Abstract
We conducted a comprehensive and in-depth assessment of body mass index (BMI) or waist circumference (WC) related to blood pressure (BP) and determined whether the association is mediated by the possible potential mediators in the cross-sectional survey of the Harbin Cohort Study on Diet, Nutrition and Chronic Non-communicable Diseases of 7094 participants aged 20–74 years, and validated the significant findings in the US National Health and Nutrition Examination Survey four cross-sectional cohorts (2005–2006, 2007–2008, 2009–2010, and 2011–2012) and the cohort data of the Harbin People’s Health Study (a median of 4.2 follow-up years). We observed that BMI or WC was positively associated with BP (all P-values < 0.0001). Mediation analyses consistently indicated that these associations were mediated mainly by insulin resistance (IR) as measured by the homeostasis model (HOMA-IR), followed by triglyceride (TG) and total cholesterol (TC), and fasting glucose (FG) in the three studies. The proportions via the mediation of insulin/HOMA-IR were 25~40%, TG and TC were 15~20%, and FG was 2~8%, respectively. These findings suggest that the mediators, insulin/insulin resistance, TG, TC, and FG, could be targeted for preventing hypertension among populations who were overweight or obesity.
Introduction
Elevated blood pressure (BP) is a leading cause of the global burden of disease1, 2, and obesity has since long been demonstrated to be associated with increased risk of elevated BP in previous studies3–6. Although activation of the sympathetic nervous system (SNS) or renin-angiotensin-aldosterone system (RAAS) has been observed to be the key mechanism in the pathogenesis of obesity-related hypertension7–10, other possible mechanism in this association remains unclear. Insulin resistance, total cholesterol (TC) or triglyceride (TG) levels has been described in normotensive and hypertensive humans in some studies11, 12, however, no studies investigated whether these factors play a mediation role in the association of body mass index (BMI) or waist circumference (WC) with BP. Further research exploring the potential mechanism relating to these factors would provide more information for the complete elucidation of the effect of BMI or WC on BP, especially which may provide novel targets for avoiding obesity-related hypertension.
Employing a cross-sectional data from the Harbin Cohort Study on Diet, Nutrition and Chronic Non-communicable Diseases (HDNNCDS), we aimed to evaluate the association of concurrently examined the association of BMI (an indicator of overall obesity) and WC (an indicator of abdominal obesity) with BP. Specifically, employing mediation analysis13, 14, we tried to quantify potential mediators of biological significance which may link BMI or WC to BP. Notably, we subsequently systematically validated the findings using independent datasets from the cross-sectional survey of the US National Health and Nutrition Examination Survey (NHANES) and a cohort study of the Harbin People’s Health Study (HPHS).
Results
The analytic procedure is summarized in Fig. 1. Descriptive characteristics of the HDNNCDS, the NHANES, and the HPHS populations at baseline are shown in Table 1.
Figure 1.
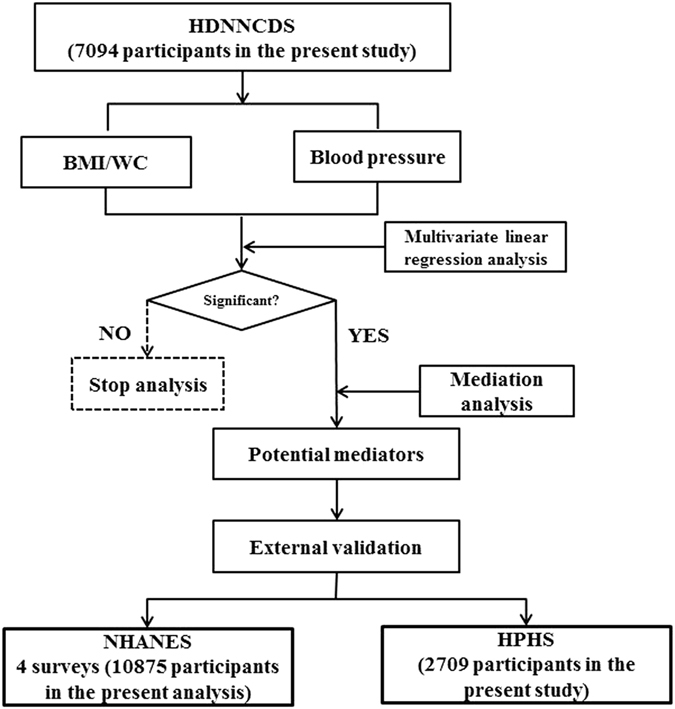
Procedure to systematically associate body mass index or waist circumference with blood pressure by potential mediators.
Table 1.
Descriptive characteristics of participants at baseline in the Harbin Cohort Study on Diet, Nutrition and Chronic Non-Communicable Diseases (HDNNCDS, 2012), the US National Health and Nutrition Examination Survey (NHANES), and the Harbin People’s Health Study (HPHS, 2008–2012).
