Abstract
There is no available blood marker for the preoperative diagnosis of adrenocortical malignancy. The objective of this study was to investigate the expression of extracellular vesicle-associated microRNAs and their diagnostic potential in plasma samples of patients suffering from adrenocortical tumors. Extracellular vesicles were isolated either by using Total Exosome Isolation Kit or by differential centrifugation/ultracentrifugation. Preoperative plasma extracellular vesicle samples of 6 adrenocortical adenomas (ACA) and 6 histologically verified adrenocortical cancer (ACC) were first screened by Taqman Human Microarray A-cards. Based on the results of screening, two miRNAs were selected and validated by targeted quantitative real-time PCR. The validation cohort included 18 ACAs and 16 ACCs. Beside RNA analysis, extracellular vesicle preparations were also assessed by transmission electron microscopy, flow cytometry and dynamic light scattering. Significant overexpression of hsa-miR-101 and hsa-miR-483-5p in ACC relative to ACA samples has been validated. Receiver operator characteristics of data revealed dCThsa-miR-483-5p normalized to cel-miR-39 to have the highest diagnostic accuracy (area under curve 0.965), the sensitivity and the specifity were 87.5 and 94.44, respectively. Extracellular vesicle-associated hsa-miR-483-5p thus appears to be a promising minimally invasive biomarker in the preoperative diagnosis of ACC but needs further validation in larger cohorts of patients.
Introduction
Adrenocortical tumors are common in humans, and their prevalence rises with age1. These are mainly represented by benign adrenocortical adenomas (ACA). The majority of ACA is hormonally inactive and thus clinically indolent, but hormone-producing ACAs secreting cortisol or aldosterone are associated with serious morbidity and increased mortality1. In contrast, adrenocortical carcinoma (ACC) is a rare, but aggressive neoplasm. The incidence of ACC is about 0.5-2 cases per million people per year2, 3. The prognosis of ACC is poor as the estimated 5-year survival ranges from 15 to 30% in advanced stages4. There is no reliable preoperative marker for distinguishing ACA from ACC at present. Imaging modalities have considerable limitations5 and biopsy is not recommended due to difficulties of histological analysis and fear for tumor spread4. Urinary steroid metabolomics is a promising approach for preoperative diagnosis of malignancy, but is not widely available and requires a 24 h urine collection6.
Recent studies have reported significantly altered expression of both tissue and circulating microRNAs (miRNAs) in ACC versus ACA7–12. MiRNAs are short (19–24 nucletide long) non-protein coding RNA molecules involved in the regulation of gene expression primarily as endogenous mediators of RNA interference13. Beside tissue miRNAs, novel studies proved the stable existence of miRNAs in different body fluids14, as well. These extracellular miRNAs could serve as minimally invasive biomarkers of malignancy and prognosis in different tumors15, 16. In our previous study, we found that circulating miRNAs isolated from whole plasma could serve as potential biomarkers for adrenocortical carcinoma10. However, the diagnostic sensitivity and specificity were not high enough for clinical applicability10. In the blood plasma, miRNAs are found both in extracellular vesicles (EV) (such as microvesicles, exosomes, apoptotic bodies) and in macromolecular complexes with lipoproteins17 or the Argonaute 2 protein18. The mechanism for cellular miRNA release is only partially understood, but the active secretion of miRNA in extracellular vesicles appears to be a regulated process19, and thus, might be directly linked to disease pathogenesis. Exosomes represent a major class of extracellular vesicles that are formed via the endo-lysosomal pathway and are 40–100 nm in diameter19. Exosomal miRNAs might even be implicated in cell-to-cell communication20. Whereas circulating miRNAs isolated from whole plasma include miRNAs released due to tissue damage or necrosis21, we hypothesize that miRNAs secreted actively in EVs could be more specific as minimally invasive biomarkers. The objective of this study was to investigate the expresssion of EV-associated miRNAs and their diagnostic potential in patients with adrenocortical tumors. To the best of our knowledge, this is the first study to investigate the biomarker potential of extracellular vesicle-associated microRNAs in adrenocortical tumors.
Results
Confirming the nature of isolated extracellular vesicles
We have used both transmission electron microscopy and flow cytometry to confirm the presence of EVs in our samples to fulfill the minimal experimental requirements for extracellular vesicles22.
Transmission electron microscopy microphotographs taken from EVs confirmed that the EV size and morphology corresponded to those described for exosomes (Fig. 1).
Figure 1.
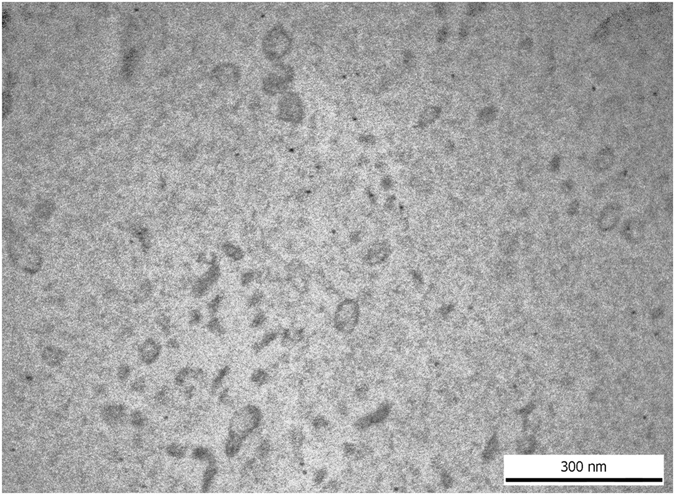
Transmission electron microscopic image of human blood plasma EVs isolated by ultracentrifugation, washed once and submitted to RNAse digestion.
Flow cytometric analysis of EVs isolated by ultracentrifugation confirmed the presence of CD9, CD63, CD81 membrane proteins and annexin V cytosolic protein (Fig. 2). In the vesicle preparations isolated by Total Exosome Isolation (from plasma) Kit, we could identify CD9, CD81 and annexin V (Fig. 2). The percentage of positive beads is presented in Supplementary Information Table S1.
Figure 2.
