Abstract
Urinary tract infections (UTIs) are one of the major sources of widespread infectious diseases in the community as well as in the hospitals which increase the cause of morbidity and mortality. Prevalence of extended-spectrum-β-lactamase (ESBL)-producing uropathogenic E. coli isolates has been found to be increased rapidly across the world. The present study was undertaken to find out the frequency of bla TEM, bla CTX-M, and bla SHV genes among E. coli isolates from UTI and detect their sensitivity pattern. A total of 112 non-repeated E. coli isolates obtained from urine samples of UTI diagnosed patients were included in this study. Antibiotic susceptibility test was done by disc diffusion method. Seventy seven (68.75%) isolates were MDR and tested for ESBL. ESBL-positive isolates were screened for bla TEM, bla CTX-M, and bla SHV genes by monoplex PCR (polymerase chain reaction). Among 46 ESBL-producing E. coli isolates, 8.69% harboured all the three bla genes. The bla TEM was the predominant (93.47%) gene followed by bla CTX-M (82.6%) and bla SHV (4.34%). It can be concluded that the prevalence of MDR (multidrug resistance) ESBL-producing E. coli appears to be high and the highest identified gene was bla TEM. The knowledge of resistance pattern can help physician’s select suitable empirical antibiotic regimens, so that antibiotics showing high-resistance pattern can be avoided.
Keywords: Urinary tract infection, Escherichia coli, blaTEM, blaSHV, blaCTX-M
Introduction
Urinary tract infections (UTIs) are one of the major sources of widespread infectious diseases in the community as well as in the hospitals which increase the cause of morbidity and mortality (Daoud et al. 2015). Various pathogens are responsible for UTIs, of which bacteria are the main contributing agents and E. coli is the prevalent organism accountable for more than 80% of infections (Jena et al. 2013). E. coli is report approximately 50% in health care settings and 85% in community acquired UTIs (Ramanath and Shafiya 2011).
Diagnosis of more than 150 million UTIs every year worldwide, reminds it as one of the most frequent communities acquired as well as nosocomial infections since long time (Gonzalez and Schae€er 1999). Overuse and misuse of antibiotics for the treatment of UTIs cause antibiotic selective pressure which results in rapid increase and spread of multidrug resistant bacteria. At present, decreased susceptibility to many antibiotics was observed against uropathogenic E. coli. This harshly situation is commonly observed in human medicine and this may lead to the increased hospital cost, prolonged stay at hospital, and also recurrent treatment failure (Pitout et al. 1997).
Among the array of antibiotics used widely, β-lactams are the most extensively used chemotherapeutic agents because of lack of significant toxicity. They account for over 50% of all systemic antibiotics in use (Bronson and Barrett 2001). Production of β-lactamases by several Gram-negative and Gram-positive organisms is possibly the most important single mechanism of resistance to β-lactam agents (Chaudhary and Aggarwal 2004).
Extended-spectrum β-lactamases (ESBLs) are enzymes which show resistance to the third generation cephalosporins. As these enzymes are plasmid encoded, spread of bacterial resistance due to these enzymes disseminate rapidly. These plasmids also carry resistance genes which encode for other antibiotics resistance genes such as aminoglycosides, fluoroquinolones, tetracyclines, chloramphenicol, and sulfamethoxazole–trimethoprim (Bagattini et al. 2006). Among ESBL-producing uropathogenic bacteria, E. coli has been reported to be the major cause of public health issues worldwide. Although ESBL genes are most commonly derived from TEM or SHV parents, since 1998, the prevalence of CTX-M types has been increased significantly in most parts of the world (Paterson and Bonomo 2014).
Treatment of UTI cases is often started empirically based on the antimicrobial resistance pattern of the urinary pathogens from existing surveillance report. Therefore, the aims of the present study were to determine the prevalence of ESBL-producing E. coli isolated from urine samples of UTI diagnosed patients, to detect their susceptibility to the antibiotics generally used for the treatment of UTI and to detect prevalence of bla SHV, bla TEM, and bla CTX-M genes.
