Abstract
There is currently no reliable and easily applicable diagnostic marker for Parkinson’s disease (PD). The aims of the present study were to compare the expression profiles of the microRNA29 family (miR-29s) in blood serum from patients with PD with healthy controls and to clarify whether the expression of miR-29s is correlated with disease severity, duration or L-dopa therapy and whether expression depends on the gender and age of patients. The levels of blood serum miR-29s in 80 patients with PD and 80 unaffected controls were assessed by reverse transcription-quantitative real-time PCR. The PCR products were confirmed by cloning and sequencing. Additionally, the expression of miR-7 in the blood serum from PD patients and control subjects was assessed. Serum miR-29 levels were significantly downregulated in PD patients compared to healthy controls. The serum miR-29 levels in female PD patients were markedly higher than in male PD patients. The expression of serum miR-29a and miR-29c expression tended to decrease with disease severity. Moreover, we found that serum miR-7 levels did not differ between PD patients and control subjects. Therefore, the reduction of serum miR-29 levels, particularly miR-29a and miR-29c, warrants further investigation of its potential serving as biomarkers for PD.
Introduction
Parkinson’s disease (PD), which is the second most common neurodegenerative disease after Alzheimer’s disease (AD), affects up to 1% of people over the age of 601, 2. Loss of dopaminergic neurons in the substantia nigra and the presence of proteinaceous inclusions termed Lewy bodies, which are primarily composed of fibrillar α-synuclein, are prominent features of PD3. Epidemiological studies have demonstrated a higher prevalence of PD in men than in women4, 5. The pathological mechanisms of PD are complex, and both genetic and epigenetic factors contribute to progressive neuronal death. MicroRNAs (miRNAs) are small non-coding RNAs of 20–25 nucleotides that mediate posttranscriptional gene repression of target RNA transcripts. The miRNA29 family (miR-29s) includes hsa-miR-29a and hsa-miR-29b-1, as well as hsa-miR-29b-2 and hsa-miR-29c, which are transcribed from two different gene clusters located on chromosome 7 and chromosome 1, respectively, of the human genome6.
Many studies have shown that miR-29s act as tumor suppressors in several types of cancer, although they can be oncogenic in other cancers7, 8. The involvement of miR-29s in the fibrosis of peripheral tissues has also been well documented9. In the central nervous system, miR-29s regulate neuronal maturation10 and dendritic spine morphology11. Dysregulation of miR-29s also has implications in aging12 and various neurological disorders such as AD13, Huntington’s disease14, amyotrophic lateral sclerosis15, multiple sclerosis16. The role of miR-29s in ischemia remains controversial17–19. In addition, two groups have reported evidence that miR-29s play a role in fine-tuning motor function20, 21. Collectively, miR-29s function in neuronal survival, proliferation, differentiation and plasticity.
However, the roles of miR-29s in PD remain unclear, and only a few studies have examined the expression of blood miR-29s in PD patients using microarray and quantitative real-time PCR (qRT-PCR)22–25. Additionally, in cellular and animal PD models, it has been found that dysregulation of some PD-related genes is attributable to the alteration in miRNAs. Among such particular miRNAs, miR-7, which is an evolutionarily conserved miRNA that represses the expression of α-synuclein, is associated with PD pathophysiology26, 27 and is downregulated in serum samples of PD patients27. In the present work, we measured the expression of serum miR-29s in a relatively large cohort of PD patients (n = 80) and healthy controls (n = 80). Additionally, all samples were used to detect the expression levels of miR-7.
Results
Blood serum miR-29s levels are significantly reduced in PD patients
miR-29b-1 and miR-29b-2 (thereafter called miR-29b) have identical mature sequences, while miR-29a and miR-29c differ by one nucleotide (Fig. 1A). Mature miR-29s are highly conserved in humans, rats, and mice. Because circulating miRNAs in serum are sufficiently stable, they can serve as clinical biomarkers28, 29. Here, blood serum miR-29s levels were determined in 80 patients with PD and 80 controls.
Figure 1.