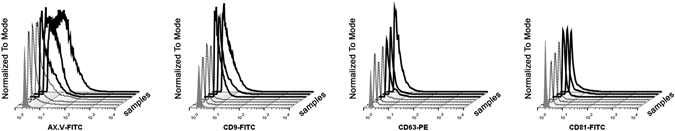
Flow cytometry detection of surface markers of human platelet free blood plasma extracellular vesicles (EVs) conjugated onto latex beads. EVs were isolated either by Total Exosome isolation kit (Life Technologies, by Thermo Fisher Scientific, Waltham, MA, USA) (empty histograms with dotted lines) or ultracentrifugation at 100,000 g (empty histograms with continuous lines) from three different human plasma samples, respectively. Antibody binding to BSA-coated latex beads is shown in gray histograms.
Size distribution of the isolated extracellular vesicles
We have used the dynamic light scattering technique to measure the size distribution of extracellular vesicles. The vesicles were isolated from 4 samples by Total Exosome Isolation (from plasma) Kit. The Z-average value of the 4 samples is 80.83 ± 19.07 nm in PBS at 25 °C. The detailed results of the dynamic light scattering are summarized in Table 1. The representative size distribution of EVs in sample 4 is presented in Fig. 3.
Table 1.
Results of dynamic light scattering.
| Sample Name | PDI | Standard deviation of PDI | Z-average (diameter nm) | Standard deviation of Z-average | Intensity mean (diameter nm) | Standard deviation of Intensity mean) |
|---|---|---|---|---|---|---|
| Sample 1 | 0.21 | 0.01 | 97 | 1.06 | 122.9 | 1.77 |
| Sample 2 | 0.2 | 0.01 | 98.6 | 0.76 | 124.1 | 2.77 |
| Sample 3 | 0.25 | 0.01 | 62.8 | 0.77 | 85.9 | 1.85 |
| Sample 4 | 0.26 | 0.01 | 65 | 0.3 | 90.8 | 2.45 |
Figure 3.
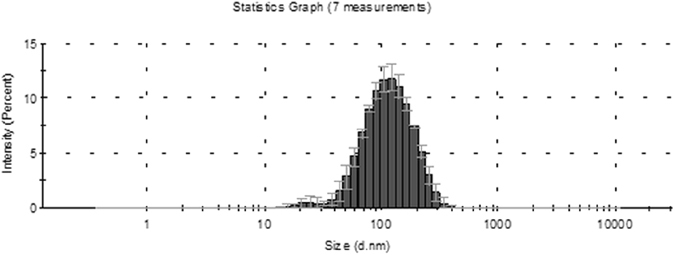
Representative size distribution of EVs isolated by Total Exosome Isolation Kit (Life Technologies, by Thermo Fisher Scientific) in sample 4 (Table 1). The y axis shows the intensity percentage of the vesicles, and the x axis shows the vesicle diameter (nm). The sizes of the EVs were approximately in the range of 16–255 nm (diameter [mean ± SD], 90.08 ± 2.45).
Screening by miRNA expression profiling
To identify differences in circulating EV-associated miRNA expression profiles of ACA and ACC patients, 12 samples have been subjected to TaqMan Array Human MicroRNA A card analysis (6 ACA and 6 ACC plasma samples). Altogether 377 human miRNA have been evaluated. By applying straightforward statistics (described in the Methods section) and Benjamini-Hochberg false discovery rate (FDR) correction, we have found no significant differences in miRNA expression between the two groups. However, we used TLDA data as an initial screening on a limited number of samples and found that 32 miRNAs are expressed in all samples of both ACA and ACC groups. We identified no miRNA expressed only in ACA or ACC groups, either. By using Fisher’s exact test, we evaluated miRNAs which were not expressed in all samples of a group and we found 2 miRNAs showing a tendency being different between ACA and ACC samples: hsa-miR-101 and hsa-miR-483–5p. These have been selected for further validation. Hsa-miR-101 was expressed in 1 of 6 ACA and 5 of 6 ACC samples, whereas hsa-miR-483-5p in 2 of 6 ACA and in all ACC samples. Raw profiling data are presented in Supplementary Dataset file 1.
miRNA Validation by RT-qPCR
The validation cohort included 18 ACAs and 16 ACCs. The widely used synthetic spike-in control RNA cel-miR-39 was applied as reference gene16, 23–25. We have found significant overexpression of hsa-miR-101 (Fig. 4a) and hsa-miR-483-5p (Fig. 4b) and in ACC relative to ACA plasma EV samples (p < 0.0001 and p < 0.0052, respectively). Raw and normalized qRT-PCR data are presented in Supplementary Dataset files 2 and 3.
Figure 4.
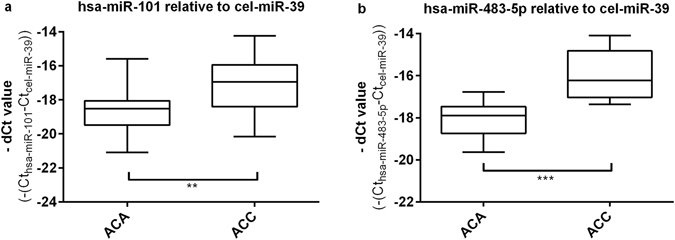
Results of RT-qPCR validation of hsa-miR-101 (a) and hsa-miR-483-5p (b) normalized to the housekeeping cel-miR-39, (mean ± SD, **p < 0.01, ***p < 0.001; unpaired t-test; n = 18 ACA, n = 16 ACC.
Diagnostic Performance of miRNA
We have evaluated the potential application of EV-associated miRNAs as minimally invasive biomarker candidates of adrenal malignancy by ROC analysis. Both, hsa-miR-101 and hsa-miR-483-5p, relative to spike-in control cel-miR-39 have been analyzed by ROC analysis. The dCThsa-miR-483-5p relative to cel-miR-39 showed the highest area under curve (AUC) value (0.965). By setting the cutoff point to 17.25, the sensitivity and the specificity of the test to discriminate ACA and ACC were 87.5 and 94.44, respectively (Fig. 5). ROC data of hsa-miR-101 have not yielded promising sensitivity and specificity values. By setting the cutoff point to 17.82 the AUC value was 0.766, with 68.75 sensitivity and 83.33 specificity.
Figure 5.
