Abstract
[68Ga]Ga-DO3A-VS-Cys40-Exendin-4/PET-CT targeting glucagon like peptide-1 receptor (GLP-1R) has previously demonstrated its potential clinical value for the detection of insulinomas. The production and accessibility of this radiopharmaceutical is one of the critical factors in realization of clinical trials and routine clinical examinations. Previously, the radiopharmaceutical was prepared manually, however larger scale of clinical trials and healthcare requires automation of the production process in order to limit the operator radiation dose as well as improve tracer manufacturing robustness and on-line documentation for enhanced good manufacturing practice (GMP) compliance. A method for 68Ga-labelling of DO3A-VS-Cys40-Exendin-4 on a commercially available synthesis platform was developed. Equipment such as 68Ge/68Ga generator, synthesis platform, and disposable cassettes for 68Ga-labelling used in the study was purchased from Eckert & Ziegler. DO3A-VS-Cys40-Exendin-4 was synthesized in-house. The parameters such as time, temperature, precursor concentration, radical scavenger, buffer concentration, pH, product purification step were investigated and optimised. Reproducible and GMP compliant automated production of [68Ga]Ga-DO3A-VS-Cys40-Exendin-4 was developed. Exendin-4 comprising methionine amino acid residue was prone to oxidation which was strongly influenced by the elevated temperature, radioactivity amount, and precursor concentration. The suppression of the oxidative radiolysis was achieved by addition of ethanol, dihydroxybenzoic acid and ascorbic acid to the reaction buffer as well as by optimizing heating temperature. The non-decay corrected radiochemical yield was 43±2% with radiochemical purity of over 90% wherein the individual impurity signals in HPLC chromatogram did not exceed 5%. Automated production and quality control methods were established for paving the pathway for broader clinical use of [68Ga]Ga-DO3A-VS-Cys40-Exendin-4.
Keywords: Exendin-4, Insulinoma, GLP-1, GMP, Gallium-68, automation
Introduction
Radiolabelled glucagon like peptide-1 analogues comprising Exendin-3 and Exendin-4 peptides demonstrated strong potential for the clinical use in detection of insulinomas [1-12] as well as for in vivo follow up of pancreatic and transplanted islets of Langenhans in diabetic patients [13]. The initial studies were conducted using 111In and 99mTc labelled analogues for the examinations with single photon emission computed tomography (SPECT) [2-4,12]. The studies were successful in localizing the lesions, however low spatial resolution of 111In/SPECT and interference of the high kidney uptake with detection of lesions in the pancreas required a second SPECT examination 3-7 days later [2,3,8,12]. Despite the lower γ-energy and shorter half-life of 99mTc as compared to 111In and potential for the improvement of the quality of images and reduction of radiation burden to the patient and medical staff, the 99mTc-labelled analogues presented similar disadvantages [4,14,15].
Further improvement can be achieved by PET technique that has inherent advantages over SPECT in terms of higher sensitivity and spatial resolution as well as accurate quantification [16] which is crucial especially considering small size of insulinomas. Amongst positron emitting radionuclides 68Ga is a very attractive one in terms of its ready availability from a simple generator system, straightforward labelling chemistry, and favorable decay characteristics. A number of clinical studies reported on successful use and superiority of 68Ga-labelled analogues. In particular, [68Ga]Ga-DO3A-VS-Cys40-Exendin-4/PET-CT targeting glucagon like peptide-1 receptor (GLP-1R) was used in a case examination of a patient with severe hypoglycaemia and localized multiple small liver metastases and paraaortal lymph node lesion while computed tomography, ultrasound, [18F]FDG/PET-CT, [11C]HTP/PET-CT, and 111In-octreoscan/SPECT failed to provide conclusive results [1]. Comparative study of [Nle14, Lys40(Ahx-DOTA-111In)NH2]exendin-4 and [Nle14, Lys40(Ahx-DOTA-68Ga)NH2]exendin-4 demonstrated superiority of the latter in terms of detection rate, resolution, and background uptake [6]. In another intrapatient comparative study [Lys40-(AHX-DOTA-68Ga)NH2] PET/CT clearly delineated pancreatic lesion while [Lys40-(AHX-DOTA-111In)NH2] SPECT/CT was not conclusive [17]. The pancreas tail insulinoma was detected by 68Ga-NOTA-MAL-Cys40-exendin-4 PET/CT [5]. High localization sensitivity of 97.7% was reported for 68Ga-NOTA-exendin-4 PET/CT [18] and lesions as small as less than 1.0 cm were detected. Even though the kidney uptake is high, unambiguous delineation can be accomplished 2-3 h post injection.
The production and accessibility of a radiopharmaceutical is one of the critical factors in realization of clinical trials and routine clinical examinations. Previously, [68Ga]Ga-DO3A-VS-Cys40-Exendin-4 was prepared manually [1], however for larger scale of clinical trials and routine clinical examinations the manual preparation would not be acceptable due to the high radiation dose to the operator and possible production variability. Thus the automation of the production process is required in order to limit the operator radiation dose as well as to improve tracer manufacturing robustness and on-line documentation for enhanced good manufacturing practice (GMP) compliance. The clinical research achievements and call for the implementation of the imaging agent into clinical practice encouraged us to develop an automated method for the production of [68Ga]Ga-DO3A-VS-Cys40-Exendin-4 using a commercially available synthesis platform.
Material and methods
Facilities, equipment, and materials
The synthesis of the precursor, DO3A-VS-Cys40-Exendin-4, was reported previously [7]. The purchased chemicals were used without further purification: HCl (ultrapure, Merck), acetate buffer (pH 4.6, Sigma-Aldrich), sterile water (Fresenius Kabi), saline (Apoteket AB), NaOH (10M, Sigma-Aldrich), ethanol (APL), water (Fluka, TraceSelect), trifluoroacetic acid (Merk, Darmstadt, Germany).
The aseptic production was conducted in a GMP grade A workstation (unidirectional laminar airflow workbench (LAFW)) situated in a cleanroom with GMP grade B air quality. The 68Ge/68Ga generator (IGG101, Eckert & Ziegler) and Modular PharmLab labelling synthesis platform (Eckert & Ziegler Eurotope, Berlin, Germany) were placed in the LAFW. A high performance liquid chromatography system (LaChrom, Hitachi, VWR) consisting of an L-2130 pump, UV detector (L-2400), and a radiation flow detector (Bioscan) coupled in series was used for product quality control. Separation of the analytes was accomplished using an endcapped analytical column with stationary reversed phase (C-4; Aeris widepore; 50×2.1 mm; particle size: 3 µm). Two systems were used: 1. A = 25 mM KH2PO4 (pH = 2.0); B = 100% acetonitrile (MeCN) with UV-detection at 220 nm; linear gradient elution: 0 min at 25% B, 0-4 min to 29% B, 4-15 min to 60% B; flow rate was 1.0 ml/min; and 2. A = 10 mM TFA; B = acetonitrile/10 mM TFA with UV-detection at 220 nm; linear gradient elution: 0-6 min from 25% to 29% B, 6-15 min from 25% to 60% B; flow rate was 1.0 ml/min. Data acquisition and handling were performed using the EZChrom Elite Software Package.
