Abstract
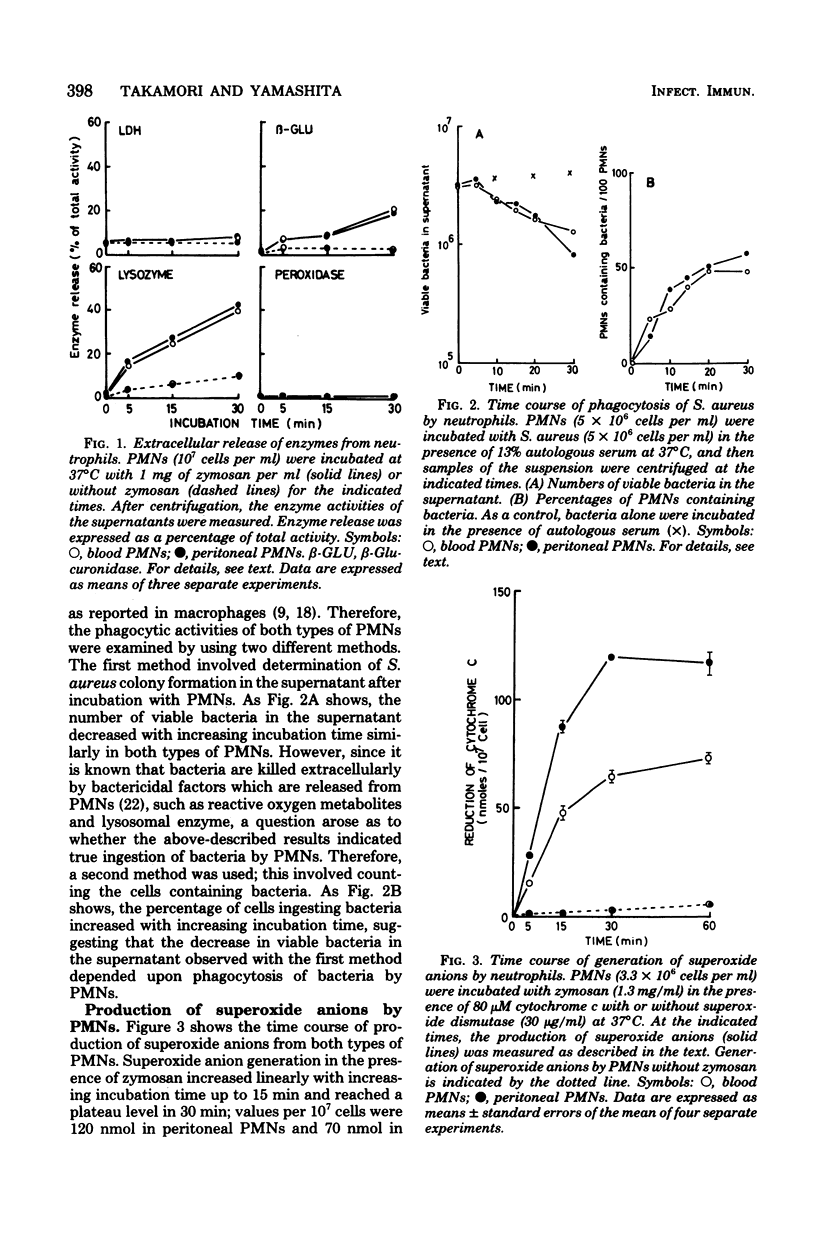
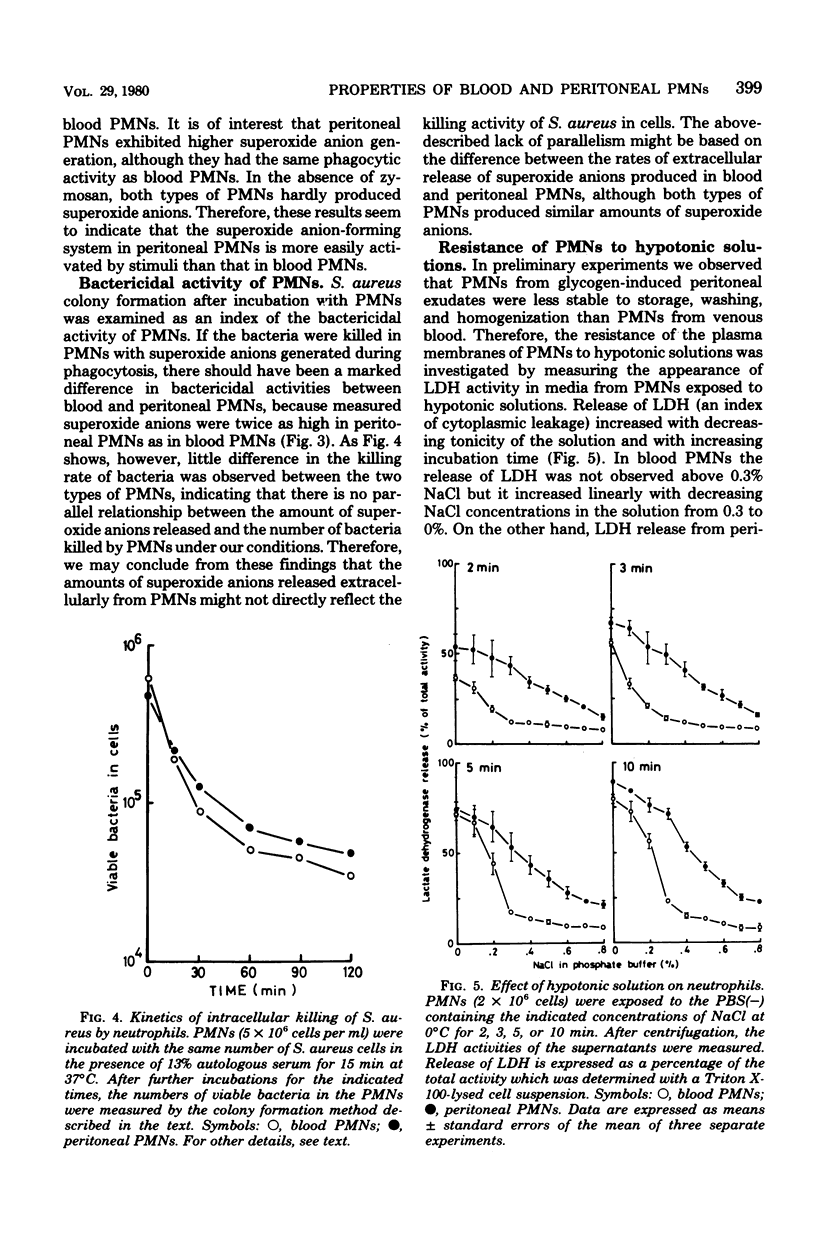
The biochemical properties of polymorphonuclear neutrophils from blood and peritoneal exudates of rabbits were compared. All enzymes measured showed almost identical activities in both types of cells, except for alkaline phosphodiesterase, the activity of which was seven times higher in peritoneal neutrophils. During phagocytosis, blood and peritoneal beta-glucuronidases were released in very similar fashions. Lysozyme, one of the enzymes concerned with killing of bacteria, as well as beta-glucuronidase, showed the same releasing pattern in both types of cells, but peroxidase was hardly released. Although superoxide anion generation in peritoneal neutrophils was two times higher than superoxide generation in blood neutrophils, phagocytic and bactericidal activities were almost the same in blood and peritoneal neutrophils. Blood neutrophils were more resistant to hypotonic lysis than were peritoneal neutrophils. These results show that there are no distinct differences in enzymatic and functional properties between blood and peritoneal polymorphonuclear neutrophils, except for alkaline phosphodiesterase activity, superoxide anion production, and osmotic fragility.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avila J. L., Convit J. Studies on human polymorphonuclear leukocyte enzymes. I. Assay of acid hydrolases and other enzymes. Biochim Biophys Acta. 1973 Feb 15;293(2):397–408. doi: 10.1016/0005-2744(73)90347-1. [DOI] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Baggiolini M., Hirsch J. G., De Duve C. Further biochemical and morphological studies of granule fractions from rabbit heterophil leukocytes. J Cell Biol. 1970 Jun;45(3):586–597. doi: 10.1083/jcb.45.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M., Hirsch J. G., De Duve C. Resolution of granules from rabbit heterophil leukocytes into distinct populations by zonal sedimentation. J Cell Biol. 1969 Feb;40(2):529–541. doi: 10.1083/jcb.40.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton D. F., Farquhar M. G. Differences in enzyme content of azurophil and specific granules of polymorphonuclear leukocytes. I. Histochemical staining of bone marrow smears. J Cell Biol. 1968 Nov;39(2):286–298. doi: 10.1083/jcb.39.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretz U., Baggiolini M. Biochemical and morphological characterization of azurophil and specific granules of human neutrophilic polymorphonuclear leukocytes. J Cell Biol. 1974 Oct;63(1):251–269. doi: 10.1083/jcb.63.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheson B. D., Curnette J. T., Babior B. M. The oxidative killing mechanisms of the neutrophil. Prog Clin Immunol. 1977;3:1–65. [PubMed] [Google Scholar]
