68.6 | 52.5 | 0.003 | 0.03 | |
| Dependency | 34 | 83.5 | 74.5 | 0.10 | 1.00 | 30 | 77.9 | 57.5 | 0.003 | 0.03 | |
| Driving | 28 | 64.9 | 51.8 | 0.05 | 1.00 | 25 | 53.0 | 12.6 | < 0.0001 | < 0.001 | |
| Color vision | 34 | 88.8 | 88.0 | 0.86 | 1.00 | 30 | 88.1 | 79.8 | 0.16 | 1.00 | 0.90 |
| Peripheral vision | 34 | 70.0 | 80.3 | 0.05 | 0.82 | 30 | 79.0 | 76.2 | 0.61 | 1.00 | 0.16 |
| Ocular pain | 34 | 82.6 | 88.7 | 0.12 | 1.00 | 30 | 85.7 | 88.8 | 0.51 | 1.00 | 0.14 |
| VFQ-39 overall score | 34 | 73.9 | 72.7 | 0.72 | — | 30 | 73.3 | 58.8 | 0.0002 | — | |
| Probably subfoveal or juxtafoveal CNV | |||||||||||
| NEI-VFQ subscale | |||||||||||
| General vision | 9 | 59.6 | 54.0 | 0.40 | 1.00 | 15 | 54.9 | 37.7 | 0.005 | 0.06 | |
| Near activities | 9 | 75.1 | 63.1 | 0.11 | 1.00 | 15 | 67.6 | 44.0 | 0.0004 | 0.005 | |
| Distance activities | 9 | 77.3 | 69.2 | 0.31 | 1.00 | 15 | 73.2 | 48.5 | 0.0005 | 0.006 | |
| Social functioning | 9 | 91.6 | 89.3 | 0.73 | 1.00 | 15 | 87.1 | 71.7 | 0.02 | 0.21 | |
| Mental health | 9 | 71.2 | 68.3 | 0.77 | 1.00 | 15 | 71.8 | 62.3 | 0.20 | 1.00 | 0.43 |
| Role difficulties | 9 | 79.1 | 69.3 | 0.30 | 1.00 | 15 | 70.3 | 44.5 | 0.0008 | 0.03 | |
| Dependency | 9 | 90.8 | 83.0 | 0.46 | 1.00 | 15 | 83.5 | 65.1 | 0.05 | 0.66 | |
| Driving | 9 | 65.9 | 69.3 | 0.77 | 1.00 | 13 | 63.7 | 13.9 | < 0.0001 | < 0.001 | |
| Color vision | 9 | 87.8 | 91.9 | 0.63 | 1.00 | 15 | 87.8 | 83.1 | 0.58 | 1.00 | 0.26 |
| Peripheral vision | 9 | 91.8 | 83.0 | 0.38 | 0.82 | 15 | 79.1 | 62.0 | 0.03 | 0.38 | |
| Ocular pain | 9 | 87.4 | 94.4 | 0.35 | 1.00 | 15 | 82.2 | 88.3 | 0.36 | 1.00 | 0.41 |
| VFQ-39 overall score | 9 | 79.8 | 76.0 | 0.54 | — | 15 | 74.9 | 57.3 | 0.001 | — | |
| Extrafoveal CNV | |||||||||||
| NEI-VFQ subscale | |||||||||||
| General vision | 20 | 60.5 | 62.4 | 0.67 | 1.00 | 28 | 61.7 | 52.4 | 0.04 | 0.44 | |
| Near activities | 20 | 67.8 | 73.2 | 0.29 | 1.00 | 28 | 70.5 | 51.4 | 0.0001 | 0.001 | |
| Distance activities | 20 | 72.5 | 74.8 | 0.67 | 1.00 | 28 | 72.4 | 60.2 | 0.02 | 0.70 | |
| Social functioning | 20 | 88.0 | 81.6 | 0.16 | 1.00 | 28 | 81.6 | 75.3 | 0.19 | 1.00 | 0.37 |
| Mental health | 20 | 71.0 | 70.9 | 0.98 | 1.00 | 28 | 66.5 | 62.5 | 0.46 | 1.00 | 0.29 |
| Role difficulties | 20 | 75.8 | 77.7 | 0.76 | 1.00 | 28 | 72.4 | 55.7 | 0.003 | 0.04 | |
| Dependency | 20 | 81.5 | 81.3 | 0.97 | 1.00 | 28 | 81.8 | 60.2 | 0.002 | 0.03 | |
| Driving | 20 | 57.3 | 62.4 | 0.50 | 1.00 | 27 | 63.8 | 30.0 | < 0.0001 | < 0.001 | |
| Color vision | 19 | 85.9 | 91.4 | 0.36 | 1.00 | 27 | 85.7 | 74.5 | 0.07 | 0.88 | 0.58 |
| Peripheral vision | 20 | 82.4 | 75.9 | 0.34 | 1.00 | 28 | 73.8 | 73.6 | 0.97 | 1.00 | 0.03 |
| Ocular pain | 20 | 86.8 | 89.6 | 0.57 | 1.00 | 28 | 83.2 | 87.5 | 0.39 | 1.00 | 0.15 |
| VFQ-39 overall score | 20 | 75.4 | 76.2 | 0.86 | — | 28 | 73.7 | 62.1 | 0.003 | — | |
Multivariable linear regression model
Bonferroni adjustment for multiple comparisons and for associated changes in physical and mental health component summaries from the SF-36 as a proxy for changes in medical comorbidities
Bold font indicates statistical significance (p value < 0.05)
Abbreviations: NEI-VFQ = National Eye Institute Visual Function Questionnaire; SST = Submacular Surgery Trials; CNV = choroidal neovascularization; SF-36 = 36-item Short Form Health Survey; NSR = non-significant results
