Abstract
Polyploidy roles on adaptive evolution and ecological novelty have been extensively studied in plants but remained unclear in vertebrates owing to the rare polyploidy incidences. Here, a huge number of 3105 specimens in Carassius species complex including 2211 hexaploids and 894 tetraploids were sampled from 34 locations through mainland China. And hexaploids had wider geographic distribution than tetraploids especially in the areas with high altitude, high latitude and low annual precipitation. Then, an approximate 1050 bp transferrin (tf) fragments were amplified from all the samples, and 526 tf alleles were identified from a total of 37260 sequences at last. Intriguingly, higher nucleotide diversity of tf alleles in hexaploids than in tetraploids was revealed. Moreover, via phylogenetic analysis of tf alleles, potential origin center of Carassius species complex was deduced to be Yangtze River basin and hexaploids should undergo multiple independent polyploidy origins from sympatric tetraploids. These findings indicate that the hexaploids might possess stronger environmental adaptation and ecological novelty than the tetraploids, which provide an association paradigm of recurrent polyploidy and ecological context in polyploid vertebrates.
Introduction
Polyploidy has been revealed to have significant potentials and evolutionary consequences for increasing allelic diversity, altering genomic complexity, introducing novel traits, and driving ecological transfiguration in plants1, because it has been found to be ubiquitous and to benefit from both physiological and genetic buffering to provide the raw materials for evolution and adaptation of diverse plants3–5. In comparison with the prevalent incidences in plants, polyploid species are rare in vertebrates2, but polyploidy events have been yet documented in a wide taxa including teleost fishes, amphibians and reptiles3–6, and polyploidy consequences are frequently associated with some unusual clonal reproduction modes, such as parthenogenesis, gynogenesis, hybridogenesis and kleptogenesis7, 8. Owing to the association with clonal reproduction without genetic recombination, polyploid vertebrates are generally considered to be evolutionary “dead-ends”9. Therefore, some key evolutionary enigmas, such as how polyploidy per se lead to ecological novelty and what extent the extant polyploids contribute to environmental adaptation, remain unknown in vertebrates.
Fishes are the most dominant and successful group of vertebrates in the world, and polyploidy events have been revealed to occur in numerous taxonomic orders including Cypriniformes, Salmoniformes, Perciformes, Siluriformes, Acipenseriformes, Gymnotiformes and Characiformes4, 10. In Cypriniformes, there are not only more than 250 recognized polyploid species across Asia, Europe, Africa and America, but also exist different ploidy of polyploids, such as triploids, tetraploids, hexaploids or even octoploids4, 10, 11. Especially in Carassius species complex of the genus Carassius that widely distribute across the Eurasian continent12–14, Carassius auratus with 100 chromosomes have been demonstrated as ancestral tetraploids that reproduce by bisexual reproduction15, 16, whereas Carassius gibelio with 156 or 162 chromosomes17 have been recognized as hexaploids that are able to reproduce by dual modes of unisexual gynogenesis and bisexual reproduction3, 18. Through evolutionary history analyses of two divergent Dmrt1 genes, tetraploids were proposed to be formed via an early polyploidy event, and hexaploids were suggested to be resulted from ancestral tetraploids via recurrent polyploidy11, 19 Therefore, the rare extant case of ancestral tetraploids and recurrent hexaploids from the same evolutionary lineage makes the Carassius species complex as an ideal model to investigate adaptive evolution and ecological novelty of recurrent polyploidy in vertebrates.
In this study, we performed a field investigation to distinguish geographic distribution patterns of recurrent hexaploid Carassius gibelio and ancestral tetraploid Carassius auratus in Carassius species complex across a wide range throughout mainland China. Moreover, phylogenetic analysis and genetic diversity were performed by using transferrin (tf) alleles, because tf allele marker had been demonstrated to be particularly valuable for elucidating origin and evolutionary history of the polyploidy Carassius species complex13, 20 due to its polymorphisms especially in wild populations. Based on these data, we further explored the evolutionary implication for environmental adaptation and ecological novelty of repeated polyploidy from the ancestral tetraploid and recurrent hexaploid complex.
Methods
Sampling
A total of 3105 individuals of the Carassius species complex were collected widely from 34 sampled sites throughout mainland China. Details about all the samples and sampling sites are given in Table S1 and Fig. 1. For all specimens sampled, caudal fin and blood were obtained. Caudal fin was preserved in 100% ethanol for subsequent DNA extraction and PCR amplification. The deposited blood cells were then washed 2–3 times with 1 × phosphate buffered saline (PBS) solution and fixed in 75% ethanol for ploidy determination. All the specimens sampled were deposited in the Institute of Hydrobiology in China. All experiments in this research were performed according to the permit guidelines established by the Institute of Hydrobiology, Chinese Academy of Sciences, and the experimental protocols were approved by the animal care and use committee of Institute of Hydrobiology, Chinese Academy of Sciences.
Figure 1.
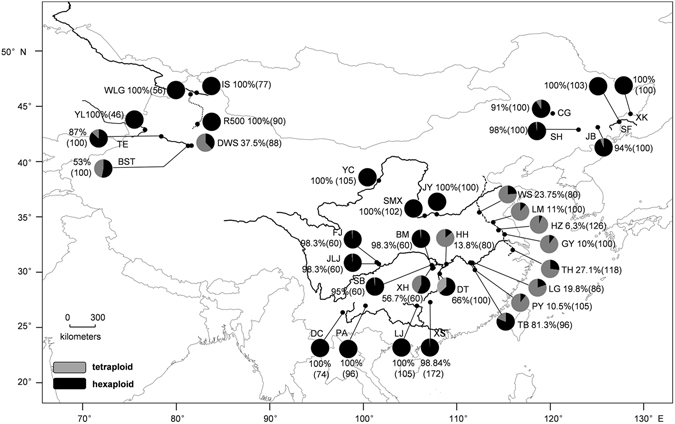
Geographical distribution of the 34 sampled populations. The detailed information of sampled sites is in Table S1. The proportions of hexaploids and tetraploids are indicated by the sizes of black and gray pie charts respectively. Percentage of hexaploids and sample size in bracket are given nearby. The map was created in ARCMAPv.9.3 (ESRI, Redlands, CA, USA) (http://www.esri.com/products).
