Abstract
Aberrant glucocorticoid secretion is implicated in the pathophysiology of stress-related disorders (i.e., depression, anxiety). Glucocorticoids exert biological effects via mineralocorticoid (MR) and glucocorticoid (GR) receptors. Previous data from our laboratory indicate that GR antagonism/modulation (i.e., mifepristone, CORT 108297) regulate endocrine, behavioral, and central stress responses. Because of the dynamic interplay between MR and GR on HPA axis regulation and emotionality, compounds targeting both receptors are of interest for stress-related pathology. We investigated the effects of CORT 118335 (a dual selective GR modulator/MR antagonist) on endocrine, behavioral, and central (c-Fos) stress responses in male rats. Rats were treated for five days with CORT 118335, imipramine (positive control), or vehicle and exposed to restraint or forced swim stress (FST). CORT 118335 dampened corticosterone responses to both stressors, without a concomitant antidepressant-like effect in the FST. Imipramine decreased corticosterone responses to restraint stress; however, the antidepressant-like effect of imipramine in the FST was independent of circulating glucocorticoids. These findings indicate dissociation between endocrine and behavioral stress responses in the FST. CORT 118335 decreased c-Fos expression only in the CA1 division of the hippocampus. Imipramine decreased c-Fos expression in the basolateral amygdala and CA1 and CA3 divisions of the hippocampus. Overall, the data indicate differential effects of CORT 118335 and imipramine on stress-induced neuronal activity invarious brain regions. The data also highlight acomplex relationship between neuronal activation instress and mood regulatory brain regions and the ensuing impact on endocrine and behavioral stress responses.
Keywords: HPA-axis, Antidepressants, Forced swim test, Restraint, Depression-like behavior, c-Fos
1. Introduction
Hypothalamic-pituitary-adrenal (HPA) axis dysfunction due to prolonged elevated glucocorticoid secretion (cortisol in humans and corticosterone in rodents) is associated with various pathological conditions including obesity, anxiety, and depression [1–3]. Despite these potentially deleterious effects, glucocorticoids are essential for survival and play an integral role in constraining excess activation of this neuroendocrine system through a negative feedback mechanism [4]. Glucocorticoids mediate their biological effects via binding to two receptor subtypes: type-1 mineralocorticoid receptors (MR) and type-2 glucocorticoid receptors (GR). Glucocorticoids bind with a higher affinity to MR over GR [5]. Because of these differences in binding affinities, it is hypothesized that MR is more important for maintaining basal HPA axis tone, while GR is necessary for neuroendocrine stress responsiveness and restoration of homeostasis via the glucocorticoid-mediated negative feedback [4]. Of note, disruption of this feedback mechanism is hypothesized to be responsible for the observed hypercortisolemia and its associated role in disorders like major depression [3].
Glucocorticoids act on forebrain regions including the medial prefrontal cortex, paraventricular nucleus of the hypothalamus (PVN), hippocampus, and amygdala to regulate HPA axis tone and emotionality in rodents [4]. Forebrain GR knockdown (KD) using genetic strategies targeting most of the aforementioned brain regions increases basal and stress-induced neuroendocrine responses in male mice [6]. This elevated neuroendocrine response is recapitulated behaviorally, as fore-brain GR-KD mice display increased depression-like behavior (immobility) in the forced swim test (FST) [6,7]. Although forebrain MR-KD does not significantly impact HPA axis tone, loss of MR in critical corticolimbic circuits impairs cognitive function and behavioral flexibility in certain tasks [8]. Furthermore, MR overexpression in the forebrain significantly decreases anxiety-like behavior in male and female mice [9]. Together, these findings using genetic mouse models highlight a role for both GR and MR on HPA axis function and emotionality.
GR and MR modulation via pharmacology also influences endocrine stress responses, as well as depression-related behaviors in rodents. For example, mifepristone, a non-selective GR antagonist, inhibits HPA axis activity, resulting in an initial suppression of corticosterone stress responses [10,11]. However, in these same studies, mifepristone consistently impairs the glucocorticoid-mediated feedback. Because complete GR antagonism may pose some adverse consequences, GR modulators, such as CORT 108297 with tissue-specific agonistic and antagonistic activity have been developed. Unlike mifepristone, CORT 108297 suppresses HPA axis stress responses without impairing HPA axis shut off [11,12]. Interestingly, the tricyclic antidepressant imipramine is associated with HPA axis attenuation [13–15] and can similarly attenuate corticosterone stress responses [11]. Relatedly, mifepristone [10], imipramine [16,17], and CORT 108297 (unpublished data) all modulate stress-induced c-Fos expression in the aforementioned brain regions, suggesting that these compounds regulate endocrine and behavioral phenotypes in response to stress via critical corticolimbic circuits.
In accordance with their stress-induced endocrine effects, mifepristone, CORT 108297, and imipramine all decrease depression-like behavior in the FST [10,11,18,19]. Interestingly, while MR antagonism with spironolactone produces similar behavioral phenotypes in mice [20], it increases basal and stress-induced glucocorticoid levels in rodents and humans [21,22]. In humans, this increase in cortisol levels was also observed with combined administration of sub-threshold levels of spironolactone and mifepristone [23]. While there is support for an interplay between MR and GR, how these two receptor subtypes interact to influence physiology, brain, and behavior is still being elucidated.
CORT 118335 has recently been developed as a putative treatment for HPA axis related disorders. This novel research compound is a dual selective GR modulator and MR antagonist, with high GR affinity and modest MR affinity [24,25]. The agonistic and antagonistic properties of CORT 118335 with regards to GR are tissue specific and dependent upon the co-regulators present. The MR antagonistic profile of CORT 118335 is highly similar to that of established MR antagonists, spironolactone and eplerenone [24]. CORT 118335 has previously been demonstrated to suppress intrinsic plasma corticosterone levels in rodents [24]. However, the effect of simultaneous selective GR modulation and MR antagonism on neuroendocrine, behavioral, and central stress responses has yet to be fully explored. Here, we investigated the effects of CORT 118335 alongside the tricyclic antidepressant imipramine on neuroendocrine stress responses as well as related behavioral phenotypes and central stress responses (c-Fos) in male rats. Tricyclic antidepressants increase GR promoter activity [26], GR mRNA expression [27], and GR binding in neuronal cell lines [28], making imipramine an appropriate positive control for an antidepressant in this study.
2. Material and methods
2.1. Subjects
Male (≈250 g) Sprague-Dawley rats (Harlan, Indianapolis) were pair-housed in standard shoebox cages and acclimated to the animal facility for at least 1 week prior to the initiation of each experiment. Animals were maintained in a temperature and humidity-controlled environment on a 12:12 light-dark cycle with food and water available ad libitum. All experimental procedures and protocols were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Animals and approved by the University of Cincinnati Institutional Care and Use Committee.
2.2. Drug administration
For each experiment, animals were randomly divided into four treatment groups. CORT 118335 (3 mg/kg or 10 mg/kg) (Corcept Therapeutics, Menlo Park, CA) was dissolved in 10% dimethyl sulfoxide (DMSO) 90% polyethylene glycol (PEG) and CORT 118335 (30 mg/kg) was dissolved in 100% DMSO. Imipramine (10 mg/kg) (Sigma-Aldrich) was dissolved insaline. CORT 118335 was administered subcutaneously (s.c.) while imipramine was given intraperitoneally (i.p.). To control for route of administration and for potential drug solvent effects, vehicle groups consisted of 10% DMSO 90%PEG (s.c) or saline (i.p.). Rats were treated with CORT 118335, imipramine, or vehicle for five days. On the fifth day, animals were injected 1 h prior to testing [11,29]. The drug doses and regimen were based on doses from previous studies using the same methodology involving related GR modulators or imipramine [10,11,30].
2.3. Experiment 1 - impact of CORT 118335 or imipramine on neuroendocrine stress responses to restraint stress in male rats
Male rats were administered CORT 118335 (10 mg/kg s.c. (n = 10); 30 mg/kg s.c.(n=10)), imipramine (10 mg/kg i.p. (n=10)), or vehicle (10% DMSO 90% PEG s.c. (n = 6) or saline i.p. (n = 4)) for five days. On the fifth day, animals were injected 1 h prior to exposure to the restraint challenge. Rats were individually placed into a well-ventilated restraint tube for 30 min. Blood samples were collected by tail clip as previously described [31] at 0, 15, 30, 60, and 120 min after restraint onset. The 60 and 120 min time points were collected from freely-moving rats. Blood sample collection for both experiments was limited to 3 min to prevent increases in stress responses due to sampling [31].