Four of the 11 NEI-VFQ subscales demonstrated no important effect of CNV development in the second eye: ocular pain, mental health, color vision, and peripheral vision. However, there were statistically and clinically significant declines in the other 7 subscales and the overall score (Table 4).
Subgroup analysis was performed based on incident CNV location (subfoveal, extrafoveal, and probably subfoveal or juxtafoveal). Subfoveal CNV resulted in declines in scores for 6 NEI-VFQ subscales compared to declines on 4 subscales for incident CNV in other locations at time of diagnosis (Table 4). For each VFQ subscale or SF-36 component summary score in Tables 4 and 5 for which the unadjusted p-value for the before and after comparison was not statistically significant, power using a type I error of 0.05 and the observed mean difference, standard deviation of the difference, and sample size was computed. Power ranged from 0.03 to 0.58.
Table 5.
SF-36 Component Summary Scores for SST Participants in Whom CNV Developed in the Second Eye before the last SST examination, Comparing Scores from (1) Baseline to prior to CNV diagnosis, and (2) Prior to and following CNV diagnosis
| Comparison of mean summary scores | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline to prior to CNV diagnosis | Prior to and following CNV diagnosis | ||||||||||
| Subgroup and SF-36 summary scale | N | Basel ine | Prior | P-value* | Adjusted p-value† | N | Prior | Following | P-value* | Adjusted p-value† | Power for NSR |
| Total CNV | 66 | 75 | |||||||||
| Physical component summary | 65.3 | 56.7 | .006 | 0.01 | 58.7 | 58.7 | 1.00 | 1.00 | 0.03 | ||
| Mental component summary | 84.4 | 85.8 | 0.53 | 1.00 | 88.6 | 80.4 | .0003 | 0.0006 | |||
| Subfoveal CNV | 34 | 30 | |||||||||
| Physical component summary | 65.1 | 57.7 | 0.11 | 0.22 | 58.8 | 57.6 | 0.79 | 1.00 | 0.05 | ||
| Mental component summary | 81.6 | 81.8 | 0.97 | 1.00 | 85.1 | 78.3 | 0.07 | 0.14 | 0.46 | ||
| Probably subfoveal or juxtafoveal CNV | 9 | 15 | |||||||||
| Physical component summary | 71.6 | 63.4 | 0.30 | 0.60 | 70.4 | 59.9 | 0.15 | 0.30 | 0.29 | ||
| Mental component summary | 94.8 | 95.5 | 1.00 | 1.00 | 93.0 | 88.2 | 0.39 | 0.78 | |||
| Extrafoveal CNV | 20 | 28 | |||||||||
| Physical component summary | 61.7 | 51.2 | 0.14 | 0.28 | 52.6 | 58.2 | 0.31 | 0.62 | 0.27 | ||
| Mental component summary | 84.3 | 88.1 | 0.22 | 0.44 | 89.8 | 80.2 | 0.002 | 0.004 | |||
Paired t-test
Bonferroni adjustment for multiple comparisons
Bold font indicates statistical significance (p value < 0.05)
Abbreviations:SF-36 = 36-item Short Form Health Survey; SST = Submacular Surgery Trials; CNV = choroidal neovascularization; NSR = non-significant results
Hospital Anxiety and Depression Scale (HADS)
Among all participants diagnosed with CNV in the second eye and within subgroups based on CNV location, none of the HADS individual item or subscale responses demonstrated a clinically or statistically significant difference between either the baseline interview and the interview prior to CNV diagnosis or before and after CNV diagnosis (Supplemental Tables 2 and 3).
SF-36 Short Form Health Survey
