Abstract
Background
Atrial fibrillation (AFib) is a common comorbidity in HF and affects patients’ outcome. We sought to assess the effects of serelaxin in patients with and without AFib.
Methods
In a post hoc analysis of the RELAX-AHF trial, we compared the effects of serelaxin on efficacy end points, safety end points and biomarkers in 1161 patients with and without AFib on admission electrocardiogram.
Results
AFib was present in 41.3% of patients. Serelaxin had a similar effect in patients with and without AFib, including dyspnea relief by visual analog scale through day 5 [mean change in area under the curve, 541.11 (33.79, 1048.44), p = 0.0366 in AFib versus 361.80 (−63.30, 786.90), p = 0.0953 in non-AFib, interaction p = 0.5954] and all-cause death through day 180 [HR = 0.42 (0.23, 0.77), p = 0.0051 in AFib versus 0.90 (0.53, 1.52), p = 0.6888 in non-AFib, interaction p = 0.0643]. Serelaxin was similarly safe in the two groups and induced similar reductions in biomarkers of cardiac, renal and hepatic damage. Stroke occurred more frequently in AFib patients (2.8 vs. 0.8%, p = 0.0116) and there was a trend for lower stroke incidence in the serelaxin arm in AFib patients (odds ratios, 0.31, p = 0.0759 versus 3.88, p = 0.2255 in non-AFib, interaction p = 0.0518).
Conclusions
Serelaxin was similarly safe and efficacious in improving short- and long-term outcomes and inducing organ protection in acute HF patients with and without AFib.
Keywords: Serelaxin, Relaxin, Acute heart failure, Atrial fibrillation
Introduction
Heart failure (HF) remains the most common reason for hospital admission in the elderly [1–4]. Although improvements in re-admission rates have been recently observed, outcomes for patients admitted for HF remain poor, with high post-discharge mortality and rehospitalization rates [2–7].
Atrial fibrillation (AFib) is a common comorbid state in HF patients, including those admitted for acute HF [8]. Acute HF registries report a prevalence of AFib ranging between 27 and 45% [2]. There is a bidirectional pathogenetic relationship linking HF with AFib [9]. On one hand, HF leads to increased atrial pressures and neurohormonal activation, resulting in structural and electrical atrial remodeling: factors that constitute the ideal substrate for the development of AFib [10, 11]. On the other hand, AFib doubles the risk for HF development. It is a frequent trigger for HF decompensation resulting in a high overall risk of cardiovascular complications, including a fivefold higher risk of stroke [2, 12]. The presence of AFib directly affects the outcomes of HF patients, predicting a worse prognosis [13].
Serelaxin, a recombinant form of human relaxin-2, improves symptoms and outcomes in patients admitted for acute HF, as reported by the RELAX-AHF trial [14–18]. In this study, 53% of patients had a history of AFib, while 41% had AFib on screening electrocardiogram performed on admission [19, 20]. A previously published sub-group analysis showed no differential effects of seralaxin based on the presence or absence of a history of AFib or AFib at screening on key study end points [19]. In the present study, we sought to expand our knowledge on the efficacy and safety of serelaxin in acute HF patients with and without AFib at the time of presentation by addressing all pre-specified efficacy and safety end points, adverse events and biomarkers of organ damage. We further analyzed the clinical profile of patients with AFib as well as the independent prognostic significance of AFib on patient outcomes.
Methods
The design and primary results of the RELAX-AHF trial are described in detail elsewhere [21]. Briefly, the study randomized 1161 AHF patients to 48-h intravenous infusion of serelaxin (30 μg/kg/day, n = 581) or placebo (n = 580) within 16 h from presentation. The study was approved by the institutional review boards and all subjects enrolled gave informed consent.
In the present analysis, we compared the effects of serelaxin versus placebo on pre-specified efficacy end points, safety end points, and biomarkers indicative of organ damage, in patients with and without AFib. The presence of AFib was defined as evidence of either atrial fibrillation or atrial flutter on the screening electrocardiogram performed on admission.
The trial’s primary efficacy end points were dyspnea improvement, defined as the area under the curve of dyspnea change from baseline on a 100-mm visual analog scale (VAS-AUC) through day 5 and the presence of moderately or markedly better breathing compared to baseline reported on a 7-point Likert scale at 6, 12 and 24 h. Adverse events (AEs) were collected through day 5, serious AEs through day 14, rehospitalizations through day 60, and vital status through day 180. Rehospitalizations and deaths were adjudicated by an independent, blinded committee. The trial’s secondary efficacy end points included cardiovascular death or rehospitalization for heart or renal failure and days alive and out of hospital through day 60. Cardiovascular death through day 180 was pre-specified as an additional efficacy end point, and all-cause death through day 180 was a pre-specified safety end point. Stroke through day 180 was defined to include any AE of stroke (through day 14), any rehospitalization for stroke (through day 60), or death due to stroke (through day 180). Biomarkers indicative of congestion and/or organ damage, including high-sensitivity troponin T (hs-TnT), N-terminal beta-type natriuretic pro-peptide (NT-proBNP), cystatin C, creatinine, alanine aminotransferase (ALT), aspartate aminotransferase (AST) and growth differentiation factor-15 (GDF-15) were assessed serially using a central core laboratory.
Statistical analysis
Baseline characteristics were compared between patients with and without AFib, without imputation for missing values, using two-sample t test for continuous variables and Chi square or Fisher’s exact test for categorical variables. Estimates of the serelaxin treatment effect (odds ratios, mean differences, or hazard ratios) for patients with and without AFib and an interaction test were obtained from separate regression models (logistic regression, analysis of covariance, or Cox proportional hazards). For the analyses of outcomes in patients with and without AFib and the analyses of treatment effects, two subjects with unknown AFib status were imputed as without AFib. Missing baseline covariates were also imputed as the mean for continuous variables or as the mode for categorical variables within the treatment group. Missing biomarker values were not imputed. Analyses were conducted on an intention-to-treat basis. All p values were two sided, and values <0.05 were considered to be statistically significant. Analyses were performed using SAS© release 9.2 (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics in patients with and without AFib
From a total 1161 patients who underwent a screening electrocardiogram on admission, 479 patients had AFib (41.3%). In addition, 602 (51.9%) patients reported a history of AFib, although this was not used as a criterion for the present analysis. Patients with AFib were significantly older with a different race and geographic distribution than those without AFib (Table 1). Patients with AFib were less likely to have an ischemic etiology of HF or a reduced ejection fraction (HFrEF), but similar New York Heart Association (NYHA) class distribution when compared to patients without AFib. AFib patients had a higher resting heart rate than non-AFib patients, but similar systolic blood pressure. Symptoms and signs of congestion did not differ between the two groups with the exception of peripheral edema, which was more frequent in patients with AFib. Several comorbid conditions including hyperlipidemia, diabetes mellitus, smoking, history of myocardial infarction and depression were less frequent in patients with AFib. A history of hypertension, lung disease and cerebrovascular or peripheral arterial disease did not differ between the two groups. Regarding cardiovascular therapies, patients with AFib were more frequently prescribed beta-blockers and digoxin, and had more frequently undergone a pacemaker implantation. However, they were less likely to have a cardiac defibrillator or a biventricular pacing system. With respect to baseline laboratory findings, renal, liver function and natriuretic peptides did not differ between the two groups. Patients with AFib, however, had lower troponin T and higher GDF-15 levels.