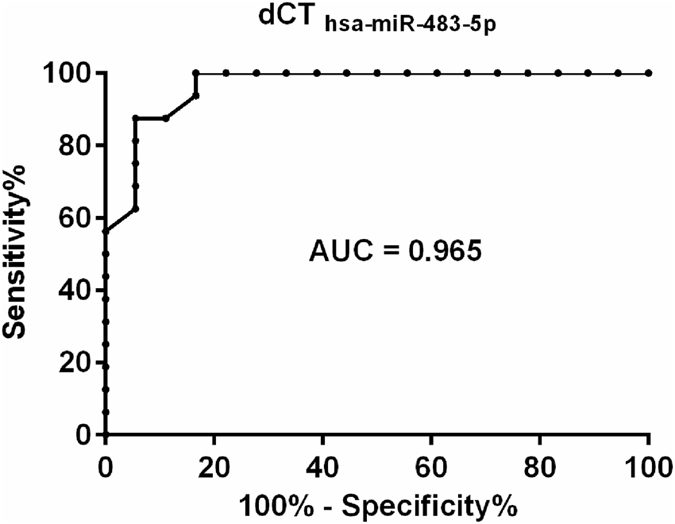
Receiver operating characteristics (ROC) curve analysis for the dCThsa-miR-4835-p exosomes miRNA signature relative to housekeeping cel-miR-39 based on the results obtained from RT-qPCR analysis of ACA (n = 18) and ACC (n = 16) plasma exosomes samples.
miRNA expression analysis in samples prepared by ultracentrifugation
We have evaluated the expression of hsa-miR-483-5p in vesicles isolated by ultracentrifugation, as well. The ultracentrifugation protocol has been performed on 4 ACA and 4 ACC patients. For data normalisation we used cel-miR-39 as reference gene. We have found significanty higher expression of hsa-miR-483-5p in exosomes in ACC relative to ACA (p = 0.0221, Fig. 6).
Figure 6.
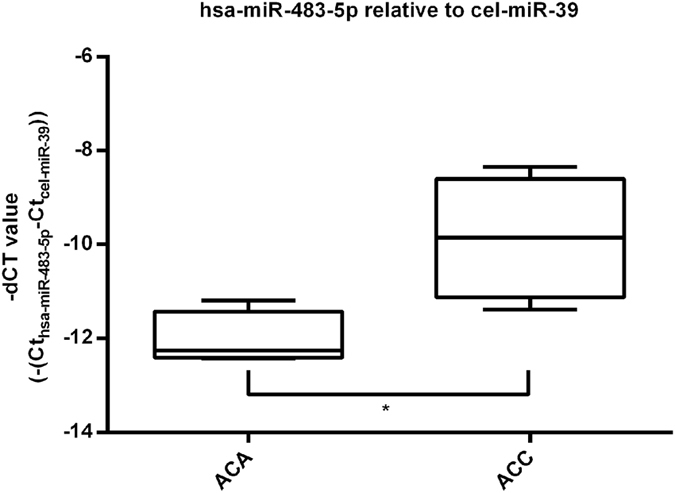
Results of ultracentrifugation with RT-qPCR of hsa-miR-483-5p normalized to the spike-in control cel-miR-39. Results are represented by – dCT (cycle threshold) (mean ± s.d., *p < 0.05; unpaired t-test; n = 4 ACA, n = 4 ACC).
Discussion
Recent studies indicated that circulating microRNAs could serve as minimally invasive biomarkers of malignancy in different tumors26. In our previous study on unfractionated plasma samples, we have found significant overexpression of 5 circulating miRNAs (hsa-miR-483-5p, hsa-miR-100, hsa-miR-181b, hsa-miR-184, hsa-miR-210) in ACC relative to ACA10. Two further studies on circulating microRNAs in unfractionated serum samples from adrenocortical tumor patients have been reported to date8, 9. Overexpressed circulating hsa-miR-483-5p in ACC has been confirmed in both, along with overexpressed hsa-miR-34a 9 and underexpressed hsa-miR-195 8. The sensitivity and specificity values for these circulating microRNAs cannot be regarded as high enough for clinical introduction at present. The best results reported by Chabre et al. for circulating hsa-miR-483-5p could only be used to differentiate aggressive and non-aggressive ACC8.
Multiple studies demonstrated that miRNA content in actively secreted EVs was quite different from that of donor cells20, 27, 28. Therefore, it can be hypothesized that, EVs-associated miRNAs might prove to be more sensitive and specific biomarkers for pathological conditions than EV-free miRNAs or miRNAs from unfractionated body fluid samples.
We aimed to investigate the the expression of EV-associated miRNAs and their potential diagnostic applicability in patients suffering from adrenocortical tumors. To the best of our knowledge, our study is the first of this kind in these tumors.
The overexpression of hsa-miR-483-5p and hsa-miR-101 in ACC has been confirmed in the validation cohort. Showing the highest AUC, specificity and sensitivity values, exosomal hsa-miR-483-5p could be a potential biomarker of adrenal malignancy using cel-miR-39 as reference gene. The significant overexpression of hsa-miR-483-5p relative to cel-miR-39 in ACC has been also confirmed by testing EVs isolated by differential ultracentrifugation, confirming the EV-association of this miRNA.
There is no broadly accepted valid reference gene for raw data normalization for circulating miRNA29. The small nuclear RNA (snRNA) U6, hsa-miR-16 and the synthetic, exogenous spike-in control cel-miR-39 are the most frequently applied reference genes29 in different studies. However, snRNA U6 has a significant interindividual variability30. In our previous study on plasma samples, we have found that hsa-miR-16 and cel-miR-39 were applicable for normalization10. In this study, we have also used cel-miR-39 as reference both for samples isolated by the kit and ultracentrifugation16, 23–25. As cel-miR-39 is not included in TLDA cards, it could not be assessed as reference in the miRNA screening. Cel-miR-39 can not be considered as a valid biological control, but definitely indicates the efficacy of miRNA isolation10.
