Summary
Oxidative stress influences stem cell behavior by promoting the differentiation, proliferation, or apoptosis of stem cells. Thus, characterizing the effects of reactive oxygen species (ROS) on stem cell behavior provides insights into the significance of redox homeostasis in stem cell-associated diseases and efficient stem cell expansion for cellular therapies. We utilized the Drosophila testis as an in vivo model to examine the effects of ROS on germline stem cell (GSC) maintenance. High levels of ROS induced by alteration in Keap1/Nrf2 activity decreased GSC number by promoting precocious GSC differentiation. Notably, high ROS enhanced the transcription of the EGFR ligand spitz and the expression of phospho-Erk1/2, suggesting that high ROS-mediated GSC differentiation is through EGFR signaling. By contrast, testes with low ROS caused by Keap1 inhibition or antioxidant treatment showed an overgrowth of GSC-like cells. These findings suggest that redox homeostasis regulated by Keap1/Nrf2 signaling plays important roles in GSC maintenance.
Keywords: redox homeostasis, Drosophila, germline stem cell, differentiation, Keap1/Nrf2 signaling, EGFR signaling, antioxidant
Highlights
-
•
Germline stem cell homeostasis in the Drosophila testis is susceptible to ROS levels
-
•
Oxidative stress decreases germline stem cell number by promoting differentiation
-
•
EGFR signaling is involved in precocious GSC differentiation caused by high ROS levels
-
•
Low levels of ROS can promote a growth of germline stem cells
Disruption of redox homeostasis can affect stem cell behavior. In the Drosophila testis, high ROS induced by altered Keap1/Nrf2 signaling decrease GSC number by promoting GSC differentiation via the activation of EGFR signaling. By contrast, testes with low ROS show an overgrowth of GSC-like cells. These findings suggest the importance of redox homeostasis in the Drosophila testis GSC maintenance.
Introduction
The tight regulation of reactive oxygen species (ROS) levels is crucial in maintaining cellular homeostasis. While the metabolic processes of a cell generate ROS such as superoxide anions and hydroxyl free radicals, their levels are kept at a low-to-moderate range which is essential for cellular proliferation, differentiation, and survival (Trachootham et al., 2009). ROS levels are modulated by a balance between pro-oxidants and antioxidants. When increased ROS are not countered by antioxidant activity or reducing equivalents, a cell is said to be in the state of oxidative stress. High levels of ROS can damage DNA, proteins, and lipids. As such, oxidative stress is linked to the pathogenesis of human diseases such as neurodegenerative disorders and cancer (Floyd and Hensley, 2002, Barnham et al., 2004, Khandrika et al., 2009, Rios-Arrabal et al., 2013). Hence, understanding the molecular mechanisms underlying the redox homeostasis and characterizing the effects of ROS on cellular homeostasis may lead to the development of effective therapeutic interventions for the treatment of ROS-associated human diseases.
In mammals, Keap1 (Kelch-like ECH-associated protein 1)/Nrf2 (NF-E2-related factor 2) signaling plays important roles in the regulation of ROS levels. Nrf2 functions in the antioxidant response element (ARE)-dependent transcriptional regulation of antioxidant and detoxification genes (Itoh et al., 1997, Itoh et al., 1999a, Itoh et al., 1999b). In the absence of oxidative stress, Nrf2 is bound to its cytoplasmic inhibitor Keap1 and prevented from translocating into the nucleus. Keap1 subsequently promotes the 26S proteosomal degradation of Nrf2 via a Cul3-based E3 ligase, negatively regulating ARE-mediated gene expression (McMahon et al., 2003, Kobayashi et al., 2004). In the presence of electrophilic and oxidative stress, reactive cysteine residues present on Keap1 are covalently modified, preventing the Keap1/Cul3 E3 ubiquitin ligase-mediated proteosomal degradation of Nrf2 (Kobayashi et al., 2004). Thus, Keap1 functions as a sensor of electrophilic and oxidative stress, and controls the release of Nrf2. Nrf2 then translocates into the nucleus, heterodimerizes with Maf, and binds to the ARE sequence located at the promoter region of its target genes (Motohashi et al., 2002). Binding of the Nrf2/Maf dimer to the multiple ARE sequences promotes the expression of an array of antioxidant and detoxification enzymes, which act to counter oxidative stress by scavenging ROS.
In Drosophila, the Keap1/Nrf2 signaling pathway was also shown to play roles in the regulation of oxidative stress tolerance, lifespan, and xenobiotic responses (Sykiotis and Bohmann, 2008, Misra et al., 2011). Keap1/Nrf2 signaling becomes activated by oxidant agents such as paraquat, followed by the induction of antioxidant and detoxification responses (Nguyen et al., 2009). CncC (cap 'n' collar isoform C) is the Drosophila homolog of Nrf2 and is known to regulate the ARE-mediated transcription of antioxidant genes (Sykiotis and Bohmann, 2008, Sykiotis and Bohmann, 2010). These findings suggest that Keap1/Nrf2 signaling is evolutionarily conserved across phyla.
Stem cells undergo an asymmetric cell division to produce one undifferentiated daughter cell and one daughter cell that differentiates into various cell types (Morrison et al., 1997). Interestingly, stem cells maintain low levels of ROS to keep their stemness and remain quiescent in mammals (Shi et al., 2012, Liang and Ghaffari, 2014). Disruptions to redox status of stem cell niche or stem cells leads to an oxidative stress, which subsequently causes abnormal stem cell behaviors by promoting the differentiation, proliferation, apoptosis, or senescence of stem cells (Scadden, 2006, Morrison and Spradling, 2008, Naka et al., 2008). For instance, when murine hematopoietic stem cells are exposed to oxidative stress, they lose self-renewal capacity and undergo the process of premature differentiation or apoptosis (Shi et al., 2012, Holmstrom and Finkel, 2014). Given the fact that redox homeostasis is implicated in stem cell maintenance, it is necessary to elucidate the effects of ROS on stem cell behavior in various systems and characterize effectors underlying ROS-mediated stem cell behavior.
In this study, we examined the role of Keap1/Nrf2 signaling in the regulation of ROS levels and assessed whether redox states can influence germline stem cell (GSC) maintenance in the Drosophila testis. We showed that elevated levels of ROS decrease GSC number by promoting GSC differentiation. In particular, high ROS appeared to facilitate GSC differentiation by enhancing the transcription of the epidermal growth factor receptor (EGFR) ligand spitz, which subsequently activates Erk1/2 signaling. Conversely, reduced levels of ROS by Keap1 knockdown resulted in an overgrowth of GSC-like cells. These observations suggest that redox status is one of key factors that determines the self-renewal and differentiation of GSCs in the Drosophila testis.