Similarly, the responses to all SF-36 items from participants with incident second eye CNV demonstrated no clinically or statistically significant difference, either from baseline to prior to CNV diagnosis or from before to after CNV diagnosis, either overall (Supplemental Table 4) or within any subgroup (Supplemental Table 5). From baseline to prior to CNV diagnosis, there was an overall statistically significant decline in the physical component summary scores (Table 5), suggesting that with aging of the patients there was a decline in physical activity level, but there was no decline in the physical component summary scores with the diagnosis of CNV. Conversely, the mental component summary scores did not change substantially from baseline to the interview prior to CNV diagnosis, but declined to a statistically significant degree from prior to and following CNV diagnosis. This change suggests that the diagnosis of CNV in the second eye had a negative effect on the mental health of affected patients although this was not reflected on the mental health subscale of the NEI-VFQ.
Discussion
We analyzed changes in self-reported visual function and mental and physical health among 92 participants with unilateral neovascular AMD who enrolled in the SST and in whose second eye CNV developed before the final SST follow-up examination. Interview results were available for comparison of responses from the baseline interview to before CNV diagnosis for 66 patients and from before and after CNV development for 75 patients. Development of CNV in the second eye resulted in a significant effect on visual functioning of these patients as evidenced by the substantial change in the NEI-VFQ scores before and after CNV development. Scores for 20 of 39 individual items and 7 of 11 subscales of the NEI-VFQ and the overall NEI-VFQ scores declined by statistically and clinically significant degrees with CNV development in the second eye. Declines in scores were 10 points or more for all 7 subscales and the overall score and greater than 20 points for 3 subscales.
Patients in the analysis population initially had good quality of life scores, likely due, at least in part, to visual acuity of 20/40 or better in 91% of eyes free of CNV at baseline. However, CNV development corresponded to a dramatic decline in visual acuity; only 29% of patients had visual acuity of 20/40 or better after CNV was diagnosed. Thus, new sequential bilateral visual impairment likely contributed substantially to the decline in quality of life scores.
As expected, patients with incident subfoveal CNV in the second eye during follow-up had larger declines in NEI-VFQ scores than those with CNV newly observed in locations that did not involve the fovea, but all subgroups based on location of CNV demonstrated changes in scores on the near activities, role difficulties, and driving subscales. To determine whether there was significant fluctuation in scores over time in the absence of CNV in second eyes, we compared interview scores from baseline to immediately prior to CNV diagnosis; no statistically significant difference in any item or subscale score of the NEI-VFQ differed between these two interview times. Our finding of no change in scores prior to CNV diagnosis supports our conclusion that CNV development contributed to these dramatic changes in perceived ability to function visually. After adjustment of scores for many covariates and Bonferroni adjustment to the p-values, the magnitude of the changes remained statistically significant and reflect the effect that new bilateral CNV and the accompanying change in visual acuity of the second eye have on patients.