In order to confirm the results obtained by using Total Exosome Isolation Kit and to fulfill the minimal experimental requirements for EVs, we have isolated EVs also by differential centrifugation/ultracentrifugation, and carried out EV analysis by using TEM and flow cytometry, as well22. Ultracentrifugation is considered to be gold standard of EV isolation to date31. TEM enabled to evaluate both the size and the morphology of EVs, simultaneously32. Using flow cytometry we detected the presence of annexin V, CD9 and CD81 on EVs isolated both by ultracentrifugation and the kit used. Annexin V and the tetraspanins CD9, CD63 and CD81 are considered to be characteristic for EVs33. We can thus conclude that the preparations that we isolated both by the kit used and by differential centrifugation/ultracentrifugation were enriched in EVs. From among the different types of EVs, exosomes are the ones that are known to be characterized by the presence of CD63, CD9 and CD81 in their membranes. Thus, we may conclude that our preparations were enriched in exosomes. The size distributon analysis of extracellular vesicles also supports the exosomal enrichment of the preparations. However, given that at present there are no universal markers to distinguish microvesicles form exosomes, in our conclusions we chose to refer to EVs rather than exosomes or microvesicles. It must be noted, however, that the expression of CD9 and CD63 was higher in the EV samples isolated by ultracentrifugation than by the kit used (CD63 was not detected in EV samples isolated by the kit). The plasma sample volumes needed for ultracentrifugation are much higher (3 mL) than the volumes needed for isolation by the kit (200 µl), thus the yield is also higher by ultracentrifugation. However, the ultracentrifugation-based protocol is very laborious and time-consuming, and not easily applicable for clinical purposes. Our aim has been to use a methodology that could be easily used in the clinical setting. Given the parallel results of miRNA expression used both protocols, we reckon that the isolation kit can be reliably used, and could be used in the clinical setting to analyze larger sets of samples.
Several studies highlight the relevance of these miRNAs in different physiological and pathological conditions. Among these, the overexpression of hsa-miR-483-5p has been described both in tissue and in blood of patients suffering from ACC7–9, 34, 35. In a previous study, we demonstrated that the expression of circulating hsa-miR-483-5p was not influenced by treatments with dexamethasone or adrenocorticotropin confirming that it could be potentially used in the preoperative diagnostic of ACC36. Furthermore, circulating hsa-miR-483-5p could represent not only a diagnostic marker, but a prognostic marker, too, since its expression predicts poor prognosis and cancer reccurence8.
To the best of our knowledge, the overexpression of hsa-miR-101 in ACC has not been reported so far. There are data on the overexpession of hsa-miR-101 in malignant pheochromocytoma compared to benign tumors37. Among others, miR-101 may have tumor suppressor role e.g. in stomach38, colorectal39, prostate40, tumors that is not suprising for a miRNA considering their tissue specific action. The same miRNA can be oncogenic or tumor suppressor depending on the cellular context41.
In our previous study using total plasma as the source of miRNAs, altogether 5 miRNAs proved to be significantly overexpressed in ACC samples10. Since only selected miRNAs based on previous studies were included without profiling, the number of significantly differentially expressed miRNA in total plasma might be even higher. In the present study using EV-associated miRNAs, only two could be validated suggesting a possibly more specific, adrenocortical origin of these miRNAs underlining the higher potential of EV-associated miRNAs than those isolated from whole plasma.
In conclusion, our results show that EV-associated miRNAs might be promising candidates to be minimally invasive biomarkers of adrenocortical cancer. We have used both high-throughput analysis for screening and targeted RT-qPCR for validation to investigate the expression of miRNAs, which seems to be the most appropriate method for blood-borne miRNA analysis42. However, the limited sizes of patient cohorts, the lack of standardization of EV isolation, miRNA extraction methods and no generally accepted RNA control for normalization makes it difficult to generalize the results of such studies. In this study, we have found high enough sensitivity and specificity for potential clinical application, however, further studies on larger numbers of patients are needed to prove this assumption.
Materials and Methods
Patients’ samples
Altogether 46 preoperative plasma samples have been collected from patients with ACA (n = 24) and ACC (n = 22) at the 2nd Department of Medicine Semmelweis University, Budapest, Hungary, the Endocrinology Unit of the Department of Medicine, University of Padua, Italy, the Department of Experimental and Clinical Biomedical Sciences, Endocrinology Unit, University of Florence, Italy and at the Division of Endocrinology, Diabetes, Metabolism and Nutrition, Department of Internal Medicine, Mayo Clinic, Rochester, USA. 6 ACA and 6 ACC samples have been included in the screening cohort, whereas 18 ACA and 16 ACC in the validation cohort. The study was approved by the Ethical Committee of the Hungarian Health Council and informed consent was obtained from all patients involved. All experiments were performed in accordance with relevant guidelines and regulations.
In every operated case, the diagnosis of ACA or ACC was determined by histological examination, whereas the diagnosis of ACA in non-operated cases was based on imaging and follow-up by abdominal computed tomography. The hormonal profile has been investigated in all cases. Tumor stage in ACC was determined based on ENSAT (European Network for the Study of Adrenal Tumors) criteria43. The clinical characteristics of both cohorts are summarized in Table 2. Patients did not receive chemo- or radiotherapy before blood collection.
Table 2.
Characteristics of patients.