Results
The Oxidant Paraquat Increases ROS Levels in the Drosophila Testis
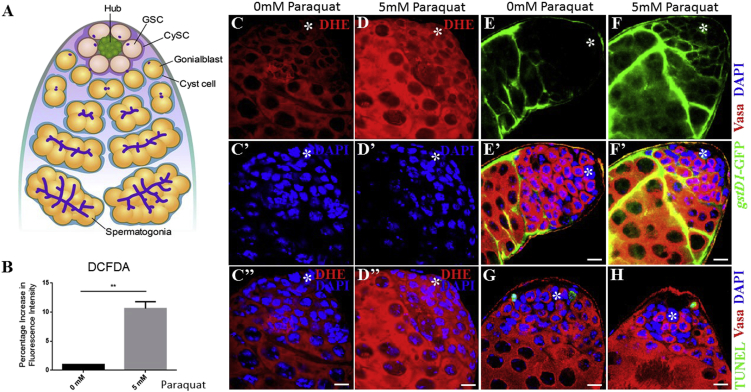
To examine the effect of redox states on GSC behavior, we tested whether treatment of flies with paraquat can induce ROS. In testis, GSCs and somatic cyst stem cells (CySCs) reside in a niche formed by a group of somatic hub cells (Figure 1A). Flies were treated with 5% sucrose alone or 5% sucrose supplemented with 5 mM paraquat. ROS levels in testes were then monitored by using CM-H2DCFDA. The intensity of fluorescence probe in treated testes was increased more than 10% compared with control, indicating that paraquat induces ROS in vivo (Figure 1B). To confirm this finding, we stained testes with dihydroethidium (DHE), which is used to monitor superoxide levels. In control testis DHE staining was detected, with a relatively low intensity at the apical tip of the testis but moderate levels at differentiating germ cells (Figures 1C and 1C″), suggesting that GSC differentiation may require moderate levels of ROS. However, paraquat-treated testis showed an increase in DHE staining throughout the testis (Figures 1D and 1D″). In Drosophila, CncC regulates the expression of gstD1 (glutathione S-transferase D1), an oxidative stress response gene. To further confirm the effects of paraquat on ROS production, we used transgenic flies carrying an independent oxidative stress reporter gene gstD1-GFP (Sykiotis and Bohmann, 2008), and assessed whether paraquat can enhance the reporter activity. Undetectable levels of GFP were observed at the apical tip of control testis (Figures 1E and 1E′), whereas we observed enhanced GFP at the apical tip of treated testis (Figures 1F and 1F′). We next examined whether paraquat treatment causes cell death by performing TUNEL assays. However, we could not detect any significant differences in the number of TUNEL-positive cells between control (1.80 per testis, n = 51) and treated testes (1.72 per testis, n = 58) (Figures 1G and 1H). Interestingly, we noticed a dramatic decrease in the number of cells with densely packed nuclei (assumed to be early-stage germ and cyst cells) positive for DAPI at the apical tip of treated testis compared with control, suggesting that altered ROS levels influence spermatogenesis (Figures 1C′ and 1D′).
Figure 1.
Paraquat Treatment Induces ROS in the Drosophila Testis
(A) Schematic of the Drosophila testis. GSC, germline stem cell; CySC, cyst stem cell.
(B–D″) ROS detection assay (B) shows an increase in ROS upon paraquat treatment. Error bar denotes SEM from three independent experiments; Student's t test (∗∗p < 0.01). ROS levels were monitored by using DHE probe in (C–C″) control testis and (D–D″) treated testis.
(E–F′) ROS levels were monitored by using the in vivo ROS reporter GstD1-GFP in (E and E′) control testis and (F and F′) treated testis.
(G and H) TUNEL assay shows that paraquat treatment does not induce cell death.
Asterisks in images indicate hub cells. Scale bars, 10 μm.
High Levels of ROS Cause a Decrease in GSC Number
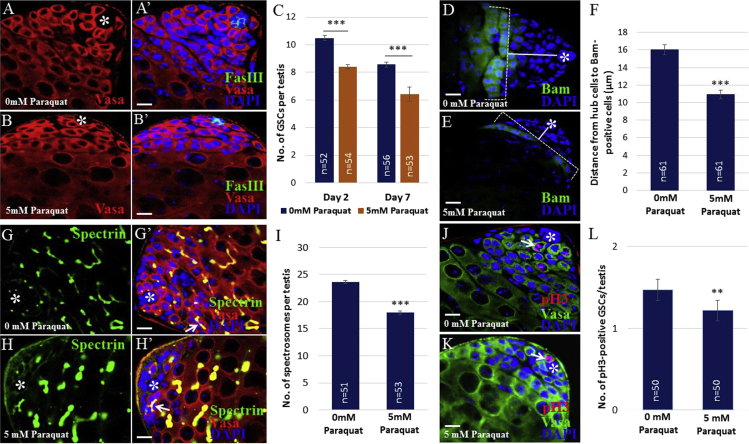
The reduction of DAPI-positive cells in paraquat-treated testis prompted us to investigate the effects of redox states on GSC number. Treated testes were stained with Vasa and FasIII antibodies, which mark germ cells and hub cells, respectively. Compared with control testes, treated testes showed a reduced number of Vasa-positive cells directly attached to hub cells, which are considered as GSCs (Figures 2A–2B′). While testes treated with paraquat for 2 days had an average of 8.3 GSCs per testis, control testes had an average of 10.4 GSCs (Figure 2C). To examine a progressive loss of GSCs, we treated testes with paraquat for 7 days and monitored GSC number. However, we could not observe any strikingly progressive loss of GSCs (Figure 2C). This may suggest that organisms started developing an endogenous antioxidant capacity upon oxidative stress and/or became adapted to high ROS environments. In addition to the decrease in GSC number, we found that differentiating spermatogonia are positioned much closer to hub cells in treated testes compared with those in control testes, suggesting that high ROS may promote GSC differentiation. Hence, we examined the expression of the differentiation-promoting factor Bam (Bag-of-marbles) using transgenic flies carrying a bam-GFP gene (a GFP signal is detected where endogenous Bam is expressed). Bam is normally expressed in 4-, 8-, and early 16-cell germline cysts located several cell diameters away from hub cells (Figure 2D). However, in paraquat-treated testes Bam-positive spermatogonia were detected closer to hub cells compared with control (Figures 2E and 2F). This finding suggests that high ROS decreased GSC number by promoting GSC differentiation. We next examined the morphology of fusome, an organelle specific for germ cells that appears spheroid in GSCs and GSC-gonialblast pairs, but appears to be interconnected branches in spermatogonia due to the incomplete cytokinesis (Hime et al., 1996) (Figure 1A). In control testes, spherical fusomes, also known as spectrosomes, were observed in GSCs attached to hub cells and in GSC-gonialblast pairs, whereas branching fusomes were detected in differentiating spermatogonia located several cell diameters away from hub cells (Figures 2G and 2G′). However, in treated testes fewer spectrosomes were observed, indicative of a decrease in early-stage germ cells. Furthermore, branching fusomes were detected closer to hub cells (Figures 2H–2I). To further confirm the decrease in GSCs by high ROS, we stained testes for phospho-histone H3 (pH3), a mitotic marker detected in actively dividing cells (Tapia et al., 2006). Both pH3- and Vasa-positive cells attached directly to hub cells would mark mitotically active GSCs. Compared with control, there was a decrease in pH3-positive GSCs in treated testes (Figures 2J and 2K). While an average of 1.5 pH3- and Vasa-positive GSCs per testis was detected in control testes, an average of 1.2 pH3-positive GSCs was observed in treated testes (Figure 2L). These findings suggest that excessive amounts of ROS cause oxidative stress, which subsequently disrupts GSC homeostasis by promoting GSC differentiation.
Figure 2.
High Levels of ROS Decrease GSC Number
(A–B′) Control (A and A′) and paraquat-treated (B and B′) testes stained with FasIII and Vasa.
(C) Paraquat-treated testes show a decrease in GSC number.
(D and E) Control (D) and treated (E) testes stained with Bam. Solid lines indicate the distance between hub cells and differentiating germ cells.
(F) Quantification of the distance between hub cells and Bam-positive germ cells.
(G–H′) Control (G and G′) and treated (H and H′) testes stained with Spectrin. Arrows indicate branching fusomes. Branching fusomes are found near hub cells in treated testis.
(I) Quantification of spectrosomes observed in control and treated testes.
(J and K) Control (J) and treated (K) testes stained with pH3, a mitotic marker. Arrows indicate pH3-positive GSCs.
(L) Treated testes show a decrease in pH3-positive GSCs.
Error bars in bar graphs denote SEM from three independent experiments; Student's t test (∗∗p < 0.01, ∗∗∗p < 0.001). Asterisks in images indicate hub cells. Scale bars, 10 μm.