The starting material, 68Ga (t1/2 = 68 min, β+ = 89%, and EC = 11%) used for the production was obtained from a pharmaceutical grade 68Ge/68Ga-generator (1850 MBq, IGG101, Eckert & Ziegler Isotope products) by elution with 0.1 M hydrochloric acid. The amount of detected metal impurities as provided by the manufacturer was less than the defined limit in the European Pharmacopeia monograph [19]. The appearance of the 68Ga eluate was clear and colorless. The 68Ge breakthrough in the eluate as a percentage of the eluted 68Ga radioactivity was calculated by measuring aliquots of the generator eluate and counting the radioactivity content using an ionization chamber with NaI(Tl) scintillation detector immediately after elution and in the well-type NaI(Tl) scintillation counter 48 h post elution. The 68Ga elution efficiency was expressed as the ratio between the obtained 68Ga and 68Ge radioactivity (in equilibrium with the 68Ga daughter nuclide) on the generator column at the time of elution. Aliquots of the generator eluate were counted immediately using an ionization chamber with NaI(Tl) scintillation detector.
Production of [68Ga]Ga-DO3A-VS-Cys40-Exendin-4
The production was conducted using commercial fully automated platform for labelling synthesis (Modular-Lab PharmTrace (Eckert & Ziegler, Eurotope, Germany) with disposable cassette system (C4-Ga68-PP, C4-Ga68-PSMA). The process included labelling synthesis, product purification, formulation, and sterile filtration as well as sterile filter integrity test. Such parameters as reaction time, temperature, and radioactivity were monitored in real time. The process included pre-concentration of the generator eluate step for which various strong cation exchange (SCX) cartridges were tested (Table 1). For the product purification various solid phase extraction (SPE) cartridges were tested. A mixture of sodium chloride (5 M) solution and hydrochloric acid (0.1 and 0.05 M) as well as mixture of acetone and 0.1 M hydrochloric acid were used for the recovery of 68Ga(III) from the SCX cartridges. The cassette was loaded with the SCX eluent solution and reaction mixture containing the reaction buffer (1 M sodium acetate buffer, 1 M NaOH, ascorbic acid, dihydroxybenzoic acid, and ethanol) and precursor (0.2-20 nmol, DO3A-VS-Cys40-Exendin-4) was added to the reactor positioned in the heating block. The reaction mixture was heated in a conventional heating block for 15 min at 75 or 85°C. After the reaction completion the crude product was cooled down by adding 3 mL of saline and then purified on a SPE cartridge eluting the final product with 1 ml of 50% ethanol. The resulting product was diluted to 6 ml with sterile saline for the formulation and the solution was passed through a 0.22 µm sterile filter into a sterile 27 ml capped glass bottle. The sterile filter integrity was controlled on the platform using a separate program. A sample was taken for the determination of the identity, radiochemical purity, peptide concentration, and pH. The total radioactivity of the product was then measured in an ionization chamber.
Table 1.
The radioactivity (68Ga(III)) adsorption and desorption values (%) using various strong cation exchange cartridges, and respective values for the purification of [68Ga]Ga-DO3A-VS-Cys40-Exendin-4 using various solid phase extraction cartridges
| Cartridge | Adsorption | Desorption | Eluent |
|---|---|---|---|
| 68Ge/68Ga eluate pre-concentration | |||
| SCX (Strata-X-C, Phenomenex) | > 99% | > 90% | Acetone/HCl |
| SCX (Strata-X-C, Phenomenex) | > 99% | < 7% | NaCl/HCl (0.1 M) |
| SCX (Bond Elut-SCX, Agilent Tech) | > 98% | > 95% | NaCl/HCl (0.1 M) |
| SCX (PS-H+, Chromafix) | > 99.9% | 86.9±1.3% | NaCl/HCl (0.1 M) |
| SCX (PS-H+, Chromafix) | > 99.9% | 84.7±1.3% | NaCl/HCl (0.05 M) |
| SCX (PS-H+, Chromafix) | > 99.9% | 74.7±2.1% | NaCl |
| Product purification | |||
| RP-SPE (C18, Waters) | > 99% | < 20% | EtOH, 50% |
| RP-SPE (C18, Waters) | > 99% | < 15% | EtOH, 80% |
| RP-SPE (C8, Waters) | > 98% | < 55% | EtOH, 50% |
| RP-SPE (C2, Waters) | > 97% | 92.5±0.8% | EtOH, 50% |
| RP-SPE (C2, Waters) | > 97% | 94.8±1.1% | EtOH, 80% |
| HLB-SPE (Oasis) | > 99% | < 65% | EtOH, 50% |
The chemical purity, radiochemical purity and amount of the peptide were determined by HPLC. A sample of the product was kept for subsequent determination of 68Ge content. The stability of the product at room temperature was monitored by UV-Radio-HPLC for 2 hours. The recovery of radioactivity from the analytical column was investigated, in order to confirm that no radioactive product or impurities were left on the column, by collecting HPLC effluent with and without analytical column and measuring the radioactivity. The tests were performed both for the product ([68Ga]Ga-DO3A-VS-Cys40-Exendin-4) and free 68Ga(III). Specificity, linearity, and precision as repeatability were validated for both UV- and Radio-detectors. Tests performed on the finished product and specifications are summarized in Table 2.
Table 2.
Summary of the product specifications and results*
| Test | Acceptance criteria | [68Ga]Ga-DO3A-VS-Cys40-Exendin-4 |
|---|---|---|
| Radiochemical purity | > 90%; no unknown impurity corresponds to > 5% | 96.9±0.6** |
| pH | 4-8.5 | 6.0 |
| Radioactivity concentration | 5-100 MBq/ml | 73.6±1.4 |
| Radioactivity | 50-500 MBq | 444±9 |
| Volume | 2-10 ml | 6.039±0.013 |
| Color | colorless | colorless |
| Specific radioactivity | 1-150 MBq/nmol | 53±10 |
| Radionuclidic purity | > 99.9% | 99.99999±0.000006 |
| 68Ge breakthrough | < 0.001% | 0.000014±0.000006 |
| Stability | RCP*** > 91% within 120 min | 96.8±0.5 |
| EtOH content | < 10% | 5.44±0.12 |
The results are presented as mean ± SD (n = 3-5).
The radiochemical purity considers the combined values for the intact and oxidized products, where 5.5±0.8% corresponded to the oxidized product.
RCP: radiochemical purity.
Results
Generator qualification
The qualification of the 68Ge/68Ga generator was conducted to control appearance, elution efficiency, and 68Ge breakthrough as well as performing labelling synthesis of a validated tracer. The appearance of the eluate was colorless and clear. The elution efficiency was estimated at the time of secular equilibrium as percentage of the eluted radioactivity of the total radioactivity expected theoretically with decay correction for 68Ge, and it corresponded to 75±5%. The 68Ge breakthrough was determined by measuring the radioactivity of the 68Ga eluate directly and 48 hours after elution resulting in < 0.00007%.
Production of [68Ga]Ga-DO3A-VS-Cys40-Exendin-4
The fully automated production of [68Ga]Ga-DO3A-VS-Cys40-Exendin-4 (Figure 1) was conducted on a commercial labelling synthesis platform, Modular-Lab PharmTrace, according to the flow chart presented in Figure 2. The distribution of the radioactivity in the cassette for the optimised production is presented in Figure 3. The rest of the radioactivity associated with the cassette manifold was calculated as the difference between the measured components and the initially expected total amount of the radioactivity entering the cassette from the 68Ge/68Ga generator. The low standard deviation of the radioactivity distribution in the cassette elements indicated high reproducibility of the production process.
Figure 1.
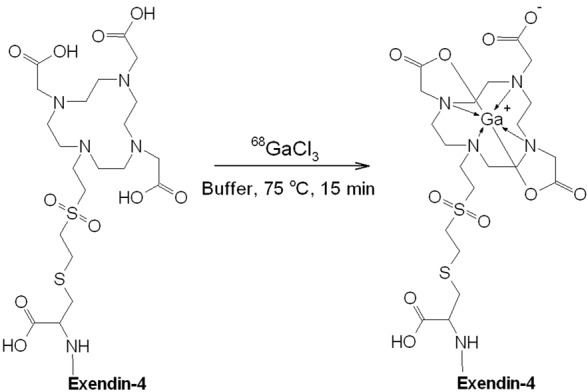
Schematic presentation of the labelling reaction for the production of [68Ga]Ga-DO3A-VS-Cys40-Exendin-4, where buffer stands for the mixture of acetate buffer, dihydroxybenzoic acid, ascorbic acid, and ethanol. Exendin-4: HGEGTFTSDLSKQMEEEAVRLFIEWLKNGGPSSGAPPPS.