Table 1.
Comparison of baseline characteristics between patients with and without atrial fibrillation (AFib) on admission
| Baseline characteristic | AFib, n = 479a | No AFib, n = 680a | p valueb |
|---|---|---|---|
| Demographics | |||
| Age (years) | 74.6 (9.5) | 70.2 (12.0) | <0.0001 |
| Male | 284 (59.3) | 440 (64.7) | 0.0608 |
| White/Caucasian | 466 (97.3) | 628 (92.4) | 0.0003 |
| Geographic region | <0.0001 | ||
| Eastern Europe | 266 (55.5) | 295 (43.4) | |
| Western Europe | 89 (18.6) | 115 (16.9) | |
| South America | 26 (5.4) | 45 (6.6) | |
| North America | 23 (4.8) | 90 (13.2) | |
| Israel | 75 (15.7) | 135 (19.9) | |
| Heart failure characteristics | |||
| Left ventricular EF | 40.3 (14.5) | 37.5 (14.5) | 0.0015 |
| EF < 40% | 217 (48.9) | 380 (58.9) | 0.0011 |
| Ischemic heart disease | 226 (47.2) | 376 (55.3) | 0.0065 |
| Time from presentation to randomization (h) | 7.6 (4.6) | 8.1 (4.7) | 0.0768 |
| CHF 1 month prior | 362 (75.6) | 497 (73.1) | 0.3414 |
| NYHA class 30 days before admission | 0.1274 | ||
| I | 128 (26.8) | 195 (29.0) | |
| II | 115 (24.1) | 187 (27.8) | |
| III | 180 (37.7) | 209 (31.1) | |
| IV | 54 (11.3) | 81 (12.1) | |
| Clinical signs | |||
| Body mass index, kg/m2 | 29.3 (5.3) | 29.2 (6.0) | 0.7501 |
| Syst. blood pressure, mmHg | 141.5 (16.2) | 142.6 (16.8) | 0.2523 |
| Diast. blood pressure, mmHg | 80.6 (13.8) | 77.9 (14.5) | 0.0018 |
| Heart rate, beat per minute | 83.0 (15.9) | 77.3 (13.8) | <0.0001 |
| Respiratory rate, breaths per minute | 21.9 (4.7) | 21.9 (4.6) | 0.8515 |
| HF hospitalization past year | 161 (33.6) | 235 (34.6) | 0.7378 |
| Congestion at baseline | |||
| Edema | 395 (83.0) | 513 (75.9) | 0.0037 |
| Orthopnea | 459 (96.4) | 645 (95.4) | 0.3962 |
| Jugular vein distension | 359 (76.7) | 489 (74.5) | 0.4054 |
| Dyspnea on exertion | 469 (99.8) | 665 (99.6) | 0.6468 |
| Dyspnea by VAS | 43.7 (20.5) | 44.5 (19.6) | 0.4915 |
| Rales | 453 (95.2) | 640 (94.5) | 0.6336 |
| Comorbidities | |||
| Hypertension | 417 (87.1) | 587 (86.3) | 0.7181 |
| Hyperlipidemia | 223 (46.6) | 392 (57.6) | 0.0002 |
| Diabetes mellitus | 196 (40.9) | 353 (51.9) | 0.0002 |
| Cigarette smoking | 35 (7.3) | 118 (17.4) | <0.0001 |
| Stroke or other cerebrovascular event | 66 (13.8) | 91 (13.4) | 0.8460 |
| Peripheral vascular disease | 62 (12.9) | 93 (13.7) | 0.7181 |
| Asthma, bronchitis, or COPD | 77 (16.1) | 106 (15.6) | 0.8229 |
| History of Atrial fibrillation or flutter | 454 (94.8) | 148 (21.8) | <0.0001 |
| History of CRT or ICD procedures | 112 (23.4) | 182 (26.8) | 0.1925 |
| Myocardial infarction | 141 (50.5) | 262 (60.0) | 0.0132 |
| Depression | 14 (2.9) | 46 (6.8) | 0.0036 |
| Medication | |||
| ACE inhibitor | 244 (50.9) | 388 (57.1) | 0.0394 |
| ACEi or ARBs | 314 (65.6) | 472 (69.4) | 0.1662 |
| Angiotensin-receptor blocker | 83 (17.3) | 101 (14.9) | 0.2563 |
| Beta-blocker | 344 (71.8) | 448 (65.9) | 0.0325 |
| Aldosterone antagonist | 166 (34.7) | 199 (29.3) | 0.0517 |
| Oral loop diuretic 30 days prior | 42.3 (59.9) | 46.4 (68.7) | 0.2900 |
| Digoxin | 152 (31.7) | 76 (11.2) | <0.0001 |
| Nitrates at randomization | 31 (6.5) | 50 (7.4) | 0.5623 |
| Devices | |||
| Pacemaker | 66 (13.8) | 55 (8.1) | 0.0018 |
| Implantable cardiac defibrillator | 42 (8.8) | 112 (16.5) | 0.0001 |
| Biventricular pacing | 33 (6.9) | 80 (11.8) | 0.0059 |
| Baseline laboratory findings | |||
| Hemoglobin, g/dL | 12.83 (1.71) | 12.77 (1.95) | 0.5721 |
| White blood cell count, ×109/L | 7.909 (2.723) | 8.370 (2.916) | 0.0082 |
| Lymphocyte, % | 18.15 (7.27) | 18.18 (8.19) | 0.9433 |
| Glucose, mmol/L | 7.29 (3.01) | 8.07 (3.89) | 0.0002 |
| BUN, mmol/L | 9.80 (4.03) | 9.75 (4.03) | 0.8340 |
| Creatinine, umol/L | 114.3 (31.5) | 118.1 (34.2) | 0.0621 |
| Cystatin C, mg/L | 1.47 (1.43, 1.51) | 1.44 (1.41, 1.47) | 0.2294 |
| eGFR, mL/min per 1.73 m2 | 53.20 (12.98) | 53.72 (13.06) | 0.5097 |
| Sodium, mmol/L | 141.0 (3.8) | 140.7 (3.4) | 0.3074 |
| Potassium, mmol/L | 4.27 (0.63) | 4.27 (0.64) | 0.9697 |
| Calcium, mmol/L | 2.26 (0.14) | 2.27 (0.16) | 0.5059 |
| Alanine aminotransferase, U/L (log transformed) | 23.4 (22.2, 24.7) | 23.7 (22.5, 24.9) | 0.7569 |
| Albumin, g/L | 40.41 (3.93) | 40.11 (4.59) | 0.2407 |
| Total cholesterol, mmol/L | 3.94 (1.07) | 4.20 (1.22) | 0.0001 |
| CRP, mg/L (log transformed) | 8.56 (7.57, 9.68) | 8.49 (7.73, 9.31) | 0.9101 |
| Uric acid, umol/L | 478.4 (132.3) | 473.8 (138.2) | 0.5751 |
| NT-proBNP, ng/L (log transformed) | 5279 (4919, 5665) | 4905 (4553, 5284) | 0.1599 |
| Troponin T, ug/L (log transformed) | 0.031 (0.028, 0.033) | 0.038 (0.036, 0.041) | <0.0001 |
| GDF-15, ng/L (log transformed) | 4598 (4329, 4883) | 4167 (3953 4392) | 0.0165 |
aMean (SD), or geometric mean (95% CI) if log transformed, presented for continuous variables, and n (%) for categorical variables (% based on the total number of patients with a non-missing value of the end point)
b p value was based on t test (with Satterthwaite correction if unequal variances), Chi-square test, or Fisher’s exact test
Efficacy and safety of serelaxin in patients with and without atrial fibrillation
Atrial fibrillation was present in 233 of 580 (40.2%) patients in the serelaxin arm and in 246 of 579 (42.5%) patients in the placebo arm (p = 0.424). Most of the study end points did not differ significantly between patients with and without AFib after multivariable adjustment, but AFib patients had a significantly higher incidence of cardiovascular mortality at 180 days (adjusted p = 0.0173, Table 2).