| Characteristics | HDNNCDS (n = 7,094) | NHANES (n = 10,875) | HPHS (n = 2,709) |
|---|---|---|---|
| Age at recruitment (mean ± SD, years) | 48.88 ± 10.34 | 45.82 ± 15.38 | 48.30 ± 10.46 |
| Male (%) | 35.54 | 19.22 | 30.57 |
| Body mearsurements (mean ± SD) | |||
| BMI (kg/m2) | 24.63 ± 3.42 | 29.02 ± 6.65 | 24.46 ± 3.27 |
| WC (cm) | 84.84 ± 10.11 | 98.53 ± 16.21 | 82.52 ± 9.94 |
| Lifestyle factors (%) | |||
| Exercised regularly | 45.46 | 12.62 | 57.37 |
| Current smokers | 17.31 | 55.05 | 18.31 |
| Current drinker | 35.49 | 74.28 | 38.90 |
| Disease history (%) | |||
| Type 2 diabetes | 13.67 | 10.73 | 11.79 |
| Coronary heart disease | 12.82 | 2.69 | 17.70 |
| Family history of hypertension | 22.26 | — | 21.17 |
| Blood pressure (mean ± SD, mmHg) | |||
| Systolic blood pressure | 131.66 ± 16.74 | 121.78 ± 17.29 | 121.73 ± 9.66 |
| Diastolic blood pressure | 80.28 ± 8.28 | 70.81 ± 12.19 | 77.33 ± 5.09 |
| Total energy intake (mean ± SD, kcal/day) | 2316.47 ± 744.03 | 2069.97 ± 763.24 | 2176.04 ± 699.77 |
| Lipid profiles (mean ± SD) | |||
| TG (mmol/L) | 1.73 ± 1.82 | 1.49 ± 1.21 | 1.68 ± 1.47 |
| TC (mmol/L) | 5.12 ± 1.03 | 5.08 ± 1.07 | 4.87 ± 0.97 |
| HDL (mmol/L) | 1.27 ± 0.33 | 1.36 ± 0.40 | 1.29 ± 0.34 |
| LDL (mmol/L) | 3.01 ± 0.85 | 3.00 ± 0.91 | 2.85 ± 1.00 |
| Glucose (mean ± SD) | |||
| FG (mmol/L) | 4.92 ± 1.65 | 5.87 ± 1.79 | 5.07 ± 2.79 |
| PG (mmol/L) | 6.24 ± 2.86 | 6.43 ± 2.81 | 6.34 ± 3.64 |
| FI (uU/L) | 8.47 ± 7.50 | 13.61 ± 15.17 | 8.37 ± 9.01 |
| PI (uU/L) | 42.68 ± 46.87 | — | 32.83 ± 27.09 |
| HOMA-IR | 1.93 ± 2.36 | 3.75 ± 5.20 | 1.95 ± 3.05 |
| HOMA-B | 268.75 ± 891.83 | 130.80 ± 145.20 | 178.37 ± 242.15 |
Abbreviation: SD, standard deviation; BMI, body mass index; WC, waist circumference; TG, triglyceride; TC, total cholesterol; HDL, high density lipoprotein; LDL, low density lipoprotein; FG, fasting glucose; PG, 2-h postprandial glucose; FI, fasting insulin; PI, 2-h postprandial insulin.
Associations between BMI or WC and BP
After adjusted for potential confounders, BMI or WC was significantly positively associated with systolic BP or diastolic BP in the HDNNCDS, the NHANES, and the HPHS (all P-values < 0.0001) (Table 2).
Table 2.
Adjusted estimated differences in systolic or diastolic blood pressure associated with BMI or WC by 1 SD in the Harbin Cohort Study on Diet, Nutrition and Chronic Non-Communicable Diseases (HDNNCDS, 2012), the Harbin People’s Health Study (HPHS, 2008–2012), and the US National Health and Nutrition Examination Survey (NHANES).
| HDNNCDS (n = 7,094) | NHANES (n = 10,875) | HPHS (n = 2,709) | ||||
|---|---|---|---|---|---|---|
| Difference–mm Hg (95% CI) | P-value | Difference–mm Hg(95% CI) | P-value | Difference–mm Hg(95% CI) | P-value | |
| SBP | ||||||
| BMI(kg/m2) | ||||||
| Model1 | 2.62 (2.22,3.03) | <0.0001 | 2.39 (2.07,2.71) | <0.0001 | 1.34 (0.73,1.96) | <0.0001 |
| Model2 | 2.16 (1.54,2.78) | <0.0001 | 2.22 (1.88,2.55) | <0.0001 | 1.23 (0.60,1.86) | 0.0001 |
| WC (cm) | ||||||
| Model1 | 3.42 (2.99,3.84) | <0.0001 | 2.11 (1.79,2.44) | <0.0001 | 1.50 (0.86,2.15) | <0.0001 |
| Model2 | 2.73 (2.06,3.40) | <0.0001 | 1.92 (1.58,2.26) | <0.0001 | 1.43 (0.75,2.10) | <0.0001 |
| DBP | ||||||
| BMI (kg/m2) | ||||||
| Model1 | 1.19 (0.98,3.84) | <0.0001 | 1.53 (1.28,1.78) | <0.0001 | 0.75 (0.41,1.09) | <0.0001 |
| Model2 | 1.11 (0.78,1.44) | <0.0001 | 1.72 (1.46,1.97) | <0.0001 | 0.74 (0.39,1.08) | <0.0001 |
| WC (cm) | ||||||
| Model1 | 1.37 (1.14,1.59) | <0.0001 | 1.44 (1.18,1.69) | <0.0001 | 0.89 (0.54,1.24) | <0.0001 |
| Model2 | 1.20 (0.85,1.56) | <0.0001 | 1.66 (1.40,1.92) | <0.0001 | 0.82 (0.45,1.18) | <0.0001 |
Abbreviation: BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure. Model1: adjusted for age and sex. Model2: adjusted for age, sex, current smoking, current drinking, exercise regularly, type 2 diabetes, cardiovascular disease, family history of hypertension, and total energy intake in the HPHS and HDNNCDS; Adjusted for age, sex, ethnicity, exercise regularly, current smoking, current drinking, type 2 diabetes, cardiovascular disease, and total energy intake in the NHANES.
Mediation analysis
In the HDNNCDS, we found that TG, TC, FG, PG, FI, and HOMA-IR were the potential mediators between BMI or WC and systolic BP or diastolic BP. Additionally, HOMA-B was the potential mediator between BMI or WC and systolic BP, and PI was the potential mediator between WC and diastolic BP. Among these mediators, the proportions via mediation by FI, HOMA-IR, and TG were the top three ones which totally accounted for more than 30% of the total effect, they were 12.0%, 10.6%, and 9% between BMI and systolic BP, 10.2%, 9.1%, and 6.6% between WC and systolic BP, 19.8%, 16.9%, and 13.4% between BMI and diastolic BP, 19.7%, 15.8%, and 11.4% between WC and diastolic BP, respectively. The proportions via mediation by other mediators were ranged from 1.8% to 4.8% (Table 3).
Table 3.