Materials and methods
Isolation and biochemical identification
Urine samples were collected, from patients of age more than 18 years, in sterile containers by the following aseptic techniques from patients suspected to have urinary tract infection from outpatient department (OPD), wards, cabins, intensive care unit (ICU), and neonatal intensive care unit (NICU) of IMS and SUM Hospital, Bhubaneswar. Urine samples were also examined microscopically especially for pus cell to confirm urinary tract infection. Urine (1 µl) was inoculated onto cysteine lactose electrolyte deficient (CLED, Hi-Media Laboratories, Mumbai, India) medium. Organisms grown in pure culture and in significant numbers (>105cfu/ml for midstream urine samples) were identified by the standard biochemical tests. Of the total 2560 urine samples obtained over a period of 1 year (March 2015–March 2016), only 290 samples were selected basing on the above criteria and processed further. Flow chart for sample selection for this study is given in Fig. 1. National Collection of Type Cultures (NCTC) strain number 10,418 (β-lactamase negative) was used as the reference strain for all experiments.
Fig. 1.
Flow chart for the sample selection for the study
Antibiotic susceptibility tests
Antibiotic sensitivity of the isolates was done by Kirby Bauer’s Method using antibiotic disks from Himedia, Mumbai. Antibiotics used were ceftazidime (30 µg) (CAZ), amikacin (30 μg) (AK), amoxyclav (30 μg) (AMC), ofloxacin (5 μg) (OF), norfloxacin (5 μg) (NX), ceftriaxone (30 μg) (CTR), piperacilin/tazobactun (100/10 μg) (PIT), co-trimoxazole (25 μg) (COT), gentamicin (10 μg) (GEN), nitrofurantoin (300 μg) (NIT), cefoperazone/sulbactum (75/30 μg) (CFS), cefepime (30 μg) (CPM), netilimicin (30 μg) (NET), imipenem (10 μg) (IPM), and colistin (10 μg) (CL).
Test for ESBL production
Those isolates which show resistance or with decreased susceptibility (intermediate by CLSI criteria) to the third generation cephalosporins were tested for ESBL production by NCCLS confirmatory test as described previously (Jena et al. 2014).
Isolation and quantification of plasmid DNA
Plasmid DNA was isolated from ESBL-producing bacterial cells by alkaline-lysis method (Sambrook et al. 1989). The plasmid DNA was stored at minus 20 °C for further use. The samples were run on 0.8% agarose gel and stained with ethidium bromide. The stained gel was examined under UV light to find the presence of plasmid bands. To evaluate the molecular weight, λ DNA double digested with hind III was used as marker.
PCR amplification for blaSHV, blaTEM, and blaCTX-M genes
ESBL-positive isolates were taken for PCR assay to ensure the presence of the bla SHV, bla TEM, and bla CTX-M genes. The PCR master mix was as follows: 2.5 μl of PCR buffer, 2.5 μl of 25 mm mgcl2, 0.2 μl of 2 mm dNTPs, 0.17 μl of Taq polymerase (Merck, Germany), 16.63 µl double distilled sterile water, and 1 μl of each of the forward and the reverse primers (Table 1). After giving out 24 μl of the master mix in the individual PCR reaction tubes, 1 μl of the extracted plasmid DNA was added in the corresponding tubes to make up the total volume to 25 μl.
Table 1.
List of primers used for the amplification of bla TEM, bla SHV, and bla CTX-M
| Target gene | Primers | Length (bp) | References |
|---|---|---|---|
| bla TEM | Forward-5′-ATGAGTATTCAACATTTCCGTG-3′ Reverse-5′-TTACCAATGCTTAATCAGTGAG-3′ |
861 | Hosoglu et al. (2007) |
| bla SHV | Forward-5′-TTATCTCCCTGTTAGCCACC-3′ Reverse- 5′- GATTTGCTGATTTCGCTCGG-3′ |
795 | Weill et al. (2004) |
| bla CTX-M | Forward- 5′-SCSATGTGCAGYACCAGTAA-3′ Reverse-5′-CCGCRATATGRTTGGTGGTG-3′ |
544 | Eckert et al. (2004) |
The reaction mixture was initially denatured for 5 min at 94 °C, subjected to 30 cycles of denaturation at 94 °C for 1 min, annealed at 55 °C for 1 min, extended at 72 °C for 1 min, finally extended at 72 °C for 10 min, and soaked to 4 °C. The amplified PCR products were analyzed using 1.5% agarose gel electrophoresis. Gels were stained with ethidium bromide and visualized by UV trans-illuminator. Figures 2, 3, and 4 show the amplified DNA bands of the bla genes upon UV trans-illumination.