Table 2.
Outcomes in patients with and without atrial fibrillation
| Clinical end points | AFib, n = 479 Estimatea |
No AFib, n = 682 Estimatea,b |
Unadjusted effect of AFib (yes versus no) | Adjusted effect of AFib (yes versus no) | ||
|---|---|---|---|---|---|---|
| (95% CI) | p valuec | (95% CI) | p valuec,d | |||
| Dyspnea improvement by VAS to day 5, mm-h | 2222.58 (1962.77, 2482.39) | 2749.22 (2538.24, 2960.20) | −526.64 (−858.50, −194.78) | 0.0019 | −166.06 (−570.24, 238.11) | 0.4207 |
| Dyspnea improvement by Likert scale at 6, 12 and 24 h | 110/479 (23.0%) | 196/682 (28.7%) | 0.74 (0.56, 0.97) | 0.0282 | – | – |
| Worsening heart failure (WHF) | 53/479 (11.1%) | 57/682 (8.4%) | 1.34 (0.92, 1.96) | 0.1258 | 1.13 (0.67, 1.91) | 0.6446 |
| Hospitalization length, days | 11.82 (10.80, 12.84) | 8.82 (8.26, 9.37) | 3.00 (1.92, 4.08) | <0.0001 | – | – |
| Cardiovascular death or HF/RF hospitalization through day 60 | 69/479 (14.5%) | 82/682 (12.1%) | 1.21 (0.88, 1.67) | 0.2414 | 1.13 (0.81, 1.59) | 0.4739 |
| All-cause death through day 180 | 51/479 (10.7%) | 56/682 (8.3%) | 1.33 (0.91, 1.95) | 0.1372 | 1.46 (0.98, 2.18) | 0.0651 |
| Cardiovascular mortality to day 180 | 43/479 (9.1%) | 45/682 (6.7%) | 1.40 (0.92, 2.12) | 0.1170 | 1.71 (1.10, 2.67) | 0.0173 |
| Stroke through day 180 | 13/479 (2.8%) | 5/682 (0.8%) | 3.77 (1.34, 10.58) | 0.0116 | – | – |
aMean (95% CI), n (K-M %) and n (%) are presented for continuous outcome, survival outcome and binary outcome, respectively
bTwo subjects with unknown AFib status were imputed as without AFib (treatment-specific mode)
cTreatment effect represents mean difference (from linear regression analysis), hazard ratio (from Cox proportional hazards model) and odds ratio (from logistic regression analysis) for continuous outcome, survival outcome and binary outcome, respectively
dDyspnea VAS AUC to day 5 is adjusted for age, US-like, weight, dyspnea on exertion, hypertension, mitral regurgitation, history of atrial fibrillation or flutter, alkaline phosphatase, sodium, body temperature (linear spline at Q1), log2 troponin (linear spline at Q2), dyspnea by VAS (cubic), uric acid (cubic); WHF is adjusted for white race, height (linear spline at 173), diastolic BP(linear spline at 70), heart rate (trichotomized: <73, [73, 85), ≥85), respiratory rate, dyspnea by VAS, mm (cubic), coronary artery bypass graft, hyperthyroidism, total bilirubin, total cholesterol, albumin, troponin (log2 transformed, linear spline at −4.2); CV death or HF/RF rehospitalization through day 60 is adjusted for white race, NYHA class 30 days before systolic BP, respiratory rate, number of HF hospitalizations past year, orthopnea (ordinal), asthma or bronchitis or COPD, hyperthyroidism, lymphocytes %, BUN, phosphate (cubic), sodium, total protein (linear spline at 68); CV mortality through day 180 is adjusted for the following variables: US-like, systolic BP, orthopnea (ordinal), angina, hyperthyroidism, mitral regurgitation, atrial fibrillation/flutter at screening, white blood cell count, lymphocytes %, BUN, sodium, potassium, calcium, total protein, log2 troponin, log2 NT-proBNP. All-cause death to day 180 is adjusted for age, CHF 1 month previously, stroke or other cerebrovascular events, respiratory rate, systolic BP, edema (2/3 versus 0/1), orthopnea (2/3 versus 0/1), lymphocytes (%), sodium, creatinine and log2 troponin