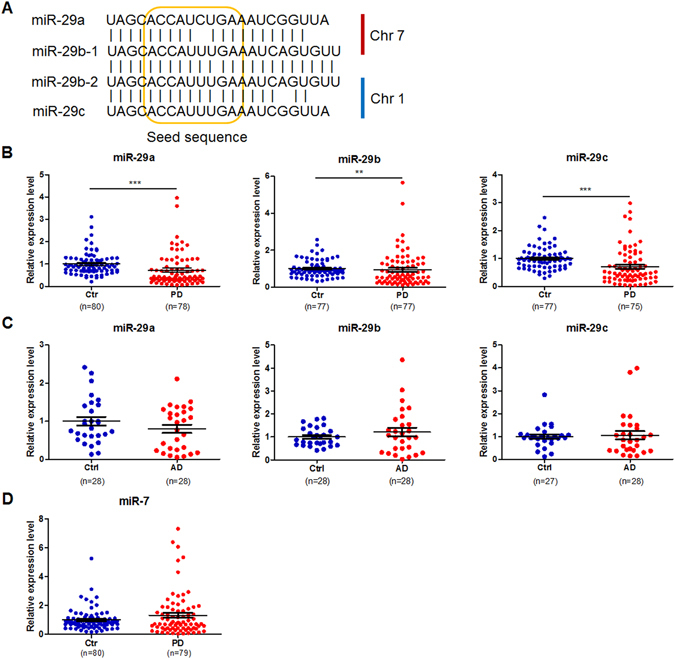
The alignment of human miR-29s (A) and the expression levels of miR-29s in the serum of control subjects and patients with PD (B) or AD (C) and the expression levels of miR-7 in the serum of controls and PD patients (D). Data are presented as the means ± SEM. Differences were analyzed by Mann-Whitney test. **p < 0.01 and ***p < 0.0001.
The main demographic and clinical characteristics of the 80 idiopathic PD patients and 80 controls recruited in this study are summarized in Table 1. PD patients ranged from early to advanced PD (Hoehn & Yahr stage 1 to 3), and their ages were comparable across groups (average age-at-examination ± SD of 64.0 ± 5.8 years in patients and 63.3 ± 5.4 years in controls). qRT-PCR analysis was performed to examine the expression of blood serum miR-29s. The expression of miR-29a, miR-29b and miR-29c was not detected in samples from 2, 3 and 5 PD patients, respectively. Both miR-29b expression and miR-29c expression was not detectable in 3 control samples. The results were summarized in Fig. 1B, which revealed a marked reduction in serum miR-29s in PD patients compared to controls. To test the relative specificity of miR-29s in PD, blood serum miR-29 levels in 30 AD patients and 30 controls were measured. The primary demographic and clinical profiles of AD patients and control subjects are summarized in Table 2. Serum levels of miR-29s were comparable between patients with AD and their controls (Fig. 1C). Additionally, all samples from control subjects and PD patients were used to measure serum miR-7 levels, which showed that serum miR-7 expression was not altered (Fig. 1D).
Table 2.
Demographic and clinical profiles of AD patients and control groups.
| AD | Controls | p Value | |
|---|---|---|---|
| No. of subjects | 30 | 30 | — |
| Age, y | 78.6 ± 9.5 | 42.6 ± 11.9 | <0.001a |
| F/M | 16/14 | 12/18 | 0.301b |
Abbreviations: AD = Alzheimer Disease.
The data are presented as mean ± SD.
ap values were calculated using two-tailed Student’s t test.
bp values were calculated using chi-square test.
Blood serum miR-29a and miR-29c tended to decrease with PD severity but not disease duration and UPDRS scores
The Hoehn & Yahr stages are widely used clinical standards for evaluating PD severity. Serum miR-29a and miR-29c tended to decrease with disease severity. The lowest expression of miR-29a and miR-29c was detected in HY-3 patients (Fig. 2A). Notably, there was a significant difference in serum miR-29b expression between control subjects and HY-3 patients. The lowest expression of miR-29b was detected in patients with disease durations longer than five years (Fig. 2B). However, there was no association between serum miR-29s and disease duration, and there was also no correlation between serum miR-29 expression and UPDRS score (Supplemental Table 1).