Mediation analysis between BMI/WC and blood pressure in the Harbin Cohort Study on Diet, Nutrition and Chronic Non-Communicable Diseases (HDNNCDS, 2012).
| Mediators (by 1 SD) | Outcomes | BMI* | WC* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total effect, estimate (95% CI) | Proportion via mediation, estimate (95% CI) | Sensitivity analysis | Total effect, estimate (95% CI) | Proportion via mediation, estimate (95% CI) | Sensitivity analysis | ||||
| R2* | R2* | ||||||||
| TG | SBP | 2.496 (2.013,2.890) | 0.090 (0.057,0.137) | 0.01 | 0.0073 | 3.254 (2.939,2.758) | 0.066 (0.040,0.090) | 0.01 | 0.0072 |
| DBP | 1.155 (0.879,1.350) | 0.134 (0.090,0.184) | 0.01 | 0.0081 | 1.345 (1.118,1.584) | 0.114 (0.087,0.155) | 0.01 | 0.0081 | |
| TC | SBP | 2.496 (2.142,2.904) | 0.018 (0.006,0.033) | 0.01 | 0.0077 | 3.254 (2.809,3.716) | 0.018 (0.009,0.034) | 0.01 | 0.0076 |
| DBP | 1.155 (0.918,1.346) | 0.021 (0.006,0.040) | 0.01 | 0.0086 | 1.345 (1.128,1.568) | 0.024 (0.009,0.039) | 0.01 | 0.0086 | |
| HDL | SBP | 2.494 (2.150,2.886) | −0.013 (−0.043,0.028) | NA | NA | 3.250 (2.899,3.767) | −0.013 (−0.042,0.019) | NA | NA |
| DBP | 1.153 (0.092,1.360) | 0.018 (−0.031,0.063) | NA | NA | 1.344 (1.137,1.479) | 0.016 (−0.023,0.058) | NA | NA | |
| LDL | SBP | 2.491 (1.965,2.864) | 0.014 (−0.001,0.032) | NA | NA | 3.251 (2.838,3.647) | 0.008 (−0.0003,0.020) | NA | NA |
| DBP | 1.149 (0.959,1.378) | 0.012 (−0.006,0.030) | NA | NA | 1.340 (1.135,1.553) | 0.008 (−0.004,0.023) | NA | NA | |
| FG | SBP | 2.491 (2.052,0.822) | 0.048 (0.031,0.070) | 0.01 | 0.0049 | 3.252 (2.856,3.638) | 0.043 (0.026,0.057) | 0.01 | 0.0048 |
| DBP | 1.154 (0.971,1.429) | 0.040 (0.021,0.065) | 0.01 | 0.0055 | 1.344 (1.124,1.568) | 0.040 (0.022,0.065) | 0.01 | 0.0055 | |
| PG | SBP | 2.561 (2.117,3.041) | 0.045 (0.019,0.079) | 0.01 | 0.0052 | 3.277 (2.866,3.815) | 0.034 (0.016,0.051) | 0.01 | 0.0051 |
| DBP | 1.218 (0.959,1.469) | 0.039 (0.017,0.066) | 0 | 0 | 1.435 (1.218,1.646) | 0.033 (0.011,0.064) | 0 | 0 | |
| FI | SBP | 2.642 (2.263,3.184) | 0.120 (0.048,0.181) | 0.01 | 0.0074 | 3.072 (2.058,3.670) | 0.102 (0.032,0.163) | 0.01 | 0.0073 |
| DBP | 1.114 (0.812,1.467) | 0.198 (0.125,0.316) | 0.01 | 0.0082 | 1.211 (0.917,1.495) | 0.197 (0.117,0.298) | 0.01 | 0.0082 | |
| PI | SBP | 2.723 (2.273,3.246) | 0.040 (−0.008,0.092) | NA | NA | 2.908 (2.300,3.400) | 0.041 (0.008,0.099) | 0 | 0 |
| DBP | 1.213 (0.967,1.556) | 0.051 (−0.006,0.134) | NA | NA | 1.305 (0.977,1.614) | 0.052 (−0.001,0.123) | NA | NA | |
| HOMA-IR | SBP | 2.647 (2.055,3.202) | 0.106 (0.064,0.152) | 0.01 | 0.0075 | 3.070 (2.584,3.553) | 0.091 (0.045,0.150) | 0.01 | 0.0075 |
| DBP | 1.115 (0.866,1.406) | 0.169 (0.098,0.262) | 0.01 | 0.0083 | 1.224 (0.961,1.567) | 0.158 (0.101,0.222) | 0.01 | 0.0084 | |
| HOMA-B | SBP | 2.509 (2.011,3.000) | −0.027 (−0.060,−0.006) | 0 | 0 | 2.937 (2.488,3.562) | −0.025 (−0.048,−0.004) | 0 | 0 |
| DBP | 1.098 (0.829,1.344) | 0.002 (−0.033,0.029) | NA | NA | 1.177 (0.881,1.451) | 0.003 (−0.030,0.040) | NA | NA | |
Abbreviation: BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglyceride; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; FG, fasting glucose; PG, 2-h postprandial glucose; FI, fasting insulin; PI, 2-h postprandial insulin.
*Adjusted for age, sex, current smoking, current drinking, exercise regularly, type 2 diabetes, cardiovascular disease, family history of hypertension, and total energy intake. R2*, the proportion of residual variances and , the proportion of original variances that were explained by the omitted confounding.
In the validation analysis, the potential mediators of TG, TC, FG, and HOMA-IR were externally validated in the cross-sectional survey of the NHANES and the cohort data of the HPHS (Table 4). In addition, PG and FI were validated in the NHANES; PI was validated in the HPHS. But HOMA-B was not validated in the two studies and PI could not be validated in the NHANES since it was not measured in this study.
Table 4.
Mediation analysis between BMI or WC and blood pressure in the US National Health and Nutrition Examination Survey (NHANES) and in the Harbin People’s Health Study (HPHS, 2008–2012).