Note: The same rats were used for Experiments 1 and 2. Rats were allowed two weeks to recover between the restraint and FST experiments. Both restraint and FST exposure occurred during the animal’s inactive period during circadian nadir (lights on). To avoid any potential carryover effects, all animals remained in the same drug condition group for both restraint and FST exposure. During the recovery period, animals were not treated with any compounds.
2.4. Experiment 2 - impact of CORT 118335 or imipramine on neuroendocrine, behavioral, and central stress responses to forced swim test in male rats
As in the restraint experiment, rats were injected with CORT 118335 (10 mg/kg or 30 mg/kg), imipramine (10 mg/kg), or vehicle for five days. On the fifth day, rats were injected 1 h prior to exposure to a modified FST [32]. The FST served as a stressor and screen for antidepressant efficacy of CORT 118335 and imipramine. Imipramine was included as a positive control as it has consistently been reported to decrease antidepressant-like effects [10,11,19,33]. Animals were exposed to the FST tank (Plexiglass cylinder 45 cm high, 20 cm in diameter, and filled with 31 cm of water (23–25 °C)) for 5 min. After removal from the FST, animals were towel dried and then returned to their home cages. Each session was recorded and scored by observers blind to treatment conditions. Behavior (time spent immobile) in the FST was scored for 5 min sessions using the Annostar Behavioral Scoring System (Clever Systems). Blood samples were collected by tail clip from freely moving rats at 15, 30, and 90 min after FST onset.
2.5. Experiment 3 - impact of CORT 118335 on open field behavior in male rats
In order to determine whether there was a dose-dependent effect of CORT 118335 on overall activity, male rats were exposed to the open field test. A separate cohort of male Sprague Dawley rats was administered CORT 118335 (3 mg/kg; 10 mg/kg; 30 mg/kg (n = 10 per group)) or vehicle (10% DMSO 90% PEG (n = 10)) using the aforementioned treatment regimen. Behavioral testing for the open field occurred during the animals’ active period (lights-off). The open field test assesses general locomotion and anxiety-like behavior. Rats were placed in a corner of the open field apparatus (36 in × 36 in Plexiglass box) and allowed to freely explore for 5 min. Each session was recorded by a camera mounted above the apparatus. Time spent in the center versus periphery, total distance traveled, time spent immobile, and velocity were calculated using the EthoVision video tracking system from Noldus Information Technology [34].
2.6. Radioimmunoassay
Plasma corticosterone concentrations were measured using 125I RIA kits (MP Biomedicals, Inc., Orangeburg, NY). All samples were run in duplicate in the same assay and the intra-assay coefficient of variation was <10%.
2.7. Tissue collection and immunohistochemistry
Ninety minutes after FST onset, animals were anesthetized with an overdose of sodium pentobarbital and transcardially perfused with 0.9% saline followed by 3.7% formaldehyde in 0.01 M phosphate buffer (PBS), pH 7.3. Brains were post-fixed in 3.7% formaldehyde fixative for 24 h at 4 °C, then stored in 30% sucrose in 0.01 M PBS at 4 °C. Brain sections were serially cut at 35 μm on a freezing microtome and alternate sections were collected into wells containing cryoprotectant solution. Coronal brain sections were transferred from cryoprotectant solution to 0.01 M PBS solution for washes and subsequent incubations. After being rinsed (5 × 5 min) in PBS at room temperature (RT), sections were incubated in 3% hydrogen peroxide diluted in PBS for 15 min at RT. Subsequently, sections were again rinsed (5 × 5 min) in PBS and incubated in blocking solution (0.1% bovine serum albumin (BSA) and 0.2% Triton X-100) for 1 h at RT. Sections were then incubated at RT overnight in blocking solution with rabbit polyclonal antibody against c-Fos (1:1000; Santa Cruz BioTechnology, Santa Cruz, CA). The next day, sections were rinsed (5 × 5 min) in PBS at RT, followed by incubation in blocking solution (no Triton X-100) with biotinylated goat anti-rabbit secondary antibody (1:400; Vector Laboratories, Burlingame, CA) for 1 h. Sections were again rinsed (5 × 5 min) in PBS and then reacted with avidin-biotin horseradish peroxidase complex (1:800; Vector Laboratories, Burlingame, CA) for 1 h at RT. Sections were rinsed once more (5 × 5 min) in PBS and then visualized with a 10 min incubation in 3,3′-diaminobenzidine (DAB) (Sigma) activated with 30% hydrogen peroxide. Sections were finally washed in PBS, mounted on gelatinized slides, allowed to dry, dehydrated with alcohol and xylene, and then coverslipped.
2.8. Quantification of Fos immunoreactivity (cell counting)
For Fos positive immunoreactivity analyses, brain sections containing areas of interest were identified using the Paxinos & Watson rat brain atlas [35] as illustrated in Fig. 1. Images were captured using a Carl Zeiss Imager Z.1 (Carl Zeiss Microimaging, Thornwood, NY). Images were exported to Image J (National Institutes of Health, Bethesda, MD) and counted using either the thresholding technique or manually counted when thresholding was found to be unreliable. Manual counts were implemented when there were differences in staining intensity and determination of stained cells was subject to trained human eye evaluation. The final Fos-positive cell counts were expressed as positive nuclei per unit area (mm2). Cell counts were quantified by observers blind to treatment conditions. For every animal counted, a total of 1–4 regions were analyzed for each area of interest and averaged to produce a mean cell-count/area. We analyzed c-Fos activation in two medial prefrontal cortex areas (prelimbic (PrL), infralimbic (IL)), the paraventricular nucleus of the hypothalamus, three amygdala subdivisions (basolateral amygdala (BLA), central amygdala (CeA), medial amygdala (MeA)), and three hippocampal subdivisions (CA1, CA2, CA3, dentate gyrus (DG)). N-sizes vary across groups and brain areas due to unexpected tissue damage during processing.
Fig. 1.
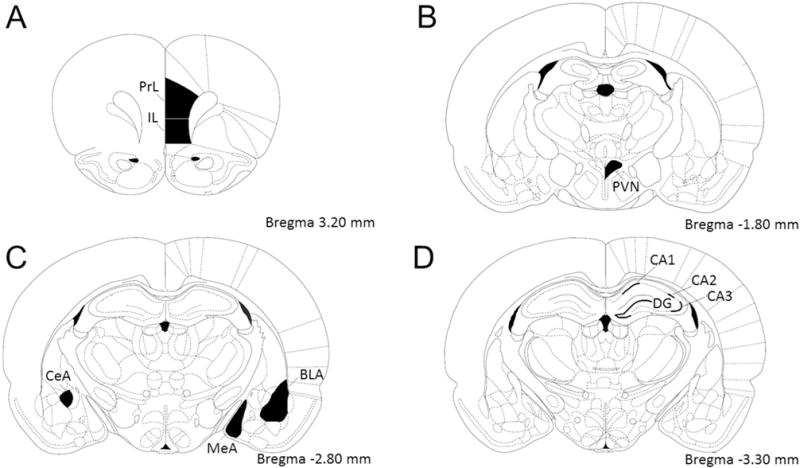
Template of brain areas analyzed for c-Fos based on Paxinos and Watson rat brain atlas. (A) Medial prefrontal cortex prelimbic (PrL) and infralimbic (IL) areas. (B) Hypothalamic paraventricular nucleus (PVN). (C) Amygdaloid basolateral (BLA), central (CeA), and medial (MeA) nuclei. (D) Hippocampal CA1, CA2, and CA3 pyramidal layers, and dentate gyrus (DG) granular layer.
2.9. Statistical analysis
Data are represented as mean ± standard error of the mean (SEM). Behavioral and cell count data were analyzed with one-way ANOVA or t-test where applicable. Hormonal data were analyzed using a two-way ANOVA with drug as a between subject factor and time as the within or repeating factor. Data points lying two standard deviations (SD) from the mean for any given condition and time point were removed as outliers prior to analysis. Non-parametric tests were used when relevant assumptions were violated in one-way ANOVAs and t-tests. Individual group differences were compared using Fisher’s LSD or Dunn’s Method when appropriate. Animals treated with vehicle (DMSO-PEG s.c. or saline i.p.) were collapsed into one group for each dependent variable (i.e., endocrine, behavioral, and c-Fos analyses for each brain region). CORT 118335 and imipramine treated animals were analyzed separately as direct comparisons between CORT 118335 and imipramine were not pre-planned. For all data, p ≤ 0.05 denotes statistical significance.