The majority of patients in the SST and in the analysis population were elderly. Limited data were collected regarding a patients’ level of activity and ability to function in various ways. Few patients were employed with an income; 90% of the patients were retired. There was no difference in the scores on either of the two HADS subscales either from baseline to prior to CNV diagnosis or before and after CNV diagnosis. The incidence of CNV in fellow eyes at risk was 26% (98 of 380), which is within the range observed in other prospective studies of patients with AMD and unilateral CNV. For example, in the randomized trials conducted by the Macular Photocoagulation Study Group, the 4-year incidence of CNV was 22% among patients with extrafoveal CNV in the study eye [40] and 36% among patients with subfoveal or juxtafoveal CNV in the study eye at baseline [41].
We observed a decline from baseline to prior to CNV in the physical component summary scores from the SF-36, suggesting that aging of the patients resulted in a decline in their physical ability. We interpret the decline in the mental component summary scores from the SF-36 prior to and following CNV diagnosis as indicating that the CNV diagnosis had psychological implications for patients. While the mental component summary score as measured by the SF-36 was affected, the mental health NEI-VFQ subscale did not show a similar effect. The NEI-VFQ mental health subscale measures different aspects of mental health compared to the SF-36 mental health summary component as reported by others [42]. It is unclear why the HADS scores for anxiety and depression were unaffected by the CNV diagnosis in the second eye. The HADS items and subscales may not be intended to detect mild symptoms of anxiety or depression. We conclude from our experience that generic patient reported outcome measures (PROMs) are not be as sensitive to diagnosis of new vision-threatening ocular conditions or changes in visual acuity as instruments specific to vision-related quality of life, such as the NEI-VFQ.
We recognize limitations to our investigation. We conducted an unplanned post-hoc analysis of the SST dataset. The applicability of the results to patients with similar vision in both eyes is likely limited since all patients in our analysis population already had CNV in one eye at baseline. Patients may not notice a loss of vision in one affected eye as long as the other eye sees well. However, the Age-Related Eye Disease Study (AREDS) investigators observed decreases of similar magnitude on the same subscales of the NEI-VFQ for patients who progressed to advanced AMD [43]. Another limitation is that the sample size was limited to 92 patients, predominantly (99%) Caucasian, residing in the United States. Visual function questions can be culture and ethnicity dependent; the applicability of our findings in different nations, ethnicities, and cultural groups could not be evaluated. In addition, these deliberately subjective questionnaires were completed by patients after diagnosis of CNV in their first eye; thus it is unclear how much patients would notice without the CNV diagnosis in the first eye. Also, we did not investigate the presence or progression of atrophy in fellow eyes. The SST was performed when available treatments for NVAMD were laser photocoagulation and photodynamic therapy (PDT). Given the significant improvement in treatment with anti-VEGF therapy, the outcomes have improved significantly for patients which could affect patient’s response to CNV diagnosis, particularly for mental health evaluations. A large, prospective cohort study of AMD patients with quality of life evaluation over several years would be a good way to evaluate these changes in CNV development given the rare occurrence of this event.
To our knowledge, this is the first study to demonstrate dramatic vision related quality of life changes associated with CNV development in second eyes of patients with AMD. As all patients already had CNV in one eye at baseline, i.e., the study eye randomized in the SST, in more than 95% of the patients the second eye was the better seeing eye at baseline [24]. Regardless of the location of incident CNV relative to the fovea, patients demonstrated increased difficulty after CNV development in near activities, role difficulties, and driving.