| Mediators (by 1 SD) | Outcomes | BMI | WC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total effect, estimate (95% CI) | Proportion via mediation, estimate (95% CI) | Sensitivity analysis | Total effect, estimate (95% CI) | Proportion via mediation, estimate (95% CI) | Sensitivity analysis | ||||
| R2* | R2* | ||||||||
| NHANES a | |||||||||
| TG | SBP | 2.311 (1.851,2.809) | 0.123 (0.070,0.182) | 0.01 | 0.0070 | 2.014 (1.577,2.506) | 0.161 (0.095,0.241) | 0.01 | 0.0071 |
| DBP | 1.763 (1.443,2.098) | 0.157 (0.101,0.223) | 0.01 | 0.0083 | 1.785 (1.449,2.155) | 0.168 (0.115,0.227) | 0.01 | 0.0083 | |
| TC | SBP | 2.221 (1.851,2.541) | 0.024 (0.011,0.038) | 0.01 | 0.0074 | 1.906 (1.599,2.170) | 0.029 (0.014,0.049) | 0.01 | 0.0075 |
| DBP | 1.749 (1.437,2.061) | 0.036 (0.014,0.056) | 0.01 | 0.0087 | 1.677 (1.449,1.905) | 0.039 (0.019,0.066) | 0.01 | 0.0087 | |
| FG | SBP | 2.330 (1.942,2.827) | 0.068 (0.032,0.097) | 0.01 | 0.0054 | 2.039 (1.471,2.508) | 0.085 (0.028,0.138) | 0.01 | 0.0054 |
| DBP | 1.739 (1.398,2.087) | 0.057 (0.021,0.096) | 0 | 0 | 1.761 (1.238,2.101) | 0.058 (0.023,0.106) | 0 | 0 | |
| PG | SBP | 2.615 (1.999,3.037) | 0.205 (0.145,0.277) | 0.01 | 0.0067 | 2.275 (1.732,2.719) | 0.259 (0.180,0.374) | 0.01 | 0.0067 |
| DBP | 1.886 (1.467,2.260) | 0.077 (0.022,0.135) | 0 | 0 | 1.800 (1.422,2.211) | 0.187 (0.084,0.327) | 0 | 0 | |
| FI | SBP | 2.394 (1.983,2.866) | 0.144 (0.056,0.245) | 0 | 0 | 2.143 (1.769,2.566) | 0.202 (0.066,0.330) | 0 | 0 |
| DBP | 1.761 (1.500,2.139) | 0.193 (0.07,0.320) | 0 | 0 | 1.800 (1.422,0.327) | 0.187 (0.084,0.327) | 0 | 0 | |
| HOMA-IR | SBP | 2.388 (1.826,2.886) | 0.119 (0.034,0.244) | 0 | 0 | 2.137 (1.678,2.598) | 0.165 (0.038,0.298) | 0 | 0 |
| DBP | 1.761 (1.365,2.128) | 0.189 (0.070,0.339) | 0.01 | 0.0073 | 1.799 (1.439,2.265) | 0.186 (0.084,0.286) | 0.01 | 0.0072 | |
| HOMA-B | SBP | 2.413 (1.885,2.912) | 0.015 (−0.071,0.111) | NA | NA | 2.157 (1.723,2.624) | 0.043 (−0.059,0.155) | NA | NA |
| DBP | 1.768 (1.399,2.085) | 0.054 (−0.047,0.176) | NA | NA | 1.808 (1.409,2.229) | 0.052 (−0.047,0.156) | NA | NA | |
| HPHS b | |||||||||
| TG | SBP | 1.306 (0.667,1.806) | 0.114 (0.046,0.306) | 0.010 | 0.0080 | 1.422 (0.754–1.949) | 0.113 (0.033,0.324) | 0.010 | 0.0077 |
| DBP | 0.728 (0.455–1.025) | 0.005 (−0.034,0.062) | NA | NA | 0.920 (0.469–1.298) | 0.162 (0.069,0.306) | 0.010 | 0.0081 | |
| TC | SBP | 1.297 (0.886,1.836) | 0.047 (−0.002,0.141) | NA | NA | 1.414 (0.774–2.004) | 0.051 (0.004,0.124) | 0.008 | 0.0070 |
| DBP | 0.702 (0.337–1.055) | 0.019 (−0.042,0.091) | NA | NA | 0.893 (0.491–1.222) | 0.016 (−0.028,0.077) | NA | NA | |
| FG | SBP | 1.299 (0.678–1.903) | 0.040 (0.001,0.103) | 0.010 | 0.0060 | 1.416 (0.699–2.082) | 0.046 (0.004,0.102) | 0.010 | 0.0060 |
| DBP | 0.717 (0.380–1.077) | 0.023 (−0.0063,0.082) | NA | NA | 0.911 (0.517–1.193) | 0.021 (−0.007,0.076) | NA | NA | |
| PG | SBP | 1.172 (0.640–1.737) | 0.002 (−0.051,0.076) | NA | NA | 1.307 (0.681–2.068) | −0.006 (−0.108,0.096) | NA | NA |
| DBP | 0.695 (0.357–1.007) | 0.005 (−0.059,0.060) | NA | NA | 0.868 (0.544–1.223) | −0.004 (−0.087,0.062) | NA | NA | |
| FI | SBP | 2.061 (1.432–2.749) | 0.103 (0.039,0.213) | 0.010 | 0.0071 | 1.918 (1.134–2.784) | 0.145 (0.042,0.298) | 0.007 | 0.0071 |
| DBP | 1.585 (1.199–2.018) | 0.151 (0.037,0.351) | 0.010 | 0.0081 | 2.616 (1.805–2.998) | 0.160 (0.054,0.311) | 0.010 | 0.0080 | |
| PI | SBP | 0.999 (0.295–1.621) | 0.098 (−0.058,0.631) | NA | NA | 1.180 (0.327–2.047) | 0.292 (0.103,0.902) | 0.010 | 0.0080 |
| DBP | 0.641 (0.202–1.083) | 0.088 (−0.043,0.428) | NA | NA | 0.824 (0.518–1.277) | 0.075 (−0.051,0.270) | NA | NA | |
| HOMA-IR | SBP | 2.346 (2.011–3.108) | 0.108 (0.043,0.281) | 0.010 | 0.0070 | 2.956 (2.494–3.419) | 0.081 (0.040,0.146) | 0 | 0.0071 |
| DBP | 1.121 (0.892–1.609) | 0.111 (0.041,0.298) | 0.010 | 0.0080 | 1.209 (0.906–1.553) | 0.143 (0.081,0.192) | 0.007 | 0.0080 | |
| HOMA-B | SBP | 1.049 (0.348–1.947) | −0.058 (−0.243,0.044) | NA | NA | 0.846 (−0.044–1.629) | −0.069 (−0.683,0.117) | NA | NA |
| DBP | 0.530 (0.103–1.018) | −0.001 (−0.177,0.205) | NA | NA | 0.054 (0.016–1.031) | 0.0001 (−0.0152,0.184) | NA | NA | |
Abbreviation: BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglyceride; TC, total cholesterol; FG, fasting glucose; PG, 2-h postprandial glucose; FI, fasting insulin. a Adjusted for age, sex, ethnicity, exercise regularly, current smoking, current drinking, diabetes mellitus, cardiovascular disease, and total energy intake. bAdjusted for age, sex, current smoking, current drinking, exercise regularly, type 2 diabetes, cardiovascular disease, family history of hypertension, and total energy intake. R2*, the proportion of residual variances and , the proportion of original variances that were explained by the omitted confounding.
In the mediation analysis of the association between BMI or WC and IR (indicated by HOMA-IR), TG, TC, FG accounted for 5.4–11.3%, 0.3–2.0%, and 9.4–16.7% of the total effect on IR due to BMI or WC in the HDNNCDS, NHANES, or HPHS, respectively (Table 5).