Figure 2.
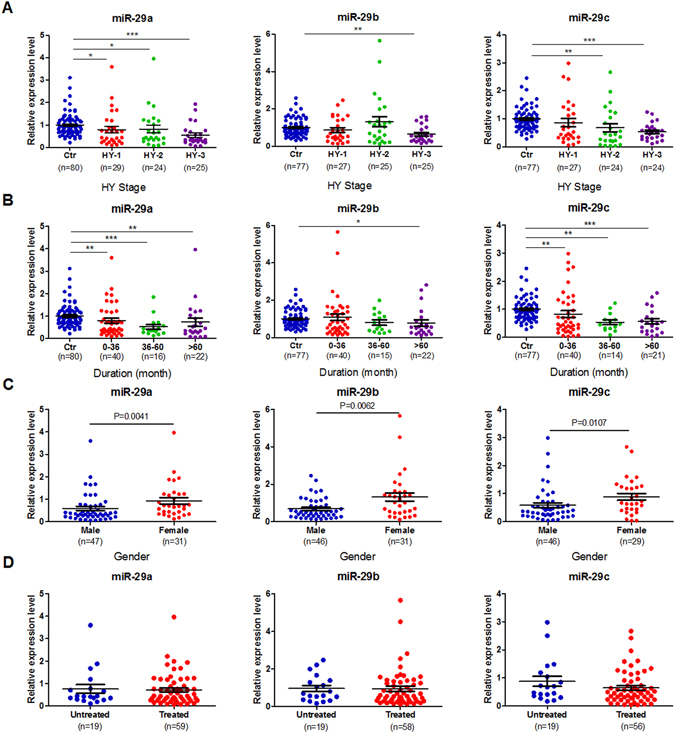
The expression levels of miR-29s in the serum of control subjects and patients with PD based on Hoehn & Yahr stages (A), disease duration (B), gender (C) and the expression levels of miR-29s in the serum of L-dopa-naïve PD patients and L-dopa-treated PD patients (D). Data are presented as the means ± SEM. Differences were analyzed by Kruskal-Wallis test in A and B or Mann-Whitney test in C and D. *p < 0.05, **p < 0.01 and ***p < 0.001.
Blood serum miR-29s show gender- but not age-dependent differences in PD patients
Although the serum miR-29 levels did not change with age in control subjects, the serum levels of miR-29a and miR-29c were markedly higher in females than in males (Supplemental Fig. 1). Similarly, in PD patients, miR-29 expression was significantly elevated in females (Fig. 2C). There were no age-dependent differences in the expression of miR-29s in patients with PD (Supplemental Table 1).
Blood serum miR-29s in L-dopa-naïve PD patients are similar to those in L-dopa-treated PD patients
In this study, 80 patients with PD were divided into two categories: L-dopa-naïve patients (n = 19) and L-dopa-treated patients (n = 61). The effects of L-dopa therapy on the serum levels of miR-29s were evaluated. As shown in Fig. 2D, L-dopa therapy did not alter serum miR-29 expression (P = 0.9073 for miR-29a; p = 0.4286 for miR-29b; p = 0.1903 for miR-29c).
Discussion
The profiles of blood miRNAs have been assessed in peripheral blood samples24, peripheral blood mononuclear cells22, 23, plasma30, and blood serum25 of idiopathic PD patients. Patient information is listed in Table 3 and includes L-dopa treatment, endogenous controls and the detected alterations in miR-29s. In this study, we focused on blood serum expression levels of miR-29s in 80 PD patients (including 19 L-dopa-naïve and 61 L-dopa-treated patients) and 80 matched controls. The serum levels of miR-29a and miR-29c were significantly decreased in PD patients and tended to reduce with disease severity. No alteration in serum miR-7 expression was detected in PD patients compared to control subjects. Our results are in agreement with those reported by Botta-Orfila et al.25 Serum miR-29b expression was also reduced in PD patients, although to a lesser extent. This result might have been due to the duplication of miR-29b in the human genome, which may affect its expression. Additionally, changes of miR-29s in PD serum are specific to some extents, as the miR-29 levels in AD serum do not differ from control serum.
