Abstract
Esophageal cancer is one of the most lethal cancers worldwide with poor survival and limited therapeutic options. The discovery of microRNAs created a new milestone in cancer research. miR-377 is located in chromosome region 14q32, which is frequently deleted in esophageal squamous cell carcinoma (ESCC), but the biological functions, clinical significance and therapeutic implication of miR-377 in ESCC are largely unknown. In this study, we found that miR-377 expression was significantly downregulated in tumor tissue and serum of patients with ESCC. Both tumor tissue and serum miR-377 expression levels were positively correlated with patient survival. Higher serum miR-377 expression was inversely associated with pathologic tumor stage, distant metastasis, residual tumor status and chemoradiotherapy resistance. The roles of miR-377 in suppressing tumor initiation and progression, and the underlying molecular mechanisms were investigated. Results of in vitro and in vivo experiments showed that miR-377 overexpression inhibited the initiation, growth and angiogenesis of ESCC tumors as well as metastatic colonization of ESCC cells, whereas silencing of miR-377 had opposite effects. Mechanistically, miR-377 regulated CD133 and VEGF by directly binding to their 3′ untranslated region. Moreover, systemic delivery of formulated miR-377 mimic not only suppressed tumor growth in nude mice but also blocked tumor angiogenesis and metastasis of ESCC cells to the lungs without overt toxicity to mice. Collectively, our study established that miR-377 plays a functional and significant role in suppressing tumor initiation and progression, and may represent a promising non-invasive diagnostic and prognostic biomarker and therapeutic strategy for patients with ESCC.
Introduction
Esophageal cancer is the eighth most common cancer worldwide and this disease is highly lethal, with a 5-year survival rate of ~14%.1, 2 Although advances in surgical techniques and pre-operative chemoradiotherapy can improve survival, the majority of patients are not eligible for surgical resection because many cases go undetected until the disease is at an advanced stage. Metastatic spread, recurrence, and resistance to chemo- and radiotherapy all contribute to the poor prognosis. A lack of robust predictive biomarkers to guide diagnostic and therapeutic selection are obstacles in achieving early remission.
MicroRNAs (miRNAs) are short (~22 nucleotides) non-protein-coding RNAs that can act as post-transcriptional regulators by binding to complementary sequences in the 3′ untranslated regions (3′-UTRs) of target mRNAs.3 Increasing evidence supports that miRNAs are critical regulators of tumorigenesis and cancer progression, as well as useful diagnostic and prognostic markers in human cancer.4, 5, 6, 7 However, our understanding of how miRNAs regulate cancer development and progression, particularly how they may affect tumor initiation and the response of cancer to chemotherapy is far from adequate. Here, we report that miR-377, a miRNA conserved among mammals, is significantly downregulated in esophageal squamous carcinoma (ESCC) cell lines, and in tumor and serum samples of patients with ESCC. The coding gene of miR-377 is located in chromosome region 14q32, which is frequently deleted in ESCC and has one of the largest miRNA clusters.8, 9 However, the biological functions and regulatory mechanisms of miR-377 in human cancer are largely unknown.
In the present study, we examined the expression profiles and prognostic value of miR-377 in ESCC and multiple other cancer types. In particular, the correlation between miR-377 expression and ESCC progression, and the role of serum miR-377 as a non-invasive biomarker in ESCC were evaluated. In addition, the roles of miR-377 in modulating chemoresistance and in inhibiting multiple aspects of tumor development including self-renewal, tumor growth and metastasis, as well as the underlying molecular mechanisms were investigated. It is widely accepted that tumors are initiated and maintained by a small population of cancer cells termed tumor-initiating cells (TICs) which have the unique abilities to renew themselves indefinitely and to resist conventional therapy, and CD133 is one of the most commonly used markers for identifying TICs.10 However, surprisingly little is known about the role or significance of CD133 in ESCC. Whether CD133 expression has prognostic significance in ESCC remains controversial,11, 12, 13, 14 and there is as yet no study showing that CD133 is a functional TIC marker for ESCC. The aggressive progression of esophageal cancer is associated with angiogenesis. Vascular endothelial growth factor (VEGF) plays a predominant angiogenic role in ESCC.15 Recent meta-analysis studies revealed that high VEGF expression is associated with worse survival in ESCC.16, 17 This study aims to examine whether the functional role of miR-377 in ESCC is attributed to its regulation of tumor initiation and angiogenesis through modulation of CD133 and VEGF expressions.
MicroRNA-based therapy is increasingly regarded as a novel and promising strategy in cancer treatment due to advances in miRNA delivery technologies.18, 19 Recent studies reported that tumor suppressor miRNA mimics could be systemically delivered using polyethylenimine-mediated delivery to inhibit cancer progression in mice.20 Whether re-expressing or replenishing miR-377 might be a useful therapeutic strategy in treatment of ESCC remains to be elucidated. We found that systemic administration of miR-377 oligonucleotide could prevent the growth of tumor xenograft and experimental metastasis of ESCC cells in mice. Our study suggests that miR-377 plays a pivotal role in tumor growth and metastasis, and might be a promising prognostic biomarker and useful therapeutic agent in the treatment of patients with ESCC.
Results
Deregulation of miR-377 expression is correlated with poor chemoresponse and poor survival in esophageal cancer
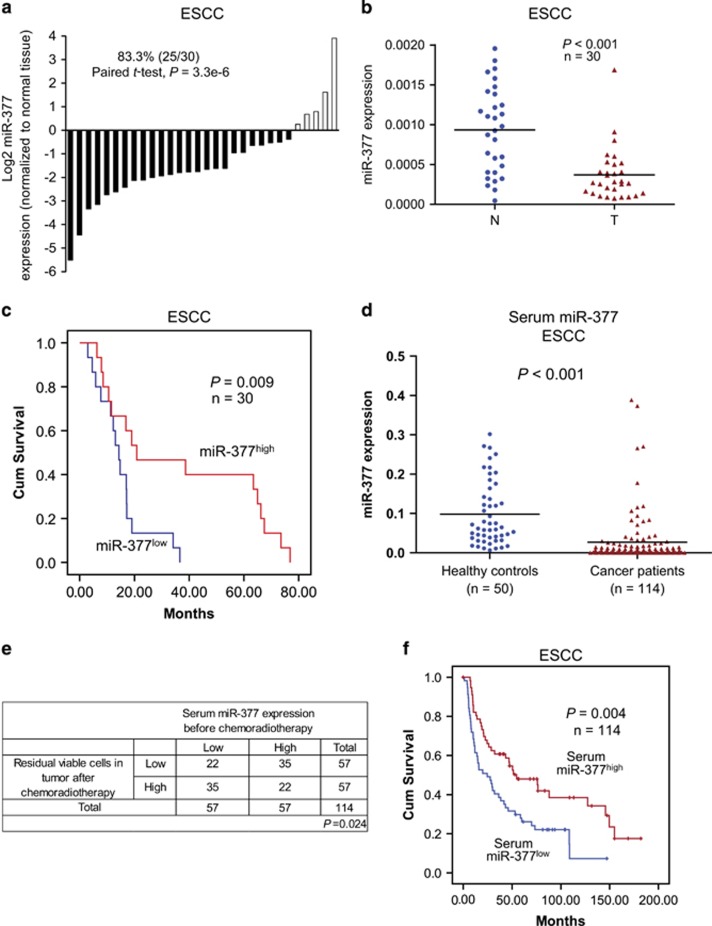
To study the clinical significance of miR-377 in human cancer, we determined the expression level of miR-377 in 30 pairs of ESCC tumor and adjacent normal tissues. As shown in Figure 1a, 25 cases (83.3%) had decreased miR-377 expression compared with corresponding normal tissues. The mean miR-377 expression in the tumor tissues was about 3-fold lower than that in the paired normal tissues (Figure 1b). The expression level of miR-377 was also examined in cell lines, and all 7 ESCC cell lines had significantly lower miR-377 expression compared with an immortalized normal esophageal epithelial cell line (Supplementary Figure S1). Analysis of gene expression profiles of several cohorts of patients from Gene Expression Omnibus (GEO) database showed that miR-377 was also significantly reduced in colon, ovarian, and breast cancers (Supplementary Figure S2a). Furthermore, although no significant correlation was found between clinicopathological parameters and tumor miR-377 expression level in the 30 ESCC patients who had upfront surgery (Supplementary Table S1), patients with low miR-377 expression in their tumor had significantly shorter survival (median survival=14.33 months) than the patients with higher tumor miR-377 expression (median survival=20.85 months; Figure 1c). Analysis of the GEO data set of ovarian cancer also indicated that low miR-377 expression in tumor may predict worse prognosis (Supplementary Figure S2b). Collectively, our results strongly suggest that miR-377 may have tumor suppressor function.
Figure 1.
Expression and prognostic significance of miR-377 in ESCC. (a) miR-377 expression in 30 tumor samples relative to the corresponding normal tissues. (b) Mean miR-377 expression was significantly downregulated in primary esophageal cancer (T) compared with the paired normal tissues (N). (c) Kaplan–Meier analysis of overall survival of 30 ESCC patients stratified according to tumor miR-377 expression level. (d) Serum miR-377 level in 114 ESCC patients and 50 healthy control subjects. (e) Correlation between miR-377 expression before chemoradiotherapy and percentage of residual viable cells in tumor after chemoradiotherapy was analyzed by χ2-test. (f) Kaplan–Meier analysis of overall survival of 114 patients with ESCC stratified according to admission serum miR-377 level.