| Sample number | Tumor type | Cohort | Sex | Age at blood taking (year) | Hormonal activity | Ki-67 (%) or mitotic index (N/10 HPF) | Weiss score | ENSAT Tumor stage |
|---|---|---|---|---|---|---|---|---|
| 1 | ACC | Screening | F | 51 | Cortisol | 25% | 9 | 1 |
| 2 | ACC | Screening | F | 62 | Subclinical testosterone | 5/10 HPF | 7 | 3 |
| 3 | ACC | Screening | F | 46 | Non-secreting | 10/10 HPF | 6 | 4 |
| 4 | ACC | Screening | M | 43 | Non-secreting | n.d. | 6 | 4 |
| 5 | ACC | Screening | F | 57 | Non-secreting | 20–25% | 5 | 4 |
| 6 | ACC | Screening | F | 36 | Cortisol | n.d. | n.d. | 4 |
| 7 | ACC | Validation | F | 67 | Cortisol | n.d. | n.d. | 4 |
| 8 | ACC | Validation | F | 39 | Subclinical cortisol | 10% | 7 | 1 |
| 9 | ACC | Validation | F | 57 | Non-secreting | n.d. | 5 | 4 |
| 10 | ACC | Validation | M | 80 | Non-secreting | n.d. | 9 | 3 |
| 11 | ACC | Validation | F | 56 | Testosterone | n.d. | 6 | 2 |
| 12 | ACC | Validation | M | 53 | Non-secreting | n.d. | 9 | 3 |
| 13 | ACC | Validation | F | 31 | Cortisol | n.d. | 6 | 2 |
| 14 | ACC | Validation | F | 58 | Testosterone | 2/10HPF | 4 | 3 |
| 15 | ACC | Validation | F | 24 | Androgens | 10% | 5 | 2 |
| 16 | ACC | Validation | F | 22 | Subclinical cortisol and androgens | 10% | 3 | 1 |
| 17 | ACC | Validation | M | 62 | Cortisol | 30% | 7 | 2 |
| 18 | ACC | Validation | M | 48 | Cortisol | 15% | 8 | 3 |
| 19 | ACC | Validation | F | 38 | Cortisol, androgens | 70% | 8 | 2 |
| 20 | ACC | Validation | F | 32 | Non-secreting | 5% | 3 | 1 |
| 21 | ACC | Validation | F | 20 | Non-secreting | 5 | 3 | 4 |
| 22 | ACC | Validation | M | 51 | Non-secreting | 2% | 4 | |
| 23 | ACA | Screening | F | 64 | Aldosterone | n.d. | n.d. | |
| 24 | ACA | Screening | F | 73 | Non-secreting | n.d. | n.d. | |
| 25 | ACA | Screening | F | 63 | Non-secreting | n.d. | n.d. | |
| 26 | ACA | Screening | F | 59 | Subclinical cortisol | n.d. | n.d. | |
| 27 | ACA | Screening | F | 77 | Non-secreting | n.d. | n.d. | |
| 28 | ACA | Screening | F | 61 | Cortisol | n.d. | n.d. | |
| 29 | ACA | Validation | F | 38 | Non-secreting | n.d. | n.d. | |
| 30 | ACA | Validation | F | 74 | Non-secreting | n.d. | n.d. | |
| 31 | ACA | Validation | F | 52 | Non-secreting | n.d. | n.d. | |
| 32 | ACA | Validation | M | 29 | Non-secreting | n.d. | n.d. | |
| 33 | ACA | Validation | M | 71 | Non-secreting | n.d. | n.d. | |
| 34 | ACA | Validation | F | 81 | Non-secreting | n.d. | n.d. | |
| 35 | ACA | Validation | M | 63 | Non-secreting | n.d. | n.d. | |
| 36 | ACA | Validation | M | 50 | Non-secreting | n.d. | n.d. | |
| 37 | ACA | Validation | F | 50 | Cortisol | n.d. | n.d. | |
| 38 | ACA | Validation | F | 69 | Cortisol | n.d. | n.d. | |
| 39 | ACA | Validation | F | 46 | Aldosterone | n.d. | n.d. | |
| 40 | ACA | Validation | M | 62 | Cortisol | n.d. | n.d. | |
| 41 | ACA | Validation | F | 33 | Cortisol | n.d. | n.d. | |
| 42 | ACA | Validation | F | 35 | Non-secreting | n.d. | n.d. | |
| 43 | ACA | Validation | F | 65 | Non-secreting | n.d. | n.d. | |
| 44 | ACA | Validation | F | 54 | Non-secreting | n.d. | n.d. | |
| 45 | ACA | Validation | F | 66 | Non-secreting | n.d. | n.d. | |
| 46 | ACA | Validation | M | 68 | Non-secreting | n.d. | n.d. |
n.d.: no data, HPF: high power field, F: female, M: male.
Sample processing and extracellular vesicle isolation
In every case, EDTA-anticoagulated blood was taken from patients and processed for plasma isolation instantly after collection. Plasma was gained by centrifuging whole blood at 1000 x g for 10 minutes at 4 °C. All extracted plasma samples were stored at −80 °C until further use. No hemolysis was observed under these storage conditions.
We used two different approaches for EV isolation. First, EVs were isolated from 200 µl plasma with Total Exosome Isolation (from plasma) Kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol.
As a second approach, we isolated EVs from blood plasma with differential centrifugation/ultracentrifugation. Cellular components were eliminated with centrifugation (2500xg, room temperature, 15 min), then the platelet-free plasma was diluted to 2x with phosphate-buffered saline (PBS), and was filtered through a 0.8 µm filter (Merck Life Science, Darmstadt, Germany) by hydrostatic pressure to remove remaining platelets and apoptotic bodies. The EVs were pelleted with ultracentrifugation at 100.000xg in an MLA-55 fixed-angle rotor (BC-OptimaTM Max-XP, MLA-55, A/D: 8, Beckman Coulter, Brea, CA, USA) for 1 h at 4 °C from 6 mL of 0.8 µm filtered and 2x PBS-diluted platelet-free plasma samples. The supernatant was discarded and the pellet was resuspended in 90 µl PBS. The solution was treated with Ribonuclease A from bovine pancreas (PN: R6513 by Merck Life Science). The RNase treatment was suspended by Ribonuclease Inhibitor Recombinant (PN: R1158-2.5KU by Merck Life Science). The solution was washed again with 0.1% Bovine serum albumin (BSA) and ProtectRNA RNase Inhibitor 500× Concentrate (PN: R7397 by Merck Life Science) and centrifuged at 100,000xg, 4 °C for 1 h (BC-OptimaTM Max-XP, MLA-55, A/D: 8, by Beckman Coulter). The solution was treated with 700 µl QIAzol Lysis Reagent (ID: 79306, Qiagen GmbH, Hilden, Germany). Samples were stored at −20 °C until further processing.
Transmission electron microscopy (TEM)
To evaluate EVs isolated by ultracentrifugation, paraformaldehyde (4%) in 0.01 M PBS was added onto the pellets for 60 min at room temperature. Following washing with PBS, we used 1% OsO4 for 30 minutes (Taab, Aldermaston, Berks, UK) for postfixation. The fixed pellets were rinsed with distilled water, then graded ethanol was used for the dehydration of the preparations. We applied 1% uranyl-acetate in 50% ethanol for 30 min for block staining, and the preparations were inlayed in Taab 812 (Taab). Ultrathin sections were made following the overnight polymerization of blocksat 60 °C. We have used Hitachi 7100 electron microscope (Hitachi Ltd., Japan) equipped with a Megaview II (lower resolution, Soft Imaging System, Germany) digital camera for the analysis of the sections.
