Abstract
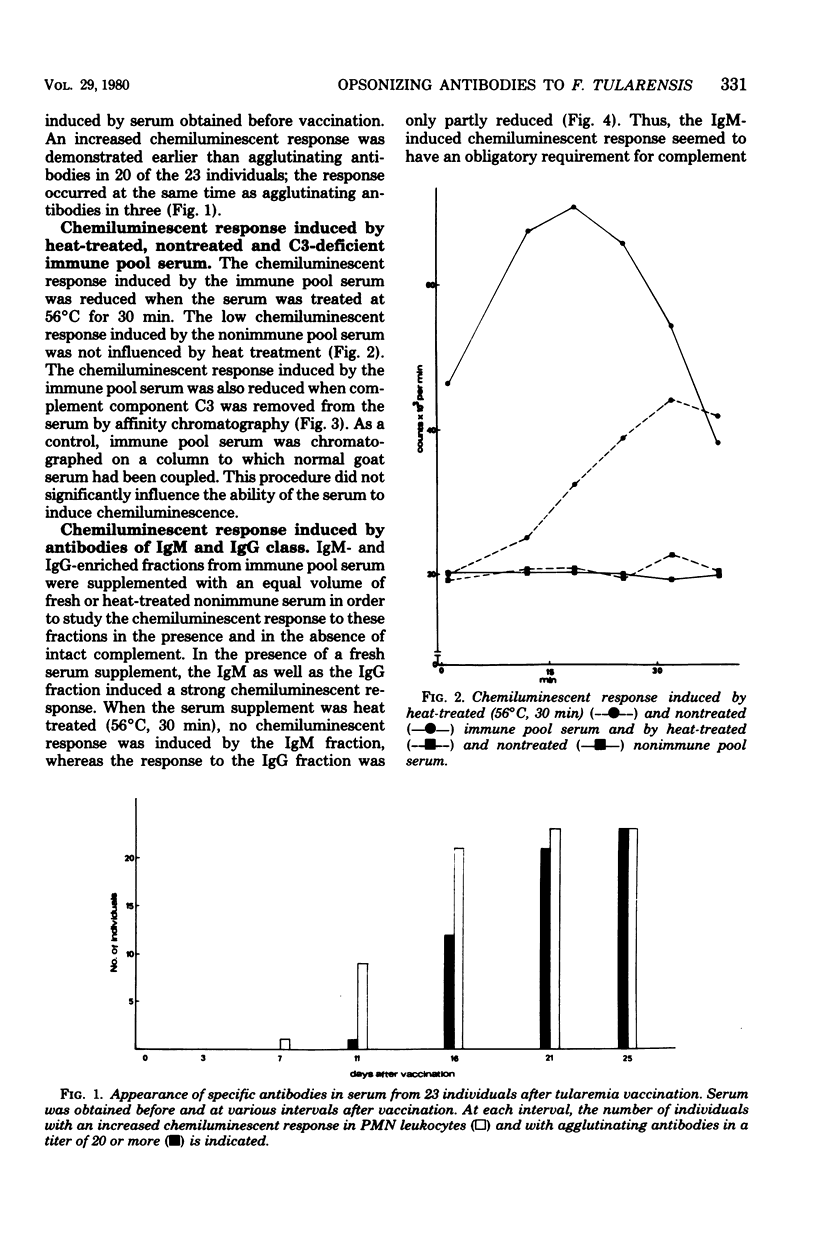
Twenty-three individuals were vaccinated with a viable attenuated strain of Francisella tularensis, and blood was collected at various time intervals during 4 weeks. To demonstrate opsonizing antibodies, a mixture of serum and vaccine bacteria was incubated, whereafter the chemiluminescence response of polymorphonuclear (PMN) leukocytes to this mixture was recorded. No opsonizing antibodies against F. tularensis were found in sera obtained before vaccination. Eleven days after vaccination, sera from nine individuals, and 21 days after vaccination, sera from all 23 individuals contained antibodies. Antibodies were demonstrated earlier with the chemiluminescent technique that with the agglutination reaction. Heat treatment (56 degrees C, 30 min) or removal of complement component C3 from immune serum reduced the chemiluminescent response of the leukocytes. A high chemiluminescent response of the leukocytes was induced by immunoglobulin G (IgG)- and IgM-enriched fractions of immune serum in the presence of complement. In the absence of complement, the IgG fraction induced a low chemiluminescent response; the IgM fraction induced no response at all.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C. Evaluation of serum opsonic capacity by quantitating the initial chemiluminescent response from phagocytizing polymorphonuclear leukocytes. Infect Immun. 1977 Mar;15(3):828–833. doi: 10.1128/iai.15.3.828-833.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Burke D. S. Immunization against tularemia: analysis of the effectiveness of live Francisella tularensis vaccine in prevention of laboratory-acquired tularemia. J Infect Dis. 1977 Jan;135(1):55–60. doi: 10.1093/infdis/135.1.55. [DOI] [PubMed] [Google Scholar]
- Canonico P. G., McManus A. T., Mangiafico J. A., Sammons L. S., McGann V. G., Dangerfield H. G. Temporal appearance of opsonizing antibody to Francisella tularensis: detection by a radiometabolic assay. Infect Immun. 1975 Mar;11(3):466–469. doi: 10.1128/iai.11.3.466-469.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M. Biological defense mechanisms. The effect of bacteria and serum on superoxide production by granulocytes. J Clin Invest. 1974 Jun;53(6):1662–1672. doi: 10.1172/JCI107717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIOGUARDI N., AGOSTONI A., FIORELLI G., LOMANTO B. Characterization of lactic dehydrogenase of normal human granulocytes. J Lab Clin Med. 1963 May;61:713–723. [PubMed] [Google Scholar]
- EIGELSBACH H. T., DOWNS C. M. Prophylactic effectiveness of live and killed tularemia vaccines. I. Production of vaccine and evaluation in the white mouse and guinea pig. J Immunol. 1961 Oct;87:415–425. [PubMed] [Google Scholar]