Table 5.
Effect of BMI or WC on insulin resistance with mediation of established biomarkers in the Harbin Cohort Study on Diet, Nutrition and Chronic Non-Communicable Diseases (HDNNCDS, 2010–2012), the US National Health and Nutrition Examination Survey (NHANES) and in the Harbin People’s Health Study (HPHS, 2008–2012)
| Mediators (by 1 SD) | BMI | WC | ||||||
|---|---|---|---|---|---|---|---|---|
| Total effect, estimate (95% CI) | Proportion via mediation, estimate (95% CI) | Sensitivity analysis | Total effect, estimate (95% CI) | Proportion via mediation, estimate (95% CI) | Sensitivity analysis | |||
| R2* | R2* | |||||||
| HDNNCDS a | ||||||||
| TG | 0.106 (0.092,0.121) | 0.074 (0.049,0.102) | 0.01 | 0.0082 | 0.107 (0.091,0.122) | 0.082 (0.055,0.114) | 0.01 | 0.0082 |
| TC | 0.106 (0.092,0.119) | 0.006 (−0.003,0.015) | NA | NA | 0.106 (0.091,0.124) | 0.001 (0.009,0.023) | 0.01 | 0.0087 |
| FG | 0.106 (0.092,0.120) | 0.094 (0.051,0.133) | 0.09 | 0.0469 | 0.107 (0.089,0.122) | 0.167 (0.069,0.154) | 0.09 | 0.0476 |
| NHANES b | ||||||||
| TG | 0.298 (0.276,0.321) | 0.054 (0.043,0.071) | 0.04 | 0.0277 | 0.295 (0.268,0.317) | 0.058 (0.044,0.076) | 0.04 | 0.0273 |
| TC | 0.298 (0.271,0.318) | 0.020 (0.011,0.028) | 0.01 | 0.0071 | 0.295 (0.275,0.319) | 0.018 (0.011,0.026) | 0.01 | 0.0071 |
| FG | 0.298 (0.274,0.322) | 0.107 (0.079,0.138) | 0.16 | 0.0748 | 0.295 (0.273,0.316) | 0.105 (0.081,0.134) | 0.16 | 0.0740 |
| HPHS a | ||||||||
| TG | 0.166 (0.118,0.215) | 0.106 (0.042,0.187) | 0.01 | 0.0072 | 0.166 (0.111,0.218) | 0.113 (0.051,0.200) | 0.04 | 0.0293 |
| TC | 0.171 (0.119,0.221) | 0.010 (−0.008,0.038) | NA | NA | 0.171 (0.126,0.220) | 0.003 (0.008,0.021) | 0.01 | 0.0083 |
| FG | 0.168 (0.166,0.218) | 0.102 (0.029,0.198) | 0.09 | 0.0481 | 0.168 (0.115,0.224) | 0.117 (0.024,0.227) | 0.09 | 0.0488 |
Abbreviation: BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglyceride; TC, total cholesterol; FG, fasting glucose; PG, 2-h postprandial glucose; FI, fasting insulin. aAdjusted for age, sex, current smoking, current drinking, exercise regularly, type 2 diabetes, cardiovascular disease, family history of hypertension, and total energy intake. b Adjusted for age, sex, ethnicity, exercise regularly, current smoking, current drinking, diabetes mellitus, cardiovascular disease, and total energy intake. R2*, the proportion of residual variances and , the proportion of original variances that were explained by the omitted confounding.
The robustness of all mediation results was supported by sensitivity analyses that showed omitted confounding effect could explain less than 10 percent of variances.
Cross-lagged path analysis
Figure 2 presents cross-lagged path analysis of BMI or WC and insulin or HOMA-IR in the HPHS, which indicated that increased BMI or WC preceded hyperinsulinemia. After adjusting for age, sex, and follow-up years, the path coefficient from baseline BMI to follow-up insulin (β2 = 0.326) was significantly greater than the path coefficient from baseline insulin to follow-up BMI (β1 = 0.0.023), with P = 0.001 for difference between β1 and β2. Autocorrelation also known as tracking correlation of BMI (r 2) were significantly greater than that of insulin (r 3). The variance (R 2) of follow-up BMI explained by baseline predictors was greater than that of follow-up insulin (Fig. 2(A)). And the path coefficients from baseline BMI to follow-up HOMA-IR, baseline WC to follow-up insulin, baseline WC to follow-up HOMA-IR were 0.113 (P < 0.0001), 0.077 (P = 0.020), and 0.032 (P = 0.001), respectively, however, the path coefficients from baseline HOMA-IR to follow-up BMI, baseline insulin to follow-up WC, baseline HOMA-IR to follow-up WC were not statistically significant (Fig. 2(B, C, and D)). These results provided stronger evidence for the results in the mediation analysis in the present study.
Figure 2.
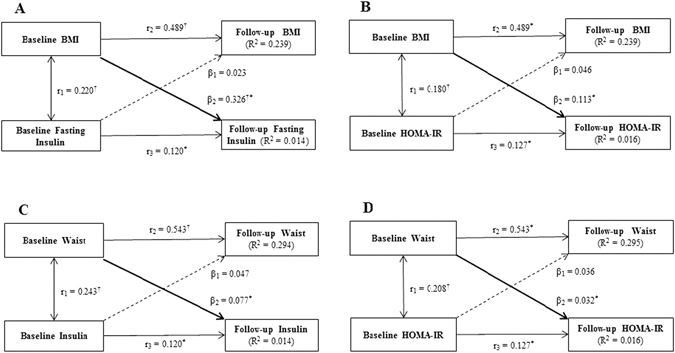
Cross-lagged path analysis of BMI or WC and insulin or HOMA-IR in the Harbin People Health Study, adjusted for age, sex, and follow-up years. β1 and β2 are cross-lagged path coefficients; r1 is synchronous correlations; r2 and r3 are tracking correlations; R2 is variance explained. Coefficients different from 0: *P < 0.01, † P < 0.001 for difference between β1 and β2.
Discussion
In the present study, we confirmed that BMI or WC was significantly positively associated with BP. By using mediation analysis, we identified insulin concentrations (FI and PI) and HOMA-IR mediated a considerable amount of the total effect of BMI or WC on BP. This effect was further mediated by TG, TC, and FG. To the best of our knowledge, this study is the first to directly provide quantifiable mechanistic evidence linking BMI or WC to BP in the three independent population-based studies.