Keap1/Nrf2 Signaling Regulates ROS Levels and Influences GSC Maintenance
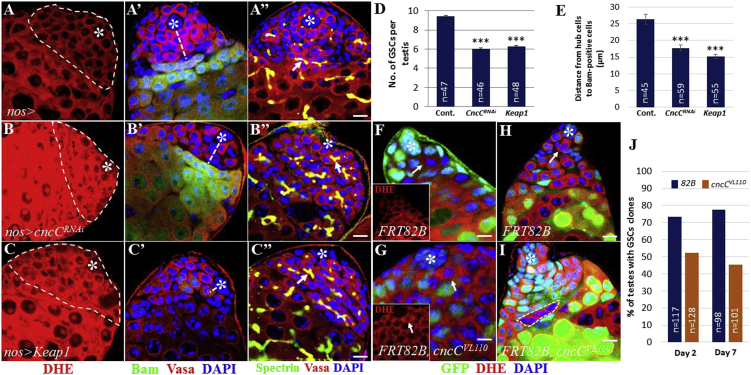
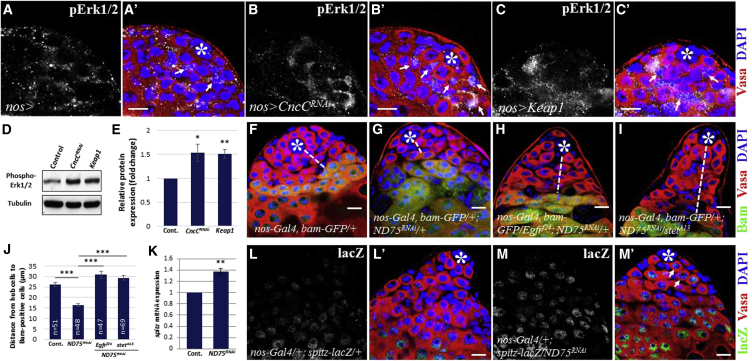
Since the Keap1/Nrf2 signaling pathway is evolutionarily conserved, we assessed whether Keap1/Nrf2 signaling can modulate ROS levels and thus affect GSC behavior in testis. We tested whether CncC knockdown or Keap1 overexpression increases ROS levels. We knocked down CncC or overexpressed Keap1 under the control of nos-Gal4, which is expressed in GSCs and early-stage germ cells. Notably, much stronger DHE staining was observed in testes expressing CncCRNAi or Keap1 compared with control (Figures 3A–3C). Since Keap1/Nrf2 activity could affect ROS levels, we expected to observe a decrease in GSCs in testes expressing CncCRNAi or Keap1. Compared with control, the resulting testes showed a reduction of GSCs attached to hub cells (Figure 3D). This is consistent with the finding that testes with high ROS induced by paraquat contain reduced GSCs (Figure 2C). We next examined the effects of high ROS caused by CncC inhibition or Keap1 overexpression on GSC differentiation. In the resulting testes Bam-positive germ cells and branching fusomes were detected much closer to hub cells compared with those in controls, indicative of premature GSC differentiation (Figures 3A′–3C″ and 3E). To further verify that high ROS promote GSC differentiation, we generated negatively marked FRT82Bwild-type or FRT82BcncCVL110 GSC clones by the FRT/FLP system. We confirmed that cncC-deficient cells show higher ROS levels than neighboring cells, indicating that CncC inhibition increases ROS levels (Figures 3F and 3G). We next counted the number of testes with at least one cncC mutant GSC remaining in the niche at 2 and 7 days after clone induction. In control testes containing FRT82Bwild-type clones, we were able to find many negatively marked GSCs in contact with hub cells (Figures 3H and 3J). However, we found a lesser number of cncC mutant GSCs compared with control, suggesting that high ROS in GSCs negatively affected their maintenance (Figures 3I and 3J). Notably, we observed GFP-negative differentiating germ cells, suggesting that cncC-deficient GSCs did not undergo apoptosis and properly differentiated. Furthermore, this suggests that cncC mutant clones can be induced in GSC populations and that high levels of ROS are deleterious to GSC self-renewal.
Figure 3.
Keap1/Nrf2 Activity Affects GSC Homeostasis by Modulating ROS Levels
(A–C″) Control testes (A–A″), cncCRNAi-expressing testes (B–B″), and Keap1-expressing testes (C–C″). (A, B, C) DHE staining. Dotted area marks the apical tip of testis in which early-stage germ and cyst cells reside. (A′, B′, C′) Bam staining. Dashed lines indicate the distance between hub cells and differentiating germ cells. (A″, B″, C″) Spectrin staining. Arrows indicate branching fusomes.
(D) Testes overexpressing Keap1 or cncCRNAi show a decrease in GSC number compared with control.
(E) Distance between hub cells and Bam-positive germ cells.
(F) Control FRT clones. Arrow indicates a GFP-negative germ cell.
(G) cncC mutant clones. Arrow indicates cncC mutant clone showing higher DHE staining than neighboring cells.
(H) Control FRT clones. Arrow indicates a GFP-negative GSC.
(I) cncC mutant clones. cncC mutant GSC is not observed, but cncC mutant germ cell clone is detected (dotted area).
(J) Clonal analysis suggests that CncC activity is required for GSCs to maintain their stemness.
Error bars in (D) and (E) denote SEM from three independent experiments; Student's t test (∗∗∗p < 0.001). Asterisks in images indicate hub cells. Scale bars, 10 μm.
Intracellular Redox State Affects GSC Maintenance
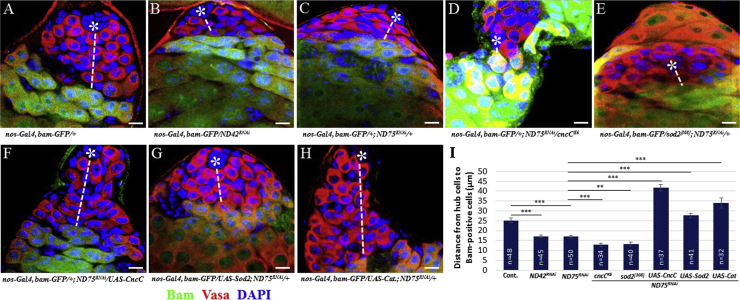
To further confirm that GSC homeostasis can be influenced by redox states, we examined the inhibitory effects of the components of complex I of the mitochondrial electron transport chain ND75 and ND42 on GSC behavior. ND75 and ND42 are the Drosophila homologs of NDUFS1 and NDUFA10, respectively. NDUFS1 and NDUFA10 are the mitochondria complex 1 subunits, which have NADH dehydrogenase and oxidoreductase activities, and function to transfer electrons from NADH to the respiratory chain. Hence, ROS levels are expected to be increased by the disruption of mitochondria complex 1. Consistent with the hypothesis that increased ROS promote GSC differentiation, knockdown of ND75 or ND42 triggered premature GSC differentiation as evidenced by the location of differentiating Bam-positive germ cells (Figures 4A–4C and 4I). We next tested whether the premature GSC differentiation phenotype caused by ND75 inhibition can be modulated by molecules involved in the ROS scavenging system. Removing one copy of cncC (cncCK6) or the antioxidant superoxide dismutase 2 (sod2[308]) gene further enhanced the phenotype (Figures 4D, 4E, and 4I). By contrast, co-expression of CncC, Sod2, or Catalase efficiently suppressed the defects observed in ND75RNAi testes (Figures 4F–4I). These findings suggest that redox homeostasis plays important roles in the maintenance of GSCs.
Figure 4.
Redox Status Influences GSC Homeostasis
(A–H) Bam-positive germ cells in testes expressing ND42RNAi or ND75RNAi (A–C) are detected closer to hub cells compared with those in control testes. Removing one copy of (D) cncC or (E) sod2 further enhances ND75RNAi phenotype. GSC differentiation induced by ND75 knockdown is suppressed by co-expression of (F) CncC, (G) Sod2, or (H) Catalase. Dashed lines indicate the distance between hub cells and differentiating germ cells. Asterisks indicate hub cells. Scale bars, 10 μm.