Serum miR-377 is a non-invasive biomarker for cancer diagnosis and prognosis
Recent studies showed that miRNAs in the circulatory system may serve as promising diagnostic and prognostic biomarkers in human cancer,19, 20 but the expression pattern of miR-377 in the blood of cancer patients and its feasibility as a non-invasive cancer biomarker have not yet been reported. To evaluate the potential of miR-377 as a non-invasive biomarker, we next analyzed the expression level of miR-377 in serum samples collected from 114 patients on admission with ESCC and 50 healthy individuals. The mean expression level of miR-377 in the serum of ESCC patients was about 3-fold lower than that in healthy controls (0.027±0.065 vs 0.098±0.082; P<0.001; Figure 1d). More importantly, we found that high miR-377 expression level in serum could predict a better response to chemoradiation with 5-fluorouracil (5-FU) and cisplatin (DDP), as indicated by the lower percentage of residual viable cells in the tumor collected during surgery (Figure 1e), and a significant inverse correlation with post-therapy pathologic T-stage, M-stage, pathological response and resection status (that is, R classification) in the ESCC patients (Table 1). In addition, higher miR-377 serum expression was positively correlated with better survival (Figure 1f). Analysis of the GEO data sets showed that expression of miR-377 was frequently downregulated in the serum of patients with other types of cancer including breast cancer, melanoma and lung cancer (Supplementary Figure S2c). Taken together, these data demonstrated that serum miR-377 may serve as a non-invasive diagnostic and prognostic biomarker for ESCC, and as a predictive marker of response to chemoradiotherapy.
Table 1. Correlation between serum miR-377 expression levels and clinicopathological parameters in ESCC.
| Variable | n |
Serum miR-377 expressiona |
P-value | |
|---|---|---|---|---|
| Low | High | |||
| Age (years) | ||||
| ⩽55 | 20 | 9 | 11 | |
| ⩾55 | 94 | 48 | 46 | 0.622 |
| Gender | ||||
| Female | 12 | 6 | 6 | |
| Male | 102 | 51 | 51 | 1.000 |
| pT-stage | ||||
| 0/1/2 | 66 | 26 | 40 | |
| 3/4 | 26 | 20 | 6 | 0.001 |
| pN-stage | ||||
| 0 | 53 | 25 | 28 | |
| 1/2/3 | 39 | 21 | 18 | 0.526 |
| pM-stage | ||||
| 0 | 84 | 38 | 46 | |
| 1 | 7 | 7 | 0 | 0.0007 |
| Pathologic stage | ||||
| pCRb | 22 | 6 | 16 | |
| Othersc/I/II/III/IV | 70 | 40 | 30 | 0.014 |
| Pathologic stage | ||||
| pCRb/Othersc | 34 | 11 | 23 | |
| I/II/III/IV | 58 | 35 | 23 | 0.009 |
| R category | ||||
| R0 | 80 | 37 | 43 | |
| R1 | 12 | 10 | 2 | 0.016 |
Abbreviations: ESCC, esophageal squamous cell carcinoma; miR, microRNA.
Expression level of miR-377 was categorized into 'high' and 'low using the median value as cutoff point.
Pathologic complete response.
pT0 with positive lymph node(s) and/or distant metastasis.
miR-377 modulates chemoresistance and inhibits tumor initiation and in vivo tumorigenicity of ESCC cells
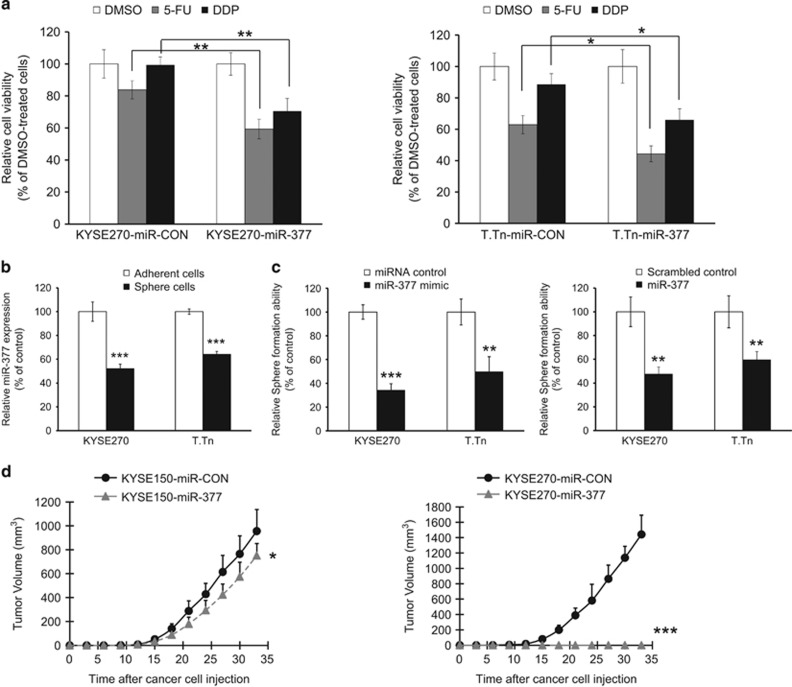
Given that ESCC patients with lower serum miR-377 level were less responsive to chemoradiotherapy (Figure 1e), we next examined the effects of miR-377 on chemoresistance and the results showed that ectopic miR-377 expression significantly enhanced the sensitivity of ESCC cells to 5-FU and DDP, which are two commonly used chemotherapeutic drugs for treating esophageal cancer (Figure 2a). Furthermore, because our data from clinical sample analysis strongly suggest that miR-377 is a tumor suppressor in ESCC, we examined its function in tumor initiation. We found that sphere-forming ESCC cells had lower miR-377 expression compared with the differentiated adherent counterparts (Figure 2b), and that ESCC cells with ectopic miR-377 expression had significantly reduced sphere formation ability (Figure 2c), which suggest that miR-377 has a regulatory role in tumor initiation. We also compared miR-377-overexpressing ESCC cell lines with respective parental cell lines expressing scrambled miRNA for ability to form tumors in nude mice. Interestingly, although a dramatic effect was observed in the group of mice inoculated with KYSE270-miR-377 cells, as indicated by the almost complete suppression of tumor growth in nude mice, ectopic miR-377 expression exerted only a modest inhibitory effect on the tumorigenic potential of KYSE150 cells (Figure 2d). This discrepancy suggests that there was a fundamental difference between the two cell lines in the downstream target(s) of miR-377 that are associated with tumor formation.
Figure 2.
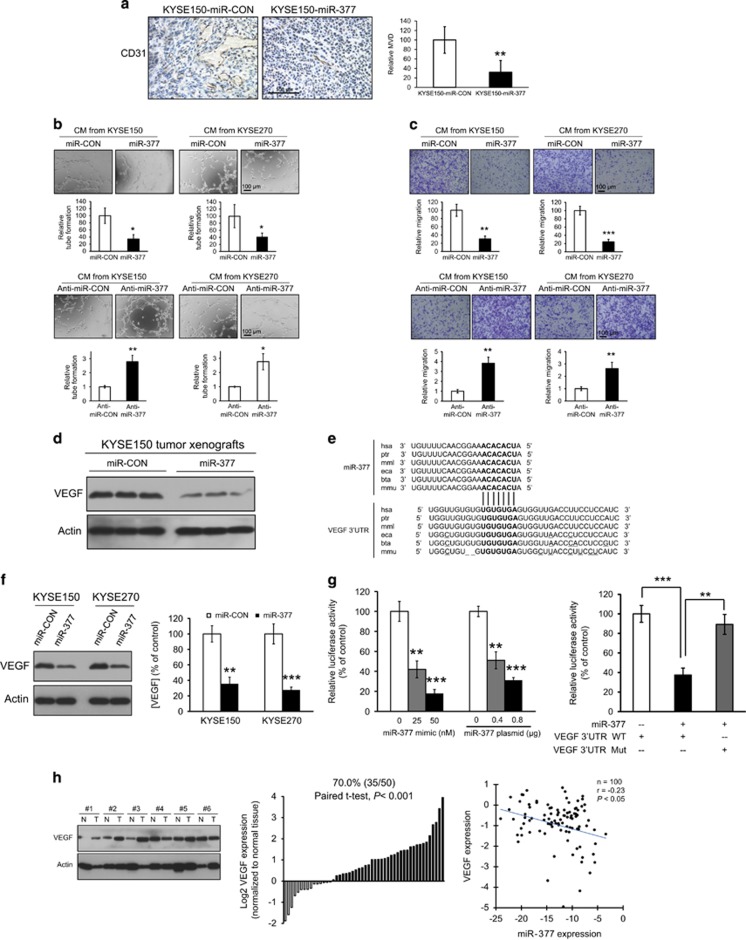
miR-377 inhibited TIC properties. (a) KYSE270 cells (left panel) and T.Tn cells (right panel) with ectopic miR-377 expression and corresponding control cells were treated with 5-FU (5 μM), DDP (20 μM) or DMSO for 72 h, and cell viability compared using MTT assay. (b) Sphere-forming ESCC cells and differentiated adherent counterparts were compared for miR-377 expression by qRT–PCR. (c) Sphere formation ability of KYSE270 and T.Tn cells transfected with 50 nm miR-377 mimic (left panel), or 0.8 μg miR-377 expressing plasmid (right panel) was compared with corresponding controls. (d) Growth curves of subcutaneous tumors formed by KYSE150-miR-377 cells (left panel) and KYSE270-miR-377 cells (right panel) compared with respective control cells expressing miR-CON (n=6/group). Bars, s.d.; *P<0.05; **P<0.01; ***P<0.001 compared with controls.