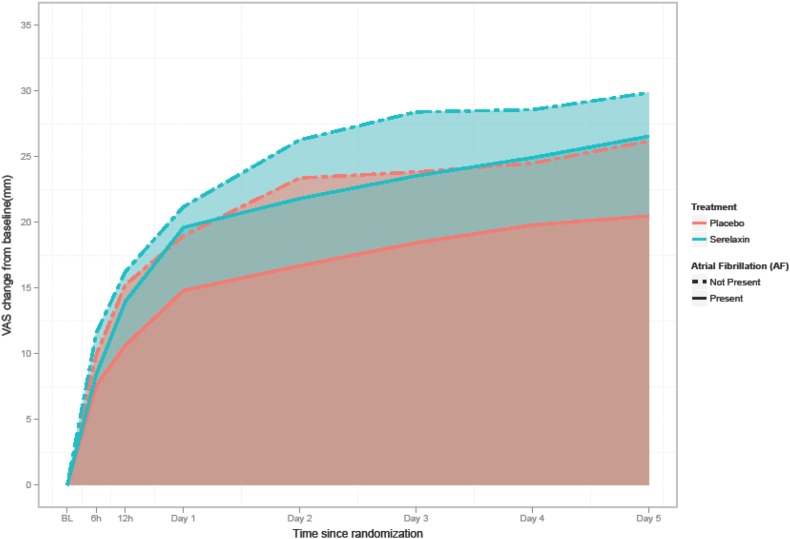
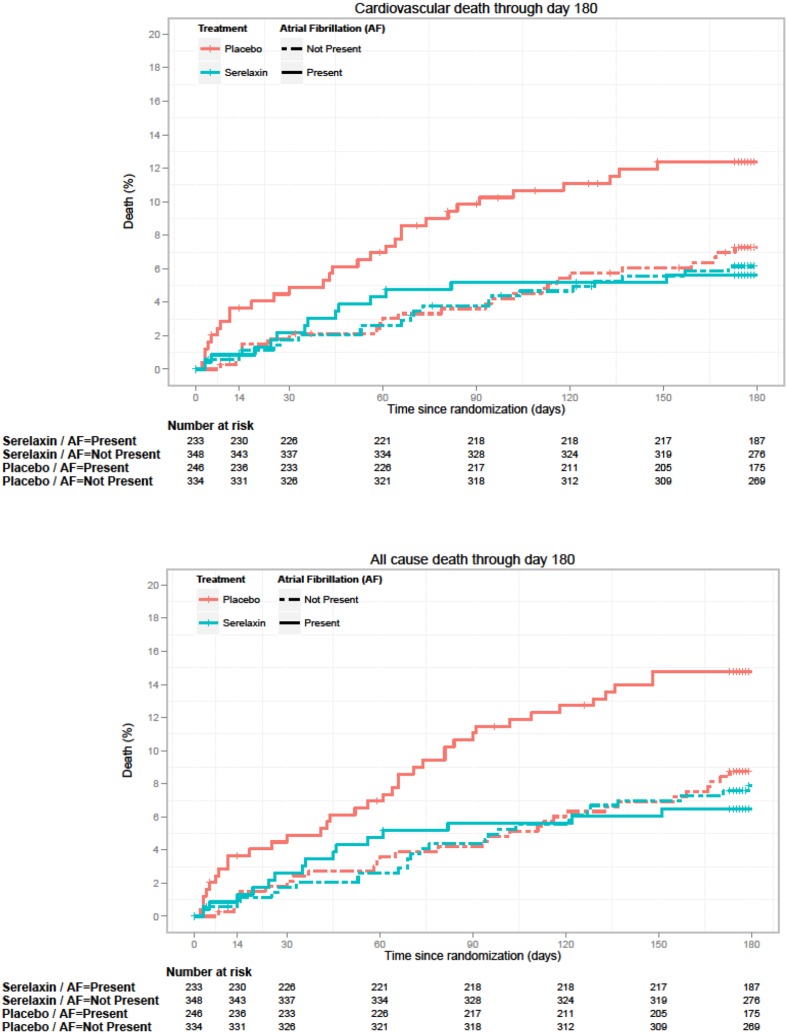
The effect of serelaxin versus placebo on several study end points in patients with and without AFib is outlined in Table 3. There was no differential effect of serelaxin on dyspnea relief according to VAS scale up to day 5 (interaction p = 0.5954; Table 3; Fig. 1) or by Likert scale at 6, 12 and 24 h (Table 3) Serelaxin induced a similar reduction in the incidence of worsening HF (interaction p = 0.7423) irrespective of the presence or absence of AFib. Similarly, the length of hospital stay did not differ (interaction p = 0.3837). Cardiovascular death or hospitalization for HF or renal failure through day 60 and all-cause death and cardiovascular mortality at 180 days were neither significantly affected by serelaxin in either of the two group interaction (interaction p = 0.1583, 0.0643 and 0.1472, respectively; Fig. 2).
Table 3.
Treatment effect (serelaxin versus placebo) on various outcomes in patients with and without atrial fibrillation (AFib)
| Outcome | AFib, n = 479 | No AFib, n = 682c | Interaction p valued | ||||
|---|---|---|---|---|---|---|---|
| Serelaxin, n = 233a | Placebo, n = 246a | Treatment effect (95% CI) p valueb |
Serelaxin, n = 348a | Placebo, n = 334a | Treatment, effect (95% CI) p valueb |
||
| Dyspnea improvement by VAS-AUC to day 5, mm-he | 2500.48 (2165.85, 2835.11) | 1959.37 (1565.60, 2353.13) | 541.11 (33.79, 1048.44) 0.0366 |
2926.41 (2654.92, 3197.90) | 2564.61 (2239.43, 2889.79) | 361.80 (−63.30, 786.90) 0.0953 |
0.5954 |
| Dyspnea improvement by Likert scale at 6, 12 and 24 h | 55 (23.6%) | 55 (22.4%) | 1.07 (0.70, 1.64) 0.7457 |
101 (29.0%) | 95 (28.4%) | 1.03 (0.74, 1.43) 0.8671 |
0.8784 |
| Worsening heart failure | 19 (8.2%) | 34 (13.8%) | 0.57 (0.32, 1.00) 0.0506 |
20 (5.8%) | 37 (11.1%) | 0.50 (0.29, 0.86) 0.0126 |
0.7423 |
| Hospitalization length, days | 11.12 (9.77, 12.48) | 12.48 (10.96, 13.99) | −1.35 (−3.00, 0.30) 0.1085 |
8.62 (7.79, 9.45) | 9.02 (8.28, 9.76) | −0.39 (−1.78, 0.99) 0.5761 |
0.3837 |
| Cardiovascular death or HF/RF hospitalization through day 60e | 30 (13.0%) | 39 (16.0%) | 0.80 (0.49, 1.28) 0.3486 |
46 (13.4%) | 36 (10.9%) | 1.27 (0.82, 1.96) 0.2866 |
0.1583 |
| All-cause death through day 180 | 15 (6.5%) | 36 (14.8%) | 0.42 (0.23, 0.77) 0.0051 |
27 (7.9%) | 29 (8.7%) | 0.90 (0.53, 1.52) 0.6888 |
0.0643 |
| Cardiovascular mortality through day 180e | 13 (5.6%) | 30 (12.4%) | 0.44 (0.23, 0.85) 0.0139 |
21 (6.1%) | 24 (7.3%) | 0.84 (0.47, 1.52) 0.5713 |
0.1472 |
| Stroke through day 180 | 3 (1.30%) | 10 (4.23%) | 0.31 (0.09, 1.13) 0.0759 |
4 (1.16%) | 1 (0.32%) | 3.88 (0.43, 34.71) 0.2255 |
0.0518 |
aMean (95% CI) presented for continuous outcome, n (K-M %) for survival outcomes, n (%) for binary outcomes
bTreatment effect represents the mean difference estimated from linear regression models for continuous outcomes, the hazard ratio from Cox regression for time-to-event outcomes, and the odds ratio from logistic regression for binary outcomes
cTwo subjects with unknown AFib status were imputed as without AFib (treatment-specific mode)
dInteraction p value is based on test of treatment by AF interaction from linear regression, Cox or logistic regression model as appropriate
eResult presented in [19]
Fig. 1.