Significant association of BMI or WC with BP has been observed consistently in different populations4, 5, 15, 16. Doll et al. found that systolic BP and diastolic BP were increased over the whole variation range of BMI and WC among populations across developed and developing countries (from the main Seychelles island (Mahe) and two Swiss regions (Vaud-Fribourg and Ticino)), in which a gain of 1.7 kg/m2 in BMI or of 4.5 cm in WC corresponded to an elevation of 1 mmHg in systolic BP for men, and the corresponding figures were 1.25 kg/m2 and 2.5 cm for women, respectively5. Bovet et al. observed that BMI was positively associated with BP in a cross-sectional survey of the entire population in five branches of Dar es Salaam (1.01 and 0.91 mmHg systolic BP per 1 kg/ m2 BMI in men and women, respectively)4. In agreement with these findings, our results showed similar positive association of BMI or WC with BP in a cross-sectional survey of the HDNNCDS, and it is particularly validated in American population in the cross-sectional surveys of the NHANES and an independent Chinese cohort of the HPHS in the present study.
Although results reported in an appreciable number of studies have supported these associations, the potential mechanisms remain to be an area of research. In addition, proximal mediators of the effect are more relevant to targeted elevated BP prevention among obesity persons yet this topic remains understudied. Mediation analysis was first proposed and has been prominent statistical analysis in psychological research17. Under appropriate causal structures justified by substantive scientific knowledge, mediation analysis addresses directly the questions of how and why the specific exposure and outcome are related by the intermediate factor through measuring its contribution to the effect of the exposure on the outcome. By using this method, we evaluated the potential mediation effects of possible mediators of BP and resulted in the observations that several mediators may be partially determined by BMI or WC and also predict BP.
The link between obesity and the development of insulin resistance has been well documented. The values of the FI and HOMA-IR have been observed significantly higher in the obese subjects in comparison with the subjects with normal weight18, and weight loss/gain correlates closely with a decrease/an increase in insulin sensitivity, respectively19. It has been proposed that obese individuals develop resistance to the cellular actions of insulin, characterized by an impaired ability of insulin to inhibit glucose output from the liver and to promote glucose uptake in fat and muscle20, 21. Meanwhile, an association between insulin metabolism/resistance and BP has been reported in previous studies as well. Insulin can cause vasodilation through increased NO production, any reduction in the functionality of the peptide will obviously have adverse effects on blood pressure. Additionally, insulin resistance causes increased blood flow to skeletal muscles to compensate for the reduced glucose delivery, which in turn also increases BP22. Insulin concentrations have been observed to be significantly higher in adult patients with hypertension and borderline hypertension than in normotensive control patients, no matter whether insulin is measured in the fasting state or in response to the oral glucose tolerance test2, 23. However, study that provided whether insulin/insulin resistance is playing a role in linking BMI or WC with BP is limited. We performed mediation analysis to examine this association. Our data appear to indicate that insulin (FI and PI) and insulin resistance (HOMA-IR) may have a significant mediating effect on the association between BMI or WC and BP, which accounted for about 25~40% of the total effect of this association. This finding adds further support to previous speculations that the association between BMI or WC and BP might be mediated by insulin/insulin resistance.
In addition, obesity has been speculated to be the main cause of the metabolic syndrome (MetS), and dyslipidemia is an important feature in MetS24. As expected, epidemiological studies have observed that both TC and TG keep increasing steadily with BMI and WC25–27. At the same time, TC and TG are usually associated with increased BP levels, and the increases in TC and TG level with BP were observed to be greater in overweight than in lean subjects28. This suggests that body mass in itself or factors associated with body mass are related to concomitant elevations of BP and blood lipids. In the present study, we found that TC and TG were mediators linking BMI or WC with BP, which explained approximately 15~20% of the total effect of this association. Moreover, BMI or WC and FG have been reported to be positively correlated in previous studies29, 30, and the association between elevated FG and risk of the development of hypertension has been described in some studies31. In the present study, we observed that FG was also a mediator linking BMI or WC and BP, which explained a small but significant amount of the total effect of BMI or WC on BP.
The strengths of our study include its base in three independent population-based surveys among Chinese and American populations and hence the ability to validate the findings in these studies, in which there is one cohort study with a high follow-up rate. Specifically, we used the novel counterfactual model-based mediation analysis rather than the traditional method that compares differences of the regression coefficients between the models with and without the mediators. Our work has limitations. First, although we adjusted for confounders, we cannot exclude the possibility of residual confounding. Second, the SNS and RAAS have also suggested being etiologically relevant in obesity related hypertension; however, we did not measure them in the present study. Thus, we cannot discuss their relevance to increased BMI or WC induced BP elevation. Third, mediating effects may not be reliable detected in the two present cross-sectional studies, but the results from the present follow-up data of the HPHS adds further support of the mediating effects of the potential mediators.
Conclusions
The present study confirmed that BMI or WC was consistently positively associated with BP, particularly across three different populations. In addition, the total effect of the association between BMI or WC and BP was found to be mainly mediated by insulin/insulin resistance, which is further explained by TG, TC and FG. These validated data provide quantifiable mechanistic evidence linking BMI or WC to BP, which may provide targets for avoiding obesity-related hypertension.
Methods
Study Populations
The HDNNCDS and the HPHS methods have been previously described in detail32.The HDNNCDS was launched in 2010 by the national key discipline, department of nutrition and food hygiene at Harbin Medical University, which recruited a total of 9734 people aged 20–74 years and the baseline survey was finished in 2012. After excluding those who were taken medications for hypertension (n = 1779), those who reported extreme values for total energy intake (>4500 or < 500 kcal/day, n = 368), and those who had missing information on BMI or WC (n = 493), 7094 participants were included in the present study. The HPHS recruited 8940 people aged 20–74 years in 2008. After finishing the baseline survey, 4515 members (about 50.5% of total participants) were randomly selected to participate in the follow-up surveys due to financial limit for this study. In 2012, 4158 participants finished the first in-person follow-up survey with a response rate of 92.1%. After excluding those who had hypertension at baseline survey (n = 1492), those who reported extreme values for total energy intake (>4500 or < 500 kcal/day, n = 177), and those who had missing information on BMI or WC (n = 137), 2709 participants were included in the present study. In both the HDNNCDS and the HPHS, detailed in-person interviews were administered by trained personnel using a structured questionnaire to collect information on demographic characteristics, dietary habits, and lifestyles and physical condition at baseline survey. Weight and height were measured with participants standing without shoes and wearing light clothing at baseline recruitment. BMI (kg/m2) was calculated as weight (kg) divided by the square of the height in meters (m2). The seated blood pressures of the subjects were measured on the right arm after 5 min of rest to the nearest 2 mmHg by using an electronic blood pressure monitor (OMRON HEM-7112), the mean of the two measurements was recorded in the HDNNCDS, and the mean BP at follow-up survey in the HPHS were used in the present study. Serum TC, TG, high density lipoprotein (HDL), and low density lipoprotein (LDL) were determined using an automatic biochemical analyzer (Hitachi 7100, Japan). An oral glucose tolerance test (OGTT) was carried out according to the World Health Organization (WHO) guidelines33. Serum insulin was measured with an auto-analyzer using commercial kits (Centaur, Bayer Corporation, Bayer Leverkusen, Germany). Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated according to the formula: FG (mmol/L) × Fasting insulin (FI) (mIU/L)/22.5, and HOMA-B was calculated with the formula: 20 × FI (mIU/L)/FG (mmol/L) −3.534.