miR-377 directly targets and inhibits CD133 expression
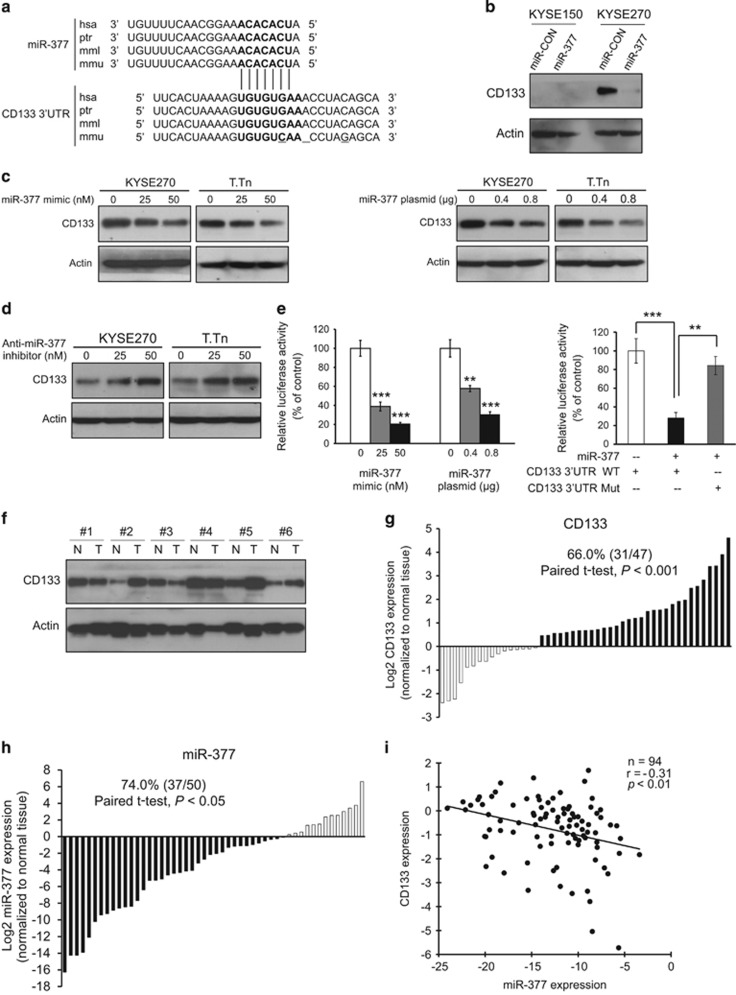
In order to characterize the molecular mechanism by which miR-377 regulates the functions of ESCC cells, in silico analysis using TargetScan and Miranda miRNA target prediction algorithms was performed to identify potential targets of miR-377. Interestingly, CD133, one of the most commonly used marker for TICs, but not CD90 and CD44, was identified as a potential target of miR-377. Since both miR-377 and its complementary binding sequence in the 3′-UTR of CD133 are highly evolutionally conserved among different species (Figure 3a), the binding of miR-377 to the CD133 3′-UTR is likely to have a significant biological function. We found that KYSE150 cells had very low CD133 expression compared with KYSE270 cells, and that there was marked decrease of CD133 expression in KYSE270-miR-377 cells (Figure 3b). This difference suggests that miR-377 can suppress tumor formation by targeting CD133 expression, and may explain the discrepancy of anti-tumor effect of miR-377 between KYSE150 and KYSE270 (Figure 2d). Western blot data showed that transfection of miR-377 mimic or miR-377-expressing plasmid reduced CD133 expression dose dependently in two ESCC cell lines, as well as in colorectal and liver cancer cell lines (Figure 3c and Supplementary Figure S3). Moreover, an increase in CD133 expression in the ESCC cells transfected with anti-miR-377 inhibitor was observed (Figure 3d). Ectopic expression of miR-377 in HEK293 cells resulted in a significant dose-dependent decrease in the relative luciferase activity of CD133 3′-UTR (Figure 3e left panel), but there was no inhibition of luciferase activity when the cells were co-transfected with miR-377 and mutant CD133 3′-UTR (Figure 3e, right panel), thus confirming that miR-377 can directly bind to and repress CD133. Furthermore, we made use of 50 pairs of ESCC tumor and adjacent normal tissues to compare the expressions of CD133 and miR-377. The significantly higher CD133 (Figures 3f and g) and lower miR-377 expression (Figure 3h) in tumor tissues compared with normal esophageal tissue suggest that CD133 has an oncogenic role whereas miR-377 has tumor suppressive function in ESCC. More importantly, the inverse correlation between miR-377 and CD133 expressions in ESCC specimens suggests that a regulatory mechanism between miR-377 and CD133 may have a functional role in tumor development (Figure 3i).
Figure 3.
Direct targeting of CD133 by miR-377 and the inverse correlation of their expression levels in ESCC. (a) Alignment of miR-377 (seed sequence in bold font) and its corresponding complementary binding sequence in CD133 3′-UTR by TargetScan bioinformatics algorithm indicated high evolutionary conservation across different species including human (hsa, Homo sapiens), chimpanzee (ptr, Pan troglodytes), rhesus macaque (mml, Macaca mulatta), and mouse (mmu, Mus musculus). The deletion and mutation sites in CD133 3′-UTR of other species, compared with human, were underlined. (b) Comparison of CD133 expression in miR-377-overexpressing KYSE150 and KYSE270 cells with the respective vector control cells by western blot. (c) Western blot analysis of the KYSE270 and T.Tn cells transfected with indicated doses of miR-377 mimic (left panel) and miR-377-expressing plasmid (right panel), respectively. (d) Effect of anti-miR-377 on CD133 expression in KYSE270 and T.Tn cells. (e) Left panel, HEK293 cells were transfected with pMir-Firefly-CD133 3′-UTR and pRL-TK, along with different concentrations of miR-377 mimic or miR-377-expressing plasmid, and the relative luciferase activity of CD133 3′-UTR was determined by dual-color luciferase assay. Right panel, miR-377 overexpression had no effect on luciferase activity of mutant CD133 3′-UTR. (f and g) CD133 and actin expressions were determined in 50 pairs of ESCC (T) and matched normal (N) tissues by western blot (six representative pairs are shown in f). The blots were quantified using ImageJ and normalized against actin. The expression level of CD133 in primary tumors relative to that of corresponding normal tissue samples are presented in G (n=47 after excluding 3 pairs which had undetectable/lack of CD133 expression in both tumor and normal tissues). (h) miR-377 expression in 50 pairs of esophageal tumor relative to that in matched normal tissues. (i) Significant inverse correlation between miR-377 and CD133 expression levels in 47 pairs of human esophageal tumor and matched normal tissues (r=−0.31, P<0.01). Bars, SD; **P<0.01; ***P<0.001 compared with controls.