Patient-reported dyspnea change (serelaxin versus placebo) in patients with and without atrial fibrillation (AF), according to visual analog scale from baseline to day 5
Fig. 2.
Kaplan–Meier curves (serelaxin versus placebo) for cardiovascular death through day 180 (upper panel) and all-cause death through day 180 (lower panel) in patients with and without atrial fibrillation (AF)
Stroke through 180 days occurred in 13 patients with AFib (2.8%) and 5 patients without AFib (0.8%, p = 0.0116). There was a trend for a lower incidence of stroke in the serelaxin arm in patients with AFib [hazard ratio serelaxin versus placebo, 0.31 (0.09, 1.13) in AFib versus 3.88 (0.43, 34.71) in patients without AFib, interaction p = 0.0518].
The effect of serelaxin versus placebo on AEs in patients with and without AFib is shown in Table 4. The overall incidence of serious AEs did not differ based on the presence or absence of AFib (interaction p = 0.3905). The same applied to the incidence of AEs indicative of hypotension or renal or hepatic impairment. It should be noted that there was no difference in anticoagulation use at baseline and from baseline through day 14 and day 60 among the study groups. In addition, CHA2DS2-VASc score was similar among the study groups (Table 5).
Table 4.
Treatment effect (serelaxin versus placebo) on adverse events (AE) in patients with and without atrial fibrillation (AFib)
| Adverse event | AFib, n = 479 | No AFib, n = 680 | Interaction p value | ||||
|---|---|---|---|---|---|---|---|
| Serelaxin, n = 233 | Placebo, n = 246 | Oddsratio(95% CI) p value |
Serelaxin, n = 347 | Placebo, n = 333 | Odds ratio (95% CI) p value |
||
| Patients with any serious AE through day 14 | 26 (11.16%) | 45 (18.29%) | 0.56 (0.32, 0.97) 0.0293 |
43 (12.39%) | 51 (15.32%) | 0.78 (0.49, 1.24) 0.3172 |
0.3905 |
| Patients with AE indicative of hypotension through day 14a | 10 (4.29%) | 9 (3.66%) | 1.18 (0.42, 3.35) 0.8166 |
18 (5.19%) | 18 (5.41%) | 0.96 (0.46, 1.99) 1.0000 |
0.7769 |
| Patients with AE indicative of renal impairment through day 14b | 4 (1.72%) | 12 (4.88%) | 0.34 (0.08, 1.15) 0.0737 |
13 (3.75%) | 20 (6.01%) | 0.61 (0.27, 1.31) 0.2116 |
0.5189 |
| Patients with AE indicative of hepatic impairment through day 14c | 1 (0.43%) | 7 (2.85%) | 0.15 (0.00, 1.16) 0.0688 |
2 (0.58%) | 4 (1.20%) | 0.48 (0.04, 3.36) 0.4422 |
0.5491 |
AE adverse events
aBlood pressure decreased, dizziness, loss of consciousness, hypotension, orthostatic hypotension, presyncope, somnolence or syncope
bAzotemia, blood creatinine increase, oliguria, proteinuria, renal failure, acute renal failure or renal impairment
cBlood bilirubin increase, cholestasis, hepatic congestion, hepatic cyst, hepatic steatosis, hyperbilirubinemia, hypoalbuminemia, INR increase or liver disorder
Table 5.
Comparison of anticoagulation therapy and CHADS2-VASc score among study groups
| AFib, n = 479 | No AFib, n = 680 | Interaction p value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Serelaxin, n = 233 | Placebo, n = 246 | Treatment effect | 95% CI | p value | Serelaxinn = 347 | Placebon = 333 | Treatment effect | 95% CI | p value | ||
| Anticoagulant use at baselinea | 119 (51.1) | 136 (55.3) | 0.84 | (0.59, 1.21) | 0.3560 | 90 (25.9) | 102 (30.6) | 0.79 | (0.57, 1.11) | 0.1744 | 0.8028 |
| Anticoagulant use through day 14a | 157 (67.4) | 179 (72.8) | 0.77 | (0.52, 1.14) | 0.1987 | 115 (33.1) | 122 (36.6) | 0.86 | (0.63, 1.18) | 0.3391 | 0.6878 |
| Anticoagulant use through day 60a | 165 (70.8) | 186 (75.6) | 0.78 | (0.52, 1.17) | 0.2364 | 124 (35.7) | 132 (39.6) | 0.85 | (0.62, 1.16) | 0.2936 | 0.7630 |
| CHADS2-VASc score at baselineb | 4.41 (4.20, 4.61) | 4.59 (4.41, 4.77) | −0.18 | (−0.47, 0.11) | 0.2171 | 4.26 (4.08, 4.44) | 4.37 (4.19, 4.54) | −0.10 | (−0.35, 0.14) | 0.3993 | 0.6863 |
Mean (95% CI) and n (%) are presented for continuous outcome and binary outcome, respectively
aTreatment effect represents odds ratio (from logistic regression analysis). Each model includes the subgroup variable (AFib versus No AFib), treatment and treatment by subgroup interaction as covariates
bTreatment effect represents mean difference (from linear regression analysis). Each model includes the subgroup variable (AFib versus No AFib), treatment and treatment by subgroup interaction as covariates. Two patients had missing AFib values at screening. Anticoagulants include acenocoumarol, dalteparin, enoxaparin, fondaparinux, heparin, heparin-fraction, nadraparin, phenprocoumon, tinzaparin, warfarin
Effects of serelaxin on biomarkers of organ damage in patients with and without atrial fibrillation
The effects of serelaxin versus placebo on biomarkers of organ damage were similar irrespective of AFib presence at baseline (Table 6; all interaction p levels were nonsignificant). There was a less pronounced increase in cystatin C with serelaxin than with placebo treatment in both AFib groups, while creatinine decreased in the serelaxin group and increased in the placebo group. There were greater reductions in NT-proBNP, AST, ALT, and GDF-15 at 48 h in the serelaxin group than in the placebo group, both in patients with and without AFib. Serelaxin induced similar reductions in relative changes in troponin T; however, in patients with AFib troponin T increased in the placebo group and remained the same in the serelaxin group, while in patients without AFib troponin T stayed the same in placebo patients and decreased in serelaxin patients.
Table 6.