In the randomized trials that demonstrated the effectiveness of intravitreal injections of anti-VEGF agents, patients and eyes were examined and affected eyes were treated at 4-week or 8-week intervals [44]. Thus, CNV-free fellow eyes also were examined at the same intervals and treated as elected by ophthalmologists and patients. More recently, various treatment and followup schedules have been proposed and adopted by some ophthalmologists. These schedules result in less frequent examination of eyes under treatment and fellow eyes. Various methods for monitoring patients for development of CNV in second eyes at risk as well as interim progression of CNV in eyes under treatment have been proposed.
Given the results from our study that scores on validated vision-related quality of life questionnaires change dramatically with CNV development in second eyes, we can envision establishing in-person or automated systems for regular, frequent monitoring of patients for changes in responses to a small set of questions, such as those that contribute to the near activities, role difficulties, and driving subscales, or even one or two items from the general vision subscale, of the NEI-VFQ. Large changes in scores between scheduled examinations would prompt immediate examination and evaluation of the need for treatment of either eye with the twin goals of preserving as much vision and vision-related functional ability as possible. Furthermore, patient responses to items on such questionnaires may allow physicians to develop better understanding of patients’ perceptions of the effects of the CNV development and loss of visual acuity on patients’ daily lives.
Supplementary Material
Figure 1.
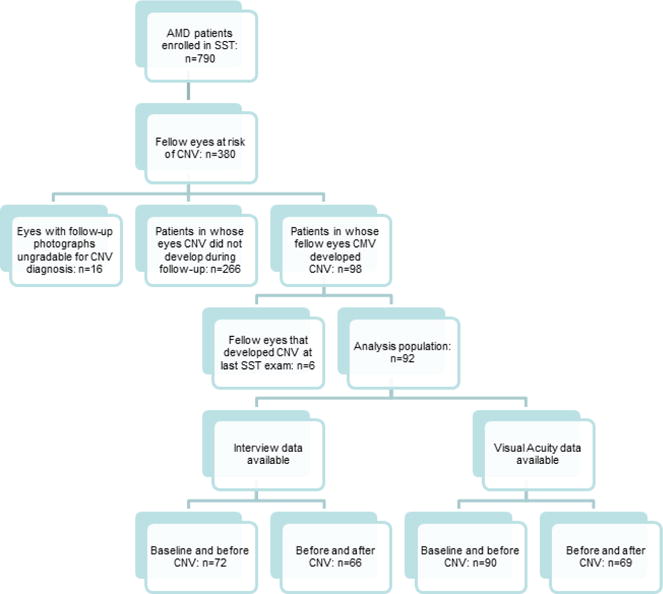
Flow diagram demonstrating the inclusion of patients in the analysis population. A total of 790 patients with choroidal neovascularization (CNV) secondary to age-related macular degeneration enrolled in the Submacular Surgery Trials. Of 380 SST participants without bilateral CNV at time of SST enrollment, 98 developed CNV in the second eye; of whom 92 were included in the analysis population after 6 participants were excluded due to CNV diagnosis at the last SST follow-up examination and thus inability to assess visual function after CNV diagnosis.
Acknowledgments
Funding was provided in part by the Heed Ophthalmic Foundation Fellows Grant (YMP) and the National Eye Institute Michigan Vision Clinician-Scientist Development Program 4K12EY022299-4 (YMP). The authors also received support from an unrestricted grant to the Wilmer Eye Institute from Research to Prevent Blindness, New York, New York. All research was performed in accordance with the Declaration of Helsinki and all local, regional, and national laws. The authors would like to thank Judith E. Goldstein, O.D., for careful review of the manuscript.
Footnotes
The findings in this manuscript were presented in part at the American Society of Retina Specialists 33rd Annual Meeting in Vienna, Austria, July 10–14, 2015.
Conflict of Interest:
The authors declare that they have no conflict of interest.