CD133+ ESCC cells possess high tumorigenic potential and stem cell-like characteristics
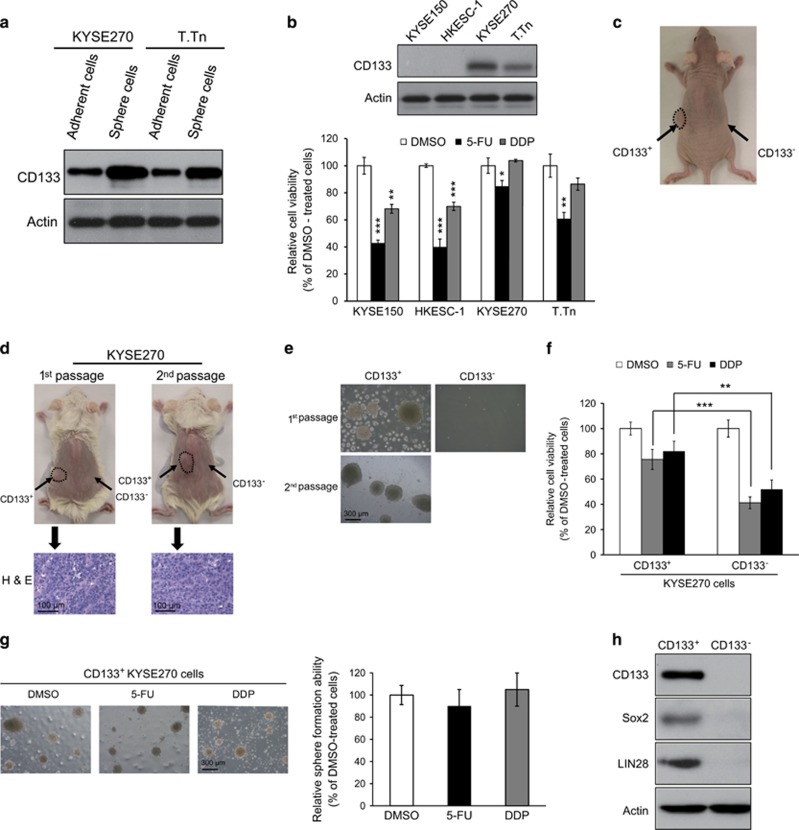
Although CD133 was reported to be a TIC marker in several cancer types,10, 11, 12, 13 the functional role of CD133 in ESCC has not been reported. We investigated the correlation between CD133 expression in ESCC cells and TIC properties. Increased CD133 expression was detected in sphere-forming ESCC cells compared with the differentiated adherent counterparts (Figure 4a). We also found a positive correlation between CD133 expression and chemoresistance, which is another important property of TICs, amongst several ESCC cell lines: the CD133-rich KYSE270 and T.Tn cells were more resistant to 5-FU and DDP, compared with CD133-low KYSE150 and HKESC-1 cells (Figure 4b). To validate that CD133 is a marker for esophageal TICs, CD133+ and CD133− cells were purified from ESCC cell lines and tumor xenografts for further investigation. The purity was generally greater than 95% (Supplementary Figure S4). The sorted CD133+ and CD133− KYSE410 cells were subcutaneously injected into the left and right flanks of nude mice, respectively, for comparison of tumor-initiating ability. Sixty days after cell injection, as few as 5000 CD133+ cells could form tumors in five out of six (83.3%) mice, whereas no tumors (none of six mice, 0%) were formed by ten times as many CD133− cells (Figure 4c and Supplementary Table S2). Higher capacity for tumorigenicity was also found in sorted CD133+ KYSE270 cells in NOD/SCID mice, compared with CD133− cells (Figure 4d and Supplementary Table S3). The ability of CD133+ cells to undergo self-renewal and display long-term tumorigenic potential was determined by in vivo serial transplantation. The primary tumor xenografts derived from CD133+ KYSE270 cells were excised from the NOD/SCID mice, re-sorted into CD133+ and CD133− cells, and re-injected into secondary mouse recipients. Consistently, only CD133+ but not CD133− ESCC cells formed tumors with the same histological morphology as the primary tumors (Figure 4d and Supplementary Table S3), thus providing direct evidence for self-renewal capacity of CD133+ ESCC cells in vivo. Sphere-formation assay showed that CD133+ ESCC cells had greater ability to form spheres, and successful serial propagation of dissociated cells from CD133+ cell-derived spheres further confirmed the self-renewal ability of CD133+ ESCC cells (Figure 4e). With respect to resistance to conventional chemotherapeutic drugs, we found that CD133+ ESCC cells were more resistant to 5-FU and DDP than CD133− cells (Figure 4f). In addition, treatment with the chemotherapeutic drugs did not reduce the size or number of spheres formed by CD133+ ESCC cells (Figure 4g). We also examined whether CD133+ cells preferentially express the stem cell-associated genes crucial for maintenance of stem cell properties, and found higher expression levels of sox2 and LIN28 in CD133+ ESCC cells (Figure 4h). Taken together, these results demonstrated that CD133 is a valid marker of ESCC TICs, and that its expression is regulated by miR-377.
Figure 4.
CD133+ ESCC cells possessed increased abilities to self-renew, initiate tumors, and resist chemotherapeutic drugs. (a) Sphere-forming ESCC cells and adherent counterparts were compared for expressions of CD133 by western blot. (b) KYSE150, HKESC-1, KYSE270 and T.Tn cells with different endogenous CD133 expression levels as determined by western blot (upper panel) were treated with 5-FU (5 μm), DDP (20 μm), or DMSO for 72 h, and cell viability determined by MTT assay (lower panel). (c) Representative example of nude mice 60 days after subcutaneous inoculation of sorted CD133+ (left flank) and CD133− (right flank) KYSE410 cells. See also Supplementary Table S2. (d) About 5000 CD133+ and CD133− cells were sorted from KYSE270-derived tumor xenografts and compared for in vivo tumorigenicity in NOD/SCID mice (left panel). The procedure was repeated for CD133+ and CD133− cells resorted from tumors derived from CD133+ KYSE270 cells (right panel). Hematoxylin and eosin (h and e) stained sections of primary and secondary CD133+ KYSE270-derived tumors showed similar morphology. (e) Comparison of sorted CD133+ and CD133− KYSE270 cells for ability to initiate and form spheres over serial passages. (f) Sorted CD133+ and CD133− KYSE270 cells were treated with 5-FU (10 μm) or DDP (40 μm) for 72 h, and their drug sensitivity compared using MTT assay. (g) CD133+ KYSE270 tumor spheres were resistant to 5-FU (10 μm) and DDP (40 μm) treatments. (h) Western blot analysis of CD133, sox2 and LIN28 in sorted CD133+ and CD133- KYSE270 cells. Actin was used as internal control. Bars, s.d.; **P<0.01; ***P<0.001.
CD133 silencing suppresses TIC properties in ESCC cells
To investigate whether CD133 is functionally crucial for maintenance of TIC properties, CD133-knockdown experiment was performed with lentiviral-based short hairpin RNA (shRNA) against CD133 (shCD133) in KYSE270 cells, which had high CD133 expression, to examine the effects on tumorigenic potential and stem cell properties. We found that CD133-knockdown reduced the expression levels of sox2 and LIN28, compared with the scrambled shRNA control (shCON) (Supplementary Figure S5a). In vivo assay showed that at a cell dose of 2 × 104, only two out of six (33.3%) mice injected with KYSE270-shCD133 cells developed tumor whereas KYSE270-shCON cells produced tumors in all of the six nude mice (100%) examined (Supplementary Figure S5b and Supplementary Table S4). In addition, inoculation of resuspended cancer cells from excised primary xenografts into secondary mouse recipients produced similar results (Supplementary Table S4). When a larger number of cells (1 × 105) were injected, both KYSE270-shCD133 and KYSE270-shCON cell lines formed tumors in all the mice in the respective groups (n=6), but the KYSE270-shCD133 tumors were significantly smaller (Supplementary Figure S5c) and with decreased expression levels of Sox2 and LIN28 (Supplementary Figure S5d). The suppressive effect of CD133 repression on self-renewal ability was further confirmed by the lower serial propagation capacity of KYSE270-shCD133 cells under sphere-forming culture conditions (Supplementary Figure S5e). We also found that CD133-knockdown significantly enhanced the sensitivity of esophageal tumor xenografts to 5-FU and DDP in vivo (Supplementary Figure S5f). Therefore, the above findings indicated that CD133 functionally contributes to tumor initiation and self-renewal capacity, as well as chemoresistance of ESCC cells.
miR-377 inhibits tumor angiogenesis by directly targeting VEGF
Since KYSE150 cells did not express CD133, the relatively modest suppressive effect of miR-377 on growth of KYSE150-derived tumor xenografts (Figure 2d) was likely to be due to mechanisms other than tumor initiation. Interestingly, histologic comparison of the tumor xenografts demonstrated significantly lower microvessel density (MVD), as determined by CD31-immunostaining, in the miR-377-overexpressing tumor xenografts, compared with tumors that expressed control vector, suggesting that miR-377 may affect tumor angiogenesis (Figure 5a). Moreover, conditioned medium (CM) from ESCC cells expressing miR-377 remarkably reduced the tube formation and migration ability of human umbilical vein endothelial cells (HUVECs) while knockdown of miR-377 in ESCC resulted in increased tube formation and migration of HUVECs (Figures 5b and c). In the mouse model, we also observed a reduction of VEGF in the tumor xenografts established by ESCC cells expressing miR-377, compared with vector control (Figure 5d). In silico analysis showed possible direct binding between miR-377 and 3′-UTR of VEGF (Figure 5e). Gain- and loss-of-function experiments using ESCC cell lines transiently transfected with miR-377 mimic/plasmid or inhibitor confirmed the inverse regulation of VEGF by miR-377 (Supplementary Figures S6a–b). ESCC cell lines that stably overexpressed miR-377 showed downregulation of VEGF in both cell lysates and CM, as determined using western blot and Enzyme-Linked Immunosorbent Assay (ELISA), respectively (Figure 5f). In the luciferase reporter assay, overexpression of miR-377 led to a dose-dependent decrease in VEGF 3′-UTR reporter expression (Figure 5g, left panel), but did not exert repressive effect on luciferase activity of mutant VEGF 3′-UTR (Figure 5g, right panel), indicating that miR-377 can directly bind to the 3′-UTR of VEGF. Analysis of 50 primary ESCC tumors and matched normal esophageal tissue samples showed upregulation of VEGF in the majority of tumor samples, and an inverse correlation between miR-377 and VEGF expressions (Figure 5h).
Figure 5.
miR-377 inhibited tumor angiogenesis by directly targeting VEGF. (a) Tumor xenografts established from KYSE150-miR-CON and KYSE150-miR-377 ESCC cells were immunostained for CD31 and compared for microvessel density (MVD). (b) Effect of conditioned medium (CM) from ESCC cells with miR-377 overexpression or knockdown on the tube-formation activity of HUVECs. (c) Effect of conditioned medium from ESCC cells with manipulated miR-377 expression on migration of HUVECs. (d) Western blot showing the expression of VEGF in tumor xenografts established from KYSE150-miR-CON and KYSE150-miR-377 cells. (e) Base pairing between miR-377 and 3′-UTR of mammalian VEGF (hsa, Homo sapiens; ptr, Pan troglodytes; mml, Macaca mulatta; eca, Equine canibus; bta, Bos taurus; mmu, Mus musculus) by TargetScan bioinformatics algorithms. (f) VEGF expression level in cell lysates and conditioned media of ESCC cells with stable expression of miR-377, determined using western blot and ELISA respectively, was compared with that of corresponding vector controls. (g) Left panel, dose-dependent effects of miR-377 plasmids and miR-377 mimic on luciferase activity in HEK293 cells. Right panel, luciferase activity in HEK293 cells co-transfected with miR-377 and wild type or mutant VEGF 3′-UTR luciferase reporter plasmids as indicated. (h) Expression levels of VEGF in 50 pairs of primary ESCC and matched normal tissues (left and middle panels); inverse correlation was found between VEGF and miR-377 expression levels (right panel). Bars, s.d.; *P<0.05; **P<0.01; ***P<0.001.