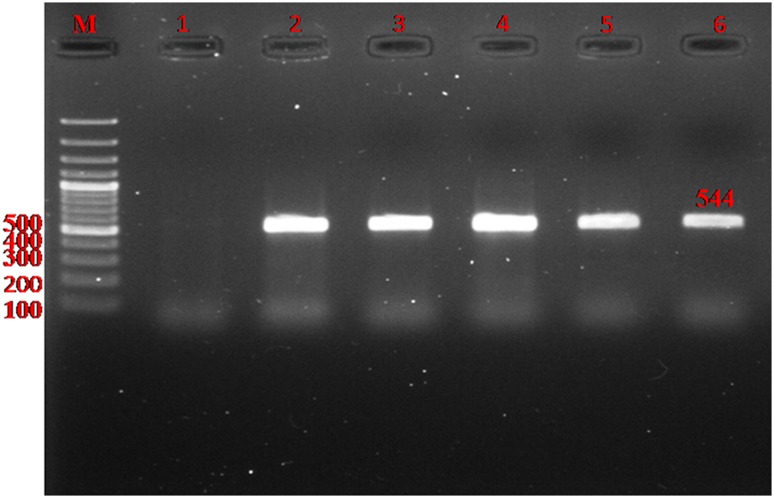
Fig. 2.
Detection of gene encoding bla CTX-M in ESBL-producing E. coli by PCR. Lane M ladder (Fermentas, UK); Lanes 2–5: The 544 bp PCR product of bla CTX-M; Lane 6 positive control (K. pneumoniae ATCC 700603); Lane 1 absent bla CTX-M gene
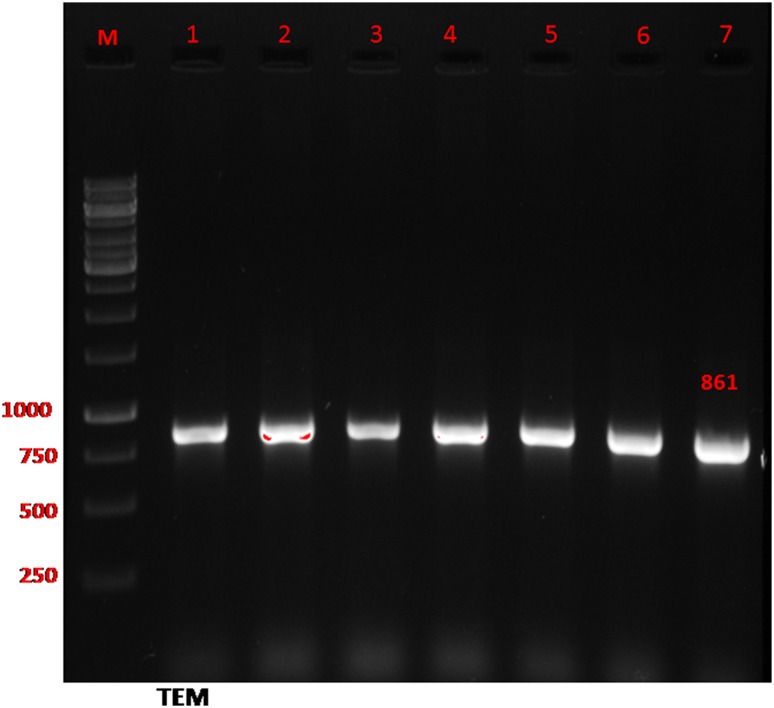
Fig. 3.
Detection of gene encoding bla TEM in ESBL-producing E. coli by PCR. Lane M ladder (Fermentas, UK); Lane 1–7 the 861 bp PCR product of bla TEM
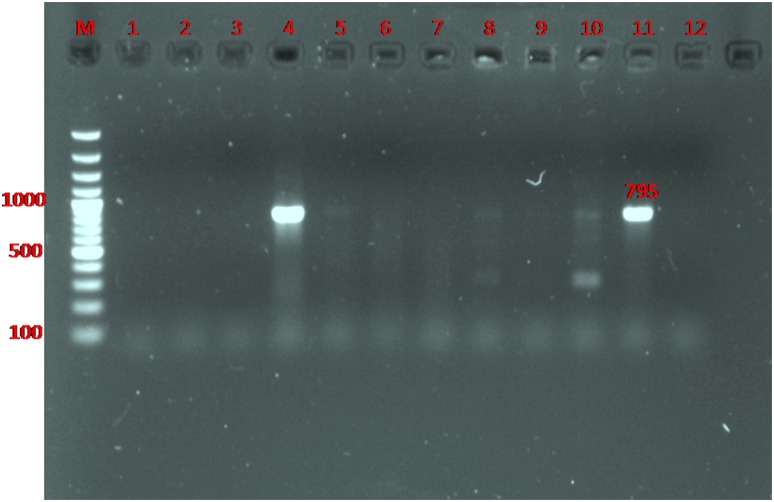
Fig. 4.