Figure 2.
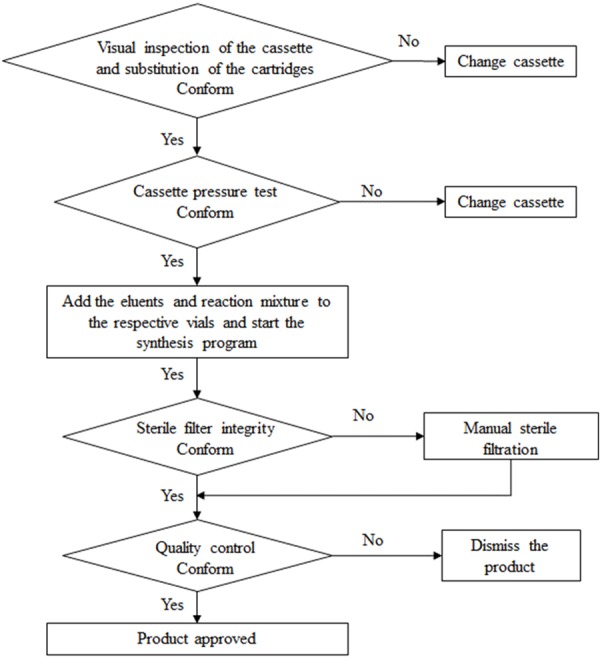
Flow chart of the production of [68Ga]Ga-DO3A-VS-Cys40-Exendin-4 on the Modular-Lab PharmTrace.
Figure 3.
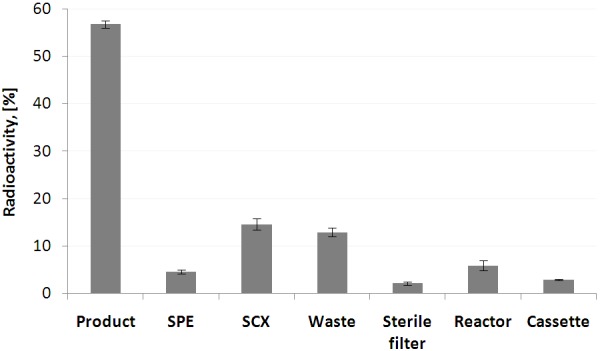
Distribution of the radioactivity on the cassette presented as fraction (%) of the total radioactivity entering cassette from the generator. Data are presented as mean ± SD (n = 5).
The developed method was GMP-compliant, reliable and reproducible. The parameters such as time, temperature, buffer content (pH) and precursor concentration were optimised. The 68Ga pre-concentration step was investigated using various SCX cartridges and controlling necessity of the pre-conditioning of the cartridges as well as optimising adsorption and desorption of 68Ga using various eluent hydrochloric acid concentrations (Table 1). Ethanol, ascorbic acid, and dihydroxybenzoic acid were tested as radical scavengers in order to supress formation of the oxidized product. The concentration of the acetate buffer was optimised in order to provide favourable and robust pH. Product purification step was optimized testing various SPE cartridges (Table 1). The product was diluted up to 6 ml on the formulation step so that the final ethanol concentration would be < 10%. The radioactivity incorporation (RAI) and formation of the oxidized product were investigated at ambient temperature and 75-100°C and dependent on the time (5-15 min). The RAI did not exceed 10% at ambient temperature within 30 min. The optimal condition was determined as 75°C for 15 min. The concentration of acetate buffer was investigated in the range of 0.1-1 M with the highest radiochemical yield and robust production obtained at 0.3 M.
The original SCX cartridge (Strata-X-C, Phenomenex) provided with the cassette was dedicated for the elution with acetone/HCl mixture and showed high recovery of > 90% (Table 1), however [68Ga]Ga-DO3A-VS-Cys40-Exendin-4 radiochemical yield was found suboptimal (< 10%). The eluent was substituted with NaCl/HCl solution, however recovery of 68Ga dropped considerably (Table 1). Another SCX cartridge (Bond Elut-SCX, Agilent Technologies) demonstrated high recovery (> 95%) in combination with NaCl/HCl eluent however the radiochemical yield was very poor (< 5%). Another commercial SCX cartridge (PS-H+) was tested and demonstrated acceptable recovery results (86.9±1.3%) and the subsequent radiolabelling reaction also worked well. Moreover, the cartridge did not require pre-conditioning. The adsorption and desorption of 68Ga was comparable for the cases with and without cartridge pre-conditioning resulting, respectively in over 99.9% of adsorption for both cases and 84±3% for desorption from the equilibrated cartridge and 84.7±1.3% for desorption from the non-equilibrated cartridge. The elution from the SCX cartridge was conducted using two concentrations of hydrochloric acid (0.05 M and 0.1 M) in sodium chloride solution (5 M), and demonstrated very similar results in terms of radioactivity recovery (Table 1), while the product radioactivity incorporation was < 70% and 87±3.2%, respectively for the eluent with 0.1 M and 0.05 M hydrochloric acid.
Various SPE cartridges for the purification of the crude product were tested (Table 1). The cassette dedicated SPE-C18 cartridge demonstrated poor recovery (< 20%) irrespectively of the strength of ethanol eluent. The substitution with SPE-C8 or SPE-HLB cartridge improved the recovery up to, respectively 55% and 65%, and further on the use of SPE-C2 cartridge resulted in the best recovery of 92.5±0.8%.
The radioactivity incorporation (RAI) was studied as a function of time and precursor concentration (Figure 4). The optimal value corresponded to 85±1% with rather low relative standard deviation of 1.14% indicating high repeatability. The radiochemical purity was 96.9±0.6% including 5.5±0.8% of the oxidized product. The product was stable at ambient temperature for at least 2 h with radiochemical purity of 96.8±0.5%. Specific radioactivity determined by the ratio of the radioactivity measured in an ionization chamber and the total amount of the peptide determined by UV-HPLC was 53±10 MBq/nmol.
Figure 4.
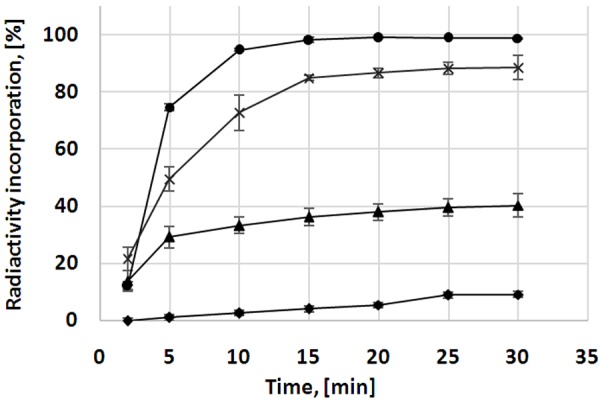
The kinetics of the radioactivity incorporation was studied for various concentrations of the precursor at 75°C: 2 µM (▲); 3 µM (×); 5 µM (•). Independent on the concentration of the precursor the radioactivity incorporation (RAI) approached the plateau after 15 min of the heating. RAI is presented as a sum of the radio-HPLC signals corresponding to the intact and oxidized products. With the heating time the fraction of the oxidized product increased (♦; 5 µM). The data is presented as mean ± SD (n = 3).