Table 3.
Summary of previous studies of miR-29s in patients with PD.
| Samples from subject Groups (n) | Age at inclusion (years; means ± SD) | Gender Men (%) | HY stage | Duration (years; means ± SD) | Normalizers | Results (relate to miR-29s) | Ref. | |
|---|---|---|---|---|---|---|---|---|
| 211(serum) | Controls(95) | 67.22 ± 10.72 | 46.3 | — | — | miR-17 | 25 | |
| IPD(95) | 67.7 ± 10.39 | 40 | 1–5 | 9.6 (CI4-12) | miR-106a | miR-29a/c ↓ | ||
| LRRK2 PD(21) | 61.83 ± 11.64 | 46.3 | — | — | miR-29a/c ↓ | |||
| 92(PBMCs) | Controls(36/10) | 67 ± 10/67 ± 7 | 39/60 | — | — | 22 | ||
| L-dopa-treated(36) | 68 ± 11 | 39 | 1–3 | 7 ± 6 | RNU24 | miR-29a ↑ | ||
| Untreated (10) | 68 ± 7 | 60 | 1–2.5 | 4 ± 3 | Z30 | miR-29a/b — | ||
| 23(blood) | Controls(8) | 67 ± 8 | — | — | 24 | |||
| Untreated(8) | 66 ± 6.7 | 50 | 1–2 | 3 ± 2.6 | NA | miR-29a ↓ | ||
| EOPD(7) | 45 ± 8.7 | 1–3 | 7.2 ± 6.6 | miR-29a — | ||||
| Treated(4)@ | — | — | — | miR-29a ↓ | ||||
| 32(PBMCs) | Controls(13) | 64.38 ± 5.92 | 38.5 | — | — | Microarrays | 23 | |
| PD(19) | 65.11 ± 4.37 | 52.6 | 1–5 | 8.7 ± 5.1 | miR-29b/c ↓ |
Abbreviations: LRRK2 PD = Patients with LRRK2-associated Parkinson’s disease carrying the heterozygous G2019S mutation; EOPD = Early-onset Parkinson’s disease; HY stage = Hoehn & Yahr stage; PBMCs = Peripheral blood mononuclear cells.
NA = Not available.
@Selected previously untreated PD patients after 97 (±39) days of the levodopa/carbidopa treatment.
Gender, but not disease duration, UPDRS score, age or L-dopa treatment, affects serum miR-29 expression. It is well known that a higher incidence rate of PD is found among men, with the relative risk being 1.5 times greater in men than in women31, 32. The neuroprotective effects of estrogens, as well as gender-specific genetic factors, may account for this difference32, 33. Additionally, the significantly reduced serum levels of miR-29s in male PD patients are consistent with the increased risk of developing PD in men in our study.
In PD pathogenesis, mitochondrial dysfunction, oxidative stress, protein mishandling, and cell death together with epigenetic abnormality play central roles1, 34. Mature miR-29s have identical seed sequences at nucleotide positions 2–7, and the predicted target genes for miR-29s heavily overlap9. Candidate targets of miR-29s include: oxidative stress sensor PARK7 (DJ-1), Parkin substrate GPR37, targets related to apoptotic processes Puma, Bim, Bak, Bcl2, IGF1 and AKT1, microglial phagocytosis-related CDC42, and the epigenetic molecules DNMT3A, DNMT3B and HDAC4. miR-29a, and miR-29c were recently found to be downregulated in the same patients with idiopathic rapid eye movement behavior disorder after they were diagnosed with PD and dementia with Lewy bodies35. Therefore, the role of miR-29s in the pathogenesis of PD and the diagnostic potential of circulating miR-29s in PD patients warrant further study.