CD133 and VEGF mediate the effects of miR-377 on cancer stemness and angiogenesis
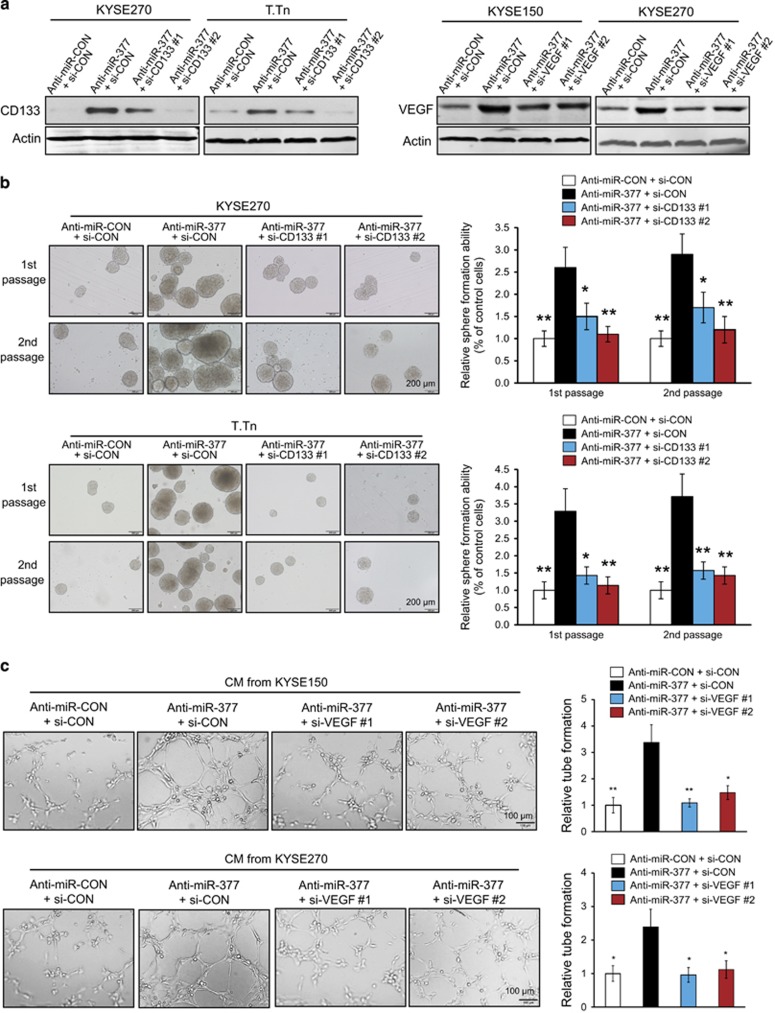
To examine the importance of CD133 and VEGF in the functional roles of miR-377 in cancer stemness and angiogenesis, the expression levels of CD133 and VEGF in the miR-377-knockdown cells were inhibited with the corresponding siRNAs (Figure 6a). Sphere-formation and tube-formation assays were then performed to determine cancer stemness and in vitro angiogenesis properties. Consistent with the findings in Figure 2c, increased sphere-forming ability was observed in the miR-377-knockdown cells, and this effect was markedly abrogated upon CD133 inhibition (Figure 6b). Moreover, our results showed that knockdown of VEGF significantly attenuated the promoting effect of miR-377 inhibition on tube-formation ability in KYSE270 and T.Tn cells (Figure 6c). Collectively, our data indicated that CD133 and VEGF are crucial for the regulatory functions of miR-377 in cancer stemness and angiogenesis.
Figure 6.
Knockdown of CD133 and VEGF abolished the promoting effects of anti-miR-377 on cancer stemness and angiogenesis. (a) The increased expression levels of CD133 and VEGF in miR-377-knockdown ESCC cells were inhibited by the siRNAs against CD133 and VEGF, respectively. (b) The miR-377-knockdown ESCC cells transfected with si-CON or two different siRNAs against CD133 were compared for ability to form spheres over serial passages. (c) Effects of conditioned medium (CM) from ESCC cells with the indicated manipulation of miR-377 and VEGF expressions on the tube-formation activity of HUVECs. Bars, s.d.; *P<0.05; **P<0.01.
miR-377 inhibits invasion and metastastic colonization of ESCC cells
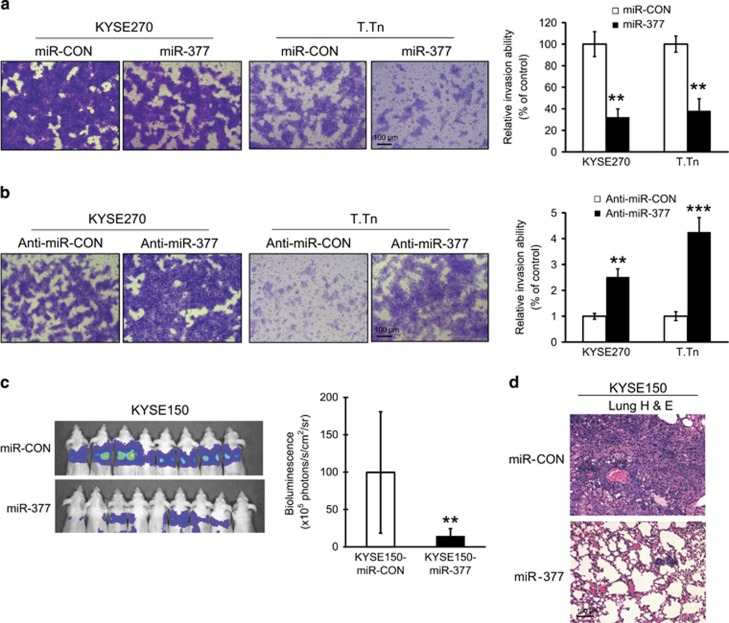
We investigated the effect of miR-377 on invasion of ESCC cells using invasion chamber assay. The results showed that overexpression of miR-377 dramatically decreased, whereas downregulation of miR-377 increased, the invasion potential of KYSE270 and T.Tn cells (Figures 7a and b). Next, KYSE150-luc cells stably expressing miR-377 were introduced intravenously into the tail vein of mice for experimental metastasis assay. Lung metastasis was evaluated and analyzed using bioluminescence imaging. As showed in Figures 7c and d, overexpression of miR-377 strongly and significantly suppressed lung metastasis. Taken together, these data suggest that miR-377 plays a role in inhibiting tumor invasion and metastasis.
Figure 7.
Overexpression of miR-377 reduced the invasion and metastasis of ESCC cells in vitro and in vivo. (a) Comparison of invasive ability between ESCC cells expressing miR-377 and vector control using Boyden chamber assay. (b) Effect of miR-377 inhibition on invasive potential of ESCC cells. (c) Bioluminescence imaging and quantification of lung metastasis in mice which were intravenously injected with KYSE150-miR-377 cells (n=9) or vector control cells (n=8/group). (d) Representative images of H&E stained lung sections. Bars, s.d.; **P<0.01; ***P<0.001.