The suppression of the oxidative radiolysis and formation of the oxidized product was achieved using such radical scavengers as ethanol, ascorbic acid and dihydroxybenzoic acid (Figures 5 and 6). Ethanol alone decreased the content of the oxidized product from 25% to 10%, and the further addition of ascorbic acid decreased it further to 3%. The further increase of the concentration of the radical scavengers reduced further the formation of the oxidized product, but impaired reaction efficiency and radioactivity incorporation (Figures 5 and 6). The final optimization resulted in the use of the mixture of all three components with 5.5±0.8% of the oxidized product.
Figure 5.
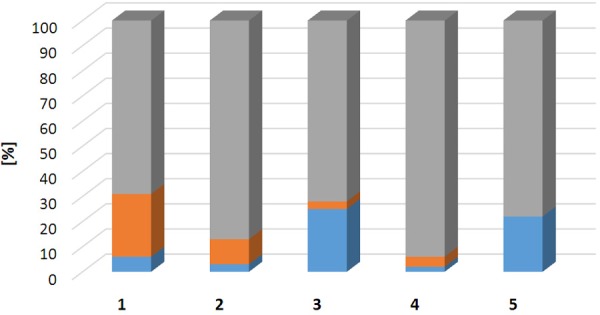
Bar graph presenting fractions of non-incorporated 68Ga(III) (blue), oxidized product (orange), and intact product (grey) dependent on the presence of radical scavengers (using 5 µM precursor): 1-without radical scavengers; 2-with EtOH; 3-with EtOH and ascorbic acid; 4-with EtOH and dihydroxybenzoic acid; 5-with EtOH, dihydroxybenzoic acid, and ascorbic acid. The data is presented as average of triplicate experiments.
Figure 6.
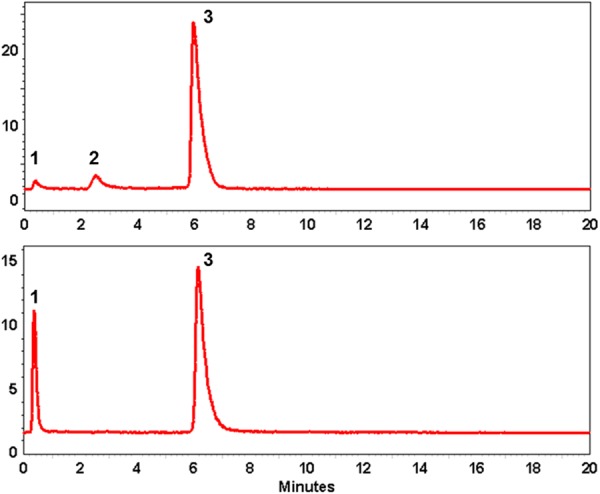
Radio-chromatograms of the crude products demonstrating influence of the scavengers on the formation of the oxidized (tR = 2.6 min; 2) and intact (tR = 6.1 min; 3) products, and RAI. Upper panel: the combination of EtOH and dihydroxybenzoic acid; lower panel: the combination of EtOH, dihydroxybenzoic acid and ascorbic acid. The front radiosignal corresponds to non-incorporated 68Ga(III) (1).
The final product [68Ga]Ga-DO3A-VS-Cys40-Exendin-4 was passed through a 0.22 µm sterile filter disk in-line and obtained in a solution of sterile saline and ethanol (5.44±0.12%) in a total volume of 6.04±0.01 ml (relative standard deviation (RSD): 0.2%) with pH of 6. The sterile filter integrity test was conducted automatically using another program sequence. The content of the peptide in the formulated product was determined by UV-HPLC using calibration plots. A sample of the product was kept for subsequent determination of 68Ge content and resulted in less than 0.00002%. Tests performed on the finished product, specifications and results are summarized in Table 2.
Quality control
The chemical purity, radiochemical purity and amount of the peptide were determined by UV-Radio-HPLC. The HPLC method was validated with respect to specificity, linearity, precision, repeatability with respect to both UV- and Radio-detectors, as well as radioactivity recovery from the HPLC column. The calibration of the UV-signal was conducted in order to enable accurate determination of the peptide content in the final product, and it resulted in high Pearson correlation coefficient (R2) of > 0.995. The range of the peptide concentration for the calibration corresponded to that expected for the product concentration. The HPLC method was developed to assure a baseline separation between the oxidized and non-oxidized products and demonstrated high specificity. The investigation of the detection limit demonstrated broad margin for the accurate detection of both UV- and radio-signals. The recovery of radioactivity from the HPLC column was found over 99% assuring adequate interpretation of the analysis results. Both HPLC methods (Materials and Methods) differing in the mobile phase and gradient demonstrated reproducible results and confirmed the identity of the product.
Discussion
Automation of labelling synthesis
The preclinical and clinical performance of [68Ga]Ga-DO3A-VS-Cys40-Exendin-4 have been studied in our laboratory and the preparation of the tracer was accomplished manually [1,20-24]. However, the manual preparation in larger scale would cause high radiation dose to the radiochemist and would be unacceptable from the radiation safety point of view. The automated synthesis reduces radiation exposure to the operator, improves robustness of the production, and provides on-line documentation of the manufacturing process thus improving GMP compliance which has outmost importance in the clinical environment involving patient examinations. In addition, the automated production of the radiopharmaceutical would improve standardization and harmonization of multicenter clinical trials and accelerate regulatory approvals for the routine clinical use [16].
Commercially available synthesis platforms are based on either stationary tubing system or disposable cassettes [25,26]. The latter are more preferable in routine clinical setup since they exclude the risk of cross-contamination, allow high frequency of the synthesis, and improve reproducibility. Modular-Lab PharmTrace platform (Eckert & Ziegler, Eurotope, Germany) meets the above mentioned requirements and also allows for the most common labelling methods involving either fractionation or pre-concentration of the generator eluate.
The translation of the manual preparation procedure to the automated one is not straightforward and it results in the loss of the flexibility of the former. The automated process is continuous and cannot be interrupted for the adjustments of the conditions, e.g. pH. Moreover, the liquid transport on the system requires larger volumes; the losses of the peptide are larger due to the liquid transport through the tubing and manifold network; all chemicals and reagents including precursors have to be mixed prior to the synthesis and loaded onto the cassette thus exposing the peptide directly to the hydrochloric acid carrying 68Ga. There is less flexibility in the liquid flow rate and thus less possibility to adjust desorption from SCX and SPE cartridges. These factors should be taken into consideration while developing an automated version of a labelling synthesis procedure. The key parameters and aspects that have to be addressed are acidity of the reaction mixture, performance of the strong cation/anion exchange and solid phase extraction cartridges, reaction time and temperature, precursor peptide concentration used for the reaction, radiochemical yield, radiochemical purity as well as repeatability, reproducibility and robustness of the process. These parameters were studied performing semi-automated experiments closely resembling conditions of the fully automated process as well as optimizing fully automated process.
Concentration of peptide precursor and specific radioactivity
Exendin-4 analogues are potent incretin-mimetics and induce insulin secretion in response to rising blood glucose already at low exposure. Serious adverse effects like hypoglycemia are rare as Exendin-4 acts in a glucose-dependent manner. However, Exendin-4 also has intestinal and centrally mediated pharmacology, and may induce dose-limiting nausea and vomiting, which may compromise patient compliance to the PET scanning protocol [27]. The incidence and severity of these adverse effects seem to be inversely correlated to the duration of Exendin-4 treatment. Thus it is of importance to control the amount of the administered peptide, especially in the case of acute intravenous injection without prior treatment with Exendin-4. The Exendin-4 based medication, Byetta, is injected subcutaneously with dose of 10 µg, and thus clinical studies conducted using various radiolabelled Exendin-4 analogues limited the dose of the intravenous administration to 10 µg [1-5,8,12,17]. The low peptide amount requires respective sufficiently high specific radioactivity of the imaging agent, wherein effective specific radioactivity (SRA) is determined as a ratio of the radioactivity in MBq and amount of the peptide in the final product associated with the measured radioactivity. There are several interplaying aspects that should be taken into consideration. The SRA should be sufficiently high in order to allow statistically viable counts for adequate signal registration while reducing the amount of the administered peptide. On the other hand, the amount of the peptide should be sufficiently high in order to allow reasonable radiochemical yield and reliable determination of the peptide concentration in the final product. SRA is a function of precursor amount, production process duration, and radioactivity incorporation magnitude. These parameters were thoroughly investigated in order to optimize the production process and outcome.