Flow cytometry
Direct measurement of (EVs) by flow cytometry is limited because of the small size of blood plasma EVs Therefore we have first adsorbed the EVs onto the surface of formaldehyde/sulfate Latex-Beads (Molecular Probes, Eugene, OR, USA). In brief, EVs were resuspended in PBS and were loaded onto 3.8 μm diameter 4% v/v% beads. Two percent BSA was used as a negative control. Then the samples were incubated overnight at 4 °C under agitation (5 × g). EV-coated beads were incubated for 1 hour with 2% BSA, and then 300 μl 0.9% NaCl solution was added and samples were centrifuged for 10 minutes at 2000xg. The beads were resuspended in 200 μl 100 mM glycerol solution and were incubated for 30 minutes at room temperature. CD9 (FITC, Sigma;#:SAB4700095), CD63 (PE, Sigma;#:SAB4700218), CD81 (FITC, molecular probes;#:A15753) and annexin V (FITC, SONY; #:3804530) were investigated. Altogether we have evaluated 6 samples (3 from ultracentrifugation and 3 isolated by Total Exosome Isolation (from plasma) Kit. Flow cytometry was perfomed by FACSCalibur (BD Biosciences, San Jose, CA, USA). For data analysis we have used FlowJo Software (Tri Star Inc., Ashland, OR, USA).
Size distribution of extracellular vesicles
The size distribution of extracellular-vesicles were characterized by dynamic light scattering (DLS) on a Zetasizer Nano S instrument (Malvern Instruments Ltd, Malvern, UK) on 4 samples isolated by Total Exosome Isolation (from plasma) Kit (Thermo Fisher Scientific). From the intensity fluctuations of a 633-nm laser light scattered at high angle from the freely moving suspended particles, their diffusion constant was obtained. Size distribution was calculated by using the Stokes-Einstein equation by the built-in algorithms of the instrument’s software. Z-average values are displayed, which represent the primary and most stable parameter produced by DLS technique44 and are recommended for quality control reports (ISO 22412:2008). Z-average value represents a good approximation of hydrodynamic diameter of well dispersed particles. Polydispersity index (PDI) is an estimate of the width of the distribution which is calculated from the cumulants analysis.
Light scattering was measured at 25 ± 1 °C. Extracellular-vesicles (refractive index: 1.370) were suspended in 1x sterile phosphate buffer (PBS). The viscosity of PBS at 25 °C was 0.8882 cP and the refractive index was 1.330. The sample was equilibrated at 25 °C for 1 min.
miRNA expression profiling
Total RNA was instantly extracted from EVs (previously isolated by Total Exosome Isolation (from plasma) Kit) using Total Exosome RNA and Protein Isolation Kit (Thermo Fisher Scientific). In the validation cohort, 5 µl of 5 nM Syn-cel-mir-39 miScript miRNA Mimic (Qiagen GmbH) as a spike-in control for purification efficiency was added before the addition of Acid-Phenol: Chloroform. The final total RNA elute was 50 µl. RNA was stored at -70 °C until further processing.
miRNA expression profiling of EVs was performed altogether on 12 samples (6 ACA and 6 ACC) using high-throughput real-time qPCR. The samples were processed strictly following the Megaplex Pools for microRNA expression Analysis protocol given by the manufacturer as previously described (PN: 4399721B, Thermo Fisher Scietific). In brief, the previously isolated RNA was reverse-transcribed using the TaqMan microRNA Reverse Transcription Kit (PN 4366597, Thermo Fisher Scientific) and Megaplex RT primers Human Pool A v2.1 (PN: 4399966, Thermo Fisher Scietific) on Proflex Base PCR System (Thermo Fisher Scientific). Before the Real-Time PCR reactions, we have run the preamplification reactions of cDNA using TaqMan Preamp Master Mix (2x) (PN: 4384266, Thermo Fisher Scietific) and Megaplex PreAmp Primers (10x), Human Pool A v2.1 (PN: 4399233, Thermo Fisher Scietific) according to the manufacturer’s instructions. The diluted PreAmp product was mixed with TaqMan Universal PCR Master Mix, No AmpErase UNG, 2x (PN: 4324018, Thermo Fisher Scietific) and was loaded on TaqMan Array Human MicroRNA Cards v2.0 pool A (PN:4398977, Thermo Fisher Scientific) and analysed on QuantStudio 7 Flex Real-Time PCR system (Thermo Fisher Scientific). Each sample was assayed with an A card resulting in the analysis of a total of 377 human miRNA.
TLDA raw data analysis, threshold settings and basic statistics were perfomed by Applied Biosystems qPCR Analysis Modules on Thermo Fisher Cloud (https://www.thermofisher.com/hu/en/home/cloud.html). Due to the lack of cel-miR-39 or other universally accepted reference for circulating miRNA studies on the TaqMan Array Human MicroRNA Cards system45, we skipped normalization of raw data applying a certain endogenous control gene on screening results, therefore the normalized quant for the reference sample then reflected the fold change indicated between the different samples directly. We used ACA samples as Reference Biological Group, confidence level was set to 95% and Benjamini-Hochberg false discovery rate correction was applied for p values. The maximum allowed CT value was set to 40 and microRNAs showing no amplification until cycle 40 were considered as not expressed. We used Fisher’s exact test to compare the prevalance of expression of those miRNAs which were detected not in all samples (expressed in more ACC samples compared to ACA and vice versa).