Detection of gene encoding bla SHV in ESBL-producing E. coli by PCR. Lane M ladder (Fermentas, UK); Lanes 4 and 11 the 795 bp PCR product of bla SHV gene
Results
Of the 290 positive cultures, 112 (38.6%) isolates were identified to be E. coli that was predominant organism followed by Staphylococcus aureus 49 (38.62%), Klebsiella spp. 34 (11.72%), Enterobacter spp. 23 (7.93%), Acinetobacter spp. 18 (6.2%), Pseudomonas spp. 11 (3.79%), Citrobacter spp.10 (3.44%), Proteus spp. 8 (2.75%), Providencia 2 (0.68%), Coagulase Negative Staphylococci (CoNS) 14 (4.82%), and Candida albicans 4 (1.37%). Seventy seven (68.75%) E. coli isolates were considered MDR as these isolates showed resistant to two or more unrelated classes of antibiotics.
Antibiotic sensitivity pattern
The drug resistance patterns of 77 MDR E. coli uropathogens were observed that ceftazidime shows the highest percentage (100%) of resistance among all other antibiotics by disc diffusion method, followed by ceftriaxone (96.1%), cefepime (96.1%), norfloxacin (94.8%), ofloxacin (93.5%), and amoxyclav (90.9%). The lowest rate of resistance was observed in colistin (3.89%) (Table 2).
Table 2.
Antibiotics sensitivity pattern of the MDR E. coli isolates
| S. no. | Antibiotics | Resistance (n = 77) | Sensitive | Intermediate |
|---|---|---|---|---|
| 1 | CL | 3.89% (3) | 96.1% (74) | 0 |
| 2 | IPM | 14.28% (11) | 80.51% (62) | 5.19% (4) |
| 3 | NIT | 24.67% (19) | 64.93% (50) | 10.38% (8) |
| 4 | CAC | 25.97% (20) | 64.93% (50) | 9.09% (7) |
| 5 | NET | 38.96% (30) | 54.54% (42) | 6.49% (5) |
| 6 | AK | 40.25% (31) | 53.24% (41) | 6.49% (5) |
| 7 | CFS | 46.75% (36) | 42.85% (33) | 10.38% (8) |
| 8 | GEN | 53.24% (41) | 42.85% (33) | 3.89% (3) |
| 9 | PIT | 55.84% (43) | 36.36% (28) | 7.79% (6) |
| 10 | COT | 67.53% (52) | 25.97% (20) | 3.89% (3) |
| 11 | AMC | 90.9% (70) | 9.09% (7) | 0 |
| 12 | OF | 93.5% (72) | 6.49% (5) | 0 |
| 13 | NX | 94.8% (73) | 5.19% (4) | 0 |
| 14 | CTR | 96.1% (74) | 2.59% (2) | 0 |
| 15 | CPM | 96.1% (74) | 2.59% (2) | 0 |
| 16 | CAZ | 100% (77) | 0 | 0 |
CL colistin, IPM imipenem, NIT nitrofurantoin, CAC ceftazidime/clavulanic acid, NET netilimicin, AK amikacin, CFS cefoperazone/sulbactum, GEN gentamicin, PIT piperacilin/tazobactun, COT co-trimoxazole, AMC amoxyclav, OF ofloxacin, NX norfloxacin, CTR ceftriaxone, CPM cefepime, CAZ ceftazidime
Prevalence of ESBL by phenotypic method
Seventy seven consecutive MDR E. coli uropathogens were tested and 46 (59.74%) isolates were found to be ESBL producers.
Plasmid profiling
Plasmid DNA was extracted from 46 ESBL-producing E. coli uropathogens by alkaline-lysis method. All the isolates showed that the presence of plasmids and multiple plasmids (bands) was found in 38 isolates. Of the 46 isolates, 8 isolates showed the presence of single plasmid. One plasmid having molecular weight 23.13 Kb was present in 31 of the isolates tested.
Prevalence of ESBL gene
All the ESBL-producing isolates confirmed by phenotypic methods were also analyzed by molecular methods (PCR) to understand the frequency of ESBL genes. All the 46 ESBL-producing E. coli isolates were found to have at least one bla gene, of which 8.69% harboured all the three bla genes. The bla TEM was the predominant (93.47%) followed by blaCTX-M (82.6%) and blaSHV (4.34%). Co-existence of two of the genes was observed at the highest range with bla TEM and bla CTX-M in 23 (50%) isolates followed by bla TEM and bla SHV in eight (17.39%) isolates and bla CTX-M and bla SHV in six (13.04%) isolates (Table 3).