The method presented herein is a compromise that on one hand allows robust production and on the other hand sufficiently high SRA that limits the amount of the administered peptide to less than 10 µg. The formulation of the product allowed adequate dilution to reduce the EtOH concentration to less than 10% while keeping the peptide concentration sufficiently high for the accurate UV-HPLC determination of the peptide content in the product and control over the injected peptide amount. It should also be mentioned that the amount of the injected peptide influences the biodistribution pattern [28-30] and consequently the determination of the injected peptide amount might be crucial for the adequate interpretation of imaging results.
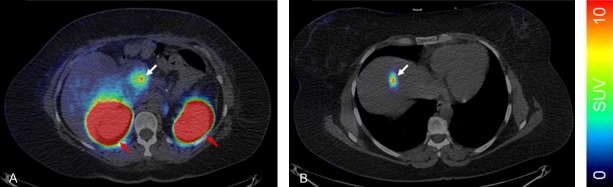
As mentioned above, SRA should assure statistically sufficient number of counts required for high quality images and accurate quantification. In this context it is worth mentioning that the scanner technology is developing and the modern scanners that are equipped with digital detectors allow higher sensitivity and thus administration of rather low radioactivity amount. Administration of 0.17 µg/kg [68Ga]Ga-DO3A-VS-Cys40-Exendin-4 yields strong accumulation in primary pancreatic insulinoma and hepatic metastasis in excess of SUV > 9 (> 0.008% ID/g, lesion-to-pancreas background ratio > 5.4) and SUV > 6 (> 0.006% ID/g, lesion-to-liver background ratio > 16.7) respectively (Figure 7) [1].
Figure 7.
Visualization of primary pancreatic insulinoma (A, white arrow) and hepatic metastasis in the same individual (B, white arrow). The kidneys are indicated by red arrows. The images are normalized to SUV = 10 according to the color bar.
Technological advances as described above in combination with the favorable organ distribution of [68Ga]Ga-DO3A-VS-Cys40-Exendin-4 may allow as little as 30-50 MBq for an examination which would correspond to less than 10 µg (< 0.05 µg/kg for an individual of 75 kg) of total injected peptide dose considering SRA of 53±10 MBq/nmol. Previous experience with [68Ga]Ga-DO3A-VS-Cys40-Exendin-4 dose escalation imaging studies in cynomolgus monkeys indicate that intravenous administration below or in the range of 0.05 µg/kg [23] induces an exposure consistent with the tracer concept (minimal GLP-1R occupancy induced by the radiolabeled substance itself) likely providing optimal lesion-to-background ratios. This would provide broad margin for safety in terms of adverse effects given the high potency of Exendin-4, while likely improving the diagnostic value of the examination.
The robustness of the production process depends on the concentration of the peptide used for the labelling reaction. It was demonstrated by investigating the radioactivity incorporation (RAI) as function of the precursor concentration wherein the relative standard deviation for the lowest and highest peptide concentration was respectively 25% (1 μM) and 0.2% (5 μM). Thus the higher amount of peptide provided higher reproducibility and robustness while allowing acceptable SRA. The increase of the precursor concentration to 5 μM improved the RAI up to over 99%, however the maximum concentration that could be used in order to allow injectable amount was limited to 3 μM. The loss of the peptide on the plastic surfaces of vials, syringes and cassette resulted in less than initially introduced amount of the peptide actually entering the reaction thus the determination of the final peptide concentration in the product was crucial for the quality control.
It is worth mentioning that SRA should not be too high either, otherwise the too low amount of peptide in vivo might be consumed by non-specific binding, circulation and excretion resulting in increased background tissue uptake, lower target uptake, and poorer image contrast [28,30]. In cases when the higher amount of Exendin-4 analogue (≥ 10 µg) has to be injected due to radiopharmaceutical production constraints or unfavorable biodistibution pattern, the episodes of severe hypoglycemia can be prevented by continuous infusion of glucose [18].
Radiolytic oxidation and formation of oxidized product
Exendin-4 contains methionine and tryptophan amino acid residues that are sensitive to radiolytic oxidation especially under labelling conditions using high amount of radioactivity making the production of radiolabeled analogues with high purity rather challenging. The formation of oxidized by-product compromising the purity of the radiopharmaceutical may occur. The change in biological activity, in particular receptor binding affinity upon the oxidation can be expected, nevertheless in case of exendin-4 analogues it has been demonstrated that the oxidized product maintains binding capability though to somewhat lesser extent as compared to the intact counterpart [31]. In the best case scenario, a single radiochemical entity is preferred. However, the comparable binding capacity would allow the calculation of the radiochemical purity as a sum of the two components. Nevertheless, attempts to decrease the formation of the oxidized product must be conducted until the options are exhausted.
The content of the oxidized product was found to be 25±7% when performing the labelling reaction in the absence of radical scavengers (Figure 5), and it increased with the time during the storage at room temperature. The suppression of the radiolytic oxidation can be achieved by the addition of radical scavengers such as ethanol, ascorbic acid, dihydroxybenzoic acid, thiols, human serum albumin, and HEPES [32,33]. In the case of an affibody molecule, [68Ga]Ga-ABY-025, which is a 7 kDa peptide, the radiolysis was fully suppressed by addition of only ethanol [30]. However, in case of DO3A-VS-Cys40-Exendin-4 addition of 10% (volume of the reaction mixture) of ethanol reduced the content of the oxidized product down to 10±3% (Figure 5). In the absence of the radical scavengers the fraction of the oxidized product increased within 2 h from 25% to 66%, while the use of ethanol solely kept the formation of the oxidized product under 15% for at least 2 hours. The mixture of ethanol and ascorbic acid (16 mM) further reduced the content of the oxidized product to 3±1%, however the RAI was also reduced by 23%. The combination of dihydroxybenzoic acid and ethanol reduced the fraction of the oxidized product to 4±1% while maintaining similar RAI. Thus the addition of dihydroxybenzoic acid did not decrease the RAI as compared to the ethanol while the formation of the oxidized product was below 5%. None of the radical scavengers alone supressed the formation of the oxidized product fully. The combination of ethanol, ascorbic acid, and dihydroxybenzoic acid (3.5 mM) with further increased concentration eliminated completely the formation of the oxidized product, but impaired reaction efficiency and RAI (Figures 5 and 6). The drop of RAI would in turn result in decrease of SRA by 22-43%. Thus the optimal balance must be found between the fraction of the oxidized product and RAI. In this study, it was preferred to allow 5.5±0.8% of the oxidized product while maintaining relatively high RAI of around 90%. The optimised method used the combination of ethanol, and lower concentration of dihydroxybenzoic acid and ascorbic acid. It should also be mentioned that ascorbic acid should be prepared freshly since it is easily oxidized in the presence of oxygen and might cause poor reproducibility of the labelling outcome.
