Emerging evidence has shown that inflammation and immunity play a central role in the genesis of hypertension. Cells of the immune system, including macrophages and T cells have been observed in tissues of humans with hypertension and experimental evidence dating to the 1960s has suggested that both the elevation in blood pressure and end-organ damage are in part mediated by this immune cell infiltration. Studies in the past decade have shown that mice lacking T cells are protected against both angiotensin II and salt-sensitive hypertension, and that adoptive transfer of T cells restores blood pressure in these animals. The T cells that accumulate in kidneys and vessels release inflammatory cytokines, including interleukin 17A, tumor necrosis factor-α, and interferon gamma (IFN-γ) that promote vascular dysfunction and renal damage.1 Macrophages and dendritic cells in tissues also release cytokines and metalloproteinases. These powerful signals affect vascular function by promoting vasoconstriction, remodeling and fibrosis, and in the kidney, locally released cytokines alter renal tubular sodium reabsorption and antagonize pressure natriuresis.
There is interest in the subsets of immune cells contributing to hypertension, with evidence supporting roles of CD8+ T cells, TH17 CD4+ T cells, B cells, monocytes and monocyte derived dendritic cells. Studies in mice and humans have emphasized a role of CD8+ T cells in hypertension, as these cells seem to be major sources of IFN-γ and an oligoclonal population of these cells accumulates in the kidney of hypertensive mice. Moreover, CD8−/− mice are partially protected against angiotensin II- and DOCA-salt induced hypertension, while CD4−/− mice are not.2 There is an increased number of circulating senescent CD8+ T cells in humans with hypertension that produce IFN-γ, TNFα, granzyme B and perforin and this is accompanied by a striking increase in plasma levels of C-X-C type 3 receptor chemokines, which are tissue homing attractants for T cells.3 We have also observed an increase in IFN-γ-producing CD8+ T cells in humans with hypertension.4
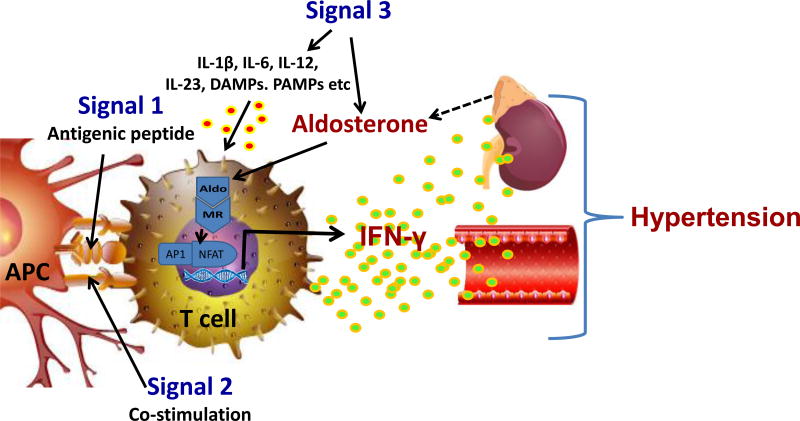
Classically, T cells require 3 signals for activation, referred to as signals 1, 2 and 3 (Figure). Signal 1 is T cell receptor recognition of specific antigenic peptides presented in the context of class I or class II major histocompatibility complexes on antigen presenting cells (APCs). Signal 2 is co-stimulation, involving interactions of ligands like CD80 and CD86 on APCs with CD28 on T cells. These two interactions occur within the immunological synapse, where they are accompanied by other modulating receptor-ligand interactions between the APC and the T cell. Signals 1 and 2 are sufficient to promote T cell proliferation, cytokine production and often mobilization from secondary lymphoid organs. Signal 3, on the other hand, involves stimulation of accessory receptors outside of the immunological synapse by hormones, cytokines and danger signals present in the inflammatory milieu. Some of these are released by the APC, while others are derived from nearby stromal cells or from the circulation. Thus cytokines like IL-12, IL-4, IL-1β, IL-23 and TGFβ skew T cells to TH1, TH2, TH17 and regulatory phenotypes by activating transcriptional pathways and epigenetic alterations that are still being elucidated. It was previously thought these commitments were irreversible, but there is ample evidence of plasticity of T cell phenotype, and that some cells can serve more than one function.
Figure. Aldosterone serves as a novel signal 3 for T cell activation.
Antigen-presenting cell (APC), including dendritic cells, B cells, macrophages and others present antigenic peptides to the T cell receptor in the context of major histocompatibility complexes (Signal 1). This is accompanied by co-stimulatory signals, including interactions between B7 ligands on the APC and CD28 on T cells (Signal 2). T cells also possess numerous receptors for cytokines, Danger Associated Molecular Patterns (DAMPs) and Pattern Associated Molecular Patterns (PAMPs) that influence the T cell phenotype (Signal 3). Sun et al show that aldosterone acting through the mineralocorticoid receptor (MR) acts as a previously unrecognized signal 3 by interacting with nuclear factor of activated T-cells 1 (NFAT1) and activator protein-1 (AP-1). This promotes production of interferon gamma (IFN-γ) and ultimately vascular dysfunction and kidney damage leading to hypertension.