Methods
Subjects
Eighty patients with PD and thirty patients with AD were recruited from the Department of Neurology, Huashan Hospital, Fudan University, and Tongde Hospital, Zhejiang Province. PD subjects were clinically examined and diagnosed by two senior investigators of movement disorders according to the UK Brain Bank criteria36. Exclusion criteria included (1) clinical signs of possible atypical Parkinsonism; (2) secondary or iatrogenic Parkinsonism; (3) patients with cognitive impairment as assessed by the Mini Mental State Examination (MMSE); and (4) patients with hepatic and/or renal dysfunction. Patients were diagnosed with probable AD based on a comprehensive evaluation by two experienced subspecialty cognitive neurologists according to NINCDS-ADRDA37 criteria and their revision38. Exclusion criteria for AD patients included metabolic diseases, large vessel strokes, head injuries, severe psychiatric illness and neuro-developmental conditions. All participants provided written informed consent in accordance with the Declaration of Helsinki. The study was approved by the Human Studies Institutional Review Board, Huashan Hospital, Fudan University, and the Human Studies Institutional Review Board, Tongde Hospital, Zhejiang Province. All methods were performed in accordance with the relevant guidelines and regulations.
Of the 80 PD patients, 61 had received medication for PD; the remaining 19 had not been previously medicated. To standardize the data on medication use, we converted the dosages of PD medications into total daily levodopa-equivalent doses. Before clinical assessment, the subjects fasted overnight and did not take anti-Parkinsonian medications for at least 12 h. The severity and stage of the patient’s Parkinsonism was evaluated using the Unified Parkinson’s Disease Rating Scale (UPDRS) motor subscore39 and the modified Hoehn and Yahr stage40. Twenty-nine patients showed unilateral motor impairment only, classified as HY-1 stage; 25 patients had presented bilateral or midline involvement without impairment of balance, classified as HY-2 stage; and 26 patients exhibited with postural reflexes impairment, classified as HY-3 stage.
Overall, 110 age- and gender-matched volunteer control subjects were recruited. All control subjects had no history of neurologic/psychiatric disorders. The demographic and clinical data of patients and controls are summarized in Tables 1 and 2.
Table1.
Demographic and clinical profiles of PD patients and control groups.
| Controls | PD | Hoehn&Yahr stage I | Hoehn&Yahr stage II | Hoehn&Yahr stage III | p Valuea | |
|---|---|---|---|---|---|---|
| No. of subjects | 80 | 80 | 29 | 25 | 26 | — |
| Age, y | 63.3 ± 5.4 | 64.0 ± 5.8 | 64.2 ± 5.9 | 63.0 ± 6.7 | 64.7 ± 5.0 | 0.777 |
| F/M | 32/48 | 32/48 | 12/17 | 12/13 | 8/18 | 0.807 |
| Disease duration, mo | — | 52.9 ± 52.2 | 23.7 ± 20.0 | 60.8 ± 63.7 | 77.8 ± 50.7d | 0.001 |
| UPDRS (motor)b | — | 27.9 ± 14.0 | 16.6 ± 6.0 | 30.8 ± 9.2d | 37.5 ± 15.6e | < 0.001 |
| Levodopa equivalent dose (mg/day) | — | 312.4 ± 319.2 | 196.7 ± 269.4 | 301.7 ± 300.9 | 451.8 ± 343.4c | 0.030 |
| No. of drug-naïve patients | — | 19 | 10 | 8 | 1 | — |
| MMSE | — | 27.4 ± 2.5 | 28.2 ± 1.4 | 26.9 ± 2.3 | 27.0 ± 3.4 | 0.331 |
Abbreviations: PD = Parkinson disease; UPDRS = Unified Parkinson’s Disease Rating Scale; MMSE = Mini Mental State Examination. The data are presented as mean ± SD.
aAnalysis of variance with the exception of chi-square for gender.
bOff-state motor ratings according to the UPDRS.
cp < 0.05 vs. Hoehn & Yahr stage I group.
dp < 0.01 vs. Hoehn & Yahr stage I group.
ep < 0.001 vs. Hoehn & Yahr stage I group.