Table 3.
Prevalence of bla TEM, bla SHV, and bla CTX-M genes in ESBL producers for E. coli (strain no. 46)
| Identified gene | No. | % | Outpatients | Inpatients |
|---|---|---|---|---|
| TEM | 43 | 93.47 | 31 | 15 |
| CTX-M | 38 | 82.60 | 29 | 9 |
| SHV | 2 | 4.34 | 0 | 2 |
| TEM + CTX-M + SHV | 2 | 4.34 | 1 | 1 |
| TEM + CTX-M | 23 | 50.00 | 15 | 8 |
| TEM + SHV | 1 | 2.17 | 1 | 0 |
| CTX-M + SHV | 0 | 0 | 0 | 0 |
Discussion
The frequency of use of antibiotics and even the dosages and period of administration vary greatly from country to country, region to region, and to some degree even in a locality. This has led to large differentials in the emergence of resistant patterns. Therefore, it is essential to study and report trends in antimicrobial resistance regularly (Shigemura et al. 2008). Multidrug resistant E. coli isolates from UTI are increasingly found worldwide, which is a serious problem in many countries. In our study, it was observed that 68.75% E. coli isolates were MDR supported by the study of Adwan et al. (2014) from Palestine reported that 76% E. coli isolates were MDR.
The present study on the susceptibility pattern of MDR isolates shows 3.89% resistance towards colistin. However, a much higher rate of colistin resistant (82%) in ESBL-producing uropathogen E. coli was reported by Rezai et al. (2015). Imipenem is highly β-lactamase stable and has an uncommon property of causing a post antibiotic effect on Gram-negative bacteria. It was observed from the present study that imipenem was resistant up to 14.28% to the MDR uropathogens, which was quite higher when compared with the analysis by Hassan et al. (2011) which is only 9%. In our study, we observed that the rate of resistance to ceftazidime was 100% which is much higher when compared with other finding from Malaysia, where resistance percentage of ceftazidime was 11% by Thong et al. (2009), and from China, where it was found to be 28% for ceftazidime (Yu et al. 2007).
Moreover, ESBL producers show resistance not only to β-lactam agents but also to other antimicrobial agents such as tetracycline, fluoroquinolones, aminoglycosides, and trimethoprim/sulfamethoxazole (Rezai et al. 2015). Similar resistance pattern was also observed in our study. There are so many factors responsible for such elevated rate of antibiotic resistance which includes enormous use and misuse of antimicrobial agents by health practitioners in hospitals in addition to the self-prescription by the community (El Bouamri et al. 2015). Colistin, imipenem, nitrofurantoin, netilimicin, ceftazidime/clavulanic acid, and amikacin constitute the reasonable treatment option for UTI, as these antibiotics showed a lower rate of resistance to the uropathogenic E. coli isolates.
UTI remains the commonest type of community acquired Gram-negative sepsis, especially in developing countries (Haque 2011). In our study, most of the ESBL producers were from outpatients 31 (67.39%) than hospital acquired 15 (32.6%). One study by Tada et al. (2012) reported that 16.7 and 30% isolates were ESBL positive from community and hospital acquired UTI, respectively.
Recommendation of proper antimicrobial regimens for the treatment of infectious diseases caused by ESBL-producing E. coli drastically get hampered due to the presence of other antibiotic resistance genes and the existence of a conjugative plasmid allied with the ESBL phenotype. In this situation, carbapenems represent a good choice for the treatment option in case of serious infection condition (Daoud et al. 2015).