Systemically delivered miR-377 mimic suppresses tumor growth and metastasis
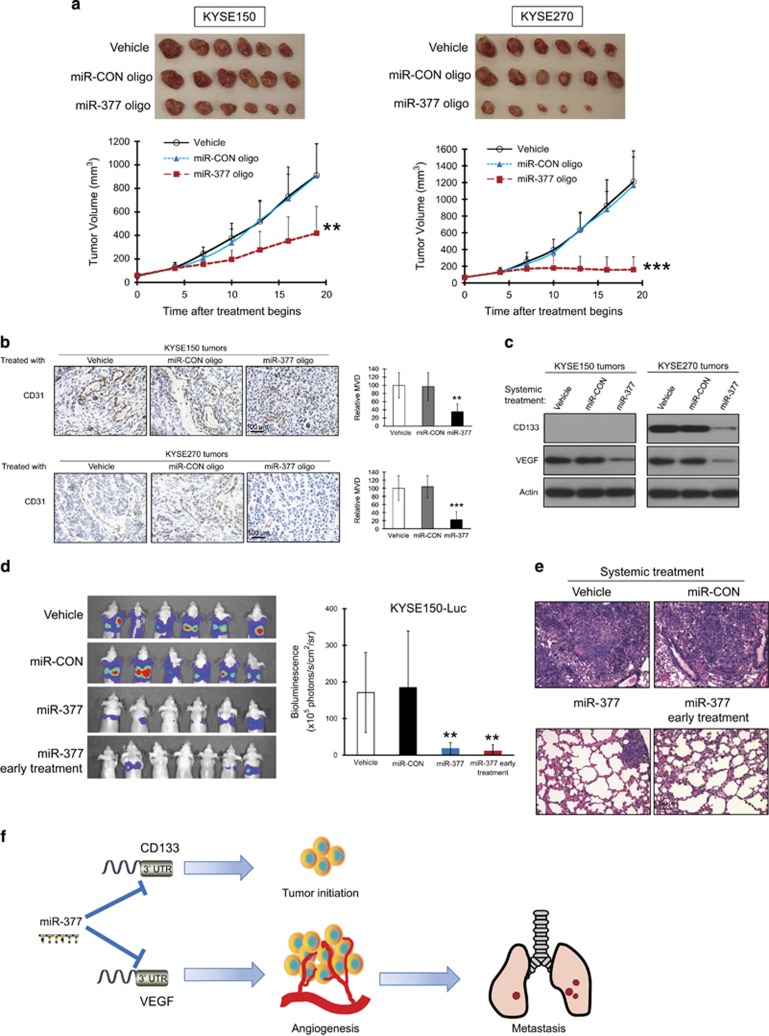
Given that VEGF-mediated angiogenesis and TICs are crucial in tumor development and maintenance, we explored the therapeutic efficacy of miR-377 using mouse models. miR-377 oligonucleotide was injected intravenously into nude mice bearing established subcutaneous tumor xenografts and the effect on tumor growth was monitored. The results showed that systemic delivery of miR-377 oligonucleotide resulted in a significant decrease in tumor burden as compared with treatment with miR-CON or vehicle (Figure 8a). The anti-tumor effect of systemically delivered miR-377 was more pronounced for KYSE270 than KYSE150 xenografts, which was consistent with that of ectopic miR-377 expression (Figure 2d). In addition, significant reduction in MVD was observed in the tumor xenografts of miR-377-treated mice (Figure 8b). Systemic miR-377 treatment reduced VEGF expression level in KYSE150 and KYSE270 tumors, as well as CD133 level in the CD133+ KYSE270 tumors (Figure 8c). Regular monitoring of body weight of mice showed that there was no obvious difference among all the groups (Supplementary Figure S7a), and histological examination of the vital organs including lungs, liver and kidneys did not reveal any overt changes in morphology (Supplementary Figure S7b), suggesting that the miR-377 treatment and the vehicle used for systemic delivery of miRNA had no toxic effects. In the experimental metastasis study, treatment with miR-377 led to significant decrease in metastatic burden in the lungs (Figures 8d and e).
Figure 8.
Systemic delivery of miR-377 oligonucleotide suppressed tumor growth, angiogenesis and metastasis in nude mice. (a–c) ESCC cells were subcutaneously injected into mice to establish tumor xenograft. Two weeks later when tumor reached ~0.5 cm diameter, the mice were randomized into three different treatment groups to receive weekly intravenous injections of 40 μg miR-377 oligonucleotide, miR-CON oligonucleotide and vehicle, respectively. (a) Images of tumors (excised 5 weeks after cell implantation) and tumor curves showing the inhibitory effect of miR-377 oligonucleotide on growth of KYSE150 (left panel) and KYSE270 (right panel)-derived tumor xenografts (n=6/group). (b) Quantification of CD31-MVD in the tumors of mice treated with miR-377 oligonucleotide, miR-CON oligonuceotide or vehicle. (c) Western blot showing the expression of CD133 and VEGF in ESCC xenografts. (d and e) KYSE150-luc cells were injected into nude mice via the tail vein and randomized into four groups. Three groups (n=6/group) were treated once every two weeks with intravenous injections of 20 μg miR-377 oligonucleotide, miR-CON oligonucleotide and vehicle, respectively, commencing one week after injection of cancer cells. The 4th group (n=7) received an additional dose of 20 μg miR-377 one day after injection of cancer cells. (d) Bioluminescence imaging and quantification of lung metastasis. (e) Histological evaluation of lung metastasis (H&E staining). (f) Schematic diagram summarizing how miR-377 suppresses tumor initiation and progression of esophageal cancer by inhibiting CD133 and VEGF. Bars, s.d.; **P<0.01; ***P<0.001.
Discussion
In this study, we have uncovered that miR-377 plays an important role in the initiation and progression of ESCC (Figure 8f). We have provided the first evidence that miR-377 expression is frequently downregulated in tumor and serum of patients with ESCC, and that lower miR-377 expression in primary tumor significantly correlates with unfavorable clinicopathological characteristics and shorter survival of patients, which strongly suggest that miR-377 may be a useful non-invasive diagnostic and prognostic biomarker. Moreover, low miR-377 concentration in admission serum may be a predictive marker for poor response to chemoradiotherapy. Deciphering the role of miR-377 in suppressing tumorigenesis not only has great mechanistic and functional significance in the study of miRNA function in human cancer, but may also facilitate development of new treatment strategies.
According to the cancer stem cell hypothesis, tumor cells are heterogeneous and only a minority of cells with self-renewal ability within a tumor (that is, TICs) is responsible for tumor initiation and maintenance.10, 21 However, identification of valid TIC markers and development of new therapies that can selectively target esophageal TICs lag behind other cancers. The ability of miRNAs to regulate stem cell fate and differentiation by attenuating the protein levels of various factors that are required for stem cell functions opens a new dimension of miRNA in regulation of TIC functions.22, 23, 24, 25 Our study was prompted by the discrepancy in the anti-tumorigenic response of KYSE270 and KYSE150 cells to ectopic miR-377 expression, with the former cell line showing complete abolition of tumorigenicity in nude mice but only a modest effect was observed in the latter (Figure 2d). We sought to identify possible gene effector(s) participating in this function which may account for this functional difference. Because our findings also showed that higher miR-377 was associated with better response of the tumors to chemoradiotherapy (Table 1), we hypothesized that miR-377 may play an important role in controlling self-renewal and chemoresistance in TICs. In silico analysis identified CD133 (Figure 3a), but not CD90 or CD44 which were previously reported as potential markers for identifying TICs,26, 27 as a possible target of miR-377, leading us to investigate whether miR-377 may suppress TIC properties through direct targeting of CD133. Here, we report for the first time that CD133 is a functional and targetable TIC marker for esophageal cancer (Figure 4). Moreover, we identified miR-377 as a direct regulator of CD133 based on several lines of evidence. First, the miR-377 binding site in the 3′-UTR of CD133 is highly conserved among mammals. Second, miR-377 was downregulated whereas CD133 was upregulated in the sphere-forming cancer cells. Third, overexpression and knockdown of miR-377 in cancer cells decreased and increased CD133 expression, respectively, in transient and stable expression experiments. Fourth, miR-377 could directly bind to the 3′-UTR of CD133 and repress its luciferase activity. Lastly and more importantly, the expressions of miR-377 and CD133 were inversely correlated in ESCC. CD133 had been shown to promote TIC or malignant phenotypes by increasing the expression of ABCB5, or activating AKT, IL-8 and MAPK/ERK pathways.28, 29, 30, 31 It was also reported that CD133 silencing can induce cancer cell cycle arrest by inhibiting expression of cytokinesis-related genes.32 Whether these or other novel mechanisms are operative in CD133+ ESCC cells remains to be explored. Likewise, whether CD133 has any advantage compared with CD44 or CD90 as a TIC marker for ESCC, and whether a combination of cell surface markers should be used to identify esophageal TICs merit further investigations.
Sustained angiogenesis is an important hallmark of cancer.33 We found that overexpression of miR-377 could suppress tumor angiogenesis (Figure 5). This was likely to be attributed to the inhibitory effect of miR-377 on VEGF, which plays a predominant role in tumor angiogenesis.34 Our results showed direct binding of miR-377 to the 3′-UTR of VEGF, and a negative correlation between miR-377 and VEGF expressions in ESCC tissue samples. Angiogenesis is required for invasive tumor growth and metastasis.35 More than 90% of mortality from cancer is due to metastasis.36, 37 We investigated the role of miR-377 in inhibiting cancer cell invasion and distant metastasis, and found that overexpression of miR-377 decreased, whereas knockdown of miR-377 increased, the invasiveness of CD133+ ESCC cells (Figures 7a and b). Furthermore, our in vivo experiment showed that ectopic expression of miR-377 inhibited metastatic colonization of KYSE150 cells despite their lack of CD133 expression (Figures 7c and d). This phenomenon might be due to the direct suppressive effect of miR-377 on VEGF in cancer cells. As mentioned in our recent review,15 VEGF plays a predominant role in angiogenesis by binding to VEGF receptors. We previously reported the significance of VEGFR1- and VEGFR2-expressing non-tumor cells in esophageal tumor angiogenesis and metastasis.38 It is possible that by targeting VEGF, miR-377 may impact on the function of VEGF-responsive non-tumor cells and deter the establishment of pre-metastatic niches at distant sites.