3. Results
3.1. Hormonal analyses
3.1.1. Exclusion of animals from hormonal statistical analyses
Some animals were removed from the endocrine analyses due to insufficient sample collection. Specifically, for the restraint area under the curve (AUC) analyses, 3 animals were removed from the vehicle group, 2 from the CORT 118335 (10 mg/kg) group, 1 from the CORT 118335 (30 mg/kg) group, and 2 from the imipramine group. For the FST AUC analyses, 3 animals were removed from the vehicle group, 1 from the CORT 118335 (10 mg/kg) group, 2 from the CORT 118335 (30 mg/kg) group, and 2 from the imipramine group. One animal was removed as a 2SD outlier from the CORT 118335 (10 mg/kg) group in the FST AUC analyses.
3.1.2. Effects of CORT 118335 on neuroendocrine stress responses in male rats
There was a significant main effect of drug, (F2, 27 = 49.723, p < 0.01), a main effect of time (F4, 96 = 138.389, p < 0.01), and a drug × time interaction (F12, 96 = 12.959, p < 0.01) on corticosterone responses to restraint stress (Fig. 2A). Relative to vehicle, CORT 118335 at both doses decreased corticosterone responses at 15 min, 30 min, and 60 min following restraint onset. Rats treated with CORT 118335 (30 mg/kg) had lower corticosterone responses relative to CORT 118335 (10 mg/kg) at 15 and 30 min time points. The stress dampening properties of CORT 118335 over time is recapitulated for both doses as a significant decrease in the integrated corticosterone responses to restraint stress (F2, 17 = 19.216, p < 0.01) (Fig. 2B). Of note, no differences were observed at 120 min between animals treated with CORT 118335 versus their vehicle counterparts. CORT 118335 attenuated corticosterone response to FST exposure (F2, 27 = 122.389, p < 0.01). There was also a main effect of time (F2, 47 = 34.366, p < 0.01), and a drug × time interaction (F4, 47 = 39.857, p < 0.01) (Fig. 2C). Animals treated with CORT 118335 at both doses had lower corticosterone responses at 15 min and 30 min relative to vehicle groups. In addition, only animals treated with CORT 118335 at 30 mg/kg had significantly lower plasma corticosterone levels compared to vehicle at the 90 min time point. Accordingly, both doses of CORT 118335 significantly decreased the integrated corticosterone response to FST stress (χ2(2) = 15.754, p < 0.001) (Fig. 2D).
Fig. 2.
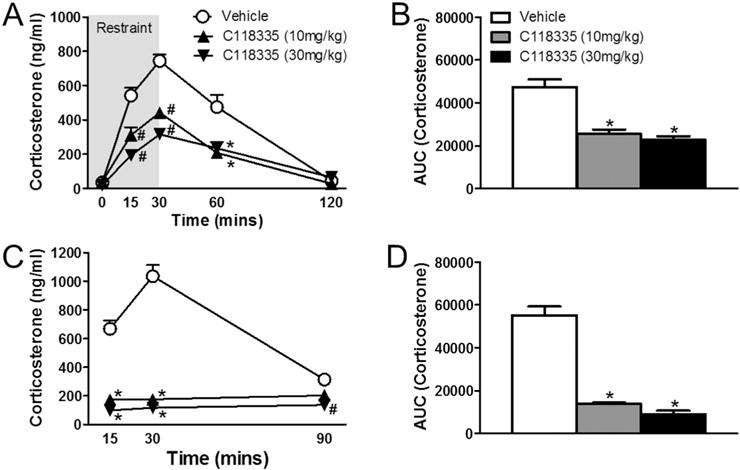
Effects of CORT 118335 on hormonal stress response in male rats. (A) Males treated with CORT 118335 at 10 mg/kg and 30 mg/kg displayed dose-dependent attenuated corticosterone levels in response to restraint stress (15 min–60 min from restraint onset) without delaying HPA axis shut off at 120 min. (B) Both doses significantly decreased integrated corticosterone response to restraint stress. (C) Males treated with CORT 118335 at both 10 mg/kg and 30 mg/kg displayed attenuated corticosterone levels in response to FST stress (15 min–30 min from FST onset) without delaying HPA axis shut off at 90 min. At 90 min, the corticosterone level for CORT 118335 at 30 mg/kg was still significantly lower compared to vehicle controls and also to the 10 mg/kg dose. (D) Both doses significantly decreased integrated corticosterone response to FST stress. Values represent mean ± SEM; n = 10 per group for corticosterone time course analyses; n = 7–9 per group for AUC analyses. * indicates significant effect compared to all vehicle treated animals only (p ≤ 0.05); # indicates significant effect compared to all other groups (p ≤ 0.05).
3.1.3. Effects of imipramine on neuroendocrine stress response in male rats
Relative to vehicle, imipramine decreased corticosterone responses to restraint stress over time (Fig. 3A). There was a significant main effect of drug (F1, 18 = 4.318, p = 0.05) and a main effect of time (F4, 65 = 83.966, p < 0.01) on neuroendocrine stress responses to restraint exposure. However, there was no drug × time interaction (F4, 65 = 1.670, p > 0.05). Imipramine had no effect on integrated corticosterone response to restraint stress (t11 = 1.152, p > 0.05) (Fig. 3B). Imipramine had no effect on corticosterone responses to FST over time (Fig. 3C). While there was a main effect of time (F2, 31 = 35.400, p < 0 0.01), there was no effect of drug (F1, 18 = 0.0923, p > 0.05), nor was there a drug × time interaction (F2, 31 = 1.270, p > 0.05). Consequently, there was no effect of imipramine on the integrated corticosterone response to FST stress (t13 = 1.267, p > 0.05) (Fig. 3D).
Fig. 3.
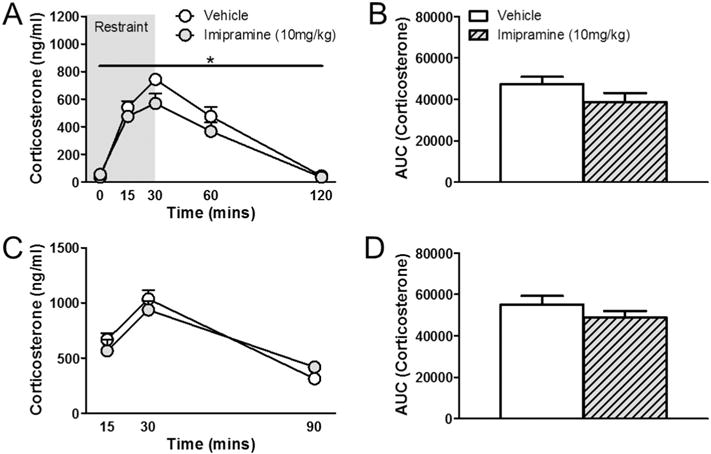
Effects of imipramine on hormonal stress response in male rats. (A) Males treated with imipramine had overall lower corticosterone levels in response to restraint stress without affecting HPA axis shut off at 120 min. (B) No differences were found in integrated corticosterone responses to restraint stress. (C) Imipramine treatment in males had no effect on FST stress response or (D) integrated corticosterone responses to FST stress. Values represent mean ± SEM; n = 10 per group for corticosterone time course analyses; n = 7–8 per group for AUC analyses. * indicates significant effect compared to all vehicle treated animals only (p ≤ 0.05).
3.2. Behavioral and locomotion analyses
3.2.1. Exclusion of animals from behavioral and locomotion statistical analyses
Some animals were removed from behavioral analyses as 2SD outliers. Specifically, for FST immobility analyses, 1 animal was removed from the CORT 118335 (10 mg/kg) group, and 1 from the CORT 118335 (30 mg/kg) group. For OFT anxiety-like behavior analyses, 1 animal was removed from the vehicle group, 1 from the CORT 118335 (3 mg/kg) group, and 2 from the CORT 118335 (30 mg/kg) group. For OFT immobility analyses, 1 animal was removed from the vehicle group, and 1 from the CORT 118335 (30 mg/kg) group. For OFT locomotion analyses, 1 animal was removed from the CORT 118335 (30 mg/kg) group.
3.2.2. Effects of CORT 118335 and imipramine on behavior and locomotion in male rats
There was no effect of CORT 118335 on immobility in the FST (F2, 26 = 1.597, p > 0.05) (Fig. 4A). However, imipramine decreased immobility in the FST (t18 = 2.261, p < 0.05) (Fig. 4B). There was no effect of CORT 118335 at any dose on anxiety-like behavior (time spent in the center) (χ2(3) = 1.665, p > 0.05) (data not shown) nor time spent immobile in the open field test (F3, 34 = 0.434, p > 0.05) (Fig. 4C). There was no effect of CORT 118335 on distance traveled (F3, 35 = 1.272, p > 0.05) (Fig. 4D) or velocity (F3, 35 = 1.273, p > 0.05) in the open field test (data not shown).