(I) Distance between hub cells and Bam-positive germ cells in testes with different genotypes. Error bar is SEM from three independent experiments; Student's t test (∗∗p < 0.01 and ∗∗∗p < 0.001).
High Levels of ROS Promote Premature GSC Differentiation by Activating EGFR Signaling
In the Drosophila gonads, EGFR signaling facilitates the differentiation of germ cells in both sexes (Shilo, 2005, Liu et al., 2010). In testis, germ cells secrete EGFR ligands including Spitz (Spi), which bind to their receptors present on neighboring cyst cells, triggering the activation of the EGFR downstream components such as Rac1 and Raf. A pair of cyst cells encloses germ cells through their cytoplasmic extensions, providing a cellular environment necessary to trigger germ cell differentiation. Consistently, mutations in Egfr, spi, or raf cause defects in encapsulating germ cells, leading to the disruption of germ cell differentiation and the accumulation of early-stage germ cells (Kiger et al., 2000, Tran et al., 2000, Sarkar et al., 2007), indicating the intercellular communication between two different stem cell populations in controlling the cell fate of one another. Thus, it is conceivable that high ROS-mediated GSC differentiation is involved in EGFR signaling. To test this hypothesis, we stained testes carrying nos>Keap1 or nos>CncCRNAi for phospho-Erk1/2 to monitor the activity of mitogen-activated protein kinase (MAPK) in cyst cells. In control testes, low levels of p-Erk1/2 were detected in CySCs as well as in differentiating cyst cells (Figure 5A). However, in CncC-knockdown or Keap1-overexpressing testes, p-Erk1/2 was significantly increased in CySCs and cyst cells (Figures 5B and 5C). To confirm this finding, we quantified p-Erk1/2 levels by western blot analysis, and found that CncC knockdown or Keap1 overexpression increases p-Erk1/2 levels (Figures 5D and 5E). This suggests that EGFR signaling became activated upon excessive ROS levels, leading to GSC differentiation. We next assessed whether inhibition of EGFR signaling can suppress GSC differentiation by high ROS. If ROS promote GSC differentiation via EGFR signaling, removing one copy of EGFR signaling components would at least in part suppress the phenotype. ND75RNAi was ectopically expressed in testes heterozygous for Egfr or stem cell tumor (stet). Reducing EGFR or Stet activity efficiently suppressed the premature GSC differentiation phenotype (Figures 5F–5J). Stet is known to be expressed in germ cells to process EGF-related ligands to generate an active diffusible form of the ligands, facilitating the ligands to bind to EGF receptors on cyst cells and activate the EGFR downstream cascade for GSC differentiation (Schulz et al., 2002). Since reducing Stet activity efficiently suppressed GSC differentiation caused by ND75 knockdown, we hypothesized that high ROS in GSCs may be involved in the expression of EGFR ligand(s) and/or Stet. qRT-PCR analysis was performed to examine the transcription of spitz, keren, grk, vein, and stet, using mRNA extracted from testes expressing ND75RNAi. We found that ROS increase the transcription of spitz (Figure 5K), but we could not detect any significant alteration in the mRNA levels of keren, grk, and vein, as well as stet (data not shown). Consistently, spitz-lacZ expression was greatly induced in early-stage germ cells within testes expressing ND75RNAi compared with that in control testes (Figures 5L–5M′).
Figure 5.
EGFR Signaling Is Involved in High ROS-Mediated GSC Differentiation
(A–C′) Control testis (A and A′), cncCRNAi-expressing testes (B and B′), and Keap1-expressing testes (C and C′). Testes with excessive ROS show an increased phospho-Erk1/2 expression compared with control. Arrows indicate p-Erk1/2-positive cyst cells.
(D and E) High levels of ROS increase phospho-Erk1/2 levels.
(F and G) Control (F) and ND75RNAi (G) testes. ND75 inhibition promotes GSC differentiation.
(H and I) ND75RNAi phenotype is suppressed by removing one copy of Egfr or stet.
(J) Distance between hub cells and Bam-positive germ cells in testes with different genotypes.
(K) High ROS enhance the transcription of spitz.
(L–M′) Control (L and L′) and ND75RNAi (M and M′) testes. spitz promoter-driven lacZ expression is highly detected in early-stage germ cells (arrows) in ND75RNAi testis.
Error bars in graphs denote SEM from three independent experiments; Student's t test (∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001). Asterisks in images indicate hub cells, and dashed lines indicate the distance between hub cells and differentiating germ cells. Scale bars, 10 μm.
Low Levels of ROS Promote the Growth of GSC-like Cells
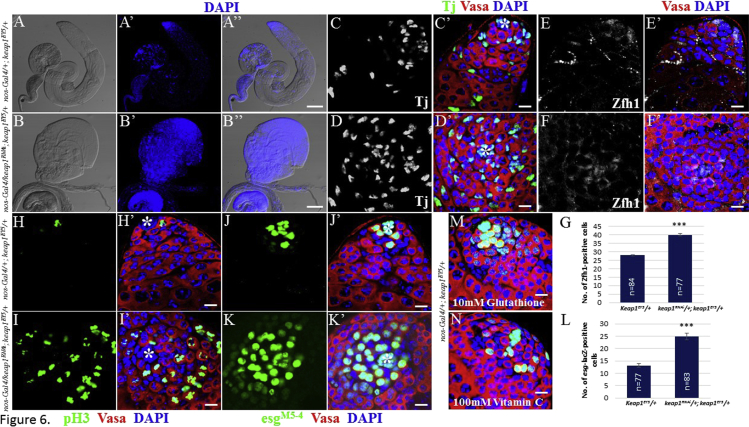
Since high ROS promote GSC differentiation, it is conceivable that low levels of ROS conversely facilitate GSC growth. Keap1 is a negative effector of Nrf2, and thus its inhibition causes the stabilization and nuclear translocalization of Nrf2 (Itoh et al., 1999a, Itoh et al., 1999b). We overexpressed keap1RNAi in a sensitized background of keap1EY5 allele to lower ROS levels and examined the effects of Keap1 inhibition on GSC behavior. Interestingly, approximately 35.5% (n = 366) of testes with reduced Keap1 activity became enlarged compared with control (compare Figures 6A–6A″ and 6B–6B″). Furthermore, a significant increase in DAPI- and Vasa-positive cells was observed in testes. Surprisingly, the number of cells positive for Traffic jam (Tj), which marks both CySCs and early-stage cyst cells, was also increased in testes with reduced Keap1 activity, suggesting the non-cell-autonomous effect of ROS on cyst cells (Figures 6C–6D′). To confirm the non-autonomous effects of ROS, we also examined the expression of Zfh1, a transcription repressor required for CySC self-renewal and is expressed in CySCs and early-stage cyst cells, and found that Zfh1-postive cell number is significantly increased in testis with reduced Keap1 activity compared with control (Figures 6E–6G). To demonstrate that the increased DAPI- and Vasa-positive cells are mitotically active, we stained testes with pH3 antibody. Compared with control where pH3-positive cells are restricted to GSCs and some of the transit amplifying germ cells, pH3-positive cells were widely distributed throughout the testes with reduced Keap1 activity (Figures 6H–6I′). Furthermore, compared with control, many of the Vasa-positive cells were positive for esg-lacZ, which marks only early-stage germ cells such as GSCs and gonialblasts, suggesting that decreased ROS levels result in a growth of early-stage germ cells (Figures 6J–6L). The effects of low ROS on germ cell growth were further confirmed by treating testes with the antioxidants glutathione and vitamin C. Although their effects on germ cell growth were not prominent compared with those of Keap1 inhibition, the resulting testes were also bigger, with more germ cells positive for Vasa and esg-lacZ compared with control testes (Figures 6M and 6N). All of these observations suggest that low ROS facilitate the growth of early-stage germ cells.
Figure 6.