Effect of treatment (serelaxin versus placebo) on biomarkers of organ damage in patients with and without atrial fibrillation (AFib)
| Biomarker | AFib, n = 479 | No AFib, n = 682c | Interaction p valued | ||||
|---|---|---|---|---|---|---|---|
| Serelaxin, n = 233a | Placebo, n = 246a | Treatment effect (95% CI) p valueb |
Serelaxin, n = 348a | Placebo, n = 334a | Treatment effect (95% CI) p valueb |
||
| Change to day 2 in cystatin C (log2 transformed) | 1.01 (0.99, 1.04) | 1.08 (1.06, 1.11) | 0.93 (0.90, 0.96) <0.0001 |
1.04 (1.02, 1.06) | 1.08 (1.05, 1.10) | 0.96 (0.94, 0.99) 0.0071 |
0.1512 |
| Change to day 2 in creatinine | −4.90 (−7.95, −1.85) | 5.34 (2.55, 8.13) | −10.25 (−14.78, −5.72) <0.0001 |
−2.17 (−5.09, 0.75) | 6.68 (3.85, 9.50) | −8.84 (−12.65, −5.04) <0.0001 |
0.6424 |
| Change to day 2 in troponin (log2 transformed) | 1.00 (0.95, 1.06) | 1.08 (1.03, 1.13) | 0.93 (0.85, 1.01) 0.0823 |
0.94 (0.89, 0.99) | 1.00 (0.95, 1.06) | 0.94 (0.87, 1.01) 0.0881 |
0.8281 |
| Change to day 2 in NT-proBNP (log2 transformed) | 0.54 (0.49, 0.58) | 0.70 (0.65, 0.76) | 0.76 (0.68, 0.86) <0.0001 |
0.46 (0.43, 0.50) | 0.54 (0.50, 0.59) | 0.85 (0.77, 0.94) 0.0019 |
0.1459 |
| Change to day 2 in GDF 15 (log2 transformed) | 0.77 (0.72, 0.82) | 0.93 (0.88, 0.99) | 0.82 (0.75, 0.90) <0.0001 |
0.80 (0.76, 0.85) | 0.88 (0.84, 0.93) | 0.91 (0.84, 0.98) 0.0091 |
0.0941 |
| Change to day 2 in ALT (log2 transformed) | 0.83 (0.80, 0.86) | 0.91 (0.86, 0.96) | 0.92 (0.87, 0.97) 0.0022 |
0.82 (0.80, 0.85) | 0.87 (0.85, 0.90) | 0.94 (0.90, 0.99) 0.0115 |
0.4777 |
| Change to day 2 in AST (log2 transformed) | 0.84 (0.80, 0.87) | 0.91 (0.86, 0.96) | 0.92 (0.86, 0.98) 0.0088 |
0.78 (0.76, 0.81) | 0.87 (0.84, 0.90) | 0.90 (0.85, 0.95) <0.0001 |
0.5810 |
cTNT cardiac troponin-T, NT-proBNP N-terminal B-type natriuretic pro-peptide, AST aspartate aminotransferase, ALT alanine aminotransferase
aMean (95% CI) change from baseline to day 2 or geometric mean (95% CI) − the ratio of day 2 to baseline − if log2 transformed
bTreatment effect represents mean difference or the ratio of the relative changes if log2 transformed
cTwo subjects with unknown AFib status were imputed as without AFib (treatment-specific mode)
dInteraction p value based on test of treatment by AF interaction from linear regression
Atrial fibrillation during follow-up
Atrial fibrillation or flutter was reported in 13 patients by day 14; the incidence was similar in the serelaxin (n = 7) and placebo (n = 6) groups. Ten patients, seven in the serelaxin group and three in the placebo group, were rehospitalized for AFib through day 60. In patients without AFib at baseline screening, there were eight episodes of AFib or flutter through day 14, including five (1.44%) in the serelaxin group and three (0.90%) in the placebo group [OR, 1.61, 95% CI (0.31, 10.4), p = 0.725].
Discussion
A 48-h serelaxin infusion in patients with acute HF improved dyspnea and congestion, reduced early HF worsening and hospital stay and improved long-term outcome in terms of cardiovascular and all-cause mortality at 6 months [14]. The effects of serelaxin versus placebo on dyspnea relief to day 5, cardiovascular death or rehospitalizations for heart or renal failure at 60 days or all-cause or cardiovascular mortality at 180 days were further shown to be generally consistent across several patient subgroups, including a history of AFib and AFIb on admission [17]. In the present analysis, we expanded those results by addressing the interaction between treatment assignment (serelaxin or placebo) and the presence or absence of AFib on admission on all efficacy and safety end points, including dyspnea improvement at 6, 12 and 24 h, worsening HF, hospitalization length, all-cause and cardiovascular death at 180 days and incidence of stroke over the same time period.
Patients with AFib on admission enrolled in the RELAX-AHF trial differed in HF etiology and phenotype as well as in baseline comorbidities compared to patients without AFib. In addition, AFIb patients had a higher adjusted incidence of cardiovascular mortality at 180 days. However, dyspnea response to therapy, HF worsening and cardiovascular death or hospitalization for HF or renal failure through day 60 as well as all-cause death through day 180 were similar in the two subgroups after multivariable adjustment. This finding suggests that worse outcomes observed in acute HF patients with AFib may be partly influenced by the different profile of those patients rather than being wholly attributable to the arrhythmia per se.
Serelaxin was similarly safe in the two groups in terms of serious adverse events or events indicative of hypotension, or renal or hepatic impairment. Not only was serelaxin safe, but it also seemed to provide organ protection, as the previously documented beneficial effect of serelaxin on biomarkers of organ damage was consistent in patients with and without AFib. In addition, although the incidence of stroke was, as expected, higher in patients with AFib, interestingly serelaxin tended to reduce its incidence in those particular patients.
Atrial fibrillation is known to confer a fivefold increase in the risk of stroke [12]. Studies have shown that even subclinical AFib episodes as short as 6 min or perioperative AFib in patients undergoing non-cardiac surgery are followed by an increased long-term risk of stroke [22–25]. Stroke may be a devastating condition associated with significant morbidity and mortality. The present post hoc analysis, despite the rather short follow-up period, confirmed a higher risk of stroke in AFib patients. Interestingly, serelaxin was followed by a lower incidence of stroke in those patients compared to placebo. Relaxin is a known vasoactive peptide that modifies beneficially arterial resistance and compliance. Regarding the cerebral vasculature, in particular, relaxin seems to have specific beneficial effects that have led to the hypothesis that it may play a protective role against ischemic stroke [26]. Experimental studies have shown that relaxin pretreatment reduced infarct size after middle cerebral artery occlusions in rats, an action accomplished through the activation of the relaxin family peptide receptor 3 (RXFP3), a process that also involved activation of the endothelial nitric oxide synthase (eNOS) pathway [27–29]. Those effects within the cerebral vascular bed may lead to vasodilation and improved brain tissue perfusion. In a small clinical study in 36 patients recovering from stroke, relaxin plus rehabilitation induced a greater recovery compared to rehabilitation alone at 20 and 40 days as indicated by measures of physical activity, cognitive function and global function [26]. It should be stressed however that the incidence rate of stroke was low and therefore those results should be interpreted with caution.