The HDNNCDS and the HPHS were approved by the institutional ethics review board of the Harbin Medical University and were conducted in accordance with the guidelines of the Declaration of Helsinki. Written informed consent was provided by all participants.
NHANES is a cross-sectional, biannual, representative health survey of the United States population35. We used data from four surveys (2005–2006, 2007–2008, 2009–2010, and 2011–2012). 24-hour food recall questionnaire were administered using the United States Department of Agriculture (USDA) and US Department of Health and Human Services (DHHS) food recall questionnaire. Self-reported data were also collected for diabetes mellitus, coronary disease status, current drinking, and current smoking. Height, weight, and 3 to 4 seated systolic blood pressure (systolic BP) and diastolic blood pressure (diastolic BP) measurements were also administered. The mean of the BP values (3–4 measurements) were used for analysis. Serum lipids profiles (including TC, TG, HDL, and LDL), OGTT, and FI were also measured. We limited the study sample to adults who were aged 20 to 74 years, were not pregnant among females, did not take extreme values for total energy intake (<500 kcal/day or >4500 kcal/day), and did not have missing or unknown information on BMI, WC, BP, current drinking, education, coronary heart disease, and type 2 diabetes for consistency across surveys and comparable to the participants in the HDNNCDS and the HPHS. At last, 10875 subjects were analyzed in the present study.
Statistical Analyses
Selected baseline characteristics were presented with mean ± standard deviation (SD) for continuous variables and percentage for categorical variables. Linear regression models were employed to evaluate the effect of BMI or WC per SD difference on BP. The two main models were as follows: Model 1 was adjusted for age at study recruitment (years) and sex (male/female). Model 2 was adjusted for age at study recruitment (years), sex (male/female), current smoking (yes/no), current drinking (yes/no), exercise regularly (yes/no), type 2 diabetes (yes/no), cardiovascular disease (yes/no), and total energy intake (kcal/day) in the NHANES and additionally adjusted for family history of hypertension (yes/no) in the HDNNCDS and HPHS. Mediation analysis was performed to evaluate the role of fasting glucose (FG), 2-h postprandial glucose (PG), FI, 2-h postprandial insulin (PI), HOMA-IR, HOMA-B, TC, TG, HDL, and LDL at baseline as potential mediators of the association of BMI or WC at baseline with BP. Mediation effect was evaluated by the degree of attenuation of the per SD increment of BMI or WC effect by further adjusting for the potential mediators in the linear regression models. The proportion of mediating effects was calculated in the risk difference scale, and the 95% CIs of the portions of effects were obtained via bootstrapping36.
In addition, the relationship between obesity and insulin has been suggested to be reciprocal37 and thus, the causal sequence between them should be figured out in longitudinal cohort in order to explain the results in the mediation analysis. The cross-lagged panel analysis is a form of path analysis that simultaneously examines reciprocal, longitudinal relationships among a set of intercorrelated variables38, 39. It was used to analyze data to provide stronger evidence for a temporal relationship between BMI or WC and insulin or HOMA-IR in longitudinal cohort of the HPHS. Pearson correlation coefficients of the Z-transformed quantitative variables of BMI or WC and insulin or HOMA-IR at baseline and follow-up were calculated, with adjustment for follow-up years.
All analyses were performed by using R version 3.0.3 (http://www.r-project.org/) and a two-sided P-value < 0.05 was considered statistically significant.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant No. 81130049 and 81573134).
Author Contributions
X.W. and C.S. wrote the manuscript. X.W., X.Y. and C.S. were involved in design, analysis, and interpretation of data. X.W., R.S., T.L., T.Z., L.N. and Y.L. were involved in data collection. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Xiaoyan Wu and Xue Yang contributed equally to this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ezzati M, Lopez AD, Rodgers A, Vander HS, Murray CJ. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 2.Mingsheng Zhou, A. W. & Yu, H. Link between insulin resistance and hypertension: What is the evidence from evolutionary biology. Diabetol Metab Syndr6 (2014). [DOI] [PMC free article] [PubMed]
- 3.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organization technical report series894, i–xii, 1–253 (2000). [PubMed]
- 4.Bovet P, et al. Distribution of blood pressure, body mass index and smoking habits in the urban population of Dar es Salaam, Tanzania, and associations with socioeconomic status. Int J Epidemiol. 2002;31:240–247. doi: 10.1093/ije/31.1.240. [DOI] [PubMed] [Google Scholar]
- 5.Doll S, Paccaud F, Bovet P, Burnier M, Wietlisbach V. Body mass index, abdominal adiposity and blood pressure: consistency of their association across developing and developed countries. Int. J. Obes. Relat. Metab. Disord. 2002;26:48–57. doi: 10.1038/sj.ijo.0801854. [DOI] [PubMed] [Google Scholar]
- 6.Kerry SM, Micah FB, Plange-Rhule J, Eastwood JB, Cappuccio FP. Blood pressure and body mass index in lean rural and semi-urban subjects in West Africa. J. Hypertens. 2005;23:1645–1651. doi: 10.1097/01.hjh.0000177536.53409.1a. [DOI] [PubMed] [Google Scholar]
- 7.Huggett RJ, Burns J, Mackintosh AF, Mary DA. Sympathetic neural activation in nondiabetic metabolic syndrome and its further augmentation by hypertension. Hypertension. 2004;44:847–852. doi: 10.1161/01.HYP.0000147893.08533.d8. [DOI] [PubMed] [Google Scholar]
- 8.Rumantir MS, et al. Neural mechanisms in human obesity-related hypertension. J. Hypertens. 1999;17:1125–1133. doi: 10.1097/00004872-199917080-00012. [DOI] [PubMed] [Google Scholar]