Fig. 4.
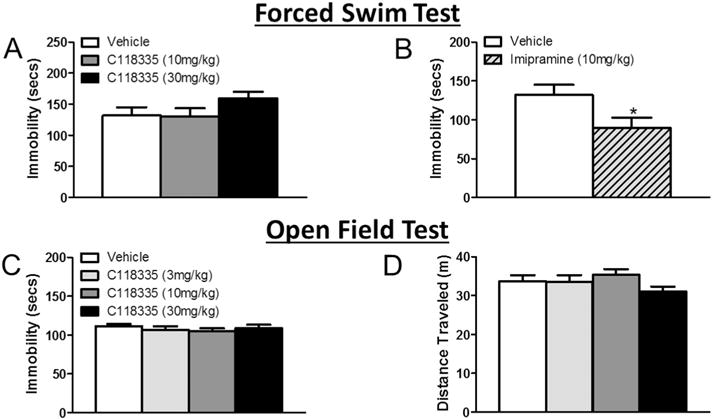
Effects of CORT 118335 and imipramine on behavior and locomotion in male rats. (A) CORT 118335 at 30 mg/kg showed a numeric increase in immobility in the FST. (B) Imipramine significantly decreased immobility in the FST. (C) CORT 118335 did not significantly affect immobility in the OFT regardless of drug concentration. (D) CORT 118335 at 30 mg/kg showed a numeric decrease in total distance traveled in the OFT. Values represent mean ± SEM; n = 9–10 per group. * indicates significant effect compared to all vehicle treated animals only (p ≤ 0.05).
3.3. c-Fos analyses in response to forced swim stress
3.3.1. Exclusion of animals from c-Fos statistical analyses
For c-Fos analyses, all groups started with 8 animals per group. Some animals were removed from c-Fos analyses due to tissue damage during processing. Specifically, for PrL analyses, 1 animal was removed from the vehicle group, and for IL analyses, 1 animal was removed from the vehicle saline group. Some animals were removed from c-Fos analyses as 2SD outliers. Specifically, for PrL analyses, 1 animal was removed from the CORT 118335 (10 mg/kg) group. For IL analyses, 1 animal was removed from the CORT 118335 (10 mg/kg) group. For PVN analyses, 1 animal was removed from the CORT 118335 (30 mg/kg) group. For CeA analyses, 1 animal was removed from the imipramine group. For CA1 analyses, 1 animal was removed from the CORT 118335 (10 mg/kg) group.
3.3.2. Medial prefrontal cortex
CORT 118335 had no effect on prelimbic (PrL) (F2, 19 = 2.326, p > 0.05) or infralimbic (IL) (F2, 19 = 2.650, p > 0.05) Fos expression (Table 1). Similarly, imipramine did not modulate neuronal activity in PrL (t13 = 0.344, p > 0.05) (Fig. 5C) or IL (t13 = −0.263, p > 0.05) (Fig. 5D).
Table 1.
Effects of CORT 118335 and imipramine on Fos expression across stress-related brain areas in male rats. Data are represented as mean ± SEM; n = 7–8 per group.
| Brain area | Vehicle | CORT 118335
|
Imip | |
|---|---|---|---|---|
| 10 mg/kg | 30 mg/kg | |||
| PRL | 101.899 ± 19.243 | 100.943 ± 10.331 | 144.521 ± 18.228 | 93.704 ± 14.619 |
| IL | 85.571 ± 19.245 | 81.261 ± 9.873 | 132.220 ± 20.728 | 91.037 ± 9.780 |
| PVN | 546.040 ± 124.844 | 522.111 ± 61.052 | 617.074 ± 59.740 | 379.502 ± 67.243 |
| BLA | 74.427 ± 6.556 | 73.985 ± 7.685 | 78.294 ± 8.147 | |
| CeA | 97.846 ± 15.837 | 103.160 ± 17.367 | 106.333 ± 13.458 | |
| MeA | 201.880 ± 23.603 | 184.977 ± 18.376 | 232.242 ± 17.161 | |
| CA2 | 367.276 ± 11.527 | 405.000 ± 18.285 | 406.724 ± 25.510 | 345.452 ± 44.445 |
| CA3 | 89.696 ± 10.743 | 99.094 ± 11.363 | 78.792 ± 8.103 | |
| DG | 155.300 ± 15.479 | 148.768 ± 17.941 | 146.816 ± 15.519 | 166.363 ± 18.235 |
Fig. 5.
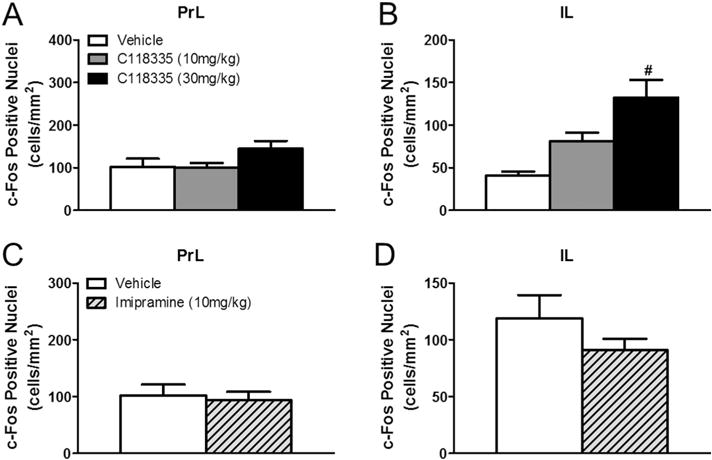
Effects of imipramine on amygdala Fos expression in male rats. (A) Representative images of c-Fos immunoreactivity in the basolateral amygdala of vehicle and (B) imipramine treated animals. (C) Imipramine treated animals showed less c-Fos activation in the basolateral amygdala. (D) Imipramine had no effect on Fos expression in the central or (E) medial amygdala. Values represent mean ± SEM, n = 7–8 per group. * indicates significant effect compared to all vehicle treated animals only (p ≤ 0.05). Scale bar 100 μm.
3.3.3. Paraventricular nucleus of the hypothalamus
Neither CORT 118335 (χ2(2) = 1.011, p > 0.05) nor imipramine (U = 25, p = 0.505) had any effect on PVN c-Fos induction in male rats (Table 1).
3.3.4. Amygdala
CORT 118335 had no effect on Fos expression in the basolateral amygdala (BLA) (F2, 21 = 0.100, p > 0.05), the central amygdala (CeA) (F2, 21 = 0.0752, p > 0.05), or the medial amygdala (MeA) (F2, 21 = 1.447, p > 0.05) (Table 1). Imipramine, however, decreased neuronal activity in the BLA (t14 = 2.312, p < 0.05) (Fig. 5A–C). Imipramine had no effect on c-Fos induction in the CeA (t13 = −0.492,p= 0.631)(Fig. 6D) or the MeA (t14 = 0.681, p = 0.507) (Fig. 5E).
Fig. 6.
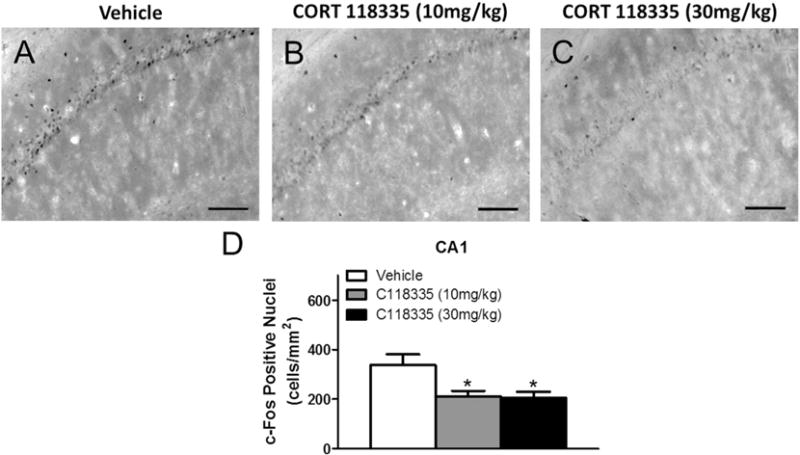
Effects of CORT 118335 on hippocampal Fos expression in male rats. (A) Representative images of c-Fos immunoreactivity in the CA1 of vehicle, (B) CORT 118335 (10 mg/kg), and (C) CORT 118335 (30 mg/kg) treated animals. (D) CORT 118335 at both 10 mg/kg and 30 mg/kg decreased CA1 c-Fos activation. Values represent mean ± SEM, n = 7–8 per group. * indicates significant effect compared to all vehicle treated animals only (p ≤ 0.05). Scale bar 100 μm.