Ploidy determination and analysis of ploidy distribution pattern
We used high speed sorting flow cytometer FACSAriaTMIII (BD) to estimate ploidy levels for each sample by measuring the relative DNA content of their fixed blood cells following the documented instructions21. Chicken blood cells with known DNA content of 2.5 pg/nucleus were used as an internally quantitative standard for each individual flow cytometry profile. The sampled blood cells of each individual were mixed with chicken blood cells and fixed in 70% pre-cooled ethanol overnight at 4 °C. The mixed cells were washed 2–3 times in 1 × PBS and then resuspended in the solution including 0.5% pepsin and 0.1 M HCl. DNA was stained with propidium iodide solution (40 g/mL) for 1–3 h at room temperature in the dark. Each sample with at least 10000 cells was measured in triplicate.
Analysis for ploidy distribution pattern
In our study, only hexaploids and tetraploids were observed in all the populations and their proportions were calculated subsequently. To evaluate the distribution pattern of the two ploidy forms in Carassius species complex, all sampling locations were divided into eight geographic areas according to the drainage systems through mainland China, including upper basin of Pearl River (LJ), upper basin of Yangtze River (DC, PA, FJ, JLJ), middle basin of Yangtze River (XS, DT, BM, XH, SB, TB, HH), lower basin of Yangtze River (LG, TH, PY), Yellow River (YC, JY, SMX), Jing-Hang Grand Canal (WS, HZ, LM, GY), northeast of China (XK, JB, SF, CG, SH), and northwest of China (BST, DWS, TE, R500, IS, WLG, YL). To examine the effect of geographic/climatic factors on distribution pattern of Carassius species complex, all the populations were assigned into four groups based on the proportions of hexaploids, including low hexaploid frequency group (hexaploid percentage ranging from 0 to 50%), sub-high hexaploid frequency group (hexaploid percentage ranging from 50% to 85%), high hexaploid frequency group (hexaploid percentage ranging from 85% to 99%) and all-hexaploid group (hexaploid percentage is 100%). The geographic/climatic factors used for analysis are longitude, latitude, altitude, average annual temperature (abbreviated as Tmean) and annual precipitation. The data of temperature, altitude and precipitation were obtained from WorldClim database22 and the data of sampling points were extracted using ARCMAPv.9.3 (ESRI, Redlands, CA, USA).
Constrained and partial canonical ordinations were used to test the influence of the above geographic/climatic variables on hexaploid and tetraploid distribution. Detrended correspondence analysis was performed to determine the appropriate type of model for direct gradient analysis23. Detrended correspondence analysis indicated that a linear model (gradient lengths < 2 standard units) would best fit the data of our study and redundancy analysis was performed subsequently.
The percentages of hexaploids and tetraploids were log (x + 1) transformed prior to analysis. Geographic/climatic variables that did not confirm the normality assumption were transformed using natural logarithms, while the other variables that were normally distributed were not transformed24. Variables with variation inflation factors > 20 were removed from the analysis to avoid high colinearity25. A Monte Carlo test with 9,999 permutations was used to determine the significance under the null hypothesis, which shows no relationship between species and environmental variables. The environmental factors showing rejection to null hypothesis (p < 0.05) in Monte Carlo test could be selected in redundancy analysis26 using the software CANOCO for Windows 4.5 version27. Based on the redundancy analysis data, the hexaploid group regression function, 0.833 × altitude + 0.681 × latitude − 0.322 × precipitation, was obtained. Then the hexaploid group index of 237 localities containing the 34 sampling points in this study were calculated and interpolated using the ordinary kriging method28 implemented in ARCMAPv.9.3 (ESRI, Redlands, CA, USA) to predict the distribution pattern of two ploidy forms throughout mainland China.
Nucleotide sequencing and sequence analysis
Genomic DNA was extracted from fin clips by DNeasy Blood & Tissue Kit (QIAGEN) following the manufacturer’s protocol. We used the following primers tf-F (CTCCTCAAAGAGCCTCGCCAT) and tf-R (TACACCTGGCCACCATCAACTG)13 to amplify an approximate 1050 bp fragment between the 7th exon to 10th exon of tf gene13, 29. PCR products were cloned into pMD-19T vector (Takara), and 12 positive clones were sequenced for each sample. M13F (−47) and M13R (−48) primers were used for bidirectional Sanger sequencing on 3730XL-96 platform (ABI).
Sequence alignments and information about nucleotide variation of tf alleles were identified using MEGA 7.030. Alleles of tf were generated by DnaSP 5.1031 software and then arranged according to their frequency. Nucleotide diversity (π) of both the 22 populations with two ploidy forms and eight geographic areas were evaluated using Arlequin version 3.532. Phylogenetic analysis of tf alleles was conducted using Bayesian inference (BI) in MRBAYES 3.1.233 and maximum-likelihood (ML) in RAXML34. We selected the best fit nucleotide substitution model using MODELTEST version 3.735. For BI tree, four independent Markov chain Monte Carlo (MCMC) chains were simultaneously run for 20,000,000 generations with sample frequency of 1000 generations. The first 25% of the trees were discarded as burn-in and the remaining tree samples were used to generate a consensus tree. For ML tree, nodal support value was assessed from 100 nonparametric bootstrap replicates. In addition, to investigate the relationship between tf alleles, a median-joining network was built using Network4.6.0.036.
To estimate divergence times in Carassius species complex, an uncorrelated relaxed molecular clock approach was implemented in Beast 1.7.537. GTR+I+G was selected as the best fit model of evolution using MODELTEST software of version 3.735. The divergence time (11.11–9.14 million years ago (Mya))38 between Cyprinus carpio and Carassius species complex was used as the calibration. The Markov Chain Monte Carlo (MCMC) analyses were run for 100,000,000 generations with sample frequency of 1,000 generations. Tracer v1.5 (http://beast.bio.ed.ac.uk/Tracer) was used to ensure adequate mixing of the MCMC with effective sample sizes (ESS) above 100. The plausible trees were summarized in the maximum clade credibility (MCC) tree after discarding first 60% of sampled generations by Tree Annotator v1.7.5, and then the results were visualized and edited in FigTree 1.4 (http://beast.bio.ed.ac.uk/FigTree).