The incidence of ESBL-producing E. coli varies from country to country and from centre to centre. Our study showed that the prevalence of ESBL was 59.74%, which was higher when compared with the study from Sudan with 30.2% ESBL producers in E. coli (Ibrahim et al. 2013). Statistics have shown that prevalence of ESBL-producing E. coli are highest in India (60%), followed by Hong Kong (48%) and Singapore (33%) as reported by Hsueh et al. (2011). Molecular characterization of β-lactamase gene would be crucial for a reliable epidemiological analysis of antimicrobial resistance (El Bouamri et al. 2015). From many different studies, it was observed that the predominant of ESBL gene was diverse. Earlier reports mentioned that the most prevalent type of ESBL genes is SHV, TEM, and CTX-M. During the past decade, TEM and SHV types were reported to be the most common types of β-lactamase genes, but recently, CTX-M type has been widespread worldwide compared to TEM and SHV genotypes (Barguigua et al. 2011). CTX-M was predominant in many regions; several reports were made from Iran (74%) (Seyedjavadi et al. 2015), Morocco, North Africa (70%) (El Bouamri et al. 2015), and India (93.7%) (Nandagopal et al. 2015). However, the present study showed that TEM-type β-lactamase gene was the predominant ESBL gene in uropathogenic E. coli which corroborated with the reports of several other previous studies (Shahcheraghi et al. 2009; Moosavian and Deiham 2012; Rezai et al. 2015; Abujnah et al. 2015). TEM-type β-lactamase gene was the predominant in Italy (45.4%) (Carattoli et al. 2008), Portugal (40.9%) (Fernandes et al. 2014), and Turkey (72.7%) (Bali et al. 2010). Our study also corroborates with another study from India, where TEM was predominant followed by SHV in E. coli and Klebsiella pneumonia from UTI and also they reported CTX-M gene was found lowest (Bajpai et al. 2017).
Several investigators reported the co-existence of various β-lactamase genes within the same isolates (Shahid et al. 2011; Manoharan et al. 2011; Sharma et al. 2013). Co-existence of all the three bla genes was observed in two (4.34%) isolates in our study, which was lower than the report from Tamilnadu, India, where they observed only 60% (12) isolates, harboured all the three genes (Ponnusamy and Nagappan 2015).
The major co-existence of both the genes was CTX-M and TEM (50%) in our isolates. This result is also in agreement with others Seyedjavadi et al. (2015) in Iran, Harada et al. (2013) in Japan, and Sharma et al. (2013) in India who reported both TEM + CTX-M as the most common type.
Conclusion
In conclusion, ESBL-producing uropathogenic E. coli is a rising problem and the dissemination occurs over the whole country. The predominant gene type was TEM, but CTX-M gene was also increasing. Colistin, imipenem, nitrofurantoin, netilimicin, ceftazidime/clavulanic acid, and amikacin constitute the reasonable treatment option for UTI as based on state of findings. Since dissemination of MDR and ESBL-producing E. coli isolates decreases the treatment options and increases the hospital cost, it is necessary to be updated with the prevailing resistant pattern of any locality which will help in appropriate antimicrobial therapy.
Acknowledgements
We are grateful to Prof. Dr. DK Roy, Dean, IMS and Sum Hospital (S‘O’A University) for extended essential laboratory facilities. Our sincere thanks are due to Prof. Dr. M.R. Nayak, Honourable President, Siksha ‘O’ Anusandhan University, Bhubaneswar, for providing financial support.
Compliance with ethical standards
Conflict of interest
We declare that we have no conflict of interest.
References
- Abujnah AA, Zorgani A, Sabri MAM, et al. Multidrug resistance and extended-spectrum β-lactamases genes among Escherichia coli from patients with urinary tract infections in Northwestern Libya. Libyan J Med. 2015;10:26412. doi: 10.3402/ljm.v10.26412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adwan K, Jarrar N, Abu-Hijleh A, et al. Molecular characterization of Escherichia coli isolates from patients with urinary tract infections in Palestine. J Med Microbiol. 2014;63:229–234. doi: 10.1099/jmm.0.067140-0. [DOI] [PubMed] [Google Scholar]
- Bagattini M, Crivaro V, Di Popolo A, et al. Molecular epidemiology of extended-spectrum β-lactamase-producing Klebsiella pneumoniae in a neonatal intensive care unit. J Antimicrob Chemother. 2006;57:979–982. doi: 10.1093/jac/dkl077. [DOI] [PubMed] [Google Scholar]