In this issue of Circulation Research Sun et al provide us with a new signal 3 that has relevance to immune activation in hypertension.5 The authors provide evidence that the Mineralocorticoid Receptor (MR) in T cells, and particularly in CD8+ T cells, modulates their production of interferon gamma (IFN-γ) and ultimately promotes hypertension. To accomplish this, the authors used Cre-Lox technology to eliminate the MR in all T cells, while leaving it intact in all other cells. Surprisingly, the resultant mice (TMRKO mice) demonstrated blunted hypertension, attenuated fibrosis in the aorta and kidneys, reduced albuminuria and preserved endothelium-dependent vasodilatation upon chronic infusion of ang II. The authors showed by flow cytometry that both CD4+ IFN-γ+ T and CD8+ IFN-γ+ T cells are reduced in kidneys and aorta of hypertensive TMRKO mice. In further experiments they found that MR mediates production of IFN-γ by CD8 T cells. MR deletion reduced CD8+ IFN-γ+ CD8+ T cells, while overexpression of MR using a lentivirus markedly increased production of IFN-γ by CD8+ T cells compared to cells infected by a control lentivirus. Moreover, Sun et al used chromatin immunoprecipitation (ChIP) assays to identify NFAT1 and AP-1 binding to specific regions of the IFN-γ gene promoter and confirmed interactions between MR and NFAT1 and between the MR and the AP-1 subunits c-Fos and c-Jun. In addition, mice engineered to overexpress MR in T cells had exacerbated hypertensive responses to Ang II. Treatment with anti-IFN-γ neutralizing antibodies during ang II infusion prevented hypertension in WT and TMRKO mice confirming that T cell MR overexpression exacerbates Ang II-induced hypertension through IFN-γ.
Classical teaching indicates that the major action of aldosterone and the MR is to promote renal sodium reabsorption, largely by increasing abundance of the epithelial sodium channel on the apical membrane of epithelial cells in the distal nephron. While this is likely its major role, there is increasing evidence that aldosterone can act on the MR in other cells to promote hypertension. Of note, McCurley et al showed that specific deletion of MR in the vascular smooth muscle blunted age-related elevations in blood pressure, vascular myogenic tone and fibrosis.6 The endothelial MR receptor contributes to the vascular stiffening and dysfunction that accompanies obesity.7 Related to the present study of Sun et al, there is also evidence that MR receptors on macrophages and dendritic cells affect their phenotype and cytokine production.8
This current study adds to our understanding of the role of IFN-γ in hypertension. While some studies have shown a protective or neutral effect of this cytokine,9 others have supported its role in hypertension. As an example, we have shown that IFN-γ−/− mice exhibit a blunted rise in blood pressure in response to ang II infusion and that that the anti-diuretic and anti-natriuretic response of ang II is virtually absent in these animals.10 This effect in part seems to be related to changes in the sodium-hydrogen exchanger 3 in the proximal nephron, which modulates pressure natriuresis.10 Sun et al observed an increase in T cells in the kidney in ang II-induced hypertension. In keeping with this, we have observed accumulation of memory T cells in the kidney and vasculature in hypertensive mice that the effector memory subset of T cells are a major source of IFN-γ in the kidney of hypertensive mice.11
An interesting finding made by Sun et al is that immunoclearing of IFN-γ more effectively lowered blood pressure than did knockout of the MR in T cells. This is almost certainly because there are factors in addition to the MR, such as the transcription factor Tbet and JAK/STAT signaling that regulate IFN-γ production. Nevertheless, it is therapeutically possible to block the MR with currently available, commonly used drugs. In this regard, MR antagonists have emerged as highly effective and likely indispensable agents to treat resistant hypertension. A recent randomized, double blind clinical trial showed that spironolactone is superior to either alpha-1 or beta-adrenergic receptor blockade as an add-on drug in the treatment of such patients, supporting a predominant role of MR activation in the pathophysiology of resistant hypertension.12 It is also possible that the mechanism proposed by Sun et al has relevance to heart failure, where T cells have been identified to play a pathogenic role,13 and MR receptor blockade clearly improves outcome. Thus the findings by Sun et al suggest a previously unknown anti-inflammatory role of MR blocking agents that might explain some of their striking therapeutic benefits beyond the simple diuretic effects of these drugs.