Results
Wider geographic distribution pattern in hexaploids than in tetraploids
A total of 3105 individuals of Carassius species complex were sampled from 34 different geographic locations through mainland China. Among the sampled specimens, 2211 (71.2%) were examined as hexaploids and 894 (28.8%) as tetraploids by flow cytometry13, 21 (Table S1). Among the 34 sampled locations, there were 12 all-hexaploid populations and 22 sympatric populations of both hexaploids and tetraploids, whereas no all-tetraploid populations were detected (Table S1 and Fig. 1). Intriguingly, all-hexaploid and highly biased-hexaploid populations were majorly distributed in northeast of China, northwest of China, Yellow River, upper basins of Pearl River and Yangtze River, whereas both hexaploids and tetraploids were mainly observed to overlap in most populations of Jing-Hang Grand Canal and middle/lower basins of Yangtze River (Table S1 and Fig. 1). The comprehensive survey data indicate a significant differential distribution pattern of Carassius species complex that the hexaploids have wider geographic distribution than the tetraploids.
Association between geographic/climatic variables and the differential distribution pattern in Carassius species complex
A total of 5 geographic/climatic variables including altitude, longitude, latitude, average annual temperature (Tmean) and annual precipitation were used to exam the association between geographic/climatic variables and the differential distribution pattern (Table S2). Via redundancy analysis, differential distribution pattern in Carassius species complex was revealed to be significantly associated with altitude (inflation factor = 2.35, p = 0.0001), latitude (inflation factor = 12.07, p = 0.024) and annual precipitation (inflation factor = 14.73, p = 0.049), whereas it was unrelated to the other variables including Tmean and longitude (Table 1). And, the hexaploid frequency was positively correlated to altitude and latitude and negatively related to annual precipitation, while the tetraploid frequency was negatively related to altitude and latitude and positively correlated to annual precipitation (Fig. 2a). Moreover, the examined 34 populations were respectively assigned to four different hexaploid frequency groups (0–50%, 50–85%, 85–99%, 100%) to clarify relative association between the three significant geographic/climatic variables and the distribution pattern (Table S2). Significantly, only 10.2% of tetraploids but 89.8% of hexaploids exist in the populations at altitude higher than 456.9 m, and all-hexaploid populations generally distribute at the over 723.0 m elevation (Fig. 2b). Moreover, 89.3% of hexaploids spread in the populations with latitude over 35.6°N, while only 10.7% of tetraploids exist in this latitude range, and all-hexaploid groups tend to occur in drainages of high latitude with 38.0°N (Fig. 2c). Additionally, a total of 77.9% of hexaploids tend to inhabit in drainage basins with annual precipitation less than 893.6 mm, while only 22.1% of tetraploids exist in the populations with the same range of annual precipitation, and all-hexaploid groups tend to occur in the area with low annual precipitation under 582.3 mm (Fig. 2d). These data indicate that hexaploids might be more tolerant to harsh geographic/climatic variables than tetraploids.
Table 1.
Redundancy analysis results of 5 geographic/climatic variables that are associated with differential geographic distribution of hexaploids and tetraploids.
| Variable | Inflation factor | P-value |
|---|---|---|
| altitude | 2.35 | 0.0001* |
| longitude | 4.37 | 0.108 |
| latitude | 12.07 | 0.024* |
| Tmean | 3.47 | 0.068 |
| Annual precipitation | 14.73 | 0.049* |
Figure 2.
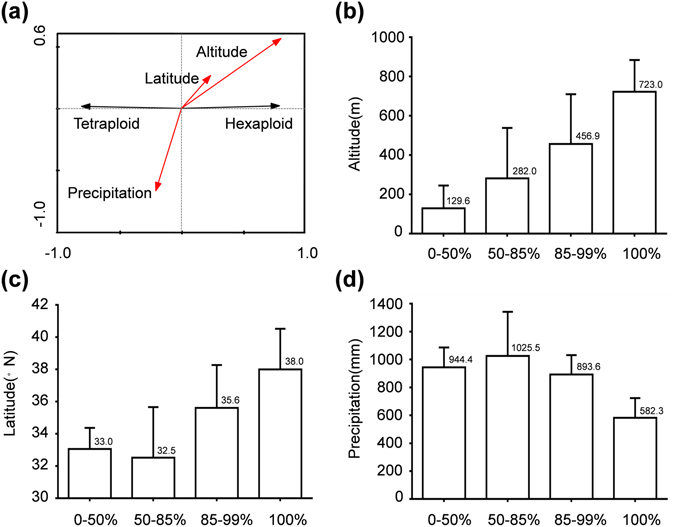
(a) The redundancy analysis on association between geographic/climatic variables and differential distribution pattern of hexaploids and tetraploids. Hexaploid and tetraploid are shown by black arrows, while altitude, latitude, and precipitation are shown by red arrows. Diagrams between different hexaploid frequencies and the correlated geographic/climatic variables including altitude (b), latitude (c) and precipitation (d). The X-axes indicate the hexaploid frequencies, and Y-axes indicate values of the correlated geographic/climatic variables.
Based on the redundancy analysis data in Fig. 2a, a hexaploid frequency group regression function, 0.833 × altitude + 0.681 × latitude − 0.322 × precipitation was obtained. To reveal further association between the ploidy distribution and the habitats, different parameters of three geographic/climatic variables in 237 localities throughout China were used to calculate the corresponding hexaploid group indexes (Table S3). We used hexaploid group indexes at Xingkai lake (−97) and Weishan lake (−176) as critical values of all-hexaploid group and low frequency (0–50%) hexaploid group through discriminant analysis, because they were closest to the lowest value of all-hexaploid frequency group and the highest value of low frequency hexaploid group respectively (Table S3). Thereby, a predicted map of hexaploid frequency distribution throughout mainland China was constructed (Fig. 3). When the hexaploid group index value of a population is higher than −97, this population would tend to be almost all-hexaploids. And, when the hexaploid group index value of a population is lower than −176, the population would tend to contain low frequency hexaploids (less than 50%). While the index of a population is between −97 and −176, this population would tend to have higher frequency of hexaploids (more than 50%). In the predicted map, all-hexaploid and highly biased-hexaploid populations were majorly distributed in northeast, northwest, upper basins of Yangtze River, Pearl River and Yellow River, whereas low frequency hexaploids were mainly observed in Jing-Hang Grand Canal and middle/lower basins of the Yangtze River (Fig. 3). Thus, actual distribution pattern of Carassius species complex (Fig. 1) is nearly concordant with the predicted map (Fig. 3), which indicates that geographic distribution of hexaploids is wider than that of tetraploids through mainland China. And hexaploids distribute predominantly in the areas of high altitude, high latitude or low precipitation (Figs 1, 2 and 3).