Validation of individual miRNAs by quantitative Real-Time PCR
Two miRNAs showing a tendency being differently expressed between ACA and ACC samples on TaqMan low density array (TLDA) cards have been subjected to validation by RT-qPCR. Among these, hsa-miR-101 (002253) and hsa-miR-483-5p (002338) were selected for validation by real-time RT-qPCR on altogether 34 samples (18 ACA and 16 ACC patients). Total RNA (5 µl) was reverse transcribed using specific TaqMan microRNA Assays (PN 442795, Thermo Fisher Scientific) and the TaqMan MicroRNA Reverse Transcription Kit (Thermo Fisher Scientific) on Proflex Base PCR System (Thermo Fisher Scientific). Quantitative RT-PCR was performed by TaqMan Fast Universal PCR Master Mix (2x) (PN: 4352042, Thermo Fisher Scientific) on a 7500 Fast Real-Time PCR system (Thermo Fisher Scientific) according to the protocol of manufacturer for TaqMan Small RNA Assays (PN 4364031E, Thermo Fisher Scientific) with minor modifications. Samples were run in triplicate. Negative control reactions did not include cDNA templates. For the evaluation of the data we used the dCT (CT) method (−dCT values = −[CT of target miRNA −CT of internal control miRNA])46 using Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA, USA).
RNA isolation from vesicles isolated by ultracentrifugation and quantitative Real-Time PCR
In the case of EVs isolated by differential centrifugation/ultracentrifugation, RNA was isolated from vesicles (n = 4 ACA, n = 4 ACC) using RNeasy Mini Kit (ID:74104, Qiagen GmbH) and RNeasy MinElute Cleanup Kit (ID:74204, Qiagen) according to the manufacturer’s protocol. The analysis of vesicular RNA was performed by capillary electrophoresis (Agilent Small RNA Kit (CN:5067-1548) on Agilent 2100 Bioanalyzer, Agilent Technologies, Santa Clara, CA, USA). 1 µl RNA in solution was analyzed according to the manufacturer’s protocol. The reverse transcription and qRT-PCR was performed as previously described above. 0.5 µl of 5 nM Syn-cel-miR-39 miScript miRNA Mimic (Qiagen GmbH) was added to the isolated RNA elution during reverse transcription. We have evaluated the expression of hsa-miR-483-5p in ACA and ACC patients relative to cel-miR-39 as reference.
Statistical analysis
Statistical analysis of high-throughput real-time PCR data and RT-qPCR data was performed by GraphPad Prism 7.02 (GraphPad Software, Inc., La Jolla, CA, USA). For the identification of diffentially expressed miRNAs between ACA and ACC groups, Student’s t-test or Mann-Whitney test were used depending on the results of Shapiro-Wilk normality test. P-values < 0.05 were considered significant.
To identify miRNA markers applicable for diagnosis, receiver operating characteristics (ROC) analysis was performed by GraphPad Prism 7.02.
Electronic supplementary material
Acknowledgements
The study has been supported by a grant from the Hungarian National Research, Development and Innovation Office (NKFIH K115398) to Dr. Peter Igaz and an Associazione Italiana Ricerca sul Cancro (AIRC) Investigator Grant to Dr. Michaela Luconi (grant #IG2015-17691). The study was performed as a collaborative study in the framework of the European Network for the Study of Adrenal Tumors (ENS@T). The authors thank prof Ambrogio Fassina, Cytopathology Unit, Department of Medicine, University of Padova, Padova, Italy for providing the Weiss scores of adrenocortical cancer samples, and Tamás Mészáros, SeroScience Ltd (Nanomedicine Research and Education Center, Budapest, Hungary) for performing the size distribution analysis of extracellular vesicles. The authors would like to thank Dr. Gábor L. Kovács and Dr. Tibor Hella (Flór F. County Hospital, Kistarcsa, Hungary) for supplying some patient samples.
Author Contributions
P.I. designed research. Research was performed by P.P., H.B., Z.N., K.P., G.N. and A.D. R.P., I.B., M.L., M.M., M.T. and M.B. provided patient’s samples. H.B., E.P., E.I.B. and A.P. were involved in data analysis. The manuscript was written by P.P. and P.I. All authors approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-05777-0
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arnaldi G, Boscaro M. Adrenal incidentaloma. Best Pract. Res. Clin. Endocrinol. Metab. 2012;26:405–19. doi: 10.1016/j.beem.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Cherradi N. microRNAs as Potential Biomarkers in Adrenocortical Cancer: Progress and Challenges. Front. Endocrinol. (Lausanne). 2015;6:195. doi: 10.3389/fendo.2015.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terzolo M, et al. Management of adrenal cancer: a 2013 update. J. Endocrinol. Invest. 2014;37:207–217. doi: 10.1007/s40618-013-0049-2. [DOI] [PubMed] [Google Scholar]
- 4.Fassnacht M, Kroiss M, Allolio B. Update in adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 2013;98:4551–64. doi: 10.1210/jc.2013-3020. [DOI] [PubMed] [Google Scholar]
- 5.Dinnes J, et al. Management Of Endocrine Disease: Imaging for the diagnosis of malignancy in incidentally discovered adrenal masses: a systematic review and meta-analysis. Eur. J. Endocrinol. 2016;175:R51–64. doi: 10.1530/EJE-16-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arlt W, et al. Urine steroid metabolomics as a biomarker tool for detecting malignancy in adrenal tumors. J. Clin. Endocrinol. Metab. 2011;96:3775–84. doi: 10.1210/jc.2011-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Igaz P, et al. MicroRNAs in adrenal tumors: relevance for pathogenesis, diagnosis, and therapy. Cell. Mol. Life Sci. 2015;72:417–28. doi: 10.1007/s00018-014-1752-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chabre O, et al. Serum miR-483-5p and miR-195 are predictive of recurrence risk in adrenocortical cancer patients. Endocr. Relat. Cancer. 2013;20:579–94. doi: 10.1530/ERC-13-0051. [DOI] [PubMed] [Google Scholar]
- 9.Patel, D. et al. MiR-34a and miR-483-5p are candidate serum biomarkers for adrenocortical tumors. Surgery154, 1224–8; discussion 1229 (2013). [DOI] [PMC free article] [PubMed]
- 10.Szabó DR, et al. Analysis of circulating microRNAs in adrenocortical tumors. Lab. Invest. 2014;94:331–9. doi: 10.1038/labinvest.2013.148. [DOI] [PubMed] [Google Scholar]
- 11.Assié G, et al. Integrated genomic characterization of adrenocortical carcinoma. Nat. Genet. 2014;46:607–12. doi: 10.1038/ng.2953. [DOI] [PubMed] [Google Scholar]