Serum isolation and storage
The PD patients refrained from taking any anti-parkinsonian medications and fasted for at least 12 h before blood samples were taken. Control subjects fasted for 12 h before blood samples were taken. First, 5 ml of whole blood was collected between 8:00 and 9:00 a.m. in tubes without anticoagulant and was preserved for 30 minutes at room temperature according to the protocols from Parkinson Progression Marker Initiative (PPMI)41. Tubes were centrifuged at 1900× g for 10 minutes at 4 °C. Serum were removed, aliquoted (200 µl/tube), flash frozen, and stored at −80 °C.
RNA extraction
Frozen sera were thawed at room temperature and centrifuged at 16000× g for 5 minutes at 4 °C. Then, 100 µl of supernatant was transferred to a new tube for the isolation of total RNA, including miRNAs, using miRNeasy Serum/Plasma Kit (Qiagen, Germany). A final 12 μl of the eluate was collected. To normalize for the miRNA content, each denatured sample was supplemented with 3.5 µl (1.6 × 108 copies/μl working solution) synthetic Caenorhabditis elegans miR-39 (cel-miR-39), as described previously42, 43.
Reverse transcription and quantitative real-time PCR
First, 5 µl of total RNA was reverse transcribed using a miRcute miRNA First-Strand cDNA Synthesis Kit (Tiangen, China). Subsequently, 2 µl of the product was used to detect miR29s expression by quantitative real-time PCR using a miRcute miRNA qPCR Detection kit (Tiangen, China). The PCR primer sequences were as follows: miR-29a (5′-TAGCACCATCTGAAATCGG-3′); miR-29b (5′-TAGCACCATTTGAAATCAGT-3′); miR-29c (5′-TAGCACCATTTGAAATCGG-3′) and miR-7 (5′-TGGAAGACTAGTGATTTTGTT-3′). Relative expression levels were calculated using the comparative ΔΔCt method with cel-miR-39 as the normalizing control. Samples with Ct values above 35 were randomly picked to run on 2.5% agarose gels. After recovering and cloning, they were confirmed by sequencing.
Statistical analysis
Data were presented as the means ± SEM. For group-wise comparisons, the Mann-Whitney test (2 groups) or Kruskal-Wallis test (n groups) was used as appropriate. The relationships between miR-29 expression and disease duration, UPDRS score and age were assessed in PD patients via a logistic regression analysis and analysis of covariance (ANCOVA) using SPSS 19.0 (Version 19.0; SPSS, Chicago, USA). The statistical analysis was performed using PRISM 5.0 (GraphPad Software Inc, USA). Significant differences were defined as P < 0.05.
Electronic supplementary material
Acknowledgements
This work was supported by grants from the Ministry of Science and Technology of China (2012CB966300) and the National Natural Science Foundation of China (31671043, 81371412, 81571232, 81371413, 81261120568 and 81400992), the Fund of Science and Technology Commission of Shanghai Municipality (13DZ2293700) and the Open Project of State Key Laboratory of Medical Neurobiology (SKLMN2015005). The authors are grateful to the study participants. The authors acknowledge Prof. Heliang Fei and Prof. Ding Ding for their valuable support in statistical issues.
Author Contributions
F.H., J.W., W.W. and J.F. proposed and supervised the study. F.H., J.W. and W.W. wrote the manuscript. Y.T., L.W., F.L. and J.N. contributed to the sample collection and clinical characterization of the patients and followed up the patients. X.B., M.Y., Z.W. and J.W. performed the experiments. X.B., Y.T., F.L., F.H. and J.W. performed the statistical analyses. X.B., Y.T. and F.L. assisted in the preparation of the manuscript. All authors contributed to the interpretation of data and the revision of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Xiaochen Bai and Yilin Tang contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-03887-3
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wei Wang, Email: wangweihz8@163.com.
Fang Huang, Email: huangf@shmu.edu.cn.
Jian Wang, Email: wangjian336@hotmail.com.