Figure 3.
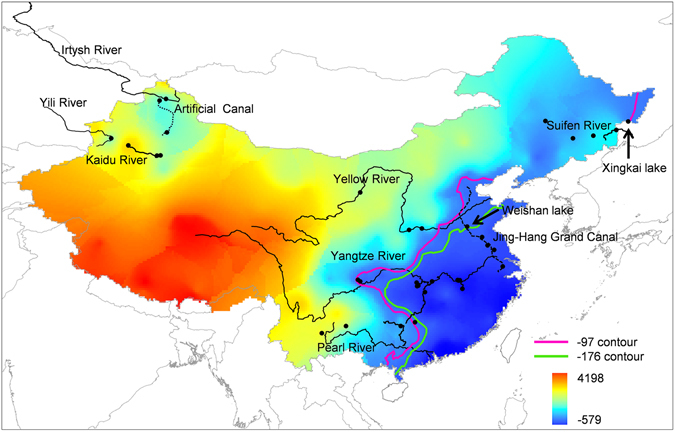
A predicted map of hexaploid frequency distribution throughout mainland China by using hexaploid group indexes. As hexaploid group indexes were of significantly difference (p = 0, < 0.05) among three assigned hexaploid frequency groups, the lowest value of all-hexaploid frequency group at Xingkai lake (−97) and the highest value of high frequency (51–99%) hexaploid group at Weishan lake (−176) were respectively used as critical values of all-hexaploid group and high frequency (51–99%) hexaploid group through discriminant analysis. The purple −97 contour line stands for the predicted boundary between all-hexaploids and high frequency (51–99%) hexaploids. The green −176 contour line represents the predicted boundary between high frequency (51–99%) hexaploids and low frequency (0–50%) hexaploids. −579 and 4198 indicate the lowest and highest values of predicted hexaploid group indexes respectively. The map was created in ARCMAPv.9.3 (ESRI, Redlands, CA, USA) (http://www.esri.com/products).
However, a few of exceptional populations with tetraploids were also observed from several lakes, such as Bositeng lake, Dawusong lake and Tian’e lake in northwest, as well as Chagan Lake, Jingbo Lake and Songhua Lake in northeast (Fig. 1). These exceptions might be resulted from human activities, as Carassius species complex was previously introduced from middle and lower basins of the Yangtze River to these lakes, especially Bositeng lake and Dawusong lake39.
Higher nucleotide diversity in hexaploids than in tetraploids
Firstly, tf fragments ranging from 1002 bp to 1108 bp were amplified to clarify their nucleotide differences among the 3105 samples. A total of 37260 clones were sequenced and 526 tf alleles were distinguished at last. Among the 526 alleles, 99 alleles (tf1–tf99) with occurrence frequencies ranging from 27.99% to 0.04% were shared by hexaploids and tetraploids, and 331 alleles (tf100–tf430) with occurrence frequencies ranging from 0.46% to 0.02% were detected only in hexaploids (Fig. S1), whereas only 96 alleles (tf431–tf526) with occurrence frequencies ranging from 0.38% to 0.02% were identified only in tetraploids (Fig. S1).
Furthermore, we analyzed and compared nucleotide diversities of these tf alleles between hexaploids and tetraploids among the 34 sampled populations (Table S4). The total nucleotide diversity of hexaploids (0.0629 ± 0.0300) is higher than that of tetraploids (0.0546 ± 0.0261) in all populations. Moreover, in the 16 populations with enough hexaploids and tetraploids (at least five specimens of each ploid form), the mean value of nucleotide diversities in hexaploids are generally higher than those of sympatric tetraploids except the populations of Taihu lake and Chagan lake (Fig. 4). The higher nucleotide diversity in hexaploids than in tetraploids implicates that hexaploids might have more advantages than tetraploids, as higher nucleotide diversity is commonly able to provide more raw materials for new gene arising and adaptive evolution.
Figure 4.

Nucleotide diversity of tf alleles in hexaploids (black) and tetraploids (grey). The X-axis shows the abbreviation of each sampled location, and Y- axis indicates value of nucleotide diversity. Geographic areas of the sampled locations are indicated by curly braces on the top. JH, Jing-Hang Grand Canal; L-YzR, lower basin of Yangtze River; M-YzR, middle basin of Yangtze River; U-YzR, upper basin of Yangtze River; NW, northwest of China; NE, northeast of China; YR, Yellow river; U-PR, upper basin of Pearl River.
Phylogenetic relationship and evolutionary history of tf alleles
Three major lineages were revealed via median-joining network analysis based on the identified 526 tf alleles (Fig. 5). Lineage A included 30 tf alleles, in which 6 tf alleles were shared by hexaploids and tetraploids, and 19 and 5 tf alleles were respectively detected only in hexaploids and in tetraploids (Fig. 5). Meanwhile, tf alleles of lineage A mainly existed in the samples of Yangtze River basin, Jingbo lake and Yili River. A total of 317 tf alleles were grouped into lineage B, in which 63 tf alleles were shared by two ploidy forms, and 190 and 64 tf alleles were respectively detected only in hexaploids and in tetraploids (Fig. 5). The highest occurrence frequency of tf1 (27.6%) was not only shared by hexaploids and tetraploids but also detected from all populations throughout mainland China (Fig. 5). The lineage C included 179 tf alleles, in which 30 tf alleles were detected in hexaploids and tetraploids, 122 tf alleles only in hexaploids, and 27 tf alleles only in tetraploids. The high frequency occurrence of alleles (tf2 and tf5) were also found in all sampled populations. The high frequency occurrence and extensive distribution implies that tf1, tf2 and tf5 might be ancient tf alleles and reflect a common origin of Carassius species complex.