There is increasing recognition that conventional treatment alone is insufficient in cancer therapy and there is an urgent need to develop new therapeutic strategies. Accumulating evidence supports the importance of miRNAs in human cancer, thus making miRNA-based cancer therapy a promising strategy. Since most target tissues or organs cannot be accessed by local administration of miRNA, systemic administration is the most widely accepted use of miRNA in the clinic. However, unmodified, naked miRNAs are relatively unstable in blood and serum, and are rapidly degraded by endo- and exonucleases. Virus-mediated gene delivery, on the other hand, may run the risks of mutagenesis due to integration of vector genome into host chromosomes39, 40 and possible toxic immune responses.41, 42 Combination of miRNA with other forefront technologies including nanoparticles, polyethylenimine, liposomes and lipid, has been developed for effective siRNA/miRNA delivery without detectable inflammatory response or toxic effects.43 For example, a recent study suggests that treatment with miR-200 members into the tumor endothelium resulted in marked reductions in metastasis and angiogenesis.44 Since miR-377 targets both CD133 and VEGF, and since systemic delivery of synthetic mimics of miR-377 had significant efficacy in suppressing tumor growth, tumor angiogenesis as well as metastatic spread (Figure 8), these findings underpin the potential of systemic miR-377 therapy as a promising strategy in the treatment of ESCC and in prevention of cancer metastasis.
Materials and methods
Cell lines and drugs
Human ESCC cell lines KYSE30, KYSE70, KYSE150, KYSE270, KYSE410 (DSMZ, Braunschweig, Germany),45 HKESC-146 and T.Tn47 were maintained in RPMI 1640 (Sigma, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS, Invitrogen, Gaithersburg, MD, USA) at 37 °C in 5% CO2. HEK293 cells (ATCC) were maintained in DMEM (Sigma) supplemented with 10% FBS. The cell lines were authenticated by short tandem repeat profiling, and tested for mycoplasma contamination. 5-Fluorouracil (5-FU) and cisplatin (DDP), purchased from Calbiochem (Darmstadt, Germany), were dissolved in dimethyl sulfoxide (DMSO).
ESCC tissue and serum samples
Fresh human ESCC and the corresponding adjacent non-tumorous esophageal samples were collected with informed consent from 50 patients who were treated with surgical resection alone between 2011 and 2012 at the Queen Mary Hospital (Hong Kong) and at the First Affiliated Hospital, Zhengzhou University (Zhengzhou, China) from 2008 to 2010. In addition, pairs of primary ESCC and matched normal tissue samples were obtained from an independent cohort of 30 patients with survival data who had surgical resection without pre-operative chemoradiotherapy at Queen Mary Hospital. All tissue specimens were snap-frozen immediately in liquid nitrogen and stored at -80ºC. Admission serum of 114 ESCC patients who subsequently received neoadjuvant chemoradiotherapy (with 5-FU and cisplatin) and serum samples from 50 healthy individuals were obtained from Queen Mary Hospital and Shantou University Medical College (Shantou, China) respectively. The percentage of residual viable cells48 in the primary tumor of the 114 ESCC patients (dichotomized into high and low with median value as cutoff value) was used as a parameter to evaluate the validity of serum miR-377 as a predictive marker for response to chemoradiotherapy. Use of all human samples was approved by the committees for ethical review of research involving human subjects at the Queen Mary Hospital, Zhengzhou University and Shantou University.
In vitro MTT, sphere formation, cell migration and invasion, endothelial tube formation, western blot, quantitative real-time PCR, immunohistochemical staining, luciferase reporter and site-directed mutagenesis
MTT, migration and invasion assays as well as immunohistochemical staining of endothelial cells using CD31 antibody (#sc-1506; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and evaluation of microvessel density (MVD) were carried out as described previously.49 The investigators were blinded to sample identity in the assessment of MVD in tumor xenograft sections. Preparation of cell and tumor lysates, and details of western blotting were described previously.50 QuikChange Lightning Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA, USA) was used to generate mutant constructs for CD133 3′-UTR and VEGF 3′-UTR. Firefly and Renilla luciferase activities were measured 48 h after transfection using Dual-Luciferase Reporter System (Promega) according to the manufacturer's instructions. More detailed experimental procedures can be found in the Supplementary Materials and Methods.
Tumorigenicity in nude mice and NOD/SCID mice and experimental metastasis model
Female BALB/c nude mice and male NOD/SCID mice aged 6–8 weeks were maintained under standard conditions and cared for according to the institutional guidelines for animal care. All the animal experiments were approved by the Committee on the Use of Live Animals in Teaching and Research of the University of Hong Kong. All animals were sex- and age-matched in the animal experiments, and littermates were used. The investigators were not blinded to the experimental groups. The experiment was performed as previously described.51
In vivo delivery of miR-377
miR-377 oligonucleotide or miR-CON (GenePharma, Shanghai, China) was formulated with a polymer-based agent (in vivo-jetPEI; Polyplus, Illkirch, France)52, 53, 54 according to manufacturer’s instructions and injected intravenously into mice.
Statistical analysis
The data were expressed as mean±SD. The expression level of miR-377 in tumor and serum samples was compared with that in normal tissues and healthy control serum, respectively, using t-test. Sample size in animal experiments was chosen on the basis of literature documentation of similar well-characterized experiments, and no statistical method was used to predetermine sample size. Correlations between miR-377 and CD133/VEGF expressions, and between miR-377 expression and clinical-pathological parameters, were assessed using Pearson’s rank correlation coefficient (two-tailed). The association between miR-377 expression level and patient survival was plotted using the Kaplan–Meier method, and statistical differences were compared using the log-rank test. P-values <0.05 were deemed significant. All in vitro experiments and assays were repeated at least three times.
Acknowledgments
We thank Professor Yutaka Shimada (University of Toyama, Toyama, Japan) and DSMZ for the KYSE cell lines; Prof G Srivastava, Department of Pathology, University of Hong Kong for the HKESC-1 cell line; Dr Hitoshi Kawamata (Dokkyo University School of Medicine, Tochigi, Japan) for the T.Tn cell line; and Mr. Yiwei Xu (Shantou University Medical College, Shantou, Guangdong, China) for collection of patient serum. We also thank Prof Quan Chen (Institute of Zoology, Chinese Academy of Sciences, Beijing, China) for CD133-knockdown and control plasmids as well as virus packaging plasmids, Prof Gregory M. Vercellotti (University of Minnesota Medical School, Minneapolis, MN) for miR-377-expressing constructs, and Dr Anna CM Tsang (School of Biomedical Sciences, University of Hong Kong) for help in flow cytometry. Flow cytometry and cell sorting were performed and analyzed using equipment maintained by the University of Hong Kong Li Ka Shing Faculty of Medicine Faculty Core Facility. This work recieves Grant Support from Research Grants Council of the Hong Kong SAR, China (GRF Project Nos. HKU 763111M and HKU 17103814); The University of Hong Kong SRT Cancer research program and CRCG (Project Nos. 201111159198, 201211159003); National Natural Science Foundation of China (Project No. 81472790). The study sponsors are not involved in study design, or collection, analysis, interpretation of data.
Footnotes
Supplementary Information accompanies this paper on the Oncogene website (http://www.nature.com/onc)
The authors declare no conflict of interest.