3.3.5. Hippocampus
CORT 118335 significantly decreased CA1 Fos expression (F2, 20 = 5.292, p < 0.05) at both 10 mg/kg (p < 0.05) and 30 mg/kg (pb 0.01) relative to vehicle (Fig. 6A–D). However, CORT 118335 had no effect on c-Fos induction in the CA2 (F2, 21 = 1.334, p > 0.05), CA3 (F2, 21 = 0.998, p > 0.05), or DG (F2, 21 = 0.0738, p > 0.05) at either dose (Table 1). Similar to CORT 118335, imipramine decreased CA1 neuronal activity (t14 = 3.353, p < 0.01) (Fig. 7A–C). Imipramine also decreased CA3 Fos expression (U = 8, p < 0.05) (Fig. 7D). However, imipramine had no effect on Fos expression in either the CA2 (U = 27, p > 0.05) or DG (t14 = 0.463, p > 0.05) (Table 1).
Fig. 7.
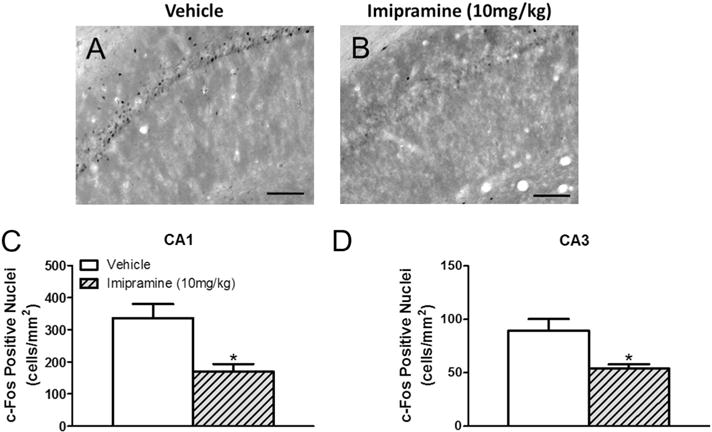
Effects of imipramine on hippocampal Fos expression in male rats. (A) Representative images of c-Fos immunoreactivity in the CA1 of vehicle and (B) imipramine treated animals. (C) Imipramine decreased c-Fos activation in CA1 and (D) CA3. Values represent mean ± SEM, n = 8 per group. * indicates significant effect compared to all vehicle treated animals only (p ≤ 0.05). Scale bar 100 μm.
4. Discussion
4.1. Overall summary
The findings of the present study indicate that CORT 118335 potently suppresses endocrine responses to two different stressors (restraint and FST) in male rats. The endocrine stress dampening properties of CORT 118335 were not accompanied by an antidepressant-like effect in the FST. Imipramine (positive control), however, decreased immobility in the FST. Given that endocrine stress responses and depression-like behavior are regulated by the CNS, we also examined stress-induced neuronal activity in critical corticolimbic circuits. CORT 118335 modulated stress-induced neuronal activity in the mPFC (IL) and hippocampus (CA1). On the other hand, imipramine affected neuronal activity in the basolateral amygdala and hippocampus (CA1, CA3). Our data illustrate dissociation between endocrine and behavioral stress responses, as the antidepressant-like effects of imipramine appear to be glucocorticoid independent. In addition, CORT 118335 mitigates excess glucocorticoid production without a concomitant impact on depression-like behavior in the forced swim test or exploratory and anxiety-like behavior in the open field.
4.2. Neuroendocrine and behavioral stress responses
4.2.1. CORT 118335 neuroendocrine and behavioral stress responses
CORT 118335 attenuated corticosterone responses to restraint and FST exposure particularly during the activational phases of the stress response in male rats. Unlike the classic GR antagonist mifepristone, CORT 118335 did not impair the HPA axis shut off responses at later time points (i.e., 90 min or 120) [10,11]. Acute administration of CORT 118335 does not appear to alter basal corticosterone in male rats as there were no differences in corticosterone concentrations between groups at the 0 min time point in the restraint studies. The stress-induced endocrine phenotype with CORT 118335 mirrors our findings using CORT 108297 [11], a GR modulator with reportedly little to no MR affinity [12]. These findings suggest the effects of CORT 118335 on stress-induced neuroendocrine responses are due to GR. Consistent with this postulate, MR antagonism alone does not modulate corticosterone responses to restraint stress [36]. Given that mifepristone, CORT 108297, and CORT 118335 all demonstrate mitigation of the stress response, it is likely that GR antagonism is driving this effect.
Despite the potent effects of CORT 118335 on HPA axis responses, there were no antidepressant-like effects of CORT 118335 in the modified FST. Our laboratory and others have previously reported dissociation between corticosterone concentrations and immobility in the FST with several compounds including mifepristone, imipramine, and CORT 108297 [11,37–39]. However, it is worth noting that CORT 108297 at higher doses exerted an antidepressant-like effect in the FST [11]. Likewise, MR antagonism alone reportedly prevents depression-like behavior in the FST in rats treated with chronic corticosterone [20]. Together, these findings suggest that CORT 118335 at least within the context of the present study, is ineffective in modulating behavioral responses to acute FST exposure.
4.2.2. Imipramine neuroendocrine and behavioral stress responses
Imipramine mildly attenuated corticosterone responses to restraint in male rats. This finding is consistent with previous reports [11]. Imipramine has a more potent effect on dampening ACTH stress responses compared to corticosterone, likely due to its effects on central HPA axis components (i.e., CRH) [15,16]. Imipramine did not impact corticosterone concentrations in response to FST exposure despite decreasing depression-like behavior in the FST. Tricyclic antidepressants have been associated with increased GR promoter activity [26], GR mRNA expression [27], and GR binding in neuronal cell lines [28]. However, the present data suggest that the antidepressant effects of imipramine may be independent of circulating glucocorticoids as well as GR and MR modulation. The GR/MR modulator CORT 118335 does not affect immobility and the two compounds have different effects on neuronal activity in some of the targeted critical corticolimbic circuits in response to FST exposure (Table 2).
Table 2.
Relative effects of CORT 118335 and imipramine on Fos expression across stress-related brain areas in male rats compared to vehicle treated animals.
| Males | |||
|---|---|---|---|
|
| |||
| Brain area | CORT 118335
|
Imipramine | |
| 10 mg/kg | 30 mg/kg | ||
| PRL | = | = | = |
| IL | = | = | = |
| PVN | = | = | = |
| BLA | = | = | ↓ |
| CEA | = | = | = |
| MEA | = | = | = |
| CA1 | ↓ | ↓ | ↓ |
| CA2 | = | = | = |
| CA3 | = | = | ↓ |
| DG | = | = | = |
4.3. c-Fos induction
4.3.1. Medial prefrontal cortex (mPFC)
The rodent mPFC is subdivided into the IL and PrL, which have opposing roles in HPA axis regulation [4]. Lesion studies propose the IL to be stress excitatory, and GR-KD in the IL has been observed to significantly increase immobility in the FST [40]. Relatedly, in male rats, mifepristone has been reported to increase IL activation following FST while decreasing endocrine stress responses [10]. However, in the present study, CORT 118335 had no effect on IL stress-induced neuronal activity. In contrast to the IL, lesion studies have repeatedly demonstrated the PrL to have a role in HPA axis inhibition [41–43]. However, in the present study, CORT 118335 had no effect on neuronal activation in the PRL despite a suppression of HPA activation in response to both restraint and FST stressors. In contrast to the current findings with CORT 118335, MR antagonism increases HPA activity [21,22,44] and exerts antidepressant-like effects [20]. Pretreatment with GR antagonists mitigates behavioral effects of MR antagonism targeted at the mPFC [45]. The GR modulating properties of CORT 118335 are eight times greater than its MR antagonism [24]. Thus, it is possible that any effects of CORT 118335’s partial MR antagonism in the mPFC is countered by the dominant GR antagonism in this brain area.
In the current study, 5-day treatment of imipramine (10 mg/kg) had no effect on c-Fos expression in the IL or the PrL in response to FST exposure. This finding is consistent with a previous study reporting no effect of 14-day imipramine treatment (30 mg/kg) on c-Fos expression in these regions in ACTH-treated rats [17]. However, a higher (20 mg/kg) dose of imipramine for 21 days decreases c-Fos expression in the IL/PRL areas while inducing antidepressant-like effects in the FST [16]. These data suggest that the antidepressant effects of imipramine, at least within the context of the present study, are independent of modulation of neuronal activation in the IL/PRL. Taken together, decreased IL/PrL neuronal activation may not be necessary for imipramine’s antidepressant effects.