Figure 5.
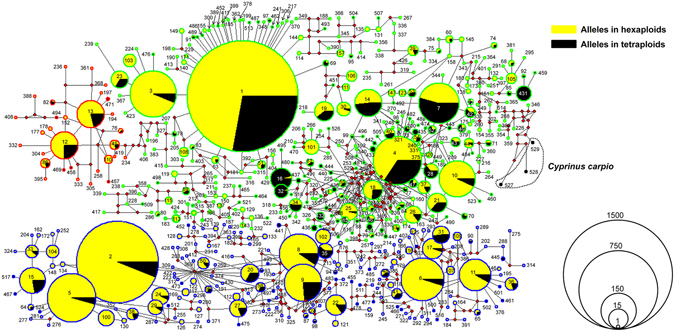
Median-joining network of 526 tf alleles identified from Carassius species complex. Three tf alleles from Cyprinus carpio are used as outgroup. Circles represent different alleles and their corresponding occurrence frequency in all sampled populations. Lineage A, B and C are exhibited in red, green and blue of border colors respectively, which also stand for phylogroups A, B and C in Fig. S2. Yellow and black colors inside the circle show the percentage of hexaploid and tetraploid respectively. Allele codes are denoted inside or beside the circles. Solid red spots represent un-sampled or predicted alleles.
Based on the 526 tf alleles, a Bayesian tree was generated (Fig. S2), and similar topology structure with MJ network was revealed. Once again, these tf alleles of hexaploids and tetraploids were intermixed in lineage A, lineage B and lineage C, and numerous alleles were shared by both hexaploids and tetraploids within each lineage (Fig. S2). Furthermore, a simplified time-calibrated phylogenetic tree of these tf alleles was constructed. As shown in Fig. 6a, lineage A was the earliest split at about 9.32 Mya, and lineage B and C were diverged around 7.88 Mya. In lineage B, sublineage B1 from 10 sampled populations and sublineage B2-B7 from individuals throughout China were separated around 6.88 Mya. In lineage C, sublineage C1 with only one tf allele (tf418) from Tian’e lake was divided around 6.62 Mya, which might be the result of human activity. Sublineage C2 and sublineage C3 occurred in all populations were split around 5.46 Mya. Significantly, the repeated polyploidy traces during the expansion of Carassius species complex could be also detected from some branch topology structures with high support in lineage C. For example, sublineage C2-6, C2-7 and C2-8 with both hexaploids and tetraploids were split at about 2.25 Mya, 1.84 Mya and 1.92 Mya respectively (Fig. 6a). The above data suggest that hexaploids might originate from tetraploids independently via multiple autopolyploidy events in Carassius species complex, because hexaploids and tetraploids are intermixed in the same lineages and they share the same alleles (Fig. 6a and Fig. S2). In addition, relative frequencies of three different lineages in all sampled populations were presented (Fig. 6b). The first separated older lineage A mainly appearing in most populations of Yangtze River basin (Fig. 6) and higher nucleotide diversities in middle/lower basins of Yangtze River than in other areas (Fig. S3) suggested that Yangtze River basin might be the potential origin center of Carassius species complex, and then the Carassius species complex distribute to other rivers and lakes throughout China.
Figure 6.
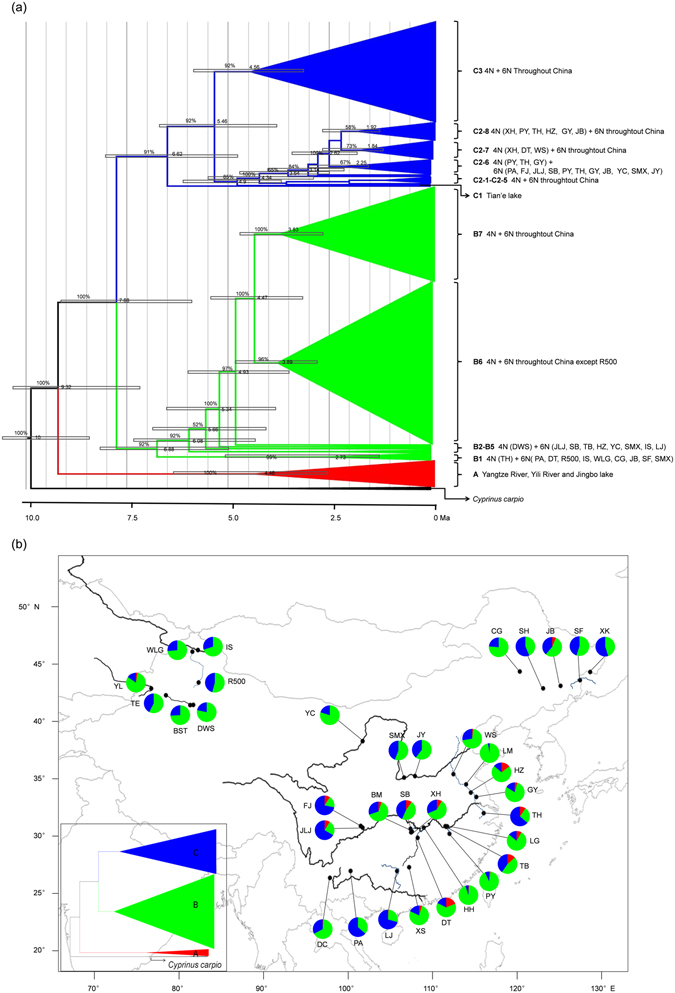
Simplified time-calibrated phylogeny tree (a) and relative frequency of three different lineages in 34 sampled populations (b). Values above branches indicate Bayesian posterior probabilities, and divergence times are shown near each node. Tf allele of Cyprinus carpio is used as outgroup. Tf alleles in each lineage are described in detail in Fig. S2. 4N and 6N are the abbreviation of tetraploids and hexaploids respectively. Each lineage is uniquely colored and is matched with the color of pie-diagrams in Fig. 5. The map was created in ARCMAPv.9.3 (ESRI, Redlands, CA, USA) (http://www.esri.com/products).