Acknowledgments
Supported by the American Heart Association grants POST290900 and SFRN204200, and National Institutes of Health grants K01HL130497, R01HL125865, R01HL039006 and P01HL129941
Literature Cited
- 1.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trott DW, Thabet SR, Kirabo A, Saleh MA, Itani H, Norlander AE, Wu J, Goldstein A, Arendshorst WJ, Madhur MS, Chen W, Li CI, Shyr Y, Harrison DG. Oligoclonal CD8+ T Cells Play a Critical Role in the Development of Hypertension. Hypertension. 2014;64:1108–1115. doi: 10.1161/HYPERTENSIONAHA.114.04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Youn JC, Yu HT, Lim BJ, Koh MJ, Lee J, Chang DY, Choi YS, Lee SH, Kang SM, Jang Y, Yoo OJ, Shin EC, Park S. Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension. 2013;62:126–133. doi: 10.1161/HYPERTENSIONAHA.113.00689. [DOI] [PubMed] [Google Scholar]
- 4.Itani HA, McMaster WG, Jr, Saleh MA, Nazarewicz RR, Mikolajczyk TP, Kaszuba AM, Konior A, Prejbisz A, Januszewicz A, Norlander AE, Chen W, Bonami RH, Marshall AF, Poffenberger G, Weyand CM, Madhur MS, Moore DJ, Harrison DG, Guzik TJ. Activation of Human T Cells in Hypertension: Studies of Humanized Mice and Hypertensive Humans. Hypertension. 2016;68:123–132. doi: 10.1161/HYPERTENSIONAHA.116.07237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun X-N, Li C, Liu Y, Du L-J, Zeng M-R, Zheng X-J, Zhang W-C, Liu Y, Zhu M, Kong D, Zhou L, Lu L, Shen Z-X, Yi Y, Du L, Qin M, Liu X, Hua Z, Sun S, Yin H, Zhou B, Yu Y, Zhang Z, Duan S-Z. T Cell Mineralocorticoid Receptor Controls Blood Pressure by Regulating Interferon Gamma. Circulation Research. 2017 doi: 10.1161/CIRCRESAHA.116.310480. XX:XXX-XXX. [DOI] [PubMed] [Google Scholar]
- 6.McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, Chambon P, Hill MA, Dorrance AM, Mendelsohn ME, Jaffe IZ. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med. 2012;18:1429–1433. doi: 10.1038/nm.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia G, Habibi J, Aroor AR, Martinez-Lemus LA, DeMarco VG, Ramirez-Perez FI, Sun Z, Hayden MR, Meininger GA, Mueller KB, Jaffe IZ, Sowers JR. Endothelial Mineralocorticoid Receptor Mediates Diet-Induced Aortic Stiffness in Females. Circ Res. 2016;118:935–943. doi: 10.1161/CIRCRESAHA.115.308269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bene NC, Alcaide P, Wortis HH, Jaffe IZ. Mineralocorticoid receptors in immune cells: emerging role in cardiovascular disease. Steroids. 2014;91:38–45. doi: 10.1016/j.steroids.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res. 2015;116:1022–1033. doi: 10.1161/CIRCRESAHA.116.303697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamat NV, Thabet SR, Xiao L, Saleh MA, Kirabo A, Madhur MS, Delpire E, Harrison DG, McDonough AA. Renal transporter activation during angiotensin-II hypertension is blunted in interferon-gamma-/- and interleukin-17A-/- mice. Hypertension. 2015;65:569–576. doi: 10.1161/HYPERTENSIONAHA.114.04975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Itani HA, Xiao L, Saleh MA, Wu J, Pilkinton MA, Dale BL, Barbaro NR, Foss JD, Kirabo A, Montaniel KR, Norlander AE, Chen W, Sato R, Navar LG, Mallal SA, Madhur MS, Bernstein KE, Harrison DG. CD70 Exacerbates Blood Pressure Elevation and Renal Damage in Response to Repeated Hypertensive Stimuli. Circ Res. 2016;118:1233–1243. doi: 10.1161/CIRCRESAHA.115.308111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams B, MacDonald TM, Morant S, Webb DJ, Sever P, McInnes G, Ford I, Cruickshank JK, Caulfield MJ, Salsbury J, Mackenzie I, Padmanabhan S, Brown MJ. British Hypertension Society's PSG. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386:2059–2068. doi: 10.1016/S0140-6736(15)00257-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laroumanie F, Douin-Echinard V, Pozzo J, Lairez O, Tortosa F, Vinel C, Delage C, Calise D, Dutaur M, Parini A, Pizzinat N. CD4+ T cells promote the transition from hypertrophy to heart failure during chronic pressure overload. Circulation. 2014;129:2111–2124. doi: 10.1161/CIRCULATIONAHA.113.007101. [DOI] [PubMed] [Google Scholar]