References
- 1.de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5:525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 2.Tieu K, et al. D-beta-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J Clin Invest. 2003;112:892–901. doi: 10.1172/JCI200318797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet (London, England) 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 4.Diamond SG, Markham CH, Hoehn MM, McDowell FH, Muenter MD. An examination of male-female differences in progression and mortality of Parkinson’s disease. Neurology. 1990;40:763–766. doi: 10.1212/WNL.40.5.763. [DOI] [PubMed] [Google Scholar]
- 5.Van Den Eeden SK, et al. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. American journal of epidemiology. 2003;157:1015–1022. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- 6.Mott JL, et al. Transcriptional suppression of mir-29b-1/mir-29a promoter by c-Myc, hedgehog, and NF-kappaB. Journal of cellular biochemistry. 2010;110:1155–1164. doi: 10.1002/jcb.22630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Zhang X, Li H, Yu J, Ren X. The role of miRNA-29 family in cancer. European journal of cell biology. 2013;92:123–128. doi: 10.1016/j.ejcb.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Yan B, et al. The role of miR-29b in cancer: regulation, function, and signaling. OncoTargets and therapy. 2015;8:539–548. doi: 10.2147/OTT.S75899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kriegel AJ, Liu Y, Fang Y, Ding X, Liang M. The miR-29 family: genomics, cell biology, and relevance to renal and cardiovascular injury. Physiological genomics. 2012;44:237–244. doi: 10.1152/physiolgenomics.00141.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kole AJ, Swahari V, Hammond SM, Deshmukh M. miR-29b is activated during neuronal maturation and targets BH3-only genes to restrict apoptosis. Genes & development. 2011;25:125–130. doi: 10.1101/gad.1975411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lippi G, et al. Targeting of the Arpc3 actin nucleation factor by miR-29a/b regulates dendritic spine morphology. The Journal of cell biology. 2011;194:889–904. doi: 10.1083/jcb.201103006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ugalde AP, et al. Aging and chronic DNA damage response activate a regulatory pathway involving miR-29 and p53. The EMBO journal. 2011;30:2219–2232. doi: 10.1038/emboj.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hebert SS, et al. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/beta-secretase expression. Proc Natl Acad Sci USA. 2008;105:6415–6420. doi: 10.1073/pnas.0710263105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson R, et al. A microRNA-based gene dysregulation pathway in Huntington’s disease. Neurobiol Dis. 2008;29:438–445. doi: 10.1016/j.nbd.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Nolan K, et al. Increased expression of microRNA-29a in ALS mice: functional analysis of its inhibition. Journal of molecular neuroscience: MN. 2014;53:231–241. doi: 10.1007/s12031-014-0290-y. [DOI] [PubMed] [Google Scholar]
- 16.Smith KM, et al. miR-29ab1 deficiency identifies a negative feedback loop controlling Th1 bias that is dysregulated in multiple sclerosis. Journal of immunology (Baltimore, Md.: 1950) 2012;189:1567–1576. doi: 10.4049/jimmunol.1103171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pandi G, Nakka VP, Dharap A, Roopra A, Vemuganti R. MicroRNA miR-29c down-regulation leading to de-repression of its target DNA methyltransferase 3a promotes ischemic brain damage. PloS one. 2013;8:e58039. doi: 10.1371/journal.pone.0058039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khanna S, et al. Loss of miR-29b following acute ischemic stroke contributes to neural cell death and infarct size. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013;33:1197–1206. doi: 10.1038/jcbfm.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi G, et al. Upregulated miR-29b promotes neuronal cell death by inhibiting Bcl2L2 after ischemic brain injury. Experimental brain research. 2012;216:225–230. doi: 10.1007/s00221-011-2925-3. [DOI] [PubMed] [Google Scholar]