Discussion
In comparison with ubiquitous existence in plants40–43, polyploidy incidences in animals are rare, especially in vertebrates including fishes, amphibians and reptiles3, 4, 6, 19, 44–46. However, our current study in Carassius species complex may provide some novel knowledge about adaptive evolution and ecological novelty of recurrent polyploidy in vertebrates from the natural evolutionary coexistence system of ancestral tetraploids and recurrent hexaploids. Through this study, we had distinguished 2211 hexaploids and 894 tetraploids in 3105 specimens of Carassius species complex sampled from 34 locations in mainland China (Fig. 1 and Table S1), and observed a close association between differential distribution pattern of hexaploids and tetraploids and geographic/climatic variables including altitude, latitude and annual precipitation (Fig. 2 and Table 1), in which hexaploids had wider distribution than tetraploids especially in the areas with higher altitude, higher latitude and lower annual precipitation (Fig. 3). Moreover, we unearthed and analyzed various tf alleles from the sampled hexaploids and tetraploids, and commonly revealed higher nucleotide diversity in hexaploids than in tetraploids. These data indicate that the hexaploids might possess stronger environmental adaptation and ecological novelty than the ancestral tetraploids. Intriguingly, similar geographic distributions and high genetic diversity have been also observed in some regional investigation of Carassius species complex in East Asia11, 13. Similar to the polyploidy in plants, which has been revealed to have significant evolutionary consequences for increasing genetic diversity, genomic complexity and ecological adaption1, 47–50, hexaploids indeed possess wider geographic distribution and higher genetic diversity than the ancestral tetraploids.
In addition, this study further helps us understand the evolutionary history and trajectory of different ploidy forms in Carassius species complex. In Carassius species complex, the ancestral tetraploids with 100 chromosomes and the recurrent hexaploids with about 150 chromosomes15, 16 were extensively found in the Eurasian continent12–14, 51, 52, and a few octoploids with about 200 chromosomes were occasionally recorded in some natural habitats and artificial populations39, 53. In this survey, only hexaploids and tetraploids were identified, and their phylogenetic relationship and evolutionary history (Figs S2 and 6) were analyzed from the identified 526 diverse tf alleles. As revealed by Figs 5 and 6, three major lineages were presented, and numerous alleles were shared by both hexaploids and tetraploids within each lineage. Based on the previous hypothesis that polyploidy occurrence might be related to glaciations and quaternary climatic changes38, 54, our current study further suggests that Yangtze River basin should be the refuge during glaciations and potential origin center of Carassius species complex, and then the Carassius species complex radiates across East Asia during this expansion, as the older lineage A mainly consists of populations from Yangtze River basin (Fig. 6) and the highest nucleotide diversity also is from the populations of Yangtze River basin (Fig. S3). The fossil of Carassius in Pliocene epoch (5.3–2.6 Mya) in north of China (Yushe, Shanxi province)55 might provide evidence for expansion of Carassius species complex. Moreover, hexaploids might originate from tetraploids independently via multiple autopolyploidy events in Carassius species complex, because hexaploids and tetraploids are intermixed in the same lineages and they share the same alleles (Fig. 6 and Fig. S2).
The evolutionary success of recurrent polyploidy has been mainly attributed to the diploidization process followed by polyploidy56–58. Based on the current findings and previous reports3, 59, 60, we believe that extant hexaploids in Carassius species complex are entering an evolutionary trajectory of diploidization or have undergone diploidization, because normal meiosis completion, multiple modes of unisexual gynogenesis and sexual reproduction, and extra microchromosomes for male determination, have been observed in some gynogenetic clones of hexaploid Carassius gibelio 59–61. However, recurrent polyploidy is not ceased in Carassius species complex as occasional octoploids have been also detected in some natural drainage systems and artificially proliferated populations31, 39. Thus the Carassius species complex provides an ideal system to better understand the consequences of recurrent polyploidy and fills in the huge gaps across the spectrum of ecology, genetics and evolution biology in polyploid vertebrates.
Electronic supplementary material
Acknowledgements
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA08030201), the earmarked fund for Modern Agro-industry Technology Research System (NYCYTX-49), the National Natural Science Foundation of China (31123001), the Special Fund for Agro-scientific Research in the Public Interest (Grant No. 200903046), and the Autonomous Project of the State Key Laboratory of Freshwater Ecology and Biotechnology (2016FBZ01), and the Autonomous Project of the Institute of Hydrobiology, Chinese Academy of Sciences (Y25A171).
Author Contributions
J.F.G. designed research, J.F.G., X.L.L., F.F.J., J.F.M., Z.W.W., L.Z. collected samples, performed laboratory work. X.L.L., X.Y.L., X.J.Z., Z.L. and F.C. contributed to data analyses and J.F.G. wrote the manuscript. All authors contributed substantially to revisions.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-05731-0
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Soltis PS, Liu X, Marchant DB, Visger CJ, Soltis DE. Polyploidy and novelty: Gottlieb’s legacy. Phil. Trans. R. Soc. B. 2014;369:20130351. doi: 10.1098/rstb.2013.0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mable BK. ‘Why polyploidy is rarer in animals than in plants’: myths and mechanisms. Biol. J. Linn. Soc. 2004;82:453–466. doi: 10.1111/j.1095-8312.2004.00332.x. [DOI] [Google Scholar]
- 3.Gui JF, Zhou L. Genetic basis and breeding application of clonal diversity and dual reproduction modes in polyploid Carassius auratus gibelio. Sci. China Life Sci. 2010;53:409–415. doi: 10.1007/s11427-010-0092-6. [DOI] [PubMed] [Google Scholar]
- 4.Mable BK, Alexandrou MA, Taylor MI. Genome duplication in amphibians and fish: an extended synthesis. J. Zool. 2011;284:151–182. doi: 10.1111/j.1469-7998.2011.00829.x. [DOI] [Google Scholar]
- 5.Schmid M, Evans BJ, Bogart JP. Polyploidy in Amphibia. Cytogenet. Genome Res. 2015;145:315–330. doi: 10.1159/000431388. [DOI] [PubMed] [Google Scholar]
- 6.Zhou, L. & Gui, J. F. Natural and artificial polyploids in aquaculture. Aquac. Fish. doi:10.1016/j.aaf.2017.04.003 (2017).