Supplementary Material
References
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127: 2893–2917. [DOI] [PubMed] [Google Scholar]
- Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet 2013; 381: 400–412. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009; 136: 215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura A, Jacks T. MicroRNAs and cancer: short RNAs go a long way. Cell 2009; 136: 586–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, Meltzer SJ. MicroRNAs in pathogenesis, diagnosis, and treatment of gastroesophageal cancers. Gastroenterology 2012; 143: 35–47. [DOI] [PubMed] [Google Scholar]
- Fang YX, Gao WQ. Roles of microRNAs during prostatic tumorigenesis and tumor progression. Oncogene 2014; 33: 135–147. [DOI] [PubMed] [Google Scholar]
- Kohlhapp FJ, Mitra AK, Lengyel E, Peter ME. MicroRNAs as mediators and communicators between cancer cells and the tumor microenvironment. Oncogene 2015; 34: 5857–5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara Y, Kato Y, Bando T, Yamagishi F, Minamimura T, Sakamoto T et al. Allelic imbalance of 14q32 in esophageal carcinoma. Cancer Genet Cytogenet 2002; 135: 177–181. [DOI] [PubMed] [Google Scholar]
- Haller F, von HA, Zhang JD, Gunawan B, Langer C, Ramadori G et al. Localization- and mutation-dependent microRNA (miRNA) expression signatures in gastrointestinal stromal tumours (GISTs), with a cluster of co-expressed miRNAs located at 14q32.31. J Pathol 2010; 220: 71–86. [DOI] [PubMed] [Google Scholar]
- Valent P, Bonnet D, De MR, Lapidot T, Copland M, Melo JV et al. Cancer stem cell definitions and terminology: the devil is in the details. Nat Rev Cancer 2012; 12: 767–775. [DOI] [PubMed] [Google Scholar]
- Hang D, Dong HC, Ning T, Dong B, Hou DL, Xu WG. Prognostic value of the stem cell markers CD133 and ABCG2 expression in esophageal squamous cell carcinoma. Dis Esophagus 2012; 25: 638–644. [DOI] [PubMed] [Google Scholar]
- Nakajima TE, Yoshida H, Okamoto N, Nagashima K, Taniguchi H, Yamada Y et al. Nucleostemin and TWIST as predictive markers for recurrence after neoadjuvant chemotherapy for esophageal carcinoma. Cancer Sci 2012; 103: 233–238. [DOI] [PubMed] [Google Scholar]
- Lu C, Xu F, Gu J, Yuan Y, Zhao G, Yu X et al. Clinical and biological significance of stem-like CD133(+)CXCR4(+) cells in esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg 2015; 150: 386–395. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Ninomiya I, Ohbatake Y, Hirose A, Tsukada T, Nakanuma S et al. Expression status of CD44 and CD133 as a prognostic marker in esophageal squamous cell carcinoma treated with neoadjuvant chemotherapy followed by radical esophagectomy. Oncol Rep 2016; 36: 3333–3342. [DOI] [PubMed] [Google Scholar]
- Xu WW, Li B, Cheung AL. The potential of targeted antiangiogenesis therapies in the treatment of esophageal cancer. Gastrointest Cancer: Targets & Therapy 2015; 5: 79–88. [Google Scholar]
- Chen M, Cai E, Huang J, Yu P, Li K. Prognostic value of vascular endothelial growth factor expression in patients with esophageal cancer: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2012; 21: 1126–1134. [DOI] [PubMed] [Google Scholar]
- Peng J, Shao N, Peng H, Chen LQ. Prognostic significance of vascular endothelial growth factor expression in esophageal carcinoma: a meta-analysis. J BUON 2013; 18: 398–406. [PubMed] [Google Scholar]
- Uchino K, Ochiya T, Takeshita F. RNAi therapeutics and applications of microRNAs in cancer treatment. Jpn J Clin Oncol 2013; 43: 596–607. [DOI] [PubMed] [Google Scholar]
- Trang P, Weidhaas JB, Slack FJ. MicroRNAs as potential cancer therapeutics. Oncogene 2008; 27(Suppl 2): S52–S57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim AF, Weirauch U, Thomas M, Grunweller A, Hartmann RK, Aigner A. MicroRNA replacement therapy for miR-145 and miR-33a is efficacious in a model of colon carcinoma. Cancer Res 2011; 71: 5214–5224. [DOI] [PubMed] [Google Scholar]
- Tirino V, Desiderio V, Paino F, De RA, Papaccio F, La NM et al. Cancer stem cells in solid tumors: an overview and new approaches for their isolation and characterization. FASEB J 2013; 27: 13–24. [DOI] [PubMed] [Google Scholar]
- Gangaraju VK, Lin H. MicroRNAs: key regulators of stem cells. Nat Rev Mol Cell Biol 2009; 10: 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceppi P, Peter ME. MicroRNAs regulate both epithelial-to-mesenchymal transition and cancer stem cells. Oncogene 2014; 33: 269–278. [DOI] [PubMed] [Google Scholar]
- Lu J, Song G, Tang Q, Yin J, Zou C, Zhao Z et al. MiR-26a inhibits stem cell-like phenotype and tumor growth of osteosarcoma by targeting Jagged1. Oncogene 2016; 36: 231–241. [DOI] [PubMed] [Google Scholar]
- Sun X, Jiao X, Pestell TG, Fan C, Qin S, Mirabelli E et al. MicroRNAs and cancer stem cells: the sword and the shield. Oncogene 2014; 33: 4967–4977. [DOI] [PubMed] [Google Scholar]
- Tang KH, Dai YD, Tong M, Chan YP, Kwan PS, Fu L et al. A CD90+ tumor-initiating cell population with an aggressive signature and metastatic capacity in esophageal cancer. Cancer Res 2013; 73: 2322–2332. [DOI] [PubMed] [Google Scholar]
- Zhao JS, Li WJ, Ge D, Zhang PJ, Li JJ, Lu CL et al. Tumor initiating cells in esophageal squamous cell carcinomas express high levels of CD44. PLoS One 2011; 6: e21419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Khattouti A, Sheehan NT, Monico J, Drummond HA, Haikel Y, Brodell RT et al. CD133(+) melanoma subpopulation acquired resistance to caffeic acid phenethyl ester-induced apoptosis is attributed to the elevated expression of ABCB5: significance for melanoma treatment. Cancer Lett 2015; 357: 83–104. [DOI] [PubMed] [Google Scholar]
- Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene 2008; 27: 1749–1758. [DOI] [PubMed] [Google Scholar]
- Tang KH, Ma S, Lee TK, Chan YP, Kwan PS, Tong CM et al. CD133(+) liver tumor-initiating cells promote tumor angiogenesis, growth, and self-renewal through neurotensin/interleukin-8/CXCL1 signaling. Hepatology 2012; 55: 807–820. [DOI] [PubMed] [Google Scholar]
- Dong L, Qi N, Ge RM, Cao CL, Lan F, Shen L. Overexpression of CD133 promotes the phosphorylation of Erk in U87MG human glioblastoma cells. Neurosci Lett 2010; 484: 210–214. [DOI] [PubMed] [Google Scholar]
- Won C, Kim BH, Yi EH, Choi KJ, Kim EK, Jeong JM et al. Signal transducer and activator of transcription 3-mediated CD133 up-regulation contributes to promotion of hepatocellular carcinoma. Hepatology 2015; 62: 1160–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol 2002; 29: 10–14. [DOI] [PubMed] [Google Scholar]
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol 2002; 29: 15–18. [DOI] [PubMed] [Google Scholar]
- Gupta GP, Massague J. Cancer metastasis: building a framework. Cell 2006; 127: 679–695. [DOI] [PubMed] [Google Scholar]
- Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med 2006; 12: 895–904. [DOI] [PubMed] [Google Scholar]
- Xu WW, Li B, Lam AK, Tsao SW, Law SY, Chan KW et al. Targeting VEGFR1- and VEGFR2-expressing non-tumor cells is essential for esophageal cancer therapy. Oncotarget 2015; 6: 1790–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai H, Montini E, Fuess S, Storm TA, Grompe M, Kay MA. AAV serotype 2 vectors preferentially integrate into active genes in mice. Nat Genet 2003; 34: 297–302. [DOI] [PubMed] [Google Scholar]
- Anson DS. The use of retroviral vectors for gene therapy-what are the risks? A review of retroviral pathogenesis and its relevance to retroviral vector-mediated gene delivery. Genet Vaccines Ther 2004; 2: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaiss AK, Liu Q, Bowen GP, Wong NC, Bartlett JS, Muruve DA. Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. J Virol 2002; 76: 4580–4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jooss K, Chirmule N. Immunity to adenovirus and adeno-associated viral vectors: implications for gene therapy. Gene Ther 2003; 10: 955–963. [DOI] [PubMed] [Google Scholar]
- Chen Y, Gao DY, Huang L. In vivo delivery of miRNAs for cancer therapy: challenges and strategies. Adv Drug Deliv Rev 2015; 81: 128–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecot CV, Rupaimoole R, Yang D, Akbani R, Ivan C, Lu C et al. Tumour angiogenesis regulation by the miR-200 family. Nat Commun 2013; 4: 2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada Y, Imamura M, Wagata T, Yamaguchi N, Tobe T. Characterization of 21 newly established esophageal cancer cell lines. Cancer 1992; 69: 277–284. [DOI] [PubMed] [Google Scholar]
- Hu Y, Lam KY, Wan TS, Fang W, Ma ES, Chan LC et al. Establishment and characterization of HKESC-1, a new cancer cell line from human esophageal squamous cell carcinoma. Cancer Genet Cytogenet 2000; 118: 112–120. [DOI] [PubMed] [Google Scholar]
- Kawamata H, Furihata T, Omotehara F, Sakai T, Horiuchi H, Shinagawa Y et al. Identification of genes differentially expressed in a newly isolated human metastasizing esophageal cancer cell line, T.Tn-AT1, by cDNA microarray. Cancer Sci 2003; 94: 699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong DK, Law S, Kwong DL, Chan KW, Lam AK, Wong KH. Histological regression of squamous esophageal carcinoma assessed by percentage of residual viable cells after neoadjuvant chemoradiation is an important prognostic factor. Ann Surg Oncol 2010; 17: 2184–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Li YY, Tsao SW, Cheung AL. Targeting NF-kappaB signaling pathway suppresses tumor growth, angiogenesis, and metastasis of human esophageal cancer. Mol Cancer Ther 2009; 8: 2635–2644. [DOI] [PubMed] [Google Scholar]
- Li B, Cheung PY, Wang X, Tsao SW, Ling MT, Wong YC et al. Id-1 activation of PI3K/Akt/NFkappaB signaling pathway and its significance in promoting survival of esophageal cancer cells. Carcinogenesis 2007; 28: 2313–2320. [DOI] [PubMed] [Google Scholar]
- Li B, Tsao SW, Chan KW, Ludwig DL, Novosyadlyy R, Li YY et al. Id1-induced IGF-II and its autocrine/endocrine promotion of esophageal cancer progression and chemoresistance—implications for IGF-II and IGF-IR-targeted therapy. Clin Cancer Res 2014; 20: 2651–2662. [DOI] [PubMed] [Google Scholar]
- Campbell M, Hanrahan F, Gobbo OL, Kelly ME, Kiang AS, Humphries MM et al. Targeted suppression of claudin-5 decreases cerebral oedema and improves cognitive outcome following traumatic brain injury. Nat Commun 2012; 3: 849. [DOI] [PubMed] [Google Scholar]
- Ebert G, Poeck H, Lucifora J, Baschuk N, Esser K, Esposito I et al. 5' Triphosphorylated small interfering RNAs control replication of hepatitis B virus and induce an interferon response in human liver cells and mice. Gastroenterology 2011; 141: 696–706. [DOI] [PubMed] [Google Scholar]
- Poeck H, Besch R, Maihoefer C, Renn M, Tormo D, Morskaya SS et al. 5'-Triphosphate-siRNA: turning gene silencing and Rig-I activation against melanoma. Nat Med 2008; 14: 1256–1263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.