- Bajpai T, Pandey M, Varma M, Bhatambare GS. Prevalence of TEM, SHV and CTX-M beta-lactamase genes in the urinary isolates of a tertiary care hospital. Avicenna J Med. 2017;7:12–16. doi: 10.4103/2231-0770.197508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bali EB, Açık L, Sultan N. Phenotypic and molecular characterization of SHV produced by Escherichia coli, Acinobacter baumannii and Klebsiella isolates in a Turkish hospital. Afr J Microbiol Res. 2010;4:650–654. [Google Scholar]
- Barguigua A, El Otmani F, Talmi M, et al. Characterization of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae isolates from the community in Morocco. J Med Microbiol. 2011;60:1344–1352. doi: 10.1099/jmm.0.032482-0. [DOI] [PubMed] [Google Scholar]
- Bronson JJ, Barrett JF. Quinolone, everninomycin, glycylcycline, carbapenem, lipopeptide and cephem antibacterials in clinical development. Curr Med Chem. 2001;8:1775–1793. doi: 10.2174/0929867013371653. [DOI] [PubMed] [Google Scholar]
- Carattoli A, García-Fernández A, Varesi P, et al. Molecular epidemiology of Escherichia coli producing extended-spectrum β-lactamases isolated in Rome, Italy. J Clin Microbiol. 2008;46:103–108. doi: 10.1128/JCM.01542-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary U, Aggarwal R. Extended spectrum β-lactamases (ESBL)-an emerging threat to clinical therapeutics. Indian J Med Microbiol. 2004;22:75–80. [PubMed] [Google Scholar]
- Daoud Z, Salem Sokhn E, Masri K, et al. Escherichia coli isolated from urinary tract infections of Lebanese patients between 2005 and 2012: epidemiology and profiles of resistance. Front Med. 2015;2:26. doi: 10.3389/fmed.2015.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert C, Gautier V, Saladin-Allard M, et al. Dissemination of CTX-M-type β-lactamases among clinical isolates of Enterobacteriaceae in Paris, France. Antimicrob Agents Chemother. 2004;48:1249–1255. doi: 10.1128/AAC.48.4.1249-1255.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Bouamri MC, Arsalane L, Zerouali K, et al. Molecular characterization of extended spectrum β-lactamase-producing Escherichia coli in a university hospital in Morocco, North Africa. Afr J Urol. 2015;21:161–166. doi: 10.1016/j.afju.2015.02.005. [DOI] [Google Scholar]
- Fernandes R, Amador P, Oliveira C, Prudêncio C. Molecular characterization of ESBL-producing Enterobacteriaceae in northern Portugal. Sci World J. 2014 doi: 10.1155/2014/782897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez CM, Schae€er AJ. Treatment of urinary tract infection: what’s old, what’s new, and what works. World J Urol. 1999;17:372–382. doi: 10.1007/s003450050163. [DOI] [PubMed] [Google Scholar]
- Haque SF. Extended spectrum β-lactamases mediated resistance in urinary tract infections “Changing profile at a teaching hospital of north India”. Int J Cur Bio Med Sci. 2011;1:103–107. [Google Scholar]
- Harada Y, Morinaga Y, Yamada K, et al. Clinical and molecular epidemiology of extended spectrum β-lactamase-producing Klebsiella pneumoniae and Escherichia coli in a Japanese tertiary hospital. J Med Microbiol Diagn. 2013;2:127. [Google Scholar]
- Hassan SA, Jamal SA, Mustafa K. Occurrence of multidrug resistant and ESBL producing E. coli causing urinary tract infections. J Basic Appl Sci. 2011;7:39–43. [Google Scholar]
- Hosoglu S, Gundes S, Kolayh F, et al. Extended spectrum β-lactamases in ceftazidime resistant Escherichia coli and Klebsiella pneumonia isolates in Turkish hospitals. Indian J Med Microb. 2007;25:346–350. doi: 10.4103/0255-0857.37336. [DOI] [PubMed] [Google Scholar]
- Hsueh PR, Hoban DJ, Carmeli Y, et al. Consensus review of the epidemiology and appropriate antimicrobial therapy of complicated urinary tract infections in Asia-Pacific region. J Infect. 2011;63:114–123. doi: 10.1016/j.jinf.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Ibrahim ME, Bilal NE, Magzoub MA, Hamid ME. Prevalence of extended-spectrum β-lactamases-producing Escherichia coli from hospitals in Khartoum State, Sudan. Oman Med J. 2013;28:116–120. doi: 10.5001/omj.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jena J, Debata NK, Subudhi E. Prevalence of extended-spectrum-β-lactamase and metallo-β-lactamase producing multi drug resistance gram-negative bacteria from urinary isolates. Indian J Med Microbiol. 2013;31:420–421. doi: 10.4103/0255-0857.118890. [DOI] [PubMed] [Google Scholar]