In addition, the kinetic study demonstrated that the RAI increased with the heating duration, however after 15 min the fraction of oxidized product was also increasing, thus the 15 min heating limit was chosen allowing balance between the formation of the oxidized product and RAI (Figure 4). Elevation of the heating temperature increased formation of the oxidized product to 50%. The fraction of the oxidized product also increased upon the reduction of the precursor concentration used for the labelling synthesis.
Exendin-4 analogues must be stored preferably in powder form and kept under -20°C after dissolving. To our experience storing the solution at 4°C did not prevent the oxidation as we observed formation of 3-5% of the oxidized product even in the presence of high concentration of scavengers most probably indicating the presence of the oxidized component already in the precursor solution, and consequently the complete elimination of the oxidized product might be difficult to achieve. Another solution has been suggested by introducing an isosteric analogue, namely norleucine amino acid residue instead of methionine [31,34,35]. Substitution of methionine with norleucine improved the chemical quality of the agent while maintaining the biological activity and even improving the binding capability [31,34,35]. Such analogue, [Nle14, Lys40(Ahx-DOTA-68Ga) NH2]exendin-4, was successfully used in a clinical study [6]. Exendin-4 also contains tryptophan which is also prone to the oxidation [36], however the corresponding by-products have not been investigated and given the fact that after the substitution of the methionine with norleucine the labelling resulted in a single product it is plausible that the oxidation of tryptophan under those conditions did not occur.
Reagents and cassette components
Although HEPES buffer is more favorable at low peptide precursor concentration [37], acetate buffer was preferred in order to reduce the validation burden, expenses and resources that would be additionally consumed, in particular on the development of the quality control and estimation of HEPES quantity required by European Pharmacopeia monograph [19]. Unfortunately, the semi-quantitative method suggested in the monograph presents large variability dependent on the operator and a more accurate HPLC method suggested by [38] presents insufficient sensitivity with regard to HEPES concentration in the final product. The use of commercially available buffers and solutions in this study excluded operator error during the preparation and contributed to the robustness of the production process. The concentration of the acetate buffer was optimized in order to avoid excess buffer that might compete with DOTA if used in excess [37] on one hand and on the other hand to assure right and stable pH as well as utilize the stabilizing function of the acetate and exclude 68Ga precipitation and colloid formation [32]. The optimized concentration in the reaction mixture was 0.3 M.
Several strong cation exchange cartridges (SCX) based either on poly(styrene-divinylbenzene) resin functionalized with sulfone groups and H+ counter ion or silica functionalized with benzene sulfonic acid were tested. They all demonstrated high adsorption of 68Ga(III), however desorption and subsequent reaction efficiency varied considerably (Table 1). The application of SCX cartridge (Strata-X-C, Pheno-menex) used in combination with acetone eluent (cassette: C4-Ga68-PP), resulted in RAI of < 35% and formation of by-products. Assuming the possible negative influence of the acetone eluent, the same SCX cartridges was tested with NaCl/HCl eluent, however the desorption of 68Ga(III) dropped considerably. The combination of SCX cartridge (Bond Elut-SCX, Agilent Tech) dedicated for the preconcentration using NaCl/HCl eluent (cassette: C4-Ga68-PSMA) demonstrated excellent adsorption and desorption characteristics, however RAI was negligible. The substitution of the cassette dedicated SCX cartridge with commercially available one (PS-H+, Chromafix) used in combination with NaCl/HCl eluent demonstrated excellent adsorption, acceptable desorption (Table 1) and RAI results. In addition, no equilibration of SCX cartridge (PS-H+, Chromafix) was required prior to use thus saving time and resources. However, it resulted in higher acidity of the reconstituted 68Ga solution which was easily compensated with higher acetate buffer concentration. It was also easy to mount the SCX cartridge (PS-H+, Chromafix) onto the cassette due to the compact dimensions and construction with luer fitting and tip. Two concentrations of HCl (0.05 and 0.1 M) in the eluent (NaCl/HCl) were tested with the objective to create milder condition and it resulted in higher ratio between the intact and oxidized products. In order to improve the labelling conditions even further, 5 M NaCl solution without HCl was also tested, however it demonstrated lower 68Ga recovery of 74.7±2.1% which would not be beneficial in terms of radiochemical yield which was reduced compared to the method using 0.05 M HCl content, respectively by 10% despite higher RAI.
The solid phase extraction cartridge (SPE)-C18 included in the commercial cassette for the purification of the final product caused very high peptide retention and poor recovery of only 20% resulting consequently in a poor radiochemical yield (RCY). In order to improve the latter, other SPE cartridges such as C-8, HLB, and tC2 were investigated (Table 1). The best results were shown by SPE-tC2 cartridge with recovery of 92.5±0.8%, thus improving the non-decay corrected (NDC) RCY up to 43±2%. The strength of the ethanol eluent for the product recovery from SPE-tC2 cartridge was also investigated. The difference in product recovery was marginal with 92.5±0.8% and 94.8±1.1%, respectively for 50% and 80% ethanol aqua solution eluents. The use of 50% ethanol was found advantageous since it allowed the lowest possible formulation dilution and thus highest possible peptide concentration that permitted the accurate determination of the latter by UV-HPLC.
The heating temperature of 75°C was optimal when using glass reactor vial, while in case of a plastic vial the RAI was reduced by 3-5%. Taking into account the possible difference in heat transfer between the glass and plastic material the heating temperature was increased up to 85°C when using the plastic vial resulting in improved RAI of 85±1%.
The low relative standard deviation values with regard to the radioactivity distribution on the cassette, adsorption/desorption of SCX and SPE cartridges (Figure 3; Table 1), radiochemical yield, and radiochemical purity indicated high repeatability as well as reproducibility and robustness of Modular-Lab PharmTrace platform and the production process. The fraction of radioactivity remained on the cassette was small and most probably associated with the labelled product. The total loss of the peptide due to the transfer was estimated to 20-25%.
The relative standard deviation of the RCY was low indicating high repeatability of the production process however it should be taken into consideration that the labelling reaction remains very sensitive to various metal cation impurities especially at low peptide precursor concentration. The content of metal cation impurities may vary in used chemicals such as sodium chloride, hydrochloric acid, acetate buffer, ascorbic acid, dihydroxybenzoic acid as well as in the resin of strong cation exchange cartridges influencing the labelling efficiency variability. Thus it is recommended to prepare the solutions for certain number of productions and store them at -20°C in order to provide high reproducibility and avoid possible operator mistakes during the preparation.
Quality control aspects
Since the generator is of pharmaceutical grade classified as a medicinal product with guaranteed quality with regard to 68Ge breakthrough, metal cation impurity content, radionuclidic purity, and 68Ga identity, the thorough generator validation [30] is not required. Nevertheless, for the qualification such parameters as eluate appearance, 68Ge breakthrough, elution yield as well as test synthesis of an established product were conducted adhering acceptance criteria defined by European Pharmacopoeia monographs [19,39]. The routine control of 68Ge breakthrough was performed monthly. Daily elution or elution 3-4 h prior to synthesis is recommended in order to keep the metal cation impurities, that may deteriorate 68Ga-labelling efficiency, at lowest possible level [32,37].
Quality control was performed using UV-Radio-HPLC with the precursor as reference standard. The accurate determination of the peptide content in the final product was enabled by UV-HPLC analysis according to a validated method and calibration plots. The tailing was observed for the HPLC signals (Figure 6) as a result of slow gradient introduced in order to allow baseline separation of the intact and oxidized products. The method reliability was confirmed, as mentioned earlier, by using two different systems with different mobile phases and elution gradients. The relatively poor symmetry of the signal did not influence the accuracy of the purity and concentration determination. The radiopharmaceutical demonstrated high stability with RCP of > 95% during 2 hours at ambient temperature. However, since the amount of the peptide that can be injected might be limited and in order to provide injected radioactivity amount statistically sufficient for high quality images and accurate quantification, the radiopharmaceutical is intended for immediate use.