Low Levels of ROS Facilitate GSC Growth
Control testes heterozygous for keap1 (A, A′, A″, C, C′, E, E′, H, H′, J, J′); testes heterozygous for keap1 that further express keap1RNAi under the control of nos-Gal4 (B, B′, B″, D, D′, F, F′, I, I′, K, K′). (B–B″) Reduced ROS cause enlarged testes containing excessive DAPI-positive cells, indicative of active cell proliferation. Keap1 inhibition results in a drastic increase in the number of (D and D′) Tj-positive cyst cells, (F, F′, G) Zfh1-positive early-stage cyst cells, (I and I′) pH3-postive germ cells, and (K, K′, L) esg-lacZ-positive early-stage germ cells. (M and N) Treatment of the antioxidants glutathione or vitamin C causes enlarged testes. Error bars in (G) and (L) denote SEM from three independent experiments; Student's t test (∗∗∗p < 0.001). Asterisks in images indicate hub cells. Scale bars, 100 μm (A″, B″) and 10 μm (C′, D′, E′, F′, H′, I′, J′, K′, M, N).
Discussion
Here, we showed that GSCs are susceptible to ROS levels and that aberrant ROS production has pronounced effects on GSC homeostasis. In particular, we found that oxidative stress induced by paraquat treatment results in a decrease in GSC number by promoting GSC differentiation. The effects of high ROS on GSC maintenance was further confirmed in the testes with altered Keap1/Nrf2 activity, which functions in antioxidant responses in Drosophila (Sykiotis and Bohmann, 2008, Hochmuth et al., 2011). Importantly, we showed that Keap1 overexpression or CncC inhibition dramatically increases ROS levels but decreases GSC number. Furthermore, we provided evidence that EGFR signaling is involved in high ROS-mediated GSC differentiation. Lastly, we showed that low ROS conversely promote a growth of GSC-like cells. These observations suggest that redox homeostasis regulated by Keap1/Nrf2 signaling plays a vital role in the maintenance of GSCs in the Drosophila testis.
Effects of High ROS on GSC Differentiation
We observed that excessive ROS production via an oxidant or altered Keap1/Nrf2 activity can effectively disturb the equilibrium of stem cell fate, favoring GSC differentiation at the expense of GSC self-renewal. Another stem cell population, CySCs, was shown to produce local signals such as Dpp (Decapentaplegic) and Gbb (Glass bottom boat), the Drosophila homologs of BMP (bone morphogenetic protein), and thus promote the self-renewal of neighboring GSCs (Kawase et al., 2004). Hence, we cannot rule out the possibility that elevated ROS levels caused by paraquat affected CySC behavior, subsequently leading to GSC differentiation. However, testis expressing Keap1 or CncCRNAi under the control of nos-Gal4 also showed a significant decrease in GSC number in a cell-autonomous manner, suggesting that excessive ROS in germ cells affected GSC-intrinsic factors required for GSC stemness. The non-cell-autonomous effects of ROS on GSCs were also examined by expressing Keap1 or CncCRNAi in the control of Tj-Gal4, which is expressed in early-stage cyst cells. Indeed, we found a significant decrease in GSC number, indicating the non-cell-autonomous effects of ROS (data not shown). Although we have not determined the cell-autonomous effects of ROS on CySCs in this study, it may be worth examining whether redox states affect CySC maintenance and elucidating mechanisms underlying the non-cell-autonomous effects of ROS on GSCs. ROS can diffuse across the cells and affect neighboring cells (Nathan and Cunningham-Bussel, 2013). Thus, it is also possible that high ROS in early-stage cyst cells diffused from the cells and affected GSC-intrinsic factors required for its maintenance.
The EGFR signaling pathway governs GSC fate by promoting GSC differentiation. EGFR ligands produced from GSCs are cleaved and activated by the transmembrane protease Stet, a Drosophila homolog of Rhomboid (Tran et al., 2000, Urban et al., 2001, Urban et al., 2002), and then bind to EGFR on CySCs, activating its downstream molecules such as Raf and MAP kinase (Erk1/2). This in turn causes the initiation of GSC differentiation by an unknown mechanism (Tran et al., 2000, Kiger et al., 2000). Our study showed an increase in phospho-Erk1/2 in early-stage cyst cells in testes expressing Keap1 or CncCRNAi, suggesting that EGFR signaling is involved in high ROS-mediated GSC differentiation. This hypothesis was verified by the fact that removal of one copy of Egfr or stet efficiently suppressed GSC differentiation caused by ND75 knockdown. Since Stet is required for the processing of EGFR ligands, our finding suggests that EGFR ligands and/or Stet itself may be involved in high ROS-mediated GSC differentiation. To test whether high ROS in GSCs can activate EGFR signaling by affecting the expression of EGFR ligands such as Spitz, and their cleavage enzyme Stet, we monitored their expression using mRNA extracted from testes with high ROS. We found that high ROS increase spitz transcription (Figure 5K). Consistently, spitz-lacZ expression was greatly induced in early-stage germ cells as ROS increased (Figures 5L–5M′).
The effects of altered redox milieu on stem cells appear to be conserved throughout evolution. In several mammalian stem cell lineages and the Drosophila lymph gland, increased ROS facilitate stem and/or progenitor cells to differentiate. In humans, the hypoxic hematopoietic stem cell (HSC) niche maintains low levels of ROS that confer HSCs to remain quiescent and have a higher self-renewal potential. However, when ROS levels become high, phosphorylated p38 MAPK levels also increase, disrupting HSC fate and promoting its differentiation (Jang and Sharkis, 2007). Similarly, high ROS promote HSC differentiation by modulating p38 activity, thus limiting HSC lifespan in mice (Ito et al., 2006). Furthermore, loss of antioxidant factor such as forkhead O was shown to cause a significant reduction in the HSC pool and a deficiency of long-term repopulating capacity of HSCs in mice, suggesting that excessive ROS negatively affects HSC stemness (Miyamoto et al., 2007, Tothova and Gilliland, 2007). On the other hand, in the Drosophila HSC niche high ROS prime hematopoietic progenitors for differentiation. In particular, elevated ROS exceeding the basal level facilitate the differentiation of hematopoietic precursors into mature blood cell types (Owusu-Ansah and Banerjee, 2009). Interestingly, pathogen infection was also shown to induce ROS production in the HSC niche, resulting in the secretion of Spitz and the upregulation of Stet, which subsequently leads to the differentiation of hematopoietic progenitors into specialized cells such as lamellocytes, suggesting that excessive ROS promote the differentiation of hematopoietic progenitors through activating EGFR signaling in Drosophila (Sinenko et al., 2011).
In the Drosophila intestinal environment, Keap1 and Nrf2 regulate intestinal stem cell (ISC) proliferation and differentiation. However, high ROS caused by paraquat treatment or CncC inhibition promote the proliferation of ISCs. This is discrepant with the observations made in testis and HSC niches where high ROS act to initiate stem cell differentiation. This could be attributed to the facts that intestinal mucosa is continually exposed to ROS-inducing ingested food substances, drugs, and toxic materials, and that the intestine is considered a highly proliferative tissue to compensate for the loss of ISCs from continuous oxidative and toxic attacks (Bach et al., 2000). Although excessive ROS give rise to different cellular outcomes in different stem cell populations, a possible contributing factor could be the primordial responsibility and role of stem cells in the specific environment. For example, spermatogenesis allows for the transmission of genetic information to ensure species survival, and hematopoietic process expands mature blood cells to enhance immune response. Importantly, both processes become facilitated in times of stress and threat to the survival of organisms. Oxidative stress in gonads could be considered as a threat to the organism's survival due to its damaging effects on biomolecules. Therefore, the premature differentiation of GSCs upon oxidative stress is possibly a protective mechanism adopted by the organism to accelerate spermatogenesis and to ensure the survival and continuation of the species. On the other hand, the proliferation of ISCs upon oxidative stress is considered as a protective mechanism that attempts to replace lost and dead cells to protect the inner intestinal lining from further oxidative attacks (Hochmuth et al., 2011). These results suggest that ROS are associated with the self-renewal and differentiation of various stem cell populations in a context-dependent manner.