References
- 1.Wong WL, Su X, Li X, Chueng CCM, Klein R, Cheng CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. The Lancet Global Health. 2014;2(2):e106–16. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 2.Friedman DS, O’Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, et al. Prevalence of age-related macular degeneration in the United States. Archives of Ophthalmology. 2004;122(4):564–72. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 3.Klein R, Klein BE. The prevalence of age-related eye diseases and visual impairment in aging: current estimates. Investigative Ophthalmology & Visual Science. 2013;54(14):ORSF5–ORSF13. doi: 10.1167/iovs.13-12789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudnicka AR, Kapetanakis VV, Jarrar Z, Wathern AK, Wormald R, Fletcher AE, et al. Incidence of late-stage age-related macular degeneration in American whites: Systematic review and meta-analysis. American Journal of Ophthalmology. 2015;160(1):85–93.e3. doi: 10.1016/j.ajo.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Bressler NM. Age-related macular degeneration is the leading cause of blindness. Journal of the American Medical Association. 2004;291(15):1900–1. doi: 10.1001/jama.291.15.1900. [DOI] [PubMed] [Google Scholar]
- 6.Boyer DS, Antoszyk AN, Awh CC, Bhisitkul RB, Shapiro H, Acharya NR, et al. Subgroup analysis of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007;114(2):246–52. doi: 10.1016/j.ophtha.2006.10.045. [DOI] [PubMed] [Google Scholar]
- 7.Rein DB, Wittenborn JS, Zhang X, Honeycutt AA, Lesesne SB, Saaddine J, et al. Vision Health Cost-Effectiveness Study Group. Forecasting age-related macular degeneration through the year 2050: the potential impact of new treatments. Archives of Ophthalmology. 2009;127(4):533–40. doi: 10.1001/archophthalmol.2009.58. [DOI] [PubMed] [Google Scholar]
- 8.Buckle M, Lee A, Mohamed Q, Fletcher E, Sallam A, Healy R, et al. Prevalence and incidence of blindness and other degrees of sight impairment in patients treated for neovascular age-related macular degeneration in a well-defined region of the United Kingdom. Eye (London) 2015;29(3):403–8. doi: 10.1038/eye.2014.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Complications of Age-Related Macular Degeneration Prevention Trial Research Group. Laser treatment in patients with bilateral large drusen: The Complications of Age-Related Macular Degeneration Prevention Trial. Ophthalmology. 2006;113(11):1974–1986. doi: 10.1016/j.ophtha.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Ying GS, Huang J, Maguire MG, Jaffe GJ, Grunwald JE, Toth C, et al. Comparison of Age-Related Macular Degeneration Treatments Trials Research Group. Baseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular age-related macular degeneration. Ophthalmology. 2013;120(1):122–9. doi: 10.1016/j.ophtha.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singer MA, Awh CC, Sadda S, Freeman WR, Antoszyk AN, Wong P, et al. HORIZON: An open-label extension trial of ranibizumab for choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology. 2012;119(6):1175–83. doi: 10.1016/j.ophtha.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Schalnus R, Meyer CH, Kuhli-Hattenbach C, Luchtenberg M. Time between symptom onset and assessment in age related macular degeneration with subfoveal choroidal neovascularization. Ophthalmologica. 2010;224(3):176–82. doi: 10.1159/000239236. [DOI] [PubMed] [Google Scholar]
- 13.Oliver-Fernandez A, Bakal J, Segal S, Shah GK, Dugar A, Sharma S. Progression of visual loss and time between initial assessment and treatment of wet age-related macular degeneration. Canadian Journal of Ophthalmology. 2005;40(3):313–9. doi: 10.1016/S0008-4182(05)80074-2. [DOI] [PubMed] [Google Scholar]
- 14.Algvere PV, Steen B, Seregard S, Kvanta A. A prospective study on intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration of different durations. Acta Ophthalmologica. 2008;86(5):482–9. doi: 10.1111/j.1600-0420.2007.01113.x. [DOI] [PubMed] [Google Scholar]