Conclusions
Fully automated GMP/GRPP-compliant, reliable and highly reproducible production with accurate determination of the product peptide concentration was developed. The formation of the oxidized product was considerably reduced. Sufficiently high NDC RCY and SRA of [68Ga]Ga-DO3A-VS-Cys40-Exendin-4 would provide possibility to obtain high quality images for accurate quantification while injecting low amount of the peptide and avoiding possible and undesirable pharmacological side effects.
Acknowledgements
Gunnar Antoni, Olle Korsgren, and Lars Johansson are acknowledged for providing funding and infrastructure. This study was funded by Göran Gustafssons Foundation, c, Diabetes fonden, and ExoDiab.
Disclosure of conflict of interest
None.
References
- 1.Eriksson O, Velikyan I, Selvaraju RK, Kandeel F, Johansson L, Antoni G, Eriksson B, Sörensen J, Korsgren O. Detection of metastatic insulinoma by positron emission tomography with [68Ga] exendin-4-A case report. J Clin Endocrinol Metab. 2014;99:1519–1524. doi: 10.1210/jc.2013-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wild D, Macke H, Christ E, Gloor B, Reubi JC. Glucagon-like peptide 1-receptor scans to localize occult insulinomas. N Engl J Med. 2008;359:766–768. doi: 10.1056/NEJMc0802045. [DOI] [PubMed] [Google Scholar]
- 3.Christ E, Wild D, Forrer F, Brandle M, Sahli R, Clerici T, Gloor B, Martius F, Maecke H, Reubi JC. Glucagon-like peptide-1 receptor imaging for localization of insulinomas. J Clin Endocrinol Metab. 2009;94:4398–4405. doi: 10.1210/jc.2009-1082. [DOI] [PubMed] [Google Scholar]
- 4.Sowa-Staszczak A, Pach D, Mikolajczak R, Macke H, Jabrocka-Hybel A, Stefanska A, Tomaszuk M, Janota B, Gilis-Januszewska A, Malecki M, Kaminski G, Kowalska A, Kulig J, Matyja A, Osuch C, Hubalewska-Dydejczyk A. Glucagon-like peptide-1 receptor imaging with [Lys40(Ahx-HYNIC-99mTc/EDDA)NH2] -exendin-4 for the detection of insulinoma. Eur J Nucl Med Mol Imaging. 2013;40:524–531. doi: 10.1007/s00259-012-2299-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo Y, Yu M, Pan Q, Wu W, Zhang T, Kiesewetter DO, Zhu Z, Li F, Chen X, Zhao Y. 68Ga-NOTA-exendin-4 PET/CT in detection of occult insulinoma and evaluation of physiological uptake. Eur J Nucl Med Mol Imaging. 2015;42:531–532. doi: 10.1007/s00259-014-2946-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antwi K, Fani M, Nicolas G, Rottenburger C, Heye T, Reubi JC, Gloor B, Christ E, Wild D. Localization of hidden insulinomas with 68Ga-DOTA-exendin-4 PET/CT: a pilot study. J Nucl Med. 2015;56:1075–1078. doi: 10.2967/jnumed.115.157768. [DOI] [PubMed] [Google Scholar]
- 7.Velikyan I, Bulenga TN, Selvaraju KR, Lubberink M, Espes D, Rosenstrom U, Eriksson O. Dosimetry of [177Lu] -DO3A-VS-Cys40-Exendin-4-impact on the feasibility of insulinoma internal radiotherapy. Am J Nucl Med Mol Imaging. 2015;5:109–126. [PMC free article] [PubMed] [Google Scholar]
- 8.Wild D, Christ E, Caplin ME, Kurzawinski TR, Forrer F, Brandle M, Seufert J, Weber WA, Bomanji J, Perren A, Ell PJ, Reubi JC. Glucagonlike peptide-1 versus somatostatin receptor targeting reveals 2 distinct forms of malignant insulinomas. J Nucl Med. 2011;52:1073–1078. doi: 10.2967/jnumed.110.085142. [DOI] [PubMed] [Google Scholar]
- 9.Schottelius M, Wester HJ. Molecular imaging targeting peptide receptors. Methods. 2009;48:161–177. doi: 10.1016/j.ymeth.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Laverman P, Sosabowski JK, Boerman OC, Oyen WJ. Radiolabelled peptides for oncological diagnosis. Eur J Nucl Med Mol Imaging. 2012;39(Suppl 1):S78–92. doi: 10.1007/s00259-011-2014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reubi JC. Old and new peptide receptor targets in cancer: future directions. Recent Results Cancer Res. 2013;194:567–576. doi: 10.1007/978-3-642-27994-2_34. [DOI] [PubMed] [Google Scholar]
- 12.Christ E, Wild D, Ederer S, Béhé M, Nicolas G, Caplin ME, Brändle M, Clerici T, Fischli S, Stettler C, Ell PJ, Seufert J, Gloor B, Perren A, Reubi JC, Forrer F. Glucagon-like peptide-1 receptor imaging for the localisation of insulinomas: a prospective multicentre imaging study. Lancet Diabetes Endocrinol. 2013;1:115–122. doi: 10.1016/S2213-8587(13)70049-4. [DOI] [PubMed] [Google Scholar]
- 13.Brom M, Woliner-van der Weg W, Joosten L, Frielink C, Bouckenooghe T, Rijken P, Andralojc K, Goke BJ, de Jong M, Eizirik DL, Behe M, Lahoutte T, Oyen WJ, Tack CJ, Janssen M, Boerman OC, Gotthardt M. Non-invasive quantification of the beta cell mass by SPECT with (1)(1)(1)In-labelled exendin. Diabetologia. 2014;57:950–959. doi: 10.1007/s00125-014-3166-3. [DOI] [PubMed] [Google Scholar]
- 14.Sowa-Staszczak A, Trofimiuk-Muldner M, Stefanska A, Tomaszuk M, Buziak-Bereza M, Gilis-Januszewska A, Jabrocka-Hybel A, Glowa B, Malecki M, Bednarczuk T, Kaminski G, Kowalska A, Mikolajczak R, Janota B, Hubalewska-Dydejczyk A. 99mTc labeled glucagon-like peptide-1-analogue (99mTc-GLP1) scintigraphy in the management of patients with occult insulinoma. PLoS One. 2016;11:e0160714. doi: 10.1371/journal.pone.0160714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pach D, Sowa-Staszczak A, Jabrocka-Hybel A, Stefanska A, Tomaszuk M, Mikolajczak R, Janota B, Trofimiuk-Muldner M, Przybylik-Mazurek E, Hubalewska-Dydejczyk A. Glucagon-like peptide-1 receptor imaging with [Lys (40) (Ahx-HYNIC-(99 m) Tc/EDDA)NH 2] -Exendin-4 for the diagnosis of recurrence or dissemination of medullary thyroid cancer: a preliminary report. Int J Endocrinol. 2013;2013:384508. doi: 10.1155/2013/384508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Velikyan I. Prospective of 68Ga-radiopharmaceutical development. Theranostics. 2014;4:47–80. doi: 10.7150/thno.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christ E, Wild D, Antwi K, Waser B, Fani M, Schwanda S, Heye T, Schmid C, Baer HU, Perren A, Reubi JC. Preoperative localization of adult nesidioblastosis using 68Ga-DOTA-exendin-4-PET/CT. Endocrine. 2015;50:821–823. doi: 10.1007/s12020-015-0633-7. [DOI] [PubMed] [Google Scholar]