Effects of Low ROS on GSC Growth
Keap1 inhibition causes sustained Nrf2 activation, which leads to the constitutive expression of phase II detoxifying enzymes and antioxidative stress enzymes. We showed that reduced Keap1 activity causes enlarged testes filled with more esg-lacZ- and pH3-positive cells compared with controls, implicating low ROS in the growth of germ cell. In support of this, testes treated with antioxidant agents such as GSH became bigger and contained more esg-lacZ-positive germ cells compared with controls. The addition of vitamin C to cell culture was shown to promote the proliferation of caprine spermatogonial stem cells (SSCs) by decreasing ROS levels, increasing anti-apoptotic Bcl-2, and decreasing apoptotic Bax and p53 (Wang et al., 2014). Similarly, the addition of the antioxidant hypotaurine in cryopreservation medium resulted in a significantly greater proliferation potential of murine SSCs (Ha et al., 2016). Furthermore, vitamin C treatment stimulated the proliferation of human mesenchymal stem cells derived from bone marrow and gingival stem cells, with an increase in the expression of the pluripotent markers such as Oct4 (Choi et al., 2008, Van Pham et al., 2016). Recent studies also suggested the role of antioxidants in promoting the cell cycle of stem cells. In the human adipose-derived mesenchymal stem cells (ADMSCs), treatment of antioxidants N-acetyl-L-cysteine or L-ascorbic acid-2-phosphate decreased cellular ROS, leading to the rapid proliferation of ADMSCs by suppressing cyclin-dependent kinase inhibitors (Sun et al., 2013). On the other hand, in the human adipose-derived stem cells vitamin C enhanced cell-cycle progression through the regulation of p53-p21 signaling, decreasing the percentage of G0/G1 phase but increasing the percentage of S and G2/M phases (Zhang et al., 2016). It is also noteworthy that keap1 mutant mice displayed proliferation and defects in the differentiation of esophageal epithelium (Wakabayashi et al., 2003). Homozygous keap1 mutant murine embryonic fibroblasts also showed higher rates of proliferation and colony formation than their wild-type counterparts by increasing oncogenic proteins such as Bcl-2 (Probst et al., 2015). Furthermore, loss of Keap1 function was shown to provide advantages for lung cancer growth (Ohta et al., 2008). Although the detailed mechanisms by which antioxidants or Keap1 inhibition induces cell proliferation are not fully understood, these observations suggest that low levels of ROS are associated with cell proliferation.
Dpp and Gbb are required for GSCs to keep their stemness (Shivdasani and Ingham, 2003, Kawase et al., 2004). Mutant clonal analyses showed that they act on GSCs and control their cell division by activating transforming growth factor β (TGF-β) signaling. We thus examined whether decreasing TGF-β signaling activity by removing one copy of thickveins (a type I receptor of Dpp) or Mothers against dpp (Mad) can suppress the effects of low ROS on germ cell growth. However, we could not detect any significant effects (data not shown). Since JAK/STAT signal transduction is required for both CySCs and GSCs to self-renew (Kiger et al., 2001, Tulina and Matunis, 2001), we also tested whether low ROS promote germ cell growth by activating STAT92E using STAT92E antibody, but failed to detect any alteration in its expression (data not shown). Hence, it may be conceivable that low ROS facilitate germ cell growth by affecting genes related to cell cycle and/or apoptosis as observed in mammalian studies. Here, we showed that extrinsic regulators such as ROS can affect the balance between the self-renewal and differentiation of GSCs in the Drosophila testis. Hence, understanding molecular mechanisms by which ROS affect stem cell behavior in various systems can shed light on the significance of redox homeostasis in the pathogenesis of stem cell-associated diseases.
Experimental Procedures
Fly Strains and Fly Husbandry
UAS-Keap1, UAS-Keap1RNAi, UAS-CncC, UAS-CncCRNAi, keap1EY5, and GstD1-GFP fly lines were obtained from D. Bohmann (Sykiotis and Bohmann, 2008). FRT82B cncCVL110 and cncCK6 lines were obtained from H. Jasper and T. Kerppola, respectively (Deng and Kerppola, 2013). UAS-Catalase was obtained from L. Liu (Yang et al., 2013). UAS-Sod2, UAS-ND75RNAi, UAS-ND42RNAi, and sod2[N308] were obtained from NIG-FLY Stock Center. Nanos (nos)-Gal4, esgM5−4-lacZ, and Tj-Gal4 lines were obtained from S. Dinardo (Terry et al., 2006), and bam-GFP transgenic line was obtained from D.M. McKearin (Chen and McKearin, 2003). stetA13 was described in our previous study (Liu et al., 2010). Egfrf24 (BL#51293), spitz-lacZ (BL#10462), Mad12 (BL#58785), and tkv8 (BL#34509) fly lines were obtained from Bloomington Drosophila Stock Center. For clonal analysis, adult males were collected for 2 days and heat shocked at 37°C for 60 min to induce clones. The following stocks were used for clonal analyses: yw, hsflp122;FRT82B EGFP, FRT82, and FRT82B cncCVL110.
Whole-Mount Immunofluorescence Staining
Testes were dissected in Dissecting Solution and fixed in 4% paraformaldehyde for 20 min. Testes were washed with 0.3% PBST (PBS with Tween 20) and incubated with primary antibodies at 4°C. Testes were then washed and incubated with secondary antibodies at room temperature for 2 hr. Testes were observed under the Olympus FluoView FV1000 Confocal Laser Scanning Biological Microscope. Distance between hub cells and Bam-expressing cells were measured by ImageJ. Primary antibodies used include: rat anti-Vasa (Developmental Studies Hybridoma Bank [DSHB], 1:100), mouse anti-Fasciclin III (DSHB, 1:1,000), mouse anti-Spectrin (DSHB, 1:170), mouse anti-β-gal (β-galactosidase) (Sigma-Aldrich #G4644, 1:200), rabbit anti-pH3 (Cell Signaling Technology #9701, 1:200), guinea pig anti-Tj (D. Godt, 1:3,000), rabbit anti-STAT92E (E. Bach and S. Hou, 1:1,000), guinea pig anti-Zfh1 (J. Skeath, 1:500), rabbit anti-GFP Alexa Fluor 488 conjugate (Thermo Fisher Scientific #A11122, 1:200), and rabbit anti-phospho-Erk1/2 (Cell Signaling #9101, 1:200). Secondary antibodies used include: Alexa Fluor 488-AffiniPure donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories #715-545-150, 1:300), Alexa Fluor 488-AffiniPure goat anti-rat immunoglobulin G (IgG) (Jackson ImmunoResearch #112-585-003, 1:300), Alexa Fluor 488-AffiniPure donkey anti-guinea pig IgG (Jackson ImmunoResearch #706-545-148, 1:300), R488 DyLight 488 IgG Fraction monoclonal mouse anti-rabbit IgG, light chain specific (Jackson ImmunoResearch #211-482-171, 1:300), Alexa Fluor 594-AffiniPure goat anti-rat IgG (Jackson ImmunoResearch #112-585-003, 1:300), and Alexa Fluor 594-AffiniPure Donkey Anti-Rabbit IgG (Jackson ImmunoResearch #111-585-144, 1:300).