4.3.2. Paraventricular nucleus of the hypothalamus (PVN)
The PVN is the “motor arm” of the HPA axis as the primary neuropeptides (CRH and AVP) that are necessary for HPA axis activation are housed within this nucleus [46]. Steroid receptor coactivator 1a (SRC-1a) represses CRH promoter activity in vitro [47], and is suggested to regulate PVN CRH expression to attenuate HPA stress responses [48]. CORT 118335 at both 10 mg/kg and 30 mg/kg attenuated the corticosterone stress response to FST, and this compound has previously been demonstrated to dose-dependently recruit the SRC-1a specific motif [49]. However, no differences were observed in PVN neuronal activation compared to the vehicle treated group. This is similar to the effects observed with mifepristone in male rats [10]. MR antagonism has been found to decrease neuronal activity in the PVN [50,51], but this cellular phenotype does not emerge with CORT 118335’s partial MR antagonism.
In the present study, 5-day treatment with imipramine had no effect on the PVN. More prolonged treatments of imipramine decreases PVN CRH mRNA levels [15] and PVN c-Fos expression [16,52]. Our findings suggest attenuation of stress-induced corticosterone responses may not be necessary for imipramine’s antidepressant effect.
4.3.3. Amygdala
The amygdala is comprised of multiple subnuclei including the BLA, CeA, and MeA. Although there are functional differences among the various nuclei, generally, the amygdala plays an excitatory role on HPA axis regulation [4]. Compared to vehicle treated controls, CORT 118335 had no effect on any subdivision of the amygdala in male rats. These results repeat the findings with mifepristone in male rats for the BLA and the MeA [10]. This suggests that GR modulation/MR antagonism at the level of the BLA and MeA is not necessary to attenuate HPA axis stress responses. CeA lesions have repeatedly been shown to decrease stress-induced neuroendocrine and central responses [53,54], suggesting the CeA to be excitatory in regards to HPA activation. While CORT 118335 had no effect on c-Fos activation in the CeA, mifepristone decreases CeA c-Fos activation in response to FST [10]. This finding suggests that the central stress dampening properties in the CeA are due to GR antagonism and not GR agonism and/or MR antagonism.
Animals exposed to acute FST exposure express higher c-Fos activation in the BLA compared to unstressed controls [10]. Imipramine decreased neuronal activation in the BLA in response to FST stress. Notably, in the absence of stress, imipramine has no effect on BLA Fos expression [17]. A targeted infusion of imipramine to the amygdala implicates this brain area as a critical site of action for its antidepressant-like effects in the FST [55]. Our data suggest that the antidepressant-like effect of imipramine may be mediated through its attenuation of BLA neuronal activity in the presence of stress.
4.3.4. Hippocampus
The dorsal and ventral hippocampus respectively mediate cognitive processing and HPA inhibition [56]. In the present study, we examined c-Fos expression in the CA1, CA2, CA3, and DG of the dorsal hippocampus, all of which may be affected by fluctuations in glucocorticoids since MR and GR are richly distributed throughout [57,58]. The CA1 in particular may be especially stress responsive given the co-expression of MR and GR within the same neuron in this area [59]. CORT 118335 at both 10 mg/kg and 30 mg/kg decreased Fos expression in the CA1 in response to the FST. Mifepristone trended towards a suppression of CA1 c-Fos activation following the FST [10], suggesting that this effect may involve GR antagonism. In support of this, CORT 118335 has been demonstrated to be predominantly antagonistic on GR in the hippocampus, blocking the transcription of GR target genes in vitro, and blocking GR-mediated corticosterone-induced memory enhancement in vivo [24]. Interestingly, while mifepristone decreased c-Fos induction in the CA2, CA3, and DG in conjunction with an attenuated neuroendocrine stress response [10], CORT 118335 did not affect any of these hippocampal subdivisions at either dose. This may be due to the differing recruitment profiles for the two GR modulators [24]. Hippocampal MR antagonism has been shown to induce anxiolytic behavior [60], but this behavioral phenotype was not observed in present study, suggesting minimal effects of partial MR antagonism by CORT 118335 in this brain area.
Imipramine decreased Fos expression in the CA1 and CA3. This finding is consistent with previous reports [52]. Excess glucocorticoid secretion is particularly damaging to the hippocampus, therefore CORT 118335 and imipramine may protect the hippocampus by decreasing stress-induced neuronal excitability in these areas. A 14-day treatment of imipramine has been shown to increase neuronal activity in the DG in an ACTH chronic stress model [17]. However, the present data suggests that this DG effect is absent for an acute stress.
5. Conclusions
Overall, our findings highlight a prominent role for CORT 118335 as a potent endocrine stress-dampening compound in male rats. However, based on its effects on neuronal activation in the brain areas investigated, it is unclear how CORT 118335 attenuates stress-induced neuroendocrine responses. Alongside the imipramine data, the present findings illuminate the complex association between HPA axis function and mood-like behavior. It is heavily reported that glucocorticoid dyshomeostasis is associated with depression. However, our results indicate dissociation between corticosterone and immobility (index of depression-like behavior) in the FST. C-Fos is used as a crude measure of neuronal activity; therefore, we do not know the phenotype of these activated neurons. Because of this limitation, we acknowledge that CORT 118335 and imipramine may not only modulate different brain regions in response to stress, but perhaps also activate different neurotransmitter and/or molecular substrates in male rats. One potential limitation of the present study is that we examined the efficacy of CORT 118335 on endocrine, behavioral, and central responses to an acute stressor in otherwise healthy animals. While these data have advanced our understanding of how this GR modulator/MR antagonist combats acute stress responses, these findings may not extend to chronic stress conditions. CORT 118335 and other GR modulating compounds are designed to mitigate pathological conditions associated with prolonged or excess glucocorticoid production. As such, future studies will explore the efficacy of this compound in established rodent models of chronic stress that induce metabolic and behavioral phenotypes of clinical relevance.
Remembering Dr. Randall Sakai
Our laboratory is honored to take part in this special issue honoring one of my mentors, Dr. Randall Sakai. My tenure at the University of Cincinnati began as a postdoctoral fellow in 2006. Although Randall was not my formal mentor, he quickly took me under his wing. He never addressed me by my first name; instead he called me Solomon. I can still hear him yelling, “Solomon, what’s wrong with you girl, are you afraid of success?” Randall pushed me to think outside of the box and constantly reassured me that Is belonged in science. I knew when he was proud of me and he didn’t mince words when he was disappointed in me. He was keenly aware that I avoided social interactions, so he made it his business to introduce me to everyone, just to aggravate me. In fact, he made it his business to introduce all of his trainees and even other mentors’ trainees to everyone that he knew.
When I transitioned from a postdoc to a faculty member at the University of Cincinnati, he completely opened up his laboratory to me. It is so fitting that we are submitting a manuscript highlighting the role of a compound targeting two receptors that Randall knew very well, GR and MR. I was thrilled to learn that my graduate student Elizabeth Nguyen (first author) won the first “Randall Sakai” Graduate Student Travel Award at the Stress Neurobiology Workshop in Irvine California in April 2016. I was simultaneously elated and saddened. I was saddened because I wanted him to be here to see this. However, that sadness was quickly replaced with laughter because I knew he was looking down frowning about how I overlooked one small detail that could potentially change the interpretation of our findings. Apart from his role in my professional life, we bonded personally over our love of great food. He was an incredible cook and an even greater entertainer. He loved entertaining people, but most of all he loved being a mentor. No matter how successful we become as independent investigators we always want to make our mentors proud. Randall, I hope that our laboratory is making you proud. Randall, we miss you today, tomorrow, and forever.

Liz wins the Randall Sakai Travel Award at the 2016 Stress Neurobiology Workshop!
Love always, Tia and Jody
HIGHLIGHTS.
CORT 118335 attenuates corticosterone stress responses in male rats.
CORT 118335 does not induce antidepressant-like effects in the forced swim test.
Antidepressant-like effects of imipramine are independent of glucocorticoids.
CORT 118335 and imipramine differentially modulate corticolimbic c-Fos activity.
Acknowledgments
This work was supported by grants from Corcept Therapeutics to MBS and the National Institutes of Health (NIH T32-NS007453) to ETN. We would like to thank Corcept Therapeutics for providing us with the drug compound CORT 118335 and Hazel Hunt for providing feedback on the manuscript. We are grateful to previous and current members of both the Solomon and Herman labs for their help with executing the behavioral testing and blood collection.