- 15.Liu TYA, Shah AR, Del Priore LV. Progression of lesion size in untreated eyes with exudative age-related macular degeneration: A meta-analysis using Lineweaver-Burk plots. JAMA Ophthalmology. 2013;131(3):335–340. doi: 10.1001/jamaophthalmol.2013.818. [DOI] [PubMed] [Google Scholar]
- 16.Vander JF, Morgan CM, Schatz H. Growth rate of subretinal neovascularization in age-related macular degeneration. Ophthalmology. 1989;96(9):1422–6. doi: 10.1016/s0161-6420(89)32740-0. [DOI] [PubMed] [Google Scholar]
- 17.Muether PS, Hermann MM, Koch K, Fauser S. Delay between medical indication to anti-VEGF treatment in age related macular degeneration can result in a loss of visual acuity. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2011;249(5):633–7. doi: 10.1007/s00417-010-1520-9. [DOI] [PubMed] [Google Scholar]
- 18.Rauch R, Weingessel B, Maca SM, Vecsei-Marlovits PV. Time to first treatment—the significance of early treatment of exudative age related macular degeneration. Retina. 2012;32(7):1260–4. doi: 10.1097/IAE.0b013e3182018df6. [DOI] [PubMed] [Google Scholar]
- 19.Weingessel B, Hintermayer G, Maca SM, Rauch R, Vecsei-Marlovits PV. The significance of early treatment of exudative age-related macular degeneration: 12 months’ results. Wiener Klinische Wochenschrift. 2012;124(21–22):750–5. doi: 10.1007/s00508-012-0249-3. [DOI] [PubMed] [Google Scholar]
- 20.Finger RP, Guymer RH, Gillies MC, Keeffe JE. The impact of antivascular endothelial growth factor treatment on quality of life in neovascular age-related macular degeneration. Ophthalmology. 2014;121(6):1246–51. doi: 10.1016/j.ophtha.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 21.Submacular Surgery Trials (SST) Research Group. Evaluation of minimum clinically meaningful changes in scores on the National Eye Institute Visual Function Questionnaire (NEI-VFQ). SST report number 19. Ophthalmic Epidemiology. 2007;14(4):205–215. doi: 10.1080/09286580701502970. [DOI] [PubMed] [Google Scholar]
- 22.Dong LM, Childs AL, Mangione CM, Bass EB, Bressler NM, Hawkins BS, Marsh MJ, Miskala P, Jaffee HA, McCaffrey LA, Submacular Surgery Trials Research Group Health- and vision-related quality of life among patients with choroidal neovascularization secondary to age-related macular degeneration at enrollment in randomized trials of submacular surgery: SST report no. 4. American Journal of Ophthalmology. 2004;138(1):91–108. doi: 10.1016/j.ajo.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Submacular Surgery Trials (SST) Research Group. Surgery for subfoveal choroidal neovascularization in age-related macular degeneration: Quality-of-life findings. SST report no. 12. Ophthalmology. 2004;111(11):1981–1992. doi: 10.1016/j.ophtha.2004.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Submacular Surgery Trials (SST) Research Group. Surgery for predominantly hemorrhagic choroidal neovascular lesions of age-related macular degeneration: Quality-of-life findings. SST report no. 14. Ophthalmology. 2004;111(11):2007–14. doi: 10.1016/j.ophtha.2004.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Alan Mason Chesney Medical Archives of the Johns Hopkins Medical Institutions. Available at: http://www.medicalarchives.jhmi.edu/ Accessed August 19, 2015.
- 26.Submacular Surgery Trials. Manual of Procedures. Spring-field, VA: National Technical Information Service; 1998. (NTIS Publication PB98-16648). [Google Scholar]
- 27.Submacular Surgery Trials (SST) Research Group. Surgery for subfoveal choroidal neovascularization in age-related macular degeneration: ophthalmic findings. SST report no. 11. Ophthalmology. 2004;111(11):1967–80. doi: 10.1016/j.ophtha.2004.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mangione CM, Berry S, Spritzer K, Janz NK, Klein R, Owsley C, et al. Identifying the content area for the National Eye Institute Vision Function Questionnaire (NEI-VFQ): results from focus groups with visually impaired persons. Archives of Ophthalmology. 1998;116(2):227–233. doi: 10.1001/archopht.116.2.227. [DOI] [PubMed] [Google Scholar]
- 29.Mangione CM. The National Eye Institute 25-Item Visual Function Questionnaire (VFQ-25) Scoring Algorithm. 2000 Version August 2000. Available at: https://nei.nih.gov/sites/default/files/nei-pdfs/manual_cm2000.pdf Accessed August 19, 2015.