Paraquat Treatment and ROS Detection
Flies were starved for 8 hr and then fed a solution containing 5% sucrose alone or 5% sucrose supplemented with 5 mM paraquat (Sigma-Aldrich) for 20 hr. Flies were then transferred to standard food for 20 hr to allow for recovery (referred to as a 2-day treatment). For time-course experiments, flies were treated with 5 mM paraquat for 6 days after starvation, followed by a 20 hr-recovery (referred to as a 7-day treatment). Testes were dissected into 1 mL of Schneider medium (SM) with 10% fetal bovine serum. One microliter of reconstituted DHE dye (Thermo Fisher) was added and rocked for 5 min in the dark. Testes were washed with SM, followed by fixation with 4% paraformaldehyde for 5–10 min. For the quantification of ROS, dissected testes were dissociated in a cocktail comprising 0.25% collagenase (Thermo Fisher) and 0.5% trypsin (Thermo Fisher) in 1× PBS for 15 min, filtered through 40-μm mesh; the reaction was stopped with SM and centrifuged for 5 min at 450 × g. The cell pellets were resuspended and stained with 10 μM CM-H2DCFDA dye (Thermo Fisher) for 30 min at 37°C in the dark. The cells were centrifuged again and resuspended in 200 μL of 1× PBS for measurement.
Western Blot Analysis and TUNEL Assay
Five micrograms of protein were loaded onto SDS-PAGE gels. Antibodies for phospho-Erk1/2 (Cell Signaling, #9101) and β-tubulin (DSHB, E7) were used. Testes were subjected to TUNEL assay (Roche, #11684795910) according to manufacturer's protocol.
Treatment of Flies with Antioxidants
Newly eclosed flies heterozygous for keap1 (keap1EY5) were kept in standard food containing 10 mM L-glutathione reduced (Sigma-Aldrich) or 100 mM ascorbic acid (vitamin C, Sigma-Aldrich) for 7 days.
qRT-PCR Analysis
qRT-PCR was performed using the AB7900HT Fast Real-Time PCR system (Applied Biosystems) and FAST SYBR Green Master Mix (Thermo Fisher). The primers used for spitz, vein, keren, and stet are as follows, with Rpl1 used as a reference gene.
spitz forward (F) 5′-TAC CAG GCA TCG AAG GTT TC-3′; reverse (R) 5′-GAC CCA GGC TCC AGT CAC TA-3′
vein F 5′-GTG AAG TTG CCT GGA TTC GT-3′; R 5′-CTA CAG GGA GCG ACT GAT GC-3′
keren F 5′-CGA GCC ATC AAT CTC CTT GT-3′; R 5′-AAC GAT GGC ACC TGC TTT AC-3′
stet F 5′-CTG CCA CTG GAG ATG GTT CAT-3′; R 5′-GTT GAG AAG CAC ATT GGC CAG-3′
Rpl1 F 5′-TCC ACC TTG AAG AAG GGC TA-3′; R 5′-TTG CGG ATC TCC TCA GAC TT-3′
Statistical Analysis
Data are presented as mean ± SEM. Significance between groups was determined using Student's t test. p < 0.05 was considered statistically significant.
Author Contributions
G.H.B. conceived and designed the experiments. S.W.S.T., Q.Y.L., and B.S.E.W. performed the experiments. G.H.B. and C.Y. analyzed the data. Q.Y.L. and G.H.B. wrote the manuscript.
Acknowledgments
We thank D. Godt, J. Skeath, E. Bach, S. Hou, and DSHB for antibodies and D. Bohmann, L. Liu, S. Dinardo, D.M. McKearin, H. Jasper, T. Kerppola, NIG-FLY Stock Center, and BDSC for fly stocks. This work was supported by MOE TIER1 (R-181-000-172-112).
Published: June 29, 2017
References
- Bach S.P., Renehan A.G., Potten C.S. Stem cells: the intestinal stem cell as a paradigm. Carcinogenesis. 2000;21:469–476. doi: 10.1093/carcin/21.3.469. [DOI] [PubMed] [Google Scholar]
- Barnham K.J., Masters C.L., Bush A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- Chen D., McKearin D.M. A discrete transcriptional silencer in the bam gene determines asymmetric division of the Drosophila germline stem cell. Development. 2003;130:1159–1170. doi: 10.1242/dev.00325. [DOI] [PubMed] [Google Scholar]
- Choi K.M., Seo Y.K., Yoon H.H., Song K.Y., Kwon S.Y., Lee H.S., Park J.K. Effect of ascorbic acid on bone marrow-derived mesenchymal stem cell proliferation and differentiation. J. Biosci. Bioeng. 2008;105:586–594. doi: 10.1263/jbb.105.586. [DOI] [PubMed] [Google Scholar]
- Deng H., Kerppola T.K. Regulation of Drosophila metamorphosis by xenobiotic response regulators. PLoS Genet. 2013;9:e1003263. doi: 10.1371/journal.pgen.1003263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R.A., Hensley K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol. Aging. 2002;23:795–807. doi: 10.1016/s0197-4580(02)00019-2. [DOI] [PubMed] [Google Scholar]
- Ha S.J., Kim B.G., Lee Y.A., Kim Y.H., Kim B.J., Jung S.E., Pang M.G., Ryu B.Y. Effect of antioxidants and apoptosis inhibitors on cryopreservation of murine germ cells enriched for spermatogonial stem cells. PLoS One. 2016;11:e0161372. doi: 10.1371/journal.pone.0161372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hime G.R., Brill J.A., Fuller M.T. Assembly of ring canals in the male germ line from structural components of the contractile ring. J. Cell Sci. 1996;109:2779–2788. doi: 10.1242/jcs.109.12.2779. [DOI] [PubMed] [Google Scholar]
- Hochmuth C.E., Biteau B., Bohmann D., Jasper H. Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell Stem Cell. 2011;8:188–199. doi: 10.1016/j.stem.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmstrom K.M., Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014;15:411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- Itoh K., Ishii T., Wakabayashi N., Yamamoto M. Regulatory mechanisms of cellular response to oxidative stress. Free Radic. Res. 1999;31:319–324. doi: 10.1080/10715769900300881. [DOI] [PubMed] [Google Scholar]
- Itoh K., Wakabayashi N., Katoh Y., Ishii T., Igarashi K., Engel J.D., Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Hirao A., Arai F., Takubo K., Matsuoka S., Miyamoto K., Ohmura M., Naka K., Hosokawa K., Ikeda Y. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat. Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- Jang Y.Y., Sharkis S.J. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawase E., Wong M.D., Ding B.C., Xie T. Gbb/Bmp signaling is essential for maintaining germline stem cells and for repressing bam transcription in the Drosophila testis. Development. 2004;131:1365–1375. doi: 10.1242/dev.01025. [DOI] [PubMed] [Google Scholar]
- Khandrika L., Kumar B., Koul S., Maroni P., Koul H.K. Oxidative stress in prostate cancer. Cancer Lett. 2009;282:125–136. doi: 10.1016/j.canlet.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiger A.A., White-Cooper H., Fuller M.T. Somatic support cells restrict germline stem cell self-renewal and promote differentiation. Nature. 2000;407:750–754. doi: 10.1038/35037606. [DOI] [PubMed] [Google Scholar]
- Kiger A.A., Jones D.L., Schulz C., Rogers M.B., Fuller M.T. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science. 2001;294:2542–2545. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- Kobayashi A., Kang M.I., Okawa H., Ohtsuji M., Zenke Y., Chiba T., Igarashi K., Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang R., Ghaffari S. Stem cells, redox signaling, and stem cell aging. Antioxid. Redox Signal. 2014;20:1902–1916. doi: 10.1089/ars.2013.5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Lim T.M., Cai Y. The Drosophila female germline stem cell lineage acts to spatially restrict DPP function within the niche. Sci. Signal. 2010;3:ra57. doi: 10.1126/scisignal.2000740. [DOI] [PubMed] [Google Scholar]
- McMahon M., Itoh K., Yamamoto M., Hayes J.D. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J. Biol. Chem. 2003;278:21592–21600. doi: 10.1074/jbc.M300931200. [DOI] [PubMed] [Google Scholar]