- 7.Choleva L, Janko K. Rise and Persistence of Animal Polyploidy: Evolutionary Constraints and Potential. Cytogenet. Genome Res. 2013;140:151–170. doi: 10.1159/000353464. [DOI] [PubMed] [Google Scholar]
- 8.Zhu HP, Gui JF. Identification of genome organization in the unusual allotetraploid form of Carassius auratus gibelio. Aquaculture. 2007;265:109–117. doi: 10.1016/j.aquaculture.2006.10.026. [DOI] [Google Scholar]
- 9.Mason AS, Pires JC. Unreduced gametes: meiotic mishap or evolutionary mechanism? Trends Genet. 2015;31:5–10. doi: 10.1016/j.tig.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Mei J, Gui JF. Genetic basis and biotechnological manipulation of sexual dimorphism and sex determination in fish. Sci. China Life Sci. 2015;58:124–136. doi: 10.1007/s11427-014-4797-9. [DOI] [PubMed] [Google Scholar]
- 11.Luo J, et al. Tempo and mode of recurrent polyploidization in the Carassius auratus species complex (Cypriniformes, Cyprinidae) Heredity. 2014;112:415–427. doi: 10.1038/hdy.2013.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abramenko MI, Nadtoka EV, Makhotkin MA, Kravchenko OV, Poltavtseva TG. Distribution and cytogenetic features of triploid male goldfish in Azov basin. Ontogenez. 2004;35:375–386. [PubMed] [Google Scholar]
- 13.Jiang FF, et al. High male incidence and evolutionary implications of triploid form in northeast Asia Carassius auratus complex. Mol. Phylogenet. Evol. 2013;66:350–359. doi: 10.1016/j.ympev.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Sakai H, Iguchi K, Yamazaki Y, Sideleva VG, Goto A. Morphological and mtDNA sequence studies on three crucian carps (Carassius: Cyprinidae) including a new stock from the Ob River system, Kazakhstan. J. Fish Biol. 2009;74:1756–1773. doi: 10.1111/j.1095-8649.2009.02203.x. [DOI] [PubMed] [Google Scholar]
- 15.Luo J, Stadler PF, He S, Meyer A. PCR survey of hox genes in the goldfish Carassius auratus auratus. J. Exp. Zool. Part. B. 2007;308:250–258. doi: 10.1002/jez.b.21144. [DOI] [PubMed] [Google Scholar]
- 16.Ohno S, Muramoto J, Christian L, Atkin NB. Diploid tetraploid relationship among Old World members of fish family Cyprinidae. Chromosoma. 1967;23:1–9. doi: 10.1007/BF00293307. [DOI] [Google Scholar]
- 17.Zhou L, Gui JF. Karyotypic diversity in polyploid gibel carp, Carassius auratus gibelio Bloch. Genetica. 2002;115:223–232. doi: 10.1023/A:1020102409270. [DOI] [PubMed] [Google Scholar]
- 18.Gui JF, Zhu ZY. Molecular basis and genetic improvement of economically important traits in aquaculture animals. Sci. Bull. 2012;57:1751–1760. doi: 10.1007/s11434-012-5213-0. [DOI] [Google Scholar]
- 19.Li XY, et al. Evolutionary history of two divergent Dmrt1 genes reveals two rounds of polyploidy origins in gibel carp. Mol. Phylogenet. Evol. 2014;78:96–104. doi: 10.1016/j.ympev.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Yang L, Yang ST, Wei XH, Gui JF. Genetic diversity among different clones of the gynogenetic silver crucian carp, Carassius auratus gibelio, revealed by transferrin and isozyme markers. Biochem. Genet. 2001;39:213–225. doi: 10.1023/A:1010297426390. [DOI] [PubMed] [Google Scholar]
- 21.Wei WH, Zhang J, Zhang YB, Zhou L, Gui JF. Genetic heterogeneity and ploidy level analysis among different gynogenetic clones of the polyploid gibel carp. Cytometry A. 2003;56:46–52. doi: 10.1002/cyto.a.10077. [DOI] [PubMed] [Google Scholar]
- 22.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005;25:1965–1978. doi: 10.1002/joc.1276. [DOI] [Google Scholar]
- 23.Correa-Metrio A, Dechnik Y, Lozano-García S, Caballero M. Detrended correspondence analysis: A useful tool to quantify ecological changes from fossil data sets. B. Soc. Geol. Mex. 2013;66:135–143. [Google Scholar]
- 24.Jiang XM, et al. Structure of Macroinvertebrate Communities in Relation to Environmental Variables in a Subtropical Asian River System. Int. Rev. Hydrobiol. 2010;95:42–57. doi: 10.1002/iroh.200811131. [DOI] [Google Scholar]
- 25.McCune, B. & Grace, J. B. Analysis of ecological communities. MjM Software Design (2002).
- 26.Magnan P, Rodriguez MA, Legendre P, Lacasse S. Dietary Variation in a freshwater fish species: relative contributions of biotic nteractions, abiotic factors, and spatia structure. Can. J. Fish Aquat. Sci. 1994;51:2856–2865. doi: 10.1139/f94-284. [DOI] [Google Scholar]
- 27.Gilliam FS, Saunders NE. Making more sense of the order: A review of Canoco for Windows 4.5, PC-ORD version 4 and SYN-TAX 2000. J. Veg. Sci. 2003;14:297–304. doi: 10.1111/j.1654-1103.2003.tb02155.x. [DOI] [Google Scholar]
- 28.Zuckerkandl, E. & Pauling, L. Evolutionary divergence and convergence in proteins. New York: Academic Press (1965).