Besides its vasodilatatory and anti-ischemic actions discussed earlier, relaxin seems to possess anti-inflamatory and antifibrotic properties [30]. As inflammation and fibrosis are thought to be important aspects in the pathophysiology of AFib, it has been postulated that relaxin may have a role in the management of AFib [31]. In an experimental study in hypertensive rats, relaxin suppressed AFib triggered by programmed stimulation [32]. The suppression of AFib was achieved by increasing conduction velocity from a combination of reversal of atrial fibrosis and hypertrophy and by increasing Na+ current density [32]. In RELAX-AHF, the occurrence of AFib during follow-up was not systematically recorded; there were only a few spontaneous reports of AFib as an adverse event. As a result, the effects of the drug on the occurrence of AFib could not be assessed, but this may be the aim of a future study.
The results of the present study should be cautiously treated as they are derived by a post hoc subgroup analysis of a randomized trial. In addition, the main RELAX-AHF study was not primarily designed and powered to assess medium and long-term prognostic outcomes and therefore the corresponding findings should be carefully interpreted.
In conclusion, serelaxin was overall similarly safe and efficacious in improving short- and long-term clinical outcomes and inducing organ protection in acute HF patients with and without AFib. However, prospective trials are required to confirm those findings.
Acknowledgements
The RELAX-AHF trial was supported by Corthera Inc., a member of the Novartis group of companies.
Compliance with ethical standards
Conflict of interest
G. F. was a member of the steering committee of RELAX-AHF. D. F. has received consultancy and speaker fees from Servier and Novartis and an educational grant from Novartis. M. M. reports receiving honoraria as a consultant for Novartis and Bayer. G. C. and B. A. D. are employees of Momentum Research Inc., which received remuneration for conducting clinical studies from Novartis, Amgen, Cardio3, Trevena, Chan RX, Laguna Pharmaceuticals and Singulex. G. M. F. has received consulting and grant support from Novartis. B. H. G. is a consultant for Novartis and Janssen. T. A. H. and T. A. S. are employees of Novartis. P.S.P. is or has been in the past year a consultant for: Janssen, Medtronic, Novartis, Trevena, scPharmaceuticals, Cardioxyl, Roche Diagnostics and Relypsa; received honoraria: Palatin Technologies; and research support: Roche and Novartis. P. P. is a consultant for Novartis, Cardiorentis and Bayer, and receives research grants from Singulex. A. A. V. was a member of the steering committee of RELAX-AHF and received consultancy, speaker fees and research grants from Novartis, and he received grants and or consultancy/speaker fees from Alere, AstraZeneca, Bayer, Cardio3Biosciences, Celladon, GSK, Merck/MSD, Servier, Stealth, Singulex, Sphingotec, Trevena and Vifor. J. R. T. receives research/consulting fees from Amgen, Madeleine, Mast Therapeutics, Novartis, Relypsa and Trevena. The rest of the authors report no conflicts of interest.
Footnotes
An erratum to this article is available at https://doi.org/10.1007/s00392-017-1139-5.
References
- 1.Alla F, Zannad F, Filippatos G. Epidemiology of acute heart failure syndromes. Heart Fail Rev. 2007;12:91–95. doi: 10.1007/s10741-007-9009-2. [DOI] [PubMed] [Google Scholar]
- 2.Farmakis D, Parissis J, Filippatos G (2015) Acute heart failure: epidemiology, classification and pathophysiology. In: The ESC textbook of intensive and acute cardiovascular care, 2nd edn. Oxford University Press, Oxford, p 800
- 3.Bueno H, Ross JS, Wang Y, Chen J, Vidan MT, Normand SL, Curtis JP, Drye EE, Lichtman JH, Keenan PS, Kosiborod M, Krumholz HM. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure 1993–2006. JAMA. 2010;303:2141–2147. doi: 10.1001/jama.2010.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mebazaa A, Yilmaz MB, Levy P, Ponikowski P, Peacock WF, Laribi S, Ristic AD, Lambrinou E, Masip J, Riley JP, McDonagh T, Mueller C, deFilippi C, Harjola VP, Thiele H, Piepoli MF, Metra M, Maggioni A, McMurray J, Dickstein K, Damman K, Seferovic PM, Ruschitzka F, Leite-Moreira AF, Bellou A, Anker SD, Filippatos G. Recommendations on pre-hospital & early hospital management of acute heart failure: a consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine. Eur J Heart Fail. 2015;17:544–558. doi: 10.1002/ejhf.289. [DOI] [PubMed] [Google Scholar]
- 5.Gheorghiade M, Filippatos G, Felker M. Diagnosis and management of acute heart failure syndromes. In: Bonnow RO, Mann DL, Zipes DP, Libby P, editors. Braunwald’s heart disease. 9. Philadelphia: Elsevier; 2012. pp. 517–542. [Google Scholar]
- 6.Meyer S, Brouwers FP, Voors AA, Hillege HL, de Boer RA, Gansevoort RT, van der Harst P, Rienstra M, van Gelder IC, van Veldhuisen DJ, van Gilst WH, van der Meer P. Sex differences in new-onset heart failure. Clin Res Cardiol. 2015;104(4):342–350. doi: 10.1007/s00392-014-0788-x. [DOI] [PubMed] [Google Scholar]
- 7.Ogah OS, Davison BA, Sliwa K, Mayosi BM, Damasceno A, Sani MU, Mondo C, Dzudie A, Ojji DB, Kouam C, Suliman A, Schrueder N, Yonga G, Ba SA, Maru F, Alemayehu B, Edwards C, Cotter G. Gender differences in clinical characteristics and outcome of acute heart failure in sub-Saharan Africa: results of the THESUS-HF study. Clin Res Cardiol. 2015;104(6):481–490. doi: 10.1007/s00392-015-0810-y. [DOI] [PubMed] [Google Scholar]
- 8.Lip GY, Laroche C, Popescu MI, Rasmussen LH, Vitali-Serdoz L, Dan GA, Kalarus Z, Crijns HJ, Oliveira MM, Tavazzi L, Maggioni AP, Boriani G. Heart failure in patients with atrial fibrillation in Europe: a report from the EURObservational Research Programme Pilot survey on Atrial Fibrillation. Eur J Heart Fail. 2015;17:570–582. doi: 10.1002/ejhf.254. [DOI] [PubMed] [Google Scholar]
- 9.Efremidis M, Pappas L, Sideris A, Filippatos G. Management of atrial fibrillation in patients with heart failure. J Card Fail. 2008;14:232–237. doi: 10.1016/j.cardfail.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 10.Richards M, Di Somma S, Mueller C, Nowak R, Peacock WF, Ponikowski P, Mockel M, Hogan C, Wu AH, Clopton P, Filippatos GS, Anand I, Ng L, Daniels LB, Neath SX, Shah K, Christenson R, Hartmann O, Anker SD, Maisel A. Atrial fibrillation impairs the diagnostic performance of cardiac natriuretic peptides in dyspneic patients: results from the BACH Study (Biomarkers in ACute Heart Failure) JACC Heart Fail. 2013;1:192–199. doi: 10.1016/j.jchf.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Letsas KP, Filippatos GS, Pappas LK, Mihas CC, Markou V, Alexanian IP, Efremidis M, Sideris A, Maisel AS, Kardaras F. Determinants of plasma NT-pro-BNP levels in patients with atrial fibrillation and preserved left ventricular ejection fraction. Clin Res Cardiol. 2009;98:101–106. doi: 10.1007/s00392-008-0728-8. [DOI] [PubMed] [Google Scholar]