References
- 1.Bose M, Olivan B, Laferrere B. Stress and obesity: the role of the hypothalamic-pituitary-adrenal axis in metabolic disease. Curr Opin Endocrinol Diabetes Obes. 2009;16:340–346. doi: 10.1097/MED.0b013e32832fa137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res. 2000;861:288–295. doi: 10.1016/s0006-8993(00)02019-9. [DOI] [PubMed] [Google Scholar]
- 3.Pariante CM. The glucocorticoid receptor: part of the solution or part of the problem? J Psychopharmacol. 2006;20:79–84. doi: 10.1177/1359786806066063. [DOI] [PubMed] [Google Scholar]
- 4.Myers B, McKlveen JM, Herman JP. Neural regulation of the stress response the many faces of feedback. Cell Mol Neurobiol. 2012;22:683–694. doi: 10.1007/s10571-012-9801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, et al. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987;237:268–275. doi: 10.1126/science.3037703. [DOI] [PubMed] [Google Scholar]
- 6.Solomon MB, Furay AR, Jones K, Packard AE, Packard BA, Wulsin AC, et al. Deletion of forebrain glucocorticoid receptors impairs neuroendocrine stress responses and induces depression-like behavior in males but not females. Neuroscience. 2012;203:135–143. doi: 10.1016/j.neuroscience.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyle MP, Brewer JA, Funatsu M, Wozniak DF, Tsien JZ, Izumi Y, et al. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc Natl Acad Sci U S A. 2005;102:473–478. doi: 10.1073/pnas.0406458102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger S, Wolfer DP, Selbach O, Alter H, Erdmann G, Reichardt HM, et al. Loss of the limbic mineralocorticoid receptor impairs behavioral plasticity. Proc Natl Acad Sci U S A. 2006;103:195–200. doi: 10.1073/pnas.0503878102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rozeboom AM, Akil H, Seasholtz AF. Mineralocorticoid receptor overexpression in forebrain decreases anxiety-like behavior and alters the stress response in mice. Proc Natl Acad Sci U S A. 2007;104:4688–4693. doi: 10.1073/pnas.0606067104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wulsin AC, Herman JP, Solomon MB. Mifepristone decreases depression-like behavior and modulates neuroendocrine and central hypothalamic-pituitary-adrenocortical axis responsiveness to stress. Psychoneuroendocrinology. 2010;35:1100–1112. doi: 10.1016/j.psyneuen.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solomon MB, Wulsin AC, Rice T, Wick D, Myers B, McKlveen J, et al. The selective glucocorticoid receptor antagonist CORT 108297 decreases neuroendocrine stress responses and immobility in the forced swim test. Horm Behav. 2014;65:363–371. doi: 10.1016/j.yhbeh.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zalachoras I, Houtman R, Atucha E, Devos R, Tijssen AM, Hu P, et al. Differential targeting of brain stress circuits with a selective glucocorticoid receptor modulator. Proc Natl Acad Sci U S A. 2013;110:7910–7915. doi: 10.1073/pnas.1219411110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frost P, Bornstein S, Ehrhart-Bornstein M, O’Kirwan F, Hutson C, Heber D, et al. The prototypic antidepressant drug, imipramine, but not Hypericum perforatum (St. John’s Wort), reduces HPA-axis function in the rat. Horm Metab Res. 2003;35:602–606. doi: 10.1055/s-2003-43507. [DOI] [PubMed] [Google Scholar]
- 14.Michelson D, Galliven E, Hill L, Demitrack M, Chrousos G, Gold P. Chronic imipramine is associated with diminished hypothalamic-pituitary-adrenal axis responsivity in healthy humans. J Clin Endocrinol Metab. 1997;82:2601–2606. doi: 10.1210/jcem.82.8.4172. [DOI] [PubMed] [Google Scholar]
- 15.Brady LS, Whitfield HJ, Jr, Fox RJ, Gold PW, Herkenham M. Long-term antidepressant administration alters corticotropin-releasing hormone, tyrosine hydroxylase, and mineralocorticoid receptor gene expression in rat brain. Therapeutic implications. J Clin Invest. 1991;87:831–837. doi: 10.1172/JCI115086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duncan GE, Knapp DJ, Johnson KB, Breese GR. Functional classification of antide-pressants based on antagonism of swim stress-induced fos-like immunoreactivity. J Pharmacol Exp Ther. 1996;277:1076–1089. [PubMed] [Google Scholar]
- 17.Li B, Suemaru K, Kitamura Y, Gomita Y, Araki H, Cui R. Imipramine-induced c-Fos expression in the medial prefrontal cortex is decreased in the ACTH-treated rats. J Biochem Mol Toxicol. 2013;27:486–491. doi: 10.1002/jbt.21510. [DOI] [PubMed] [Google Scholar]
- 18.Lim DW, Lee MS, Her S, Cho S, Lee CH, Kim IH, et al. Antidepressant-like effects of Lindera obtusiloba extracts on the immobility behavior of rats in the forced swim test. Molecules. 2016;21:277. doi: 10.3390/molecules21030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barros HM, Ferigolo M. Ethopharmacology of imipramine in the forced-swimming test: gender differences. Neurosci Biobehav Rev. 1998;23:279–286. doi: 10.1016/s0149-7634(98)00029-3. [DOI] [PubMed] [Google Scholar]
- 20.Wu TC, Chen HT, Chang HY, Yang CY, Hsiao MC, Cheng ML, et al. Mineralocorticoid receptor antagonist spironolactone prevents chronic corticosterone induced depression-like behavior. Psychoneuroendocrinology. 2013;38:871–883. doi: 10.1016/j.psyneuen.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Ratka A, Sutanto W, Bloemers M, de Kloet ER. On the role of brain mineralocorticoid (type I) and glucocorticoid (type II) receptors in neuroendocrine regulation. Neuroendocrinology. 1989;50:117–123. doi: 10.1159/000125210. [DOI] [PubMed] [Google Scholar]
- 22.Young EA, Lopez JF, Murphy-Weinberg V, Watson SJ, Akil H. The role of mineralocorticoid receptors in hypothalamic-pituitary-adrenal axis regulation in humans. J Clin Endocrinol Metab. 1998;83:3339–3345. doi: 10.1210/jcem.83.9.5077. [DOI] [PubMed] [Google Scholar]
- 23.Mattsson C, Reynolds RM, Simonyte K, Olsson T, Walker BR. Combined receptor antagonist stimulation of the hypothalamic-pituitary-adrenal axis test identifies impaired negative feedback sensitivity to cortisol in obese men. J Clin Endocrinol Metab. 2009;94:1347–1352. doi: 10.1210/jc.2008-2054. [DOI] [PubMed] [Google Scholar]
- 24.Atucha E, Zalachoras I, van den Heuvel JK, van Weert LT, Melchers D, Mol IM, et al. A mixed glucocorticoid/mineralocorticoid selective modulator with dominant antagonism in the male rat brain. Endocrinology. 2015;156:4105–4114. doi: 10.1210/en.2015-1390. [DOI] [PubMed] [Google Scholar]
- 25.Hunt HJ, Ray NC, Hynd G, Sutton J, Sajad M, O’Connor E, et al. Discovery of a novel non-steroidal GR antagonist with in vivo efficacy in the olanzapine-induced weight gain model in the rat. Bioorg Med Chem Lett. 2012;22:7376–7380. doi: 10.1016/j.bmcl.2012.10.074. [DOI] [PubMed] [Google Scholar]
- 26.Pepin MC, Govindan MV, Barden N. Increased glucocorticoid receptor gene promoter activity after antidepressant treatment. Mol Pharmacol. 1992;41:1016–1022. [PubMed] [Google Scholar]
- 27.Pepin MC, Beaulieu S, Barden N. Antidepressants regulate glucocorticoid receptor messenger RNA concentrations in primary neuronal cultures. Brain Res Mol Brain Res. 1989;6:77–83. doi: 10.1016/0169-328x(89)90031-4. [DOI] [PubMed] [Google Scholar]
- 28.Okugawa G, Omori K, Suzukawa J, Fujiseki Y, Kinoshita T, Inagaki C. Long-term treatment with antidepressants increases glucocorticoid receptor binding and gene expression in cultured rat hippocampal neurones. J Neuroendocrinol. 1999;11:887–895. doi: 10.1046/j.1365-2826.1999.00405.x. [DOI] [PubMed] [Google Scholar]