- 9.Engeli S, et al. Weight loss and the renin-angiotensin-aldosterone system. Hypertension. 2005;45:356–362. doi: 10.1161/01.HYP.0000154361.47683.d3. [DOI] [PubMed] [Google Scholar]
- 10.Umemura S, et al. Plasma angiotensinogen concentrations in obese patients. Am. J. Hypertens. 1997;10:629–633. doi: 10.1016/S0895-7061(97)00053-8. [DOI] [PubMed] [Google Scholar]
- 11.Masuo K, Mikami H, Ogihara T, Tuck ML. Weight gain-induced blood pressure elevation. Hypertension. 2000;35:1135–1140. doi: 10.1161/01.HYP.35.5.1135. [DOI] [PubMed] [Google Scholar]
- 12.Bonaa KH, Thelle DS. Association between blood pressure and serum lipids in a population. The Tromso Study. Circulation. 1991;83:1305–1314. doi: 10.1161/01.CIR.83.4.1305. [DOI] [PubMed] [Google Scholar]
- 13.De Stavola BL, Daniel RM, Ploubidis GB, Micali N. Mediation analysis with intermediate confounding: structural equation modeling viewed through the causal inference lens. Am. J. Epidemiol. 2015;181:64–80. doi: 10.1093/aje/kwu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradham WS, et al. Insulin resistance is associated with increased concentrations of NT-proBNP in rheumatoid arthritis: IL-6 as a potential mediator. Inflammation. 2014;37:801–808. doi: 10.1007/s10753-013-9799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danon-Hersch N, Chiolero A, Shamlaye C, Paccaud F, Bovet P. Decreasing association between body mass index and blood pressure over time. Epidemiology. 2007;18:493–500. doi: 10.1097/EDE.0b013e318063eebf. [DOI] [PubMed] [Google Scholar]
- 16.Kaufman JS, et al. Relationship between blood pressure and body mass index in lean populations. Hypertension. 1997;30:1511–1516. doi: 10.1161/01.HYP.30.6.1511. [DOI] [PubMed] [Google Scholar]
- 17.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elbassuoni E. Better association of waist circumference with insulin resistance and some cardiovascular risk factors than body mass index. Endocr Regul. 2013;47:3–14. doi: 10.4149/endo_2013_01_3. [DOI] [PubMed] [Google Scholar]
- 19.Steinberger J, Daniels SR. Obesity, insulin resistance, diabetes, and cardiovascular risk in children: an American Heart Association scientific statement from the Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young) and the Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism) Circulation. 2003;107:1448–1453. doi: 10.1161/01.CIR.0000060923.07573.F2. [DOI] [PubMed] [Google Scholar]
- 20.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 21.Hribal ML, Oriente F, Accili D. Mouse models of insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2002;282:E977–981. doi: 10.1152/ajpendo.00561.2001. [DOI] [PubMed] [Google Scholar]
- 22.Sowers JR. Insulin resistance and hypertension. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H1597–1602. doi: 10.1152/ajpheart.00026.2004. [DOI] [PubMed] [Google Scholar]
- 23.Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev. 2007;21:1443–1455. doi: 10.1101/gad.1550907. [DOI] [PubMed] [Google Scholar]
- 24.Klop B, Elte JW, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013;5:1218–1240. doi: 10.3390/nu5041218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu C, et al. Association between serum lipids, blood pressure, and simple anthropometric measures in an adult Chinese population. Arch. Med. Res. 2008;39:610–617. doi: 10.1016/j.arcmed.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Schröder H, Marrugat J, Elosua R, Covas MI. Relationship between body mass index, serum cholesterol, leisure-time physical activity, and diet in a Mediterranean Southern-Europe population. Br. J. Nutr. 2003;90:431–439. doi: 10.1079/BJN2003899. [DOI] [PubMed] [Google Scholar]
- 27.Brenner DR, Tepylo K, Eny KM, Cahill LE, El-Sohemy A. Comparison of body mass index and waist circumference as predictors of cardiometabolic health in a population of young Canadian adults. Diabetol Metab Syndr. 2010;2:28. doi: 10.1186/1758-5996-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choudhury KN, Mainuddin AK, Wahiduzzaman M, Islam SM. Serum lipid profile and its association with hypertension in Bangladesh. Vasc Health Risk Manag. 2014;10:327–332. doi: 10.2147/VHRM.S61019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O Innocent, O., O. T. & EO Sandra, I. E. J. Correlation between body mass index and blood glucose levels among some Nigerian undergraduates. HOAJ Biol (2013).
- 30.S. P. Patil, S. S. & A Bhate, A. M. Correlation of Blood sugar with waist circumference and Body mass index in an Indian population. (2012).
- 31.Morio M, Inoue M, Inoue K, Akimoto K. Impaired fasting glucose as an independent risk factor for hypertension among healthy middle-aged Japanese subjects with optimal blood pressure: the Yuport Medical Checkup Centre retrospective cohort study. Diabetol Metab Syndr. 2013;5:81. doi: 10.1186/1758-5996-5-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Na L, et al. The Harbin Cohort Study on Diet, Nutrition and Chronic Non-communicable Diseases: study design and baseline characteristics. PLoS ONE. 2015;10:e0122598. doi: 10.1371/journal.pone.0122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diabetes mellitus. Report of a WHO Study Group. World Health Organization technical report series727, 1–113 (1985). [PubMed]
- 34.Matthews DR, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 35.Centers, f. D. C. a. P. (. National Health and Nutrition Examination Survey. http://www.cdc.gov/nchs/nhanes/
- 36.K Imai, L. K. & Yamamoto, T. Identification, inference and sensitivity analysis for causal mediation effects. Statistical Science (2010).
- 37.Gustafson B, Hedjazifar S, Gogg S, Hammarstedt A, Smith U. Insulin resistance and impaired adipogenesis. Trends Endocrinol. Metab. 2015;26:193–200. doi: 10.1016/j.tem.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Kenny, D. A. Cross-lagged panel correlation: A test for spuriousness. Psychol Bull (1975).
- 39.M Kivimäki, T. F. & J Vahtera, J. E. N. Sense of coherence and health: evidence from two cross-lagged longitudinal samples. Soc Sci Med (2000). [DOI] [PubMed]