- 30.Ware JE, Jr, Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF-36), I. Conceptual framework and item selection. Medical Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 31.SF-36.org: A community for measuring health outcomes using SF tools. Available at: http://www.sf-36.org/ Accessed August 19, 2015.
- 32.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica. 1983;67(6):361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 33.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. Journal of Psychosomatic Research. 2002;52(2):69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 34.Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD, National Eye Institute Visual Function Questionnaire Field Test Investigators Development of the 25-item National Eye Institute Visual Function Questionnaire. Archives of Ophthalmology. 2001;119(7):1050–8. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 35.Submacular Surgery Trials Research Group. Responsiveness of the National Eye Institute Visual Function Questionnaire to changes in visual acuity: findings in patients with subfoveal choroidal neovascularization–SST Report No. 1. Archives of Ophthalmology. 2003;121(4):531–9. doi: 10.1001/archopht.121.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Likert R. A Technique for the Measurement of Attitudes. Archives of Psychology. 1932;140:1–55. [Google Scholar]
- 37.Submacular Surgery Trials (SST) Research Group. Incident choroidal neovascularization in fellow eyes of patients with unilateral subfoveal choroidal neovascularization secondary to age-related macular degeneration: SST report No. 20 from the Submacular Surgery Trials Research Group. Archives of Ophthalmology. 2007;125(10):1323–30. doi: 10.1001/archopht.125.10.1323. [DOI] [PubMed] [Google Scholar]
- 38.Miskala PH, Bressler NM, Meinert CL. Relative contributions of reduced vision and general health to NEI-VFQ scores in patients with neovascular age-related macular degeneration. Archives of Ophthalmology. 2004;122(5):758–766. doi: 10.1001/archopht.122.5.758. [DOI] [PubMed] [Google Scholar]
- 39.Miskala PH, Bressler NM, Meinert CL. Is adjustment of National Eye Institute Visual Function Questionnaire scores for general health necessary in randomized trials? American Journal of Ophthalmology. 2004;137(5):961–963. doi: 10.1016/j.ajo.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 40.Macular Photocoagulation Study Group. Five-year follow-up of fellow eyes of patients with age-related macular degeneration and unilateral extrafoveal choroidal neovascularization. Archives of Ophthalmology. 1993;111(9):1189–99. doi: 10.1001/archopht.1993.01090090041018. [DOI] [PubMed] [Google Scholar]
- 41.Macular Photocoagulation Study Group. Risk factors for choroidal neovascularization in the second eye of patients with juxtafoveal or subfoveal choroidal neovascularization secondary to age-related macular degeneration. Archives of Ophthalmology. 1997;115(6):741–7. doi: 10.1001/archopht.1997.01100150743009. [DOI] [PubMed] [Google Scholar]
- 42.Massof RW, Ahmadian L. What do different visual function questionnaires measure? Ophthalmic Epidemiology. 2007;14(4):198–204. doi: 10.1080/09286580701487883. [DOI] [PubMed] [Google Scholar]
- 43.Lindblad AS, Clemons TE. Responsiveness of the National Eye Institute Visual Function Questionnaire to progression to advanced age-related macular degeneration, vision loss, and lens opacity: AREDS Report no. 14. Archives of Ophthalmology. 2005;123(9):12071214. doi: 10.1001/archopht.123.9.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, MARINA Study Group Ranibizumab for neovascular age-related macular degeneration. New England Journal of Medicine. 2006;355(14):1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