- 29.Yang L, Gui JF. Positive selection on multiple antique allelic lineages of transferrin in the polyploid Carassius auratus. Mol. Biol. Evol. 2004;21:1264–1277. doi: 10.1093/molbev/msh121. [DOI] [PubMed] [Google Scholar]
- 30.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- 32.Excoffier L, Lischer HEL. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010;10:564–567. doi: 10.1111/j.1755-0998.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- 33.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 34.Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML Web Servers. Syst. Biol. 2008;57:758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- 35.Posada D, Crandall KA. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- 36.Bandelt HJ, Forster P, Rohl A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999;16:37–48. doi: 10.1093/oxfordjournals.molbev.a026036. [DOI] [PubMed] [Google Scholar]
- 37.Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007;7:8. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao Y, et al. Quaternary palaeoenvironmental oscillations drove the evolution of the Eurasian Carassius auratus complex (Cypriniformes, Cyprinidae) J. Biogeogr. 2012;39:2264–2278. doi: 10.1111/j.1365-2699.2012.02755.x. [DOI] [Google Scholar]
- 39.Gui JF, Liang SC, Zhu LF, Jiang YG. Discovery of multiple tetraploids in artificially propagated populations of allogynogenetic Silver crucian carp and their breeding potentialities. Sci. Bull. 1993;38:327–331. [Google Scholar]
- 40.Guo YP, Saukel J, Mittermayr R, Ehrendorfer F. AFLP analyses demonstrate genetic divergence, hybridization, and multiple polyploidization in the evolution of Achillea (Asteraceae-Anthemideae) New Phytol. 2005;166:273–289. doi: 10.1111/j.1469-8137.2005.01315.x. [DOI] [PubMed] [Google Scholar]
- 41.Jiao Y, et al. Ancestral polyploidy in seed plants and angiosperms. Nature. 2011;473:97–100. doi: 10.1038/nature09916. [DOI] [PubMed] [Google Scholar]
- 42.Robertson A, et al. Hybridization and polyploidy as drivers of continuing evolution and speciation in Sorbus. Mol. Ecol. 2010;19:1675–1690. doi: 10.1111/j.1365-294X.2010.04585.x. [DOI] [PubMed] [Google Scholar]
- 43.Segraves KA, Thompson JN, Soltis PS, Soltis DE. Multiple origins of polyploidy and the geographic structure of Heuchera grossulariifolia. Mol. Ecol. 1999;8:253–262. doi: 10.1046/j.1365-294X.1999.00562.x. [DOI] [Google Scholar]
- 44.Gregory, T. R. & Mable, B. K. Polyploidy in Animals. Genome Biol. Evol. 427–517 (2005).
- 45.Sick K. Haemoglobin polymorphism of cod in the North Sea and the North Atlantic ocean. Hereditas. 1965;54:49–69. doi: 10.1111/j.1601-5223.1965.tb02005.x. [DOI] [PubMed] [Google Scholar]
- 46.Xu P, et al. Genome sequence and genetic diversity of the common carp. Cyprinus carpio. Nature Genet. 2014;46:1212–1219. doi: 10.1038/ng.3098. [DOI] [PubMed] [Google Scholar]
- 47.Doyle JJ, et al. Evolutionary genetics of genome merger and doubling in plants. Annu. Rev. Genet. 2008;42:443–461. doi: 10.1146/annurev.genet.42.110807.091524. [DOI] [PubMed] [Google Scholar]
- 48.Parisod C, Holderegger R, Brochmann C. Evolutionary consequences of autopolyploidy. New Phytol. 2010;186:5–17. doi: 10.1111/j.1469-8137.2009.03142.x. [DOI] [PubMed] [Google Scholar]
- 49.Ramsey J, Ramsey TS. Ecological studies of polyploidy in the 100 years following its discovery. Phil. Trans. R. Soc. B. 2014;369:5042–5050. doi: 10.1098/rstb.2013.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.te Beest M, et al. The more the better? The role of polyploidy in facilitating plant invasions. Ann. Bot. 2012;109:19–45. doi: 10.1093/aob/mcr277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takada M, et al. Biogeography and evolution of the Carassius auratus-complex in East Asia. BMC Evol. Biol. 2010;10:18. doi: 10.1186/1471-2148-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toth B, Varkonyi E, Hidas A, Meleg EE, Varadi L. Genetic analysis of offspring from intra- and interspecific crosses of Carassius auratus gibelio by chromosome and RAPD analysis. J. Fish Biol. 2005;66:784–797. doi: 10.1111/j.0022-1112.2005.00644.x. [DOI] [Google Scholar]
- 53.Xiao J, et al. Coexistence of diploid, triploid and tetraploid crucian carp (Carassius auratus) in natural waters. BMC Genet. 2011;12:15. doi: 10.1186/1471-2156-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Comes HP, Kadereit JW. The effect of Quaternary climatic changes on plant distribution and evolution. Trends Plant Sci. 1998;3:432–438. doi: 10.1016/S1360-1385(98)01327-2. [DOI] [Google Scholar]
- 55.Liu XT, Su DZ. Pliocene fishes from Yushe basin, Shansi. Vert. PalAsiat. 1962;6:1–125. [Google Scholar]
- 56.Soltis PS, Marchant DB, Van dPY, Soltis DE. Polyploidy and genome evolution in plants. Curr. Opin. Genet. Dev. 2015;35:119–125. doi: 10.1016/j.gde.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 57.Wendel JF. The wondrous cycles of polyploidy in plants. Am. J. Bot. 2015;102:1753. doi: 10.3732/ajb.1500320. [DOI] [PubMed] [Google Scholar]
- 58.Zhou, L. & Gui, J. F. Natural and artificial polyploids in aquaculture. Aqua. Fish., doi:10.1016/j.aaf.2017.04.003 (2017).
- 59.Li XY, et al. Extra microchromosomes play male determination role in polyploid gibel carp. Genetics. 2016;203:1415–1424. doi: 10.1534/genetics.115.185843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang J, et al. Meiosis completion and various sperm responses lead to unisexual and sexual reproduction modes in one clone of polyploid Carassius gibelio. Sci. Rep. 2015;5:10898. doi: 10.1038/srep10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li XY, et al. A novel male-specific setdm domain-containing gene setdm identified from extra microchromosomes of gibel carp males. Sci. Bull. 2017;62:528–536. doi: 10.1016/j.scib.2017.04.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