- 12.Zheng S, et al. Comprehensive Pan-Genomic Characterization of Adrenocortical Carcinoma. Cancer Cell. 2016;29:723–736. doi: 10.1016/j.ccell.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 14.Weber JA, et al. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010;56:1733–41. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarry J, Schadendorf D, Greenwood C, Spatz A, van Kempen LC. The validity of circulating microRNAs in oncology: five years of challenges and contradictions. Mol. Oncol. 2014;8:819–29. doi: 10.1016/j.molonc.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell PS, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA. 2008;105:10513–8. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011;13:423–33. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arroyo JD, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. USA. 2011;108:5003–8. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, et al. Exosome and Exosomal MicroRNA: Trafficking, Sorting, and Function. Genomics. Proteomics Bioinformatics. 2015;13:17–24. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 21.Turchinovich A, Tonevitsky AG, Burwinkel B. Extracellular miRNA: A Collision of Two Paradigms. Trends Biochem. Sci. 2016;41:883–92. doi: 10.1016/j.tibs.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Lötvall J, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J. Extracell. vesicles. 2014;3:26913. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonald JS, Milosevic D, Reddi HV, Grebe SK, Algeciras-Schimnich A. Analysis of circulating microRNA: preanalytical and analytical challenges. Clin. Chem. 2011;57:833–40. doi: 10.1373/clinchem.2010.157198. [DOI] [PubMed] [Google Scholar]
- 24.Fornari F, et al. Circulating microRNAs, miR-939, miR-595, miR-519d and miR-494, Identify Cirrhotic Patients with HCC. PLoS One. 2015;10:e0141448. doi: 10.1371/journal.pone.0141448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brase JC, et al. Circulating miRNAs are correlated with tumor progression in prostate cancer. Int. J. Cancer. 2011;128:608–616. doi: 10.1002/ijc.25376. [DOI] [PubMed] [Google Scholar]
- 26.Schwarzenbach H, Nishida N, Calin GA, Pantel K. Clinical relevance of circulating cell-free microRNAs in cancer. Nat. Rev. Clin. Oncol. 2014;11:145–56. doi: 10.1038/nrclinonc.2014.5. [DOI] [PubMed] [Google Scholar]
- 27.Guduric-Fuchs J, et al. Selective extracellular vesicle-mediated export of an overlapping set of microRNAs from multiple cell types. BMC Genomics. 2012;13:357. doi: 10.1186/1471-2164-13-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pigati L, et al. Selective release of microRNA species from normal and malignant mammary epithelial cells. PLoS One. 2010;5:e13515. doi: 10.1371/journal.pone.0013515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Occhipinti G, Giulietti M, Principato G, Piva F. The choice of endogenous controls in exosomal microRNA assessments from biofluids. Tumour Biol. 2016;37:11657–11665. doi: 10.1007/s13277-016-5164-1. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, et al. Identification of Endogenous Controls for Analyzing Serum Exosomal miRNA in Patients with Hepatitis B or Hepatocellular Carcinoma. Dis. Markers. 2015;2015:1–12. doi: 10.1155/2015/893594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Witwer, K. W. et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles2 (2013). [DOI] [PMC free article] [PubMed]
- 32.György B, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell. Mol. Life Sci. 2011;68:2667–88. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Escola JM, et al. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J. Biol. Chem. 1998;273:20121–7. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- 34.Patterson EE, Holloway AK, Weng J, Fojo T, Kebebew E. MicroRNA profiling of adrenocortical tumors reveals miR-483 as a marker of malignancy. Cancer. 2011;117:1630–9. doi: 10.1002/cncr.25724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soon PSH, et al. miR-195 and miR-483-5p Identified as Predictors of Poor Prognosis in Adrenocortical Cancer. Clin. Cancer Res. 2009;15:7684–7692. doi: 10.1158/1078-0432.CCR-09-1587. [DOI] [PubMed] [Google Scholar]
- 36.Igaz I, et al. Analysis of Circulating MicroRNAs In Vivo following Administration of Dexamethasone and Adrenocorticotropin. Int. J. Endocrinol. 2015;2015:589230. doi: 10.1155/2015/589230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zong L, Meng L, Shi R. Role of miR-101 in pheochromocytoma patients with SDHD mutation. Int J Clin Exp Pathol. 2015;8:1545–1554. [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H-J, et al. MicroRNA-101 is down-regulated in gastric cancer and involved in cell migration and invasion. Eur. J. Cancer. 2010;46:2295–2303. doi: 10.1016/j.ejca.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 39.Strillacci A, et al. MiR-101 downregulation is involved in cyclooxygenase-2 overexpression in human colon cancer cells. Exp. Cell Res. 2009;315:1439–1447. doi: 10.1016/j.yexcr.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 40.Hao Y, et al. Enforced expression of miR-101 inhibits prostate cancer cell growth by modulating the COX-2 pathway in vivo. Cancer Prev. Res. (Phila). 2011;4:1073–83. doi: 10.1158/1940-6207.CAPR-10-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen C-Z. MicroRNAs as oncogenes and tumor suppressors. N. Engl. J. Med. 2005;353:1768–71. doi: 10.1056/NEJMp058190. [DOI] [PubMed] [Google Scholar]
- 42.Chakraborty C, Das S. Profiling cell-free and circulating miRNA: a clinical diagnostic tool for different cancers. Tumor Biol. 2016;37:5705–5714. doi: 10.1007/s13277-016-4907-3. [DOI] [PubMed] [Google Scholar]
- 43.Fassnacht M, et al. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma. Cancer. 2009;115:243–250. doi: 10.1002/cncr.24030. [DOI] [PubMed] [Google Scholar]
- 44.Frisken BJ. Revisiting the method of cumulants for the analysis of dynamic light-scattering data. Appl. Opt. 2001;40:4087–91. doi: 10.1364/AO.40.004087. [DOI] [PubMed] [Google Scholar]
- 45.Butz H, Patócs A. Technical Aspects Related to the Analysis of Circulating microRNAs. EXS. 2015;106:55–71. doi: 10.1007/978-3-0348-0955-9_3. [DOI] [PubMed] [Google Scholar]
- 46.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