- Jena J, Sahoo RK, Subudhi E, Debata NK. Prevalence of ESBL, MBL and Ampc-β-lactamases producing multidrug resistance gram negative bacteria in a tertiary care hospital. J Pure Appl Microbiol. 2014;8:4099–4105. [Google Scholar]
- Manoharan A, Premalatha K, Chatterjee S, Mathai D. Correlation of TEM, SHV and CTX-M extended-spectrum beta lactamases among Enterobacteriaceae with their in vitro antimicrobial susceptibility. Indian J Med Microbiol. 2011;29:161–164. doi: 10.4103/0255-0857.81799. [DOI] [PubMed] [Google Scholar]
- Moosavian M, Deiham B. Distribution of TEM, SHV and CTX-M Genes among ESBL-producing Enterobacteriaceae isolates in Iran. African J Microbiol Res. 2012;6:5433–5439. [Google Scholar]
- Nandagopal B, Sankar S, Sagadevan K, et al. Frequency of extended spectrum β-lactamase producing urinary isolates of gram-negative bacilli among patients seen in a multispecialty hospital in Vellore district, India. Indian J Med Microbiol. 2015;33:282–285. doi: 10.4103/0255-0857.150896. [DOI] [PubMed] [Google Scholar]
- Paterson DL, Bonomo RA. Extended-spectrum β-lactamases: a clinical update. Clin Microbiol Rev. 2014;18:1–36. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitout JD, Sanders CC, Sanders WE. Antimicrobial resistance with focus on beta-lactam resistance in gram-negative bacilli. Am J Med. 1997;103:51–59. doi: 10.1016/S0002-9343(97)00044-2. [DOI] [PubMed] [Google Scholar]
- Ponnusamy P, Nagappan R. Molecular characterization of bla CTX-M, bla TEM, bla SHV- beta lactamase produced by uropathogenic Escherichia coli isolates. Int J Microbiol Res. 2015;6:67–73. [Google Scholar]
- Ramanath KV, Shafiya SB. Prescription pattern of antibiotic usage for urinary tract infection treated in a rural tertiary care hospital. Indian J Pharma Pract. 2011;4:57–63. [Google Scholar]
- Rezai MS, Salehifar E, Rafiei A, et al. Characterization of multidrug resistant extended-spectrum beta-lactamase-producing Escherichia coli among uropathogens of pediatrics in North of Iran. Biomed Res Int. 2015 doi: 10.1155/2015/309478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Seyedjavadi SS, Goudarzi M, Sabzehali F. Relation between blaTEM, blaSHV and blaCTX-M genes and acute urinary tract infections. J Acute Dis. 2015;5:1–6. [Google Scholar]
- Shahcheraghi F, Nasiri S, Noveiri H. Detection of extended-spectrum β-lactamases (ESBLs) in Escherichia coli, Iran. J Clin Infect Dis. 2009;4:65–70. [Google Scholar]
- Shahid M, Singh A, Sobia F, et al. blaCTX-M, blaTEM, and blaSHV in Enterobacteriaceae from North-Indian tertiary hospital: high occurrence of combination genes. Asian Pac J Trop Med. 2011;4:101–105. doi: 10.1016/S1995-7645(11)60046-1. [DOI] [PubMed] [Google Scholar]
- Sharma M, Pathak S, Srivastava P. Prevalence and antibiogram of extended spectrum β-lactamase (esbl) producing gram negative bacilli and further molecular characterization of ESBL producing Escherichia coli and Klebsiella spp. J Clin Diagn Res. 2013;7:2173–2177. doi: 10.7860/JCDR/2013/6460.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemura K, Arakawa S, Miura T, et al. Significance of fluoroquinolone resistant Escherichia coli in urinary tract infections. Jpn J Infect Dis. 2008;61:226–228. [PubMed] [Google Scholar]
- Tada DG, Gandhi PJ, Patel KN. A study on antibiotic related resistance in UTI patients: a comparison between community acquired and hospital acquired E. coli. Natl J Community Med. 2012;3:255–258. [Google Scholar]
- Thong KL, Lim KT, Yasin R, et al. Characterization of multidrug resistant ESBL-producing Escherichia coli isolates from hospitals in malaysia. J Biomed Biotechnol. 2009 doi: 10.1155/2009/165637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weill FX, Demartin M, Tande D, et al. SHV-12 like extended spectrum β-lactamase producing strains of Salmonella enteric serotypes Babelsberg and Enteritidis isolated in France among infants adopted from Mali. J Clin Microbiol. 2004;42:2432–2437. doi: 10.1128/JCM.42.6.2432-2437.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Ji S, Chen Y, et al. Resistance of strains producing extended-spectrum beta-lactamases and genotype distribution in China. J Infect. 2007;54:53–57. doi: 10.1016/j.jinf.2006.01.014. [DOI] [PubMed] [Google Scholar]