- 18.Luo Y, Pan Q, Yao S, Yu M, Wu W, Xue H, Kiesewetter DO, Zhu Z, Li F, Zhao Y, Chen X. Glucagon-like peptide-1 receptor PET/CT with 68Ga-NOTA-Exendin-4 for detecting localized insulinoma: a prospective cohort study. J Nucl Med. 2016;57:715–720. doi: 10.2967/jnumed.115.167445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.IGallium (68Ga) edotreotide injection. European Pharmacopoeia. 2014:1062. [Google Scholar]
- 20.Nalin L, Selvaraju RK, Velikyan I, Berglund M, Andreasson S, Wikstrand A, Ryden A, Lubberink M, Kandeel F, Nyman G, Korsgren O, Eriksson O, Jensen-Waern M. Positron emission tomography imaging of the glucagon-like peptide-1 receptor in healthy and streptozotocininduced diabetic pigs. Eur J Nucl Med Mol Imaging. 2014;41:1800–1810. doi: 10.1007/s00259-014-2745-3. [DOI] [PubMed] [Google Scholar]
- 21.Selvaraju R, Bulenga TN, Espes D, Lubberink M, Sörensen J, Eriksson B, Estrada S, Velikyan I, Eriksson O. Dosimetry of [68Ga] Ga-DO3A-VS-Cys40-Exendin-4 in rodents, pigs, non-human primates and human-repeated scanning in human is possible. Am J Nucl Med Mol Imaging. 2015;5:259–269. [PMC free article] [PubMed] [Google Scholar]
- 22.Selvaraju RK, Velikyan I, Asplund V, Johansson L, Wu Z, Todorov I, Shively J, Kandeel F, Eriksson B, Korsgren O, Eriksson O. Pre-clinical evaluation of [68Ga] Ga-DO3A-VS-Cys40-Exendin-4 for imaging of insulinoma. Nucl Med Biol. 2014;41:471–476. doi: 10.1016/j.nucmedbio.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 23.Selvaraju RK, Velikyan I, Johansson L, Wu Z, Todorov I, Shively J, Kandeel F, Korsgren O, Eriksson O. In vivo imaging of the glucagonlike peptide 1 receptor in the pancreas with 68Galabeled DO3A-exendin-4. J Nucl Med. 2013;54:1458–1463. doi: 10.2967/jnumed.112.114066. [DOI] [PubMed] [Google Scholar]
- 24.Ryden A, Nyman G, Nalin L, Andreasson S, Velikyan I, Korsgren O, Eriksson O, Jensen-Waern M. Corrigendum to “Cardiovascular side-effects and insulin secretion after intravenous administration of radiolabeled Exendin-4 in pigs” [Nucl Med Biol 43 (2016) 397-402] . Nucl Med Biol. 2016;43:742. doi: 10.1016/j.nucmedbio.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Decristoforo C, Knopp R, von Guggenberg E, Rupprich M, Dreger T, Hess A, Virgolini I, Haubner R. A fully automated synthesis for the preparation of 68Ga-labelled peptides. Nucl Med Commun. 2007;28:870–875. doi: 10.1097/MNM.0b013e3282f1753d. [DOI] [PubMed] [Google Scholar]
- 26.Boschi S, Malizia C, Lodi F. Overview and perspectives on automation strategies in 68Ga radiopharmaceutical preparations. Recent Results Cancer Res. 2012;194:17–31. doi: 10.1007/978-3-642-27994-2_2. [DOI] [PubMed] [Google Scholar]
- 27.Fineman MS, Shen LZ, Taylor K, Kim DD, Baron AD. Effectiveness of progressive doseescalation of exenatide (exendin-4) in reducing dose-limiting side effects in subjects with type 2 diabetes. Diabetes Metab Res Rev. 2004;20:411–417. doi: 10.1002/dmrr.499. [DOI] [PubMed] [Google Scholar]
- 28.Velikyan I, Sundin A, Eriksson B, Lundqvist H, Sorensen J, Bergstrom M, Langstrom B. In vivo binding of [68Ga] -DOTATOC to somatostatin receptors in neuroendocrine tumours--impact of peptide mass. Nucl Med Biol. 2010;37:265–275. doi: 10.1016/j.nucmedbio.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Sörensen J, Velikyan I, Sandberg D, Wennborg A, Feldwisch J, Tolmachev V, Orlova A, Sandström M, Lubberink M, Olofsson H, Carlsson J, Lindman H. Measuring HER2-Receptor Expression In Metastatic Breast Cancer Using [68Ga] ABY-025 Affibody PET/CT. Theranostics. 2016;6:262–271. doi: 10.7150/thno.13502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Velikyan I, Wennborg A, Feldwisch J, Lindman H, Carlsson J, Sorensen J. Good manufacturing practice production of [(68)Ga] Ga-ABY-025 for HER2 specific breast cancer imaging. Am J Nucl Med Mol Imaging. 2016;6:135–153. [PMC free article] [PubMed] [Google Scholar]
- 31.Janota B, Karczmarczyk U, Laszuk E, Garnuszek P, Mikolajczak R. Oxidation of methionineis it limiting the diagnostic properties of 99mTc-labeled Exendin-4, a Glucagon-Like Peptide-1 receptor agonist? Nucl Med Rev Cent East Eur. 2016;19:104–110. doi: 10.5603/NMR.2016.0021. [DOI] [PubMed] [Google Scholar]
- 32.Velikyan I. 68Ga-based radiopharmaceuticals: production and application relationship. Molecules. 2015;20:12913–12943. doi: 10.3390/molecules200712913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jodal A, Lankat-Buttgereit B, Brom M, Schibli R, Behe M. A comparison of three (67/68) Ga-labelled exendin-4 derivatives for beta-cell imaging on the GLP-1 receptor: the influence of the conjugation site of NODAGA as chelator. EJNMMI Res. 2014;4:31. doi: 10.1186/s13550-014-0031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sako T, Hasegawa K, Nishimura M, Kanayama Y, Wada Y, Hayashinaka E, Cui Y, Kataoka Y, Senda M, Watanabe Y. Positron emission tomography study on pancreatic somatostatin receptors in normal and diabetic rats with 68Ga-DOTA-octreotide: a potential PET tracer for beta cell mass measurement. Biochem Biophys Res Commun. 2013;442:79–84. doi: 10.1016/j.bbrc.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Kirsi M, Yim CB, Veronica F, Tamiko I, Viki-Veikko E, Johan R, Jori J, Tiina S, Tuula T, Marko T, Eleni G, Martin B, Martin G, Claude RJ, Helmut M, Anne R, Olof S, Pirjo N. 64Cuand 68Ga-Labelled [Nle14, Lys 40(Ahx-NODAGA) NH2] -Exendin-4 for pancreatic beta cell imaging in rats. Mol Imaging Biol. 2014;16:255–263. doi: 10.1007/s11307-013-0691-2. [DOI] [PubMed] [Google Scholar]
- 36.Gebhardt P, Opfermann T, Saluz HP. Computer controlled Ga-68 milking and concentration system. Appl Radiat Isot. 2010;68:1057–1059. doi: 10.1016/j.apradiso.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 37.Velikyan I, Beyer GJ, Langstrom B. Microwave-supported preparation of 68Ga-bioconjugates with high specific radioactivity. Bioconjugate Chem. 2004;15:554–560. doi: 10.1021/bc030078f. [DOI] [PubMed] [Google Scholar]
- 38.Sasson R, Vaknin D, Bross A, Lavie E. Determination of HEPES in 68Ga-labeled peptide solutions. Journal of Radioanalytical and Nuclear Chemistry. 2010;283:753–756. [Google Scholar]
- 39.Pharmacopeia E. Gallium (68Ga) chloride solution for radiolabelling. Ph Eur. 2014:1060. [Google Scholar]