- 12.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 13.Cheng M, Lu X, Huang J, Zhang J, Zhang S, Gu D. The prognostic significance of atrial fibrillation in heart failure with a preserved and reduced left ventricular function: insights from a meta-analysis. Eur J Heart Fail. 2014;16:1317–1322. doi: 10.1002/ejhf.187. [DOI] [PubMed] [Google Scholar]
- 14.Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF, Dorobantu MI, Grinfeld LR, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin TM, Metra M. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013;381:29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- 15.Metra M, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF, Dorobantu MI, Grinfeld L, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Prescott MF, Edwards C, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin T, Teerlink JR, Investigators RA. Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the Relaxin in Acute Heart Failure (RELAX-AHF) development program: correlation with outcomes. J Am Coll Cardiol. 2013;61:196–206. doi: 10.1016/j.jacc.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Felker GM, Teerlink JR, Butler J, Hernandez AF, Miller AB, Cotter G, Davison BA, Filippatos G, Greenberg BH, Ponikowski P, Voors AA, Hua TA, Severin TM, Unemori E, Metra M. Effect of serelaxin on mode of death in acute heart failure: results from the RELAX-AHF study. J Am Coll Cardiol. 2014;64:1591–1598. doi: 10.1016/j.jacc.2014.05.071. [DOI] [PubMed] [Google Scholar]
- 17.Liu LC, Voors AA, Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Chen Y, Greenberg BH, Ponikowski P, Pang PS, Prescott MF, Hua TA, Severin TM, Metra M. Effects of serelaxin in acute heart failure patients with renal impairment: results from RELAX-AHF. Clin Res Cardiol. 2016;105(9):727–737. doi: 10.1007/s00392-016-0979-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmieder RE, Mitrovic V, Hengstenberg C. Renal impairment and worsening of renal function in acute heart failure: can new therapies help? The potential role of serelaxin. Clin Res Cardiol. 2015;104(8):621–631. doi: 10.1007/s00392-015-0839-y. [DOI] [PubMed] [Google Scholar]
- 19.Metra M, Ponikowski P, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Hua TA, Severin T, Unemori E, Voors AA, Teerlink JR. Effects of serelaxin in subgroups of patients with acute heart failure: results from RELAX-AHF. Eur Heart J. 2013;34:3128–3136. doi: 10.1093/eurheartj/eht371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miró Ò, Gil V, Müller C, Mebazaa A, Bueno H, Martín-Sánchez FJ, Herrero P, Jacob J, Llorens P. How does a clinical trial fit into the real world? The RELAX-AHF study population into the EAHFE registry. Clin Res Cardiol. 2015;104(10):850–860. doi: 10.1007/s00392-015-0854-z. [DOI] [PubMed] [Google Scholar]
- 21.Ponikowski P, Metra M, Teerlink JR, Unemori E, Felker GM, Voors AA, Filippatos G, Greenberg B, Teichman SL, Severin T, Mueller-Velten G, Cotter G, Davison BA. Design of the RELAXin in acute heart failure study. Am Heart J 2012;163:149–55 e1. [DOI] [PubMed]
- 22.Gialdini G, Nearing K, Bhave PD, Bonuccelli U, Iadecola C, Healey JS, Kamel H. Perioperative atrial fibrillation and the long-term risk of ischemic stroke. JAMA. 2014;312:616–622. doi: 10.1001/jama.2014.9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Healey JS, Connolly SJ, Gold MR, Israel CW, van Gelder IC, Capucci A, Lau CP, Fain E, Yang S, Bailleul C, Morillo CA, Carlson M, Themeles E, Kaufman ES, Hohnloser SH, Investigators A. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–129. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 24.Brambatti M, Connolly SJ, Gold MR, Morillo CA, Capucci A, Muto C, Lau CP, van Gelder IC, Hohnloser SH, Carlson M, Fain E, Nakamya J, Mairesse GH, Halytska M, Deng WQ, Israel CW, Healey JS, Investigators A. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation. 2014;129:2094–2099. doi: 10.1161/CIRCULATIONAHA.113.007825. [DOI] [PubMed] [Google Scholar]
- 25.Farmakis D, Parissis J, Filippatos G. Insights into onco-cardiology: atrial fibrillation in cancer. J Am Coll Cardiol. 2014;63:945–953. doi: 10.1016/j.jacc.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 26.Milia P, Caserio M, Bani D, Rastelli TF, Sonaglia F, Bigazzi B, Bigazzi M. Efficacy of relaxin on functional recovery of post stroke patients. Ital J Anat Embryol. 2013;118:92–97. [PubMed] [Google Scholar]
- 27.Wilson BC, Connell B, Saleh TM. Relaxin-induced reduction of infarct size in male rats receiving MCAO is dependent on nitric oxide synthesis and not estrogenic mechanisms. Neurosci Lett. 2006;393:160–164. doi: 10.1016/j.neulet.2005.09.059. [DOI] [PubMed] [Google Scholar]
- 28.Wilson BC, Milne P, Saleh TM. Relaxin pretreatment decreases infarct size in male rats after middle cerebral artery occlusion. Ann N Y Acad Sci. 2005;1041:223–228. doi: 10.1196/annals.1282.035. [DOI] [PubMed] [Google Scholar]
- 29.Bergeron LH, Willcox JM, Alibhai FJ, Connell BJ, Saleh TM, Wilson BC, Summerlee AJ. Relaxin peptide hormones are protective during the early stages of ischemic stroke in male rats. Endocrinology. 2015;156:638–646. doi: 10.1210/en.2014-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teichman SL, Unemori E, Teerlink JR, Cotter G, Metra M. Relaxin: review of biology and potential role in treating heart failure. Curr Heart Fail Rep. 2010;7:75–82. doi: 10.1007/s11897-010-0010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao Z, Ng CY, Liu T, Li H, Li G. Relaxin as novel strategy in the management of atrial fibrillation: potential roles and future perspectives. Int J Cardiol. 2014;171:e72–e73. doi: 10.1016/j.ijcard.2013.11.103. [DOI] [PubMed] [Google Scholar]
- 32.Parikh A, Patel D, McTiernan CF, Xiang W, Haney J, Yang L, Lin B, Kaplan AD, Bett GC, Rasmusson RL, Shroff SG, Schwartzman D, Salama G. Relaxin suppresses atrial fibrillation by reversing fibrosis and myocyte hypertrophy and increasing conduction velocity and sodium current in spontaneously hypertensive rat hearts. Circ Res. 2013;113:313–321. doi: 10.1161/CIRCRESAHA.113.301646. [DOI] [PMC free article] [PubMed] [Google Scholar]