- Misra J.R., Horner M.A., Lam G., Thummel C.S. Transcriptional regulation of xenobiotic detoxification in Drosophila. Genes Dev. 2011;25:1796–1806. doi: 10.1101/gad.17280911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K., Araki K.Y., Naka K., Arai F., Takubo K., Yamazaki S., Matsuoka S., Miyamoto T., Ito K., Ohmura M. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Morrison S.J., Spradling A.C. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S.J., Shah N.M., Anderson D.J. Regulatory mechanisms in stem cell biology. Cell. 1997;88:287–298. doi: 10.1016/s0092-8674(00)81867-x. [DOI] [PubMed] [Google Scholar]
- Motohashi H., O'Connor T., Katsuoka F., Engel J.D., Yamamoto M. Integration and diversity of the regulatory network composed of Maf and CNC families of transcription factors. Gene. 2002;294:1–12. doi: 10.1016/s0378-1119(02)00788-6. [DOI] [PubMed] [Google Scholar]
- Naka K., Muraguchi T., Hoshii T., Hirao A. Regulation of reactive oxygen species and genomic stability in hematopoietic stem cells. Antioxid. Redox Signal. 2008;10:1883–1894. doi: 10.1089/ars.2008.2114. [DOI] [PubMed] [Google Scholar]
- Nathan C., Cunningham-Bussel A. Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nat. Rev. Immunol. 2013;13:349–361. doi: 10.1038/nri3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T., Nioi P., Pickett C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T., Iijima K., Miyamoto M., Nakahara I., Tanaka H., Ohtsuji M., Suzuki T., Kobayashi A., Yokota J., Sakiyama T. Loss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growth. Cancer Res. 2008;68:1303–1309. doi: 10.1158/0008-5472.CAN-07-5003. [DOI] [PubMed] [Google Scholar]
- Owusu-Ansah E., Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461:537–541. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst B.L., McCauley L., Trevino I., Wigley W.C., Ferguson D.A. Cancer cell growth is differentially affected by constitutive activation of NRF2 by KEAP1 deletion and pharmacological activation of NRF2 by the synthetic triterpenoid, RTA 405. PLoS One. 2015;10:e0135257. doi: 10.1371/journal.pone.0135257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios-Arrabal S., Artacho-Cordon F., Leon J., Roman-Marinetto E., Del Mar Salinas-Asensio M., Calvente I., Nunez M.I. Involvement of free radicals in breast cancer. Springerplus. 2013;2:404. doi: 10.1186/2193-1801-2-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A., Parikh N., Hearn S.A., Fuller M.T., Tazuke S.I., Schulz C. Antagonistic roles of Rac and Rho in organizing the germ cell microenvironment. Curr. Biol. 2007;17:1253–1258. doi: 10.1016/j.cub.2007.06.048. [DOI] [PubMed] [Google Scholar]
- Scadden D.T. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- Schulz C., Wood C.G., Jones D.L., Tazuke S.I., Fuller M.T. Signaling from germ cells mediated by the rhomboid homolog stet organizes encapsulation by somatic support cells. Development. 2002;129:4523–4534. doi: 10.1242/dev.129.19.4523. [DOI] [PubMed] [Google Scholar]
- Shi X., Zhang Y., Zheng J., Pan J. Reactive oxygen species in cancer stem cells. Antioxid. Redox Signal. 2012;16:1215–1228. doi: 10.1089/ars.2012.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilo B.Z. Regulating the dynamics of EGF receptor signaling in space and time. Development. 2005;132:4017–4027. doi: 10.1242/dev.02006. [DOI] [PubMed] [Google Scholar]
- Shivdasani A.A., Ingham P.W. Regulation of stem cell maintenance and transit amplifying cell proliferation by TGF-beta signaling in Drosophila spermatogenesis. Curr. Biol. 2003;13:2065–2072. doi: 10.1016/j.cub.2003.10.063. [DOI] [PubMed] [Google Scholar]
- Sinenko S.A., Shim J., Banerjee U. Oxidative stress in the haematopoietic niche regulates the cellular immune response in Drosophila. EMBO Rep. 2011;13:83–89. doi: 10.1038/embor.2011.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L.Y., Pang C.Y., Li D.K., Liao C.H., Huang W.C., Wu C.C., Chou Y.Y., Li W.W., Chen S.Y., Liu H.W. Antioxidants cause rapid expansion of human adipose-derived mesenchymal stem cells via CDK and CDK inhibitor regulation. J. Biomed. Sci. 2013;20:53. doi: 10.1186/1423-0127-20-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykiotis G.P., Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev. Cell. 2008;14:76–85. doi: 10.1016/j.devcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykiotis G.P., Bohmann D. Stress-activated cap'n'collar transcription factors in aging and human disease. Sci. Signal. 2010;3:re3. doi: 10.1126/scisignal.3112re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia C., Kutzner H., Mentzel T., Savic S., Baumhoer D., Glatz K. Two mitosis-specific antibodies, MPM-2 and phospho-histone H3 (Ser28), allow rapid and precise determination of mitotic activity. Am. J. Surg. Pathol. 2006;30:83–89. doi: 10.1097/01.pas.0000183572.94140.43. [DOI] [PubMed] [Google Scholar]
- Terry N.A., Tulina N., Matunis E., DiNardo S. Novel regulators revealed by profiling Drosophila testis stem cells within their niche. Dev. Biol. 2006;294:246–257. doi: 10.1016/j.ydbio.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Tothova Z., Gilliland D.G. FoxO transcription factors and stem cell homeostasis: insights from the hematopoietic system. Cell Stem Cell. 2007;1:140–152. doi: 10.1016/j.stem.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Trachootham D., Alexandre J., Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- Tran J., Brenner T.J., DiNardo S. Somatic control over the germline stem cell lineage during Drosophila spermatogenesis. Nature. 2000;407:754–757. doi: 10.1038/35037613. [DOI] [PubMed] [Google Scholar]
- Tulina N., Matunis E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science. 2001;294:2546–2549. doi: 10.1126/science.1066700. [DOI] [PubMed] [Google Scholar]
- Urban S., Lee J.R., Freeman M. Drosophila rhomboid-1 defines a family of putative intramembrane serine proteases. Cell. 2001;107:173–182. doi: 10.1016/s0092-8674(01)00525-6. [DOI] [PubMed] [Google Scholar]
- Urban S., Lee J.R., Freeman M. A family of Rhomboid intramembrane proteases activates all Drosophila membrane-tethered EGF ligands. EMBO J. 2002;21:4277–4286. doi: 10.1093/emboj/cdf434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pham P., Tran N.Y., Phan N.L., Vu N.B., Phan N.K. Vitamin C stimulates human gingival stem cell proliferation and expression of pluripotent markers. In Vitro Cell. Dev. Biol. Anim. 2016;52:218–227. doi: 10.1007/s11626-015-9963-2. [DOI] [PubMed] [Google Scholar]
- Wakabayashi N., Itoh K., Wakabayashi J., Motohashi H., Noda S., Takahashi S., Imakado S., Kotsuji T., Otsuka F., Roop D.R. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat. Genet. 2003;35:238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- Wang J., Cao H., Xue X., Fan C., Fang F., Zhou J., Zhang Y., Zhang X. Effect of vitamin C on growth of caprine spermatogonial stem cells in vitro. Theriogenology. 2014;81:545–555. doi: 10.1016/j.theriogenology.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Yang Y., Hou L., Li Y., Ni J., Liu L. Neuronal necrosis and spreading death in a Drosophila genetic model. Cell Death Dis. 2013;4:e723. doi: 10.1038/cddis.2013.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Li J., Qi Y., Zou Y., Liu L., Tang X., Duan J., Liu H., Zeng G. Vitamin C promotes the proliferation of human adipose-derived stem cells via p53-p21 pathway. Organogenesis. 2016;12:143–151. doi: 10.1080/15476278.2016.1194148. [DOI] [PMC free article] [PubMed] [Google Scholar]