- 29.Budziszewska B, Jaworska-Feil L, Kajta M, Lason W. Antidepressant drugs inhibit glucocorticoid receptor-mediated gene transcription - a possible mechanism. Br J Pharmacol. 2000;130:1385–1393. doi: 10.1038/sj.bjp.0703445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peeters BW, Tonnaer JA, Groen MB, Broekkamp CL, van der Voort HA, Schoonen WG, et al. Glucocorticoid receptor antagonists: new tools to investigate disorders characterized by cortisol hypersecretion. Stress. 2004;7:233–241. doi: 10.1080/10253890400019672. [DOI] [PubMed] [Google Scholar]
- 31.Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, et al. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab. 2005;289:E823–E828. doi: 10.1152/ajpendo.00122.2005. [DOI] [PubMed] [Google Scholar]
- 32.Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Gutierrez-Garcia AG, Contreras CM. Stressors can affect immobility time and response to imipramine in the rat forced swim test. Pharmacol Biochem Behav. 2008;91:542–548. doi: 10.1016/j.pbb.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Spink AJ, Tegelenbosch RAJ, Buma MOS, Noldus LPJJ. The ethovision video tracking system: a tool for behavioral phenotyping of transgenic mice.pdf. Physiol Behav. 2001;73:731–744. doi: 10.1016/s0031-9384(01)00530-3. [DOI] [PubMed] [Google Scholar]
- 35.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 1998 doi: 10.1016/0165-0270(80)90021-7. [DOI] [PubMed] [Google Scholar]
- 36.Pace TW, Spencer RL. Disruption of mineralocorticoid receptor function increases corticosterone responding to a mild, but not moderate, psychological stressor. Am J Physiol Endocrinol Metab. 2005;288:E1082–E1088. doi: 10.1152/ajpendo.00521.2004. [DOI] [PubMed] [Google Scholar]
- 37.Walker CD, Trottier G, Rochford J, Lavallee D. Dissociation between behavioral and hormonal responses to the forced swim stress in lactating rats. J Neuroendocrinol. 1995;7:615–622. doi: 10.1111/j.1365-2826.1995.tb00799.x. [DOI] [PubMed] [Google Scholar]
- 38.Rogoz Z, Kabzinski M, Sadaj W, Rachwalska P, Gadek-Michalska A. Effect of cotreatment with fluoxetine or mirtazapine and risperidone on the active behaviors and plasma corticosterone concentration in rats subjected to the forced swim test. Pharmacol Rep. 2012;64:1391–1399. doi: 10.1016/s1734-1140(12)70936-2. [DOI] [PubMed] [Google Scholar]
- 39.De Kloet ER, De Kock S, Schild V, Veldhuis HD. Antiglucocorticoid RU 38486 attenuates retention of a behaviour and disinhibits the hypothalamic-pituitary adrenal axis at different brain sites. Neuroendocrinology. 1988;47:109–115. doi: 10.1159/000124900. [DOI] [PubMed] [Google Scholar]
- 40.McKlveen JM, Myers B, Flak JN, Bundzikova J, Solomon MB, Seroogy KB, et al. Role of prefrontal cortex glucocorticoid receptors in stress and emotion. Biol Psychiatry. 2013;74:672–679. doi: 10.1016/j.biopsych.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP. The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. Eur J Neurosci. 2003;18:2357–2364. doi: 10.1046/j.1460-9568.2003.02932.x. [DOI] [PubMed] [Google Scholar]
- 43.Radley JJ, Gosselink KL, Sawchenko PE. A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci. 2009;29:7330–7340. doi: 10.1523/JNEUROSCI.5924-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heuser I, Deuschle M, Weber B, Stalla GK, Holsboer F. Increased activity of the hypothalamus-pituitary-adrenal system after treatment with the mineralocorticoid receptor antagonist spironolactone. Psychoneuroendocrinology. 2000;25:513–518. doi: 10.1016/s0306-4530(00)00006-8. [DOI] [PubMed] [Google Scholar]
- 45.Reis FM, Almada RC, Fogaca MV, Brandao ML. Rapid activation of glucocorticoid receptors in the prefrontal cortex mediates the expression of contextual conditioned fear in rats. Cereb Cortex. 2016;26:2639–2649. doi: 10.1093/cercor/bhv103. [DOI] [PubMed] [Google Scholar]
- 46.Goncharova ND. Stress responsiveness of the hypothalamic-pituitary-adrenal axis: age-related features of the vasopressinergic regulation. Front Endocrinol (Lausanne) 2013;4:26. doi: 10.3389/fendo.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Laan S, Lachize SB, Vreugdenhil E, de Kloet ER, Meijer OC. Nuclear receptor coregulators differentially modulate induction and glucocorticoid receptor-mediated repression of the corticotropin-releasing hormone gene. Endocrinology. 2008;149:725–732. doi: 10.1210/en.2007-1234. [DOI] [PubMed] [Google Scholar]
- 48.Lachize S, Apostolakis EM, van der Laan S, Tijssen AM, Xu J, de Kloet ER, et al. Steroid receptor coactivator-1 is necessary for regulation of corticotropin-releasing hormone by chronic stress and glucocorticoids. Proc Natl Acad Sci U S A. 2009;106:8038–8042. doi: 10.1073/pnas.0812062106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zalachoras I. Targeting the Brain under Stress: Selective Glucocorticoid Receptor Modulation. Leiden Univeristy Medical Center; Leiden, The Netherlands: 2014. [Google Scholar]
- 50.Zhang ZH, Francis J, Weiss RM, Felder RB. The renin-angiotensin-aldosterone system excites hypothalamic paraventricular nucleus neurons in heart failure. Am J Physiol Heart Circ Physiol. 2002;283:H423–H433. doi: 10.1152/ajpheart.00685.2001. [DOI] [PubMed] [Google Scholar]
- 51.Kang YM, Zhang ZH, Johnson RF, Yu Y, Beltz T, Johnson AK, et al. Novel effect of mineralocorticoid receptor antagonism to reduce proinflammatory cytokines and hypothalamic activation in rats with ischemia-induced heart failure. Circ Res. 2006;99:758–766. doi: 10.1161/01.RES.0000244092.95152.86. [DOI] [PubMed] [Google Scholar]
- 52.de Medeiros MA, Carlos Reis L, Eugenio Mello L. Stress-induced c-Fos expression is differentially modulated by dexamethasone, diazepam and imipramine. Neuropsychopharmacology. 2005;30:1246–1256. doi: 10.1038/sj.npp.1300694. [DOI] [PubMed] [Google Scholar]
- 53.Beaulieu S, Di Paolo T, Barden N. Control of ACTH secretion by the central nucleus of the amygdala: implication of the serotoninergic system and its relevance to the glucocorticoid delayed negative feedback mechanism. Neuroendocrinology. 1986;44:247–254. doi: 10.1159/000124652. [DOI] [PubMed] [Google Scholar]
- 54.Feldman S, Conforti N, Itzik A, Weidenfeld J. Differential effect of amygdaloid lesions on CRF-41, ACTH and corticosterone responses following neural stimuli. Brain Res. 1994;658:21–26. doi: 10.1016/s0006-8993(09)90005-1. [DOI] [PubMed] [Google Scholar]
- 55.Duncan GE, Breese GR, Criswell H, Stumpf WE, Mueller RA, Covey JB. Effects of antidepressant drugs injected into the amygdala on behavioral responses of rats in the forced swim test. J Pharmacol Exp Ther. 1986;238:758–762. [PubMed] [Google Scholar]
- 56.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Medina A, Seasholtz AF, Sharma V, Burke S, Bunney W, Jr, Myers RM, et al. Glucocorticoid and mineralocorticoid receptor expression in the human hippocampus in major depressive disorder. J Psychiatr Res. 2013;47:307–314. doi: 10.1016/j.jpsychires.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herman JP, Patel PD, Akil H, Watson SJ. Localization and regulation of glucocorticoid and mineralocorticoid receptor messenger RNAs in the hippocampal formation of the rat. Mol Endocrinol. 1989;3:1886–1894. doi: 10.1210/mend-3-11-1886. [DOI] [PubMed] [Google Scholar]
- 59.De Kloet R, Wallach G, McEwen BS. Differences in corticosterone and dexamethasone binding to rat brain and pituitary. Endocrinology. 1975;96:598–609. doi: 10.1210/endo-96-3-598. [DOI] [PubMed] [Google Scholar]
- 60.Smythe JW, Murphy D, Timothy C, Costall B. Hippocampal mineralocorticoid, but not glucocorticoid, receptors modulate anxiety-like behavior in rats. Pharmacol Biochem Behav. 1997;56:507–513. doi: 10.1016/s0091-3057(96)00244-4. [DOI] [PubMed] [Google Scholar]