- 20.Roshan R, et al. Brain-specific knockdown of miR-29 results in neuronal cell death and ataxia in mice. RNA (New York, N.Y.) 2014;20:1287–1297. doi: 10.1261/rna.044008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papadopoulou AS, et al. Deficiency of the miR-29a/b-1 cluster leads to ataxic features and cerebellar alterations in mice. Neurobiol Dis. 2014;73C:275–288. doi: 10.1016/j.nbd.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Serafin, A. et al. Overexpression of blood microRNAs 103a, 30b, and 29a in l-dopa-treated patients with PD. Neurology, doi:10.1212/wnl.0000000000001258 (2015). [DOI] [PubMed]
- 23.Martins M, et al. Convergence of miRNA expression profiling, alpha-synuclein interacton and GWAS in Parkinson’s disease. PloS one. 2011;6:e25443. doi: 10.1371/journal.pone.0025443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Margis R, Margis R, Rieder CR. Identification of blood microRNAs associated to Parkinsonis disease. Journal of biotechnology. 2011;152:96–101. doi: 10.1016/j.jbiotec.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 25.Botta-Orfila T, et al. Identification of blood serum micro-RNAs associated with idiopathic and LRRK2 Parkinson’s disease. J Neurosci Res. 2014;92:1071–1077. doi: 10.1002/jnr.23377. [DOI] [PubMed] [Google Scholar]
- 26.Junn E, et al. Repression of alpha-synuclein expression and toxicity by microRNA-7. Proc Natl Acad Sci USA. 2009;106:13052–13057. doi: 10.1073/pnas.0906277106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Y, et al. MicroRNA-7 targets Nod-like receptor protein 3 inflammasome to modulate neuroinflammation in the pathogenesis of Parkinson’s disease. Molecular neurodegeneration. 2016;11:28. doi: 10.1186/s13024-016-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilad S, et al. Serum microRNAs are promising novel biomarkers. PloS one. 2008;3:e3148. doi: 10.1371/journal.pone.0003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell PS, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khoo SK, et al. Plasma-based circulating MicroRNA biomarkers for Parkinson’s disease. Journal of Parkinson’s disease. 2012;2:321–331. doi: 10.3233/JPD-012144. [DOI] [PubMed] [Google Scholar]
- 31.Berg D, et al. MDS research criteria for prodromal Parkinson’s disease. Movement disorders: official journal of the Movement Disorder Society. 2015;30:1600–1611. doi: 10.1002/mds.26431. [DOI] [PubMed] [Google Scholar]
- 32.Wooten GF, Currie LJ, Bovbjerg VE, Lee JK, Patrie J. Are men at greater risk for Parkinson’s disease than women? J Neurol Neurosurg Psychiatry. 2004;75:637–639. doi: 10.1136/jnnp.2003.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Latourelle JC, Dybdahl M, Destefano AL, Myers RH, Lash TL. Risk of Parkinson’s disease after tamoxifen treatment. BMC neurology. 2010;10:23. doi: 10.1186/1471-2377-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lardenoije R, et al. The epigenetics of aging and neurodegeneration. Progress in neurobiology. 2015;131:21–64. doi: 10.1016/j.pneurobio.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandez-Santiago R, et al. MicroRNA association with synucleinopathy conversion in rapid eye movement behavior disorder. Ann Neurol. 2015;77:895–901. doi: 10.1002/ana.24384. [DOI] [PubMed] [Google Scholar]
- 36.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKhann G, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/WNL.34.7.939. [DOI] [PubMed] [Google Scholar]
- 38.Dubois B, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 39.Patrick SK, Denington AA, Gauthier MJ, Gillard DM, Prochazka A. Quantification of the UPDRS Rigidity Scale. IEEE transactions on neural systems and rehabilitation engineering: a publication of the IEEE Engineering in Medicine and Biology Society. 2001;9:31–41. doi: 10.1109/7333.918274. [DOI] [PubMed] [Google Scholar]
- 40.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/WNL.17.5.427. [DOI] [PubMed] [Google Scholar]
- 41.The Parkinson Progression Marker Initiative (PPMI). Progress in neurobiology95, 629–635, doi:10.1016/j.pneurobio.2011.09.005 (2011). [DOI] [PMC free article] [PubMed]
- 42.Fichtlscherer S, et al. Circulating microRNAs in patients with coronary artery disease. Circulation research. 2010;107:677–684. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 43.De Rosa S, et al. Transcoronary concentration gradients of circulating microRNAs. Circulation. 2011;124:1936–1944. doi: 10.1161/CIRCULATIONAHA.111.037572. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