- Cohn Z. A. Activation of mononuclear phagocytes: fact, fancy, and future. J Immunol. 1978 Sep;121(3):813–816. [PubMed] [Google Scholar]
- Dewald B., Baggiolini M., Curnutte J. T., Babior B. M. Subcellular localization of the superoxide-forming enzyme in human neutrophils. J Clin Invest. 1979 Jan;63(1):21–29. doi: 10.1172/JCI109273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald B., Rindler-Ludwig R., Bretz U., Baggiolini M. Subcellular localization and heterogeneity of neutral proteases in neutrophilic polymorphonuclear leukocytes. J Exp Med. 1975 Apr 1;141(4):709–723. doi: 10.1084/jem.141.4.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDBARG J. A., RUTENBURG A. M. The colorimetric determination of leucine aminopeptidase in urine and serum of normal subjects and patients with cancer and other diseases. Cancer. 1958 Mar-Apr;11(2):283–291. doi: 10.1002/1097-0142(195803/04)11:2<283::aid-cncr2820110209>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M., Hoffstein S. T., Weissmann G. Mechanisms of lysosomal enzyme release from human polymorphonuclear leukocytes. Effects of phorbol myristate acetate. J Cell Biol. 1975 Sep;66(3):647–652. doi: 10.1083/jcb.66.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I., Hoffstein S., Gallin J., Weissmann G. Mechanisms of lysosomal enzyme release from human leukocytes: microtubule assembly and membrane fusion induced by a component of complement. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2916–2920. doi: 10.1073/pnas.70.10.2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Lint T. F., George W. J. Hormonal control of lysosomal enzyme release from human neutrophils. Effects of autonomic agents on enzyme release, phagocytosis, and cylic nucleotide levels. J Exp Med. 1974 Jun 1;139(6):1395–1414. doi: 10.1084/jem.139.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Godzik C. A., Cohn Z. A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978 Jul 1;148(1):115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIND P. R., KING E. J. Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine. J Clin Pathol. 1954 Nov;7(4):322–326. doi: 10.1136/jcp.7.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky M. L. Chronic granulomatous disease--pieces of a cellular and molecular puzzle. Fed Proc. 1973 Apr;32(4):1527–1533. [PubMed] [Google Scholar]
- Karnovsky M. L., Lazdins J. K. Biochemical criteria for activated macrophages. J Immunol. 1978 Sep;121(3):809–813. [PubMed] [Google Scholar]