- Harvath L., Amirault H. J., Andersen B. R. Chemiluminescence of human and canine polymorphonuclear leukocytes in the absence of phagocytosis. J Clin Invest. 1978 May;61(5):1145–1154. doi: 10.1172/JCI109029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostiala A. A., McGregor D. D., Logie P. S. Tularaemia in the rat. I. The cellular basis on host resistance to infection. Immunology. 1975 May;28(5):855–869. [PMC free article] [PubMed] [Google Scholar]
- Lehmeyer J. E., Snyderman R., Johnston R. B., Jr Stimulation of neutrophil oxidative metabolism by chemotactic peptides: influence of calcium ion concentration and cytochalasin B and comparison with stimulation by phorbol myristate acetate. Blood. 1979 Jul;54(1):35–45. [PubMed] [Google Scholar]
- Löfgren S., Tärnvik A., Carlsson J. Influence of complement on the chemiluminescent response of human leukocytes to immune complex. Infect Immun. 1980 Aug;29(2):335–341. doi: 10.1128/iai.29.2.335-341.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. K., Verhoef J., Sabath L. D., Quie P. G. Extracellular and bacterial factors influencing staphylococcal phagocytosis and killing by human polymorphonuclear leukocytes. Infect Immun. 1976 Aug;14(2):496–501. doi: 10.1128/iai.14.2.496-501.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor R. A., White J. D., Ayala E., Canonico P. G. Phagocytosis of Francisella tularensis by Rhesus monkey peripheral leukocytes. Infect Immun. 1975 Jan;11(1):146–151. doi: 10.1128/iai.11.1.146-151.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Health Weekly Reports for JUNE 25, 1926. Public Health Rep. 1926 Jun 25;41(26):1273–1339. [PMC free article] [PubMed] [Google Scholar]
- Quie P. G., White J. G., Holmes B., Good R. A. In vitro bactericidal capacity of human polymorphonuclear leukocytes: diminished activity in chronic granulomatous disease of childhood. J Clin Invest. 1967 Apr;46(4):668–679. doi: 10.1172/JCI105568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKOOG W. A., BECK W. S. Studies on the fibrinogen, dextran and phytohemagglutinin methods of isolating leukocytes. Blood. 1956 May;11(5):436–454. [PubMed] [Google Scholar]
- Tärnvik A., Löfgren S. Stimulation of human lymphocytes by a vaccine strain of Francisella tularensis. Infect Immun. 1975 Nov;12(5):951–957. doi: 10.1128/iai.12.5.951-957.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN METRE T. E., Jr, KADULL P. J. Laboratory-acquired tularemia in vaccinated individuals: a report of 62 cases. Ann Intern Med. 1959 Mar;50(3):621–632. doi: 10.7326/0003-4819-50-3-621. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Zurier R. B., Spieler P. J., Goldstein I. M. Mechanisms of lysosomal enzyme release from leukocytes exposed to immune complexes and other particles. J Exp Med. 1971 Sep 1;134(3 Pt 2):149s–165s. [PubMed] [Google Scholar]
- van Oss C. J. Phagocytosis as a surface phenomenon. Annu Rev Microbiol. 1978;32:19–39. doi: 10.1146/annurev.mi.32.100178.000315. [DOI] [PubMed] [Google Scholar]