- Klebanoff S. J. Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol. 1975 Apr;12(2):117–142. [PubMed] [Google Scholar]
- Klebanoff S. J., Hamon C. B. Role of myeloperoxidase-mediated antimicrobial systems in intact leukocytes. J Reticuloendothel Soc. 1972 Aug;12(2):170–196. [PubMed] [Google Scholar]
- Nagaoka I., Yamashita T. Leucine aminopeptidase as an echo-enzyme of polymorphonuclear neutrophils. Biochim Biophys Acta. 1980 May 8;598(1):169–172. doi: 10.1016/0005-2736(80)90274-6. [DOI] [PubMed] [Google Scholar]
- Okamura N., Ishibashi S., Takano T. Evidence for bactericidal activity of polymorphonuclear leukocytes without phagocytosis. J Biochem. 1979 Aug;86(2):469–475. doi: 10.1093/oxfordjournals.jbchem.a132546. [DOI] [PubMed] [Google Scholar]
- Touster O., Aronson N. N., Jr, Dulaney J. T., Hendrickson H. Isolation of rat liver plasma membranes. Use of nucleotide pyrophosphatase and phosphodiesterase I as marker enzymes. J Cell Biol. 1970 Dec;47(3):604–618. doi: 10.1083/jcb.47.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright D. G., Malawista S. E. The mobilization and extracellular release of granular enzymes from human leukocytes during phagocytosis. J Cell Biol. 1972 Jun;53(3):788–797. doi: 10.1083/jcb.53.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Imaizumi N., Yuasa S. Effect of endocellular cryoprotectant upon polymorphonuclear neutrophil function during storage at low temperature. Cryobiology. 1979 Apr;16(2):112–117. doi: 10.1016/0011-2240(79)90020-8. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Takamori K., Tanaka Y. A possible preferential inhibition of chemotaxis of polymorphonuclear neutrophils by a chemical modification. Experientia. 1979 Oct 15;35(10):1345–1347. doi: 10.1007/BF01963999. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Tanaka Y., Horigome T., Fujikawa E. Dependence on the lipophilicity of maleimide derivatives in their inhibitory action upon chemotaxis in neutrophils. Experientia. 1979 Aug 15;35(8):1054–1056. doi: 10.1007/BF01949937. [DOI] [PubMed] [Google Scholar]
- Zurier R. B., Hoffstein S., Weissmann G. Cytochalasin B: effect on lysosomal enzyme release from human leukocytes. Proc Natl Acad Sci U S A. 1973 Mar;70(3):844–848. doi: 10.1073/pnas.70.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurier R. B., Hoffstein S., Weissmann G. Mechanisms of lysosomal enzyme release from human leukocytes. I. Effect of cyclic nucleotides and colchicine. J Cell Biol. 1973 Jul;58(1):27–41. doi: 10.1083/jcb